Abstract
The parafacial respiratory group (pFRG) is thought to be involved in respiratory rhythm generation in neonates. This subgroup expresses the transcription factor, Phox2b, and contains intrinsically CO2 sensitive neurons. Calcium imaging has been widely used for analysis of neuronal activity at the cellular and network level. In the present study, we applied calcium imaging to analyze neuronal activity of the most-rostral pFRG in an in vitro brainstem–spinal cord preparation from neonatal rats. We detected strong pre-inspiratory neuron activity in the most rostral pFRG, suggesting that significant numbers of pre-inspiratory neurons are localized in the ventrolateral medulla near the rostral end of the medulla. We show that usage of calcium imaging would be very useful for analysis of neuronal activity over different time scales, and discuss the advantages and disadvantages of this method.
Electronic supplementary material
The online version of this article (doi:10.1007/s12576-011-0179-2) contains supplementary material, which is available to authorized users.
Keywords: Parafacial, Respiratory, Calcium imaging, Medulla, Phox2b, Newborn rat, In vitro
Introduction
Optical recordings have been widely used for monitoring neuronal activity. Respiratory neuron activity has been analyzed by voltage and calcium imaging. Optical recording with voltage sensitive dye is very useful for macroscopic analysis of respiratory neuron network activity in brain slices or block preparations [1–4]. In contrast, calcium imaging is more advantageous for detection of neuronal activity at the single cell level [5–7]. Voltage imaging has been applied to analysis of the spatio-temporal pattern of respiratory neuron network activity in brainstem–spinal cord preparations in vitro, and the parafacial respiratory group (pFRG) has been found in the rostral ventral medulla [1] using this technique. Application of calcium imaging to such block preparations would be helpful for further optical analysis of the pFRG at the cellular level.
In the present study, we applied calcium imaging to analyze respiratory neuron activity in brainstem–spinal cord preparations from newborn rats. In particular, we targeted the most rostral pFRG, because previous electrophysiological analysis suggested that there were many pre-inspiratory (Pre-I) neurons in the rostral pFRG that could be easily approached via the rostral cut surface after a transverse section at the level of the anterior inferior cerebellar artery (AICA) [8]. We discuss the advantages and disadvantages of the application of calcium imaging for analysis of rostral pFRG activity.
Methods
Experiments were approved by the Animal Ethics Committee of Showa University in accordance with the Japanese Government Law No. 105 for the care and use of laboratory animals. The 0- to 2-day-old Wistar rats were placed under deep ether anesthesia until disappearance of the nociceptive reflex (induced by tail pinch) before dissection. Then brainstem–spinal cord preparations were isolated in modified Krebs solution [9] composed of (in mM) 124 NaCl, 5.0 KCl, 1.2 KH2PO4, 2.4 CaCl2, 1.3 MgCl2, 26 NaHCO3, 30 glucose, equilibrated with 95% O2 and 5% CO2; pH 7.4. The preparations were cut transversely at a level just rostral to the AICA with a custom-made vibratome (Fig. 1). This cut was equivalent to a position approximately 0.5 mm rostral to the caudal end of the facial nucleus that corresponded to a level of the Xth cranial nerve root [7, 10]. During the experiments, each preparation was placed in a 2-ml recording chamber and was superperfused continuously at the flow rate of 2.5–3 ml/min with the modified Krebs solution at a temperature of 25–26°C. Inspiratory activity correlating with phrenic nerve activity was monitored from the fourth cervical ventral root (C4) [9].
Fig. 1.
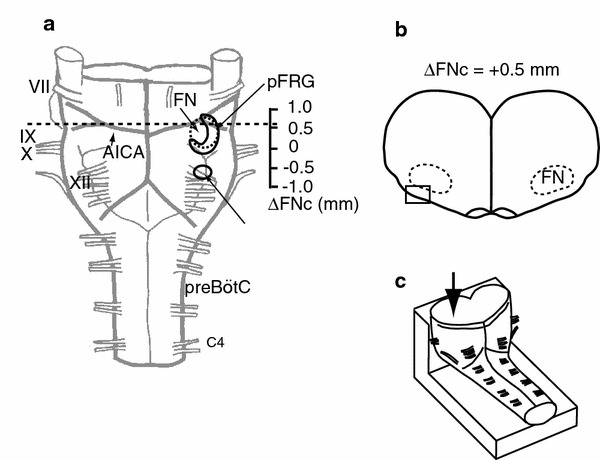
The level of the transverse section used for optical recordings. a Ventral view of a brainstem–spinal cord preparation from a neonatal rat. The preparation was cut at the level of the dotted line. b Rostral cut surface view of a preparation at a level of 0.5 mm rostral to the caudal end of the facial nucleus (ΔFNc). The square denotes the approximate recording area. c Schematic representation of the preparation in the recording chamber. The preparation was fixed onto a rubber block by pins with cut surface facing up for observation from the cut surface of the cross-section (arrow). AICA anterior inferior cerebellar artery, FN facial nucleus, pFRG parafacial respiratory group, preBötC preBötzinger complex, VII–XII cranial nerves, C4 the fourth cervical ventral root
Neuronal activity in the preparation was detected as changes in the fluorescence of the calcium-sensitive dye by means of an optical recording apparatus (MiCAM02; Brain Vision, Tsukuba, Japan). Fluctuation of intracellular Ca2+ concentration corresponds to single-cell activity, and this method has been successfully used in rhythmic in vitro slice preparations [5, 6, 11, 12]. Recently, Ca2+ imaging has been applied to analyze respiratory (motor-) neuron activity in en bloc brainstem–spinal cord preparations from newborn mice [13]. Since activity-dependent changes of intracellular Ca2+ concentration trigger (up to) a 10-fold increase in fluorescence emissions [14], Ca2+ imaging did not require averaging. A major problem has been the loading of Ca2+-sensitive dyes by passive diffusion into the neurons because of the slow diffusion rate. In the present study, we use Fluo-8 (Quest Fluo-8™ AM; AAT Bioquest, Sunnyvale, CA, USA) as a Ca2+-sensitive dye, because this dye is suggested to be more readily loaded into cells and brighter than Fluo-3 or Fluo-4 (AAT Bioquest Product Technical Information Sheet). We examined two methods of dye staining: (1) injection method; the preparation was injected with the calcium sensitive dye, 5–10 μM Fluo-8, using glass micropipettes (50–100 μm tip diameter) that were inserted into sites of about 50 μm depth from the rostral cut surface. Approximately 0.5-μl injection volume per site was applied at 4–5 overlapping sites of the exposed ventral superficial region corresponding to the rostral pFRG; and (2) incubation method; the preparation was incubated in the modified Krebs solution including 2–5 μM Fluo-8 for 60 min. After the injection or incubation, the preparation was superperfused with the modified Krebs solution for at least 30 min before measurement. The fluorescence change, in correlation with the change in intracellular calcium concentration, was measured with two different recording modes of a BV-Analyzer (Brain Vision): (1) conventional continuous recording mode (sampling rate of 20–100 ms, 512 or 1024 frames); and (2) short exposure (SE) mode for long-term recordings (sampling rate: every 1 s for 512 frames with 100 ms exposure time in each frame, total recording period of 8.5 min). In some cases, the fluorescence change was averaged over 5–10 trials by use of C4 inspiratory activity as the trigger signal. The optical path contained a light-emitting diode (LED5W-B; Brain Vision) as the light source, a 460–495 nm excitation filter, a dichroic mirror, and a 510-nm emission filter (U-MWIB3 mirror unit; Olympus Optical, Tokyo, Japan). The fluorescence changes were expressed as a ratio (percentage, fractional changes) (i.e., the fluorescence intensity against that of the reference image). The differential image, processed with a software-spatial filter for 3 × 3 pixels and with bleaching compensation, was represented by a pseudocolor display in which red corresponded to a fluorescence increase. Values are presented as mean ± SD.
After optical recording, preparations were fixed for >48 h at 4°C in Lillie solution (10% formalin in phosphate buffer). Transverse 50-μm slices were then cut with a custom-made vibratome and stained with 1% neutral red (Wako Pure Chemical Industries, Tokyo, Japan) or with NeuroTrace (435/455 blue fluorescence; Molecular Probes/Invitrogen, Carlsbad, CA) for Nissl staining.
Results
Continuous recordings
It was expected that calcium imaging could identify fluctuations of intracellular Ca2+ concentrations in Pre-I neurons without signal averaging. The calcium signals recorded from the rostral cut surface of the pFRGs in dye-injected preparations (Fig. 2a, b; Supplementary Video 1) closely corresponded to the membrane potential trajectory of single Pre-I neurons. In the membrane potential recordings, more than 70% of Pre-I neurons in this region showed only weak inspiratory inhibition (Fig. 2d). The group data from 14 experiments were: C4 burst rate 5.9 ± 1.5/min, pre-inspiratory phase duration 897 ± 590 ms, inspiratory phase 917 ± 101 ms, and the post-inspiratory phase duration 3593 ± 825 ms. This post-inspiratory phase duration was comparable to that of previous electrophysiological measurements [10]. In the present measurements, we could not clearly focus on individual stained cells because the dye injection method used here was effective in labeling cells located in regions slightly deeper than the cut surface (approximately 50 μm depth), but not for cells in the rostral cut surface plane due to insertion of dye-containing glass micropipettes into the tissue. Therefore, it is possible that calcium signals might originate from multiple cells.
Fig. 2.
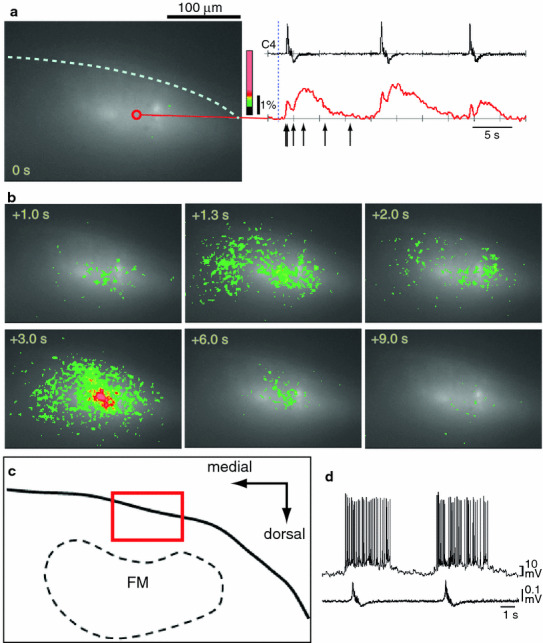
Calcium imaging of respiratory neuron activity in the ventral medulla of the rostral cut surface following the dye-injection method. Results are from a single sweep continuous recording with a 100 ms sampling rate and 512 frames (total recording period of 51.2 s). a An optical image of the rostral cut surface at the time point represented by the dotted vertical line (i.e. time 0 s) in the right column where C4 activity and fluorescence change at one position (red open circle) is indicated. b Optical images of respiratory neuron activity. The images are arranged in a time course from left to right and top to bottom, as indicated by the numeric values. Each image corresponds to arrows in (a). c Illustration of the cut surface of the rostral medulla at lower magnification. The red square denotes the area of the optical recording. FN Facial nucleus. d A typical example of a parafacial pre-inspiratory neuron recorded in a similar area of another preparation
Short exposure mode recordings
This mode of optical recording is suitable for long-term monitoring of the fluorescence change by using a low sampling rate. We tested this mode for responses to high K+ stimulation and high CO2 stimulation in the presence of tetrodotoxin (TTX). Figure 3a shows continuous control recordings in the rostral pFRG that indicated a Pre-I neuron burst. Fluorescence change in response to high K+ stimulation (+10 mM K+) is shown in Fig. 3c. An increase in the fluorescence probably corresponding to an increase of intracellular Ca2+ concentration was observed in the Fluo-8 stained region following application of high K+ solution. The rostral pFRG is thought to contain CO2-sensitive neurons and overlaps at least partly with the retrotrapezoid nucleus (RTN), which is considered to play a role in central chemoreception [15–17]. Therefore, we tested the change in fluorescence in this region following hypercapnic stimulation in the presence of TTX. When the perfusate was changed from 2 to 8% CO2 solution, we observed an initial transient increase (2.8 ± 1.5%, n = 4; Fig. 3d, e) and a subsequent large decrease (9.3 ± 2.1%; Fig. 3d) in fluorescence intensity.
Fig. 3.
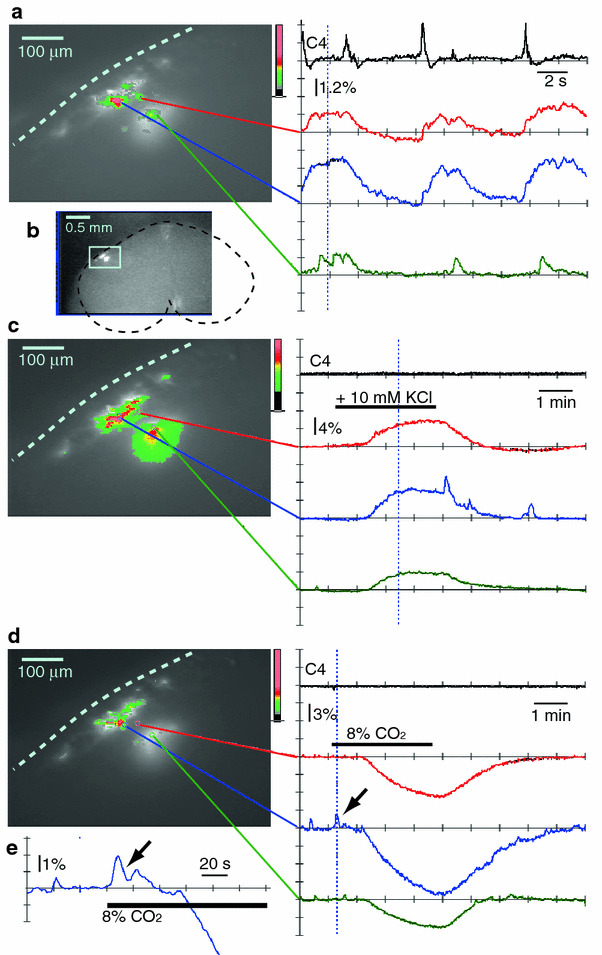
Long-term monitoring of respiratory neuron activity in the ventral medulla of the rostral cut surface with calcium imaging following the dye-injection method. Optical images of the rostral cut surface in the left column (a, c, d) show the time points represented by the dotted vertical lines in the right column. a Results from a single sweep continuous recording with a 40 ms sampling rate and 512 frames (total recording period of 20.5 s). b An optical image of the cut surface of the rostral medulla at lower magnification. The light blue square denotes the area of the optical recording. c Long-term monitoring of the fluorescence change in response to high K+ stimulation (+10 mM) in the presence of 0.5 μM TTX. Results are from a single sweep recording with the short exposure (SE) mode; 100 ms sampling at every second for 8.5 min (512 frames). Note the large increase in the fluorescence intensity during exposure to the high K+ solution. d Long-term monitoring of the fluorescence change in response to high CO2 stimulation (2→8%) in the presence of 0.5 μM TTX. Note that high CO2 stimulation initially induced a transient increase in the fluorescence intensity (arrow, e) followed by a large decrease
Visualization of individual cells
To visualize individual cells in the cut surface, we stained the cells by incubation of the preparation in the dye-containing solution. A typical example of the rostral cut surface observation is shown in Fig. 4. We found several active spots that generated spontaneous calcium oscillation indicating a Pre-I burst pattern in the rostral pFRG. Each spot may correspond to single neurons or multiple neurons that are located in close proximity. Although the oscillation correlated with the C4 burst, the burst activity of some cells did not appear in some respiratory cycles (Fig. 4a, orange trace), probably due to partial disruption of neuronal connections in the cut surface. Five to ten times averaging revealed cells with a clear Pre-I neuron burst pattern (Fig. 4c). The group data from 6 cells in three preparations were: pre-inspiratory phase duration 680 ± 570 ms, inspiratory phase 937 ± 275 ms, post-inspiratory phase duration 4,490 ± 2,942 ms and the C4 burst rate 4.4 ± 1.5/min. The burst rate and burst duration were comparable to the population activity of the dye-injected preparation.
Fig. 4.
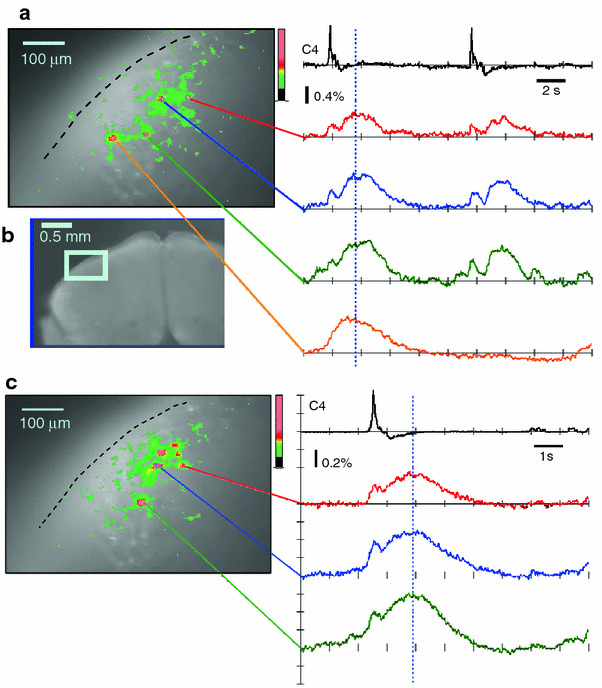
Calcium imaging of respiratory neuron activity in the ventral medulla of the rostral cut surface after cell staining by the incubation method. Optical images of the rostral cut surface in the left column (a, c) show the time points represented by the dotted vertical lines in the right column. a Results from a single sweep continuous recording with a 40 ms sampling rate and 512 frames (total recording period of 20.5 s). b An optical image of the cut surface of the rostral medulla at lower magnification. The light blue square denotes the area of the optical recording. c Results from 10 times averaging where the C4 burst was used to trigger the acquired images (20 ms sampling rate, 512 frames)
Discussion
We tested two different dye-staining methods (injection and incubation with Ca2+ sensitive dyes) for calcium imaging, and detected clear Pre-I neuron activity in the most rostral pFRG. In the dye-injection method, the respiratory-related activity could be clearly detected if the injection points were correct, although it was difficult to clearly identify individual stained cells. In the dye-incubation method, the number of neurons that indicated clear respiratory activity was less than expected. The Ca2+ imaging has been mainly used in in vitro slice preparations [5, 6, 11, 12], while voltage-sensitive dye imaging has been previously used for en bloc brainstem–spinal cord or in situ perfused brainstem preparations [1, 18]. The present study on brainstem–spinal cord preparations from neonatal rats showed that the use of targeted microinjection of the Ca2+-sensitive dyes into areas of interest enabled the detection of multiple neuron activity. Imaging of Ca2+ using a ventral approach to the ponto-medullary respiratory column in in situ perfused brainstem preparations [18, 19] or in in vivo preparations are future approaches for analyzing the specific temporal dynamics of rhythmogenic neural networks.
The present optical recording system was also useful for long-term monitoring of the change in fluorescence intensity during the short exposure mode as well as during short-term continuous recording with a fast sampling rate. Long-term recording could be used for monitoring of neuronal responses to various drugs that are bath-applied. In our previous studies, we have shown that pFRG Pre-I neurons in the ventral superficial region of the neonatal rat medulla express Phox2b and are CO2 sensitive in the presence of TTX [15]. Thus, it was interesting to investigate the neuronal response of the rostral pFRG during hypercapnic stimulation. The present results showed that an increase in the fluorescence intensity was detected only during the initial period when the CO2 concentration was increased, and this response was followed by a large decrease in the fluorescence intensity. The mechanism of this late response is not clear. It may be attributable to pH sensitivity of the calcium sensitive dye used (Fluo-8) but not due to a decrease in the intracellular calcium concentration (AAT Bioquest Product Technical Information Sheet). Although physiological range pH has a relatively small effect on the fluorescence signals of Fluo-3 or Fura red [14, 20], Fluo-8 should be used carefully when examining neuronal responses under stimulation in association with pH change.
The monitoring of single cell activity may be possible after dye staining by the incubation method. One issue with this method is that it is currently difficult to determine the precise location of recorded cells after histological examination. Since it is hypothesized that the rostral pFRG Pre-I neurons express Phox2b [15, 16], this problem could be resolved by introducing a fluorescent reporter gene into Phox2b regulatory regions [21, 22]. The distribution of Phox2b-expressing cells in the parafacial region was consistent with the idea that optical signaling detected by imaging the Ca2+-sensitive dye in the pFRG originates from ventral superficial Phox2b-ir Pre-I neurons. Indeed, the embryonic parafacial rhythm generator that was suggested as a forerunner of neonatal pFRG has been shown to express Phox2b [23]. However, future studies are required to clarify what proportion of Phox2b-expressing cells are indeed Pre-I neurons in the neonatal rat.
In conclusion, our findings indicate that a certain number of Pre-I neurons exist in the most rostral parafacial region of the neonatal rat ventral medulla, and these observations are consistent with previous electrophysiological characterizations of Phox2b-ir neurons [15, 16]. The physiological significance of the rostral cluster of Phox2b-ir Pre-I neurons is not presently clear. It is possible that they play a role as a central chemoreceptor and may also contribute to respiratory rhythm generation during the neonatal period. Calcium imaging of the rostral pFRG is a useful method for analysis of neuronal activity in the brainstem–spinal cord preparation of newborn rats. Furthermore, this recording system could cover analysis of neuronal activity over different time scales (milliseconds to minutes).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Video 1. Calcium imaging of respiratory neuron activity in the ventral medulla of the rostral cut surface following the dye-injection method, corresponding to Fig. 2 (MOV 998 kb)
Video 2. Calcium imaging of respiratory neuron activity in the ventral medulla of the rostral cut surface following dye staining by the incubation method, corresponding to Fig. 4a (MOV 794 kb)
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (KAKENHI: 19500277, 22500296) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kashiwagi M, Osaka Y, Onimaru H, Takeda J. Optical imaging of propofol-induced central respiratory depression in medulla-spinal cord preparations from newborn rats. Clin Exp Pharmacol Physiol. 2011;38:186–191. doi: 10.1111/j.1440-1681.2011.05480.x. [DOI] [PubMed] [Google Scholar]
- 3.Fujii M, Umezawa K, Arata A. Dopamine desynchronizes the pace-making neuronal activity of rat respiratory rhythm generation. Eur J Neurosci. 2006;23:1015–1027. doi: 10.1111/j.1460-9568.2006.04622.x. [DOI] [PubMed] [Google Scholar]
- 4.Okada Y, Masumiya H, Tamura Y, Oku Y. Respiratory and metabolic acidosis differentially affect the respiratory neuronal network in the ventral medulla of neonatal rats. Eur J Neurosci. 2007;26:2834–2843. doi: 10.1111/j.1460-9568.2007.05891.x. [DOI] [PubMed] [Google Scholar]
- 5.Koshiya N, Smith JC. Neuronal pacemaker for breathing visualized in vitro. Nature. 1999;400:360–363. doi: 10.1038/22540. [DOI] [PubMed] [Google Scholar]
- 6.Funke F, Dutschmann M, Muller M. Imaging of respiratory-related population activity with single-cell resolution. Am J Physiol Cell Physiol. 2007;292:C508–C516. doi: 10.1152/ajpcell.00253.2006. [DOI] [PubMed] [Google Scholar]
- 7.Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Onimaru H, Ikeda K, Kawakami K. Phox2b expressing neurons in the most rostral medulla of newborn rats. Adv Exp Med Biol. 2010;669:87–90. doi: 10.1007/978-1-4419-5692-7_18. [DOI] [PubMed] [Google Scholar]
- 9.Suzue T. Respiratory rhythm generation in the in vitro brain stem-spinal cord preparation of the neonatal rat. J Physiol. 1984;354:173–183. doi: 10.1113/jphysiol.1984.sp015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballanyi K, Ruangkittisakul A, Onimaru H. Opioids prolong and anoxia shortens delay between onset of preinspiratory (pFRG) and inspiratory (preBötC) network bursting in newborn rat brainstems. Pflugers Arch. 2009;458:571–587. doi: 10.1007/s00424-009-0645-3. [DOI] [PubMed] [Google Scholar]
- 11.Funke F, Müller M, Dutschmann M. Reconfiguration of respiratory-related population activity in a rostrally tilted transversal slice preparation following blockade of inhibitory neurotransmission in neonatal rats. Pflugers Arch. 2008;457:185–195. doi: 10.1007/s00424-008-0509-2. [DOI] [PubMed] [Google Scholar]
- 12.Ruangkittisakul A, Schwarzacher SW, Secchia L, Ma Y, Bobocea N, Poon BY, Funk GD, Ballanyi K. Generation of eupnea and sighs by a spatiochemically organized inspiratory network. J Neurosci. 2008;28:2447–2458. doi: 10.1523/JNEUROSCI.1926-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson K, Rekling JC. Population calcium imaging of spontaneous respiratory and novel motor activity in the facial nucleus and ventral brainstem in newborn mice. J Physiol. 2011;589:2543–2558. doi: 10.1113/jphysiol.2011.207225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minta A, Kao JP, Tsien RY. Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 15.Onimaru H, Ikeda K, Kawakami K. CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci. 2008;28:12845–12850. doi: 10.1523/JNEUROSCI.3625-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onimaru H, Ikeda K, Kawakami K. Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol. 2009;168:13–18. doi: 10.1016/j.resp.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Guyenet PG, Bayliss DA, Stornetta RL, Fortuna MG, Abbott SB, DePuy SD. Retrotrapezoid nucleus, respiratory chemosensitivity and breathing automaticity. Respir Physiol Neurobiol. 2009;168:59–68. doi: 10.1016/j.resp.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Potts JT, Paton JF. Optical imaging of medullary ventral respiratory network during eupnea and gasping in situ. Eur J Neurosci. 2006;23:3025–3033. doi: 10.1111/j.1460-9568.2006.04809.x. [DOI] [PubMed] [Google Scholar]
- 19.Dutschmann M, Paton JF. Whole cell recordings from respiratory neurones in an arterially perfused in situ neonatal rat preparation. Exp Physiol. 2003;88:725–732. doi: 10.1113/eph8802639. [DOI] [PubMed] [Google Scholar]
- 20.Kurebayashi N, Harkins AB, Baylor SM. Use of fura red as an intracellular calcium indicator in frog skeletal muscle fibers. Biophys J. 1993;64:1934–1960. doi: 10.1016/S0006-3495(93)81564-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corpening JC, Cantrell VA, Deal KK, Southard-Smith EM. A Histone2BCerulean BAC transgene identifies differential expression of Phox2b in migrating enteric neural crest derivatives and enteric glia. Dev Dyn. 2008;237:1119–1132. doi: 10.1002/dvdy.21498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazarenko RM, Milner TA, Depuy SD, Stornetta RL, West GH, Kievits JA, Bayliss DA, Guyenet PG. Acid sensitivity and ultrastructure of the retrotrapezoid nucleus in Phox2b-EGFP transgenic mice. J Comp Neurol. 2009;517:69–86. doi: 10.1002/cne.22136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thoby-Brisson M, Karlén M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the preBötzinger complex. Nat Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. Calcium imaging of respiratory neuron activity in the ventral medulla of the rostral cut surface following the dye-injection method, corresponding to Fig. 2 (MOV 998 kb)
Video 2. Calcium imaging of respiratory neuron activity in the ventral medulla of the rostral cut surface following dye staining by the incubation method, corresponding to Fig. 4a (MOV 794 kb)


