Abstract
Little is known about the effects of glucagon-like peptide 1 (GLP-1) on the pancreatic exocrine gland. In the gland, secretagogues induce amylase release. That signal transduction is evoked mainly by an increase in intracellular Ca2+ levels and activation of protein kinase C (PKC). We previously demonstrated that myristoylated alanine-rich C kinase substrate (MARCKS), a PKC substrate, is involved in pancreatic amylase release. Here, we studied the effects of GLP-1 on MARCKS phosphorylation and amylase release in rat pancreatic acini. GLP-1 induced amylase release and MARCKS phosphorylation in isolated pancreatic acini. Inhibitors of cAMP-dependent protein kinase (PKA) suppressed those effects. Furthermore, a MARCKS-related peptide inhibited the GLP-1-induced amylase release. These findings suggest that GLP-1 induces amylase release through MARCKS phosphorylation via activation of PKA in isolated pancreatic acini.
Keywords: GLP-1, MARCKS, Amylase, PKA, Exocytosis
Introduction
An incretin hormone, glucagon-like peptide-1 (GLP-1), is secreted from intestinal L cells [1] and enhances glucose-dependent insulin secretion by activating GLP-1 receptor (GLP-1R) in pancreatic islet β cells [2]. GLP-1 binds to GLP-1R, leading to G protein-mediated elevation of intracellular cAMP levels and activation of cAMP-dependent protein kinase (PKA) [2]. This GLP-1R signal transduction has been shown to be involved in other nonglycemic actions, such as regulation of inflammation [3], lipid metabolism [4], and blood pressure [5]. However, several roles of GLP-1/GLP-1R have been unclear in the pancreas as an exocrine gland. In guinea pig pancreatic acinar cells, GLP-1 binds to a specific receptor, GLP-1R [6]. GLP-1R has been detected in human [7], mouse, and rat [8] pancreatic acinar cells. Taken together, GLP-1 may induce certain cell functions in the pancreatic exocrine gland.
In pancreatic acinar cells, a secretagogue, such as cholecystokinin (CCK), binds to receptors, thereby increasing intracellular Ca2+ levels and activating protein kinase C (PKC). This signal transduction is involved in amylase release in pancreatic acini [9]. We previously reported that myristoylated alanine-rich C kinase substrate (MARCKS), a major cellular substrate of PKC [10], is involved in CCK-induced amylase release in acini isolated from rat pancreas [11]. We had also demonstrated involvement of MARCKS in cAMP-dependent amylase release in rat parotid acinar cells [12]. Interestingly, involvement of MARCKS in amylase release is a shared feature between Ca2+- and cAMP-dependent pathways in pancreatic and parotid acinar cells, respectively.
Phosphorylated MARCKS (p-MARCKS) is translocated from the membrane to the cytosol [13]. This translocation has been implicated in membrane trafficking, such as exocytosis. The NH2-terminal sequence peptide of MARCKS (MANS) has been shown to be a useful inhibitor of MARCKS function [14]. This peptide inhibited amylase release in rat pancreatic acini [11] and parotid acinar cells [12]. It also inhibited mucin secretion in a mouse model of asthma [15] and a human bronchial epithelial cell line (NHBE cells) [14]. Furthermore, MARCKS phosphorylation was involved in secretion of oxytocin in bovine luteal cells [16] and norepinephrine in bovine chromaffin cells [17]. We previously proposed that lipid rafts, which release MARCKS, contribute to exocytotic amylase release in pancreatic acini [11]. Taken together, these findings suggest that MARCKS and MARCKS phosphorylation regulate secretory function in the pancreatic exocrine gland.
We previously demonstrated that GLP-1 induced release of insulin, but not amylase, in rat pancreas preparations using the organ bath technique [18]. This experimental design is a sensitive and reproducible method for assessing the pharmacological properties of hypoglycemic agents. However, it is unfit for observing signal transduction in acini. The pancreas has two types of tissue, i.e., endocrine islets and exocrine glands. Interaction between the two tissues makes it difficult to investigate the effects of GLP-1 using the organ bath technique. In the present study, we used isolated acini from rat pancreas and were able to demonstrate the effects of GLP-1 on MARCKS phosphorylation and amylase release.
Materials and methods
Reagents
Bovine serum albumin (BSA) and collagenase A were purchased from Roche (Basel, Switzerland). RIPA buffer, a protease inhibitor cocktail, and a phosphatase inhibitor cocktail were purchased from ATTO (Tokyo, Japan). Rabbit anti-MARCKS and rabbit anti-p-MARCKS antibodies were purchased from Millipore (Temecula, CA, USA). Anti-rabbit IgG horseradish peroxidase-linked antibody was purchased from Beckman Coulter (Fullerton, CA, USA). H89 and 8-bromo-cAMP were purchased from Cayman Chemical (Ann Arbor, MI, USA). GLP-1 (amide fragment 7–36, human) was purchased from Sigma Aldrich (St. Louis, MO, USA). Cyclic AMPS-Rp was purchased from Tocris (Bristol, UK). Trypsin inhibitor was purchased from Sigma Aldrich. ECL Western blotting detection reagents were purchased from GE (Piscataway, NJ, USA). Skim milk was purchased from Morinaga-Nyugyo (Tokyo, Japan). The Bio-Rad protein assay kit was purchased from Bio-Rad Laboratories (Hercules, CA, USA).
MARCKS-related peptide (MANS)
MANS and a random NH2-terminal sequence (RNS) peptide were synthesized as previously described [14] at Scrum (Tokyo, Japan). The RNS peptide was used as the control.
Acini treatment
Pancreatic acini from Sprague–Dawley rats (males, 200–250 g) were prepared as described previously [11]. The collected acini were finally suspended in appropriate amounts of modified KRB solution [11] containing 0.5% BSA and 0.02% trypsin inhibitor and incubated at 37 °C under an atmosphere of 95% O2–5% CO2. The acini were stimulated with GLP-1 (25 nM) or 8-bromo-cAMP (1 mM) for the indicated times. When the effects of inhibitors were examined, cells were pretreated with H89 (10 μM, 10 min), cAMPS-Rp (15 μM, 15 min), MANS peptide (50 μM, 15 min), and RNS peptide (50 μM, 15 min), and then GLP-1 was added. The acini viability was kept more than 95% determined by trypan blue exclusion.
Preparation of lysate
Incubated acini were immediately transferred onto ice and lysed with ice-cold RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 5 mM sodium deoxycholate, and 20 mM HEPES) containing protease inhibitors (pepstatin A, aprotinin, and leupeptin) and phosphatase inhibitors (sodium fluoride, sodium orthovanadate, and sodium glycerophosphate) for 15 min on ice. The lysates were then spun at 14,000 g for 10 min. Supernatants were collected, and protein concentrations were determined with a Bio-Rad protein assay kit using BSA as a standard [19]. The supernatants were used for Western blotting.
Western blotting
The protein samples were separated by SDS-PAGE using a Mini-Protean 3 Cell system (Bio-Rad). After electrophoresis, the separated proteins were transferred onto a PVDF filter using a Trans-Blot Turbo System (Bio-Rad). The blots were blocked at room temperature for 50 min in BSA or skim milk and then probed for 120 min with a primary antibody, i.e., anti-MARCKS (diluted 1:1000) and anti-p-MARCKS (diluted 1:2000). The blots were washed three times with Tris buffered saline (pH 7.6) containing 0.05% Tween 20 and then probed for 90 min with anti-rabbit IgG (diluted 1:10,000). Immunoreactivity was determined using ECL Western blotting detection reagents. Images were acquired using Light-Capture II (ATTO, Tokyo, Japan). The intensity of p-MARCKS was measured with CS Analyzer 3.0 (ATTO).
Amylase release
The suspensions of acini were passed through a filter paper to separate the medium and the cells. The acini were then homogenized in phosphate buffer (pH 6.9) containing 0.01% Triton X-100 for measurement of the total amylase activity. Amylase activity in the medium and the homogenates was assayed in accordance with the method described by Bernfeld [20]. The integrated value of amylase release at the indicated time was expressed as a percentage of the total amylase in the cells, as shown below.
Statistical analysis
Either one-way analysis of variance (ANOVA) followed by Bonferroni correction (Figs. 1a, c, d, 2c, 3, 4) or Student’s t test (Figs. 1b, 2d) was employed to test for statistical significance, and a P value of less than 0.05 was considered significant. The P values are indicated with asterisks. All statistical analyses were performed using GraphPad Prism7 (GraphPad Software, La Jolla, CA, USA).
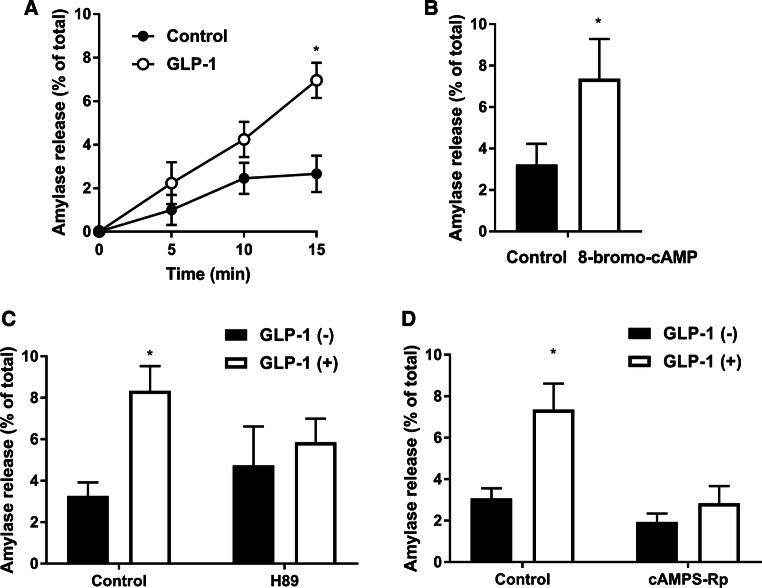
Fig. 1.
Effect of GLP-1 on amylase release. a Isolated pancreatic acini were incubated with or without GLP-1 (25 nM) for the indicated times. b Acini were incubated with or without 8-bromo-cAMP (1 mM) for 15 min. c, d After pretreatment with H89 (10 μM) for 10 min (c) or cAMPS-Rp (15 μM) for 15 min (d), acini were incubated with or without GLP-1 (25 nM) for 15 min. Amylase release was expressed as the percentage of the total amylase activity. Values are shown as means ± SE from three independent experiments. The asterisks indicate a significant difference (P < 0.05) compared with the corresponding period for the control (a) or the control in the absence of 8-bromo-cAMP (b) or GLP-1 (c, d)
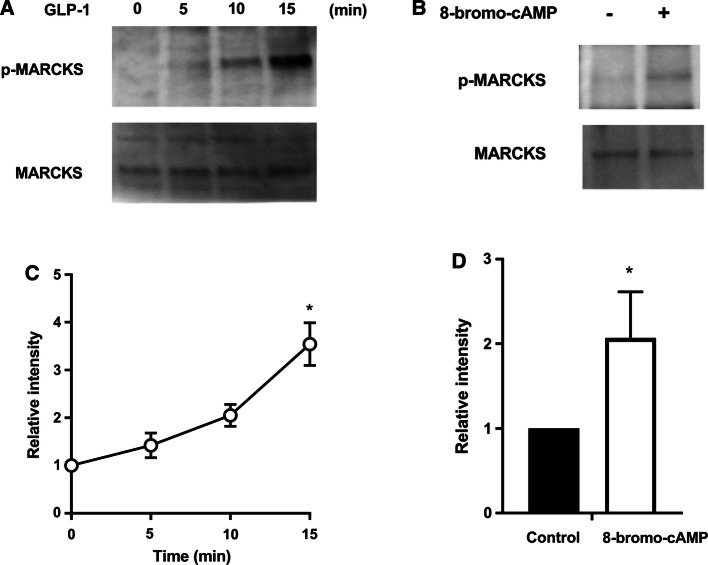
Fig. 2.
Effect of GLP-1 on MARCKS phosphorylation. a, b MARCKS and p-MARCKS in lysates (20 μg protein) were detected by Western blotting analysis using anti-MARCKS and anti-p-MARCKS antibodies, respectively. a Acini were stimulated with GLP-1 (25 nM) for the indicated times. b Acini were stimulated with 8-bromo-cAMP (1 mM) for 15 min. c, d The Western blotting data for p-MARCKS were normalized against stimulation at 0 min. Values show the means ± SE from three independent experiments. The asterisks indicate a significant difference (P < 0.05) compared with stimulation at 0 min (a) or the control (b)
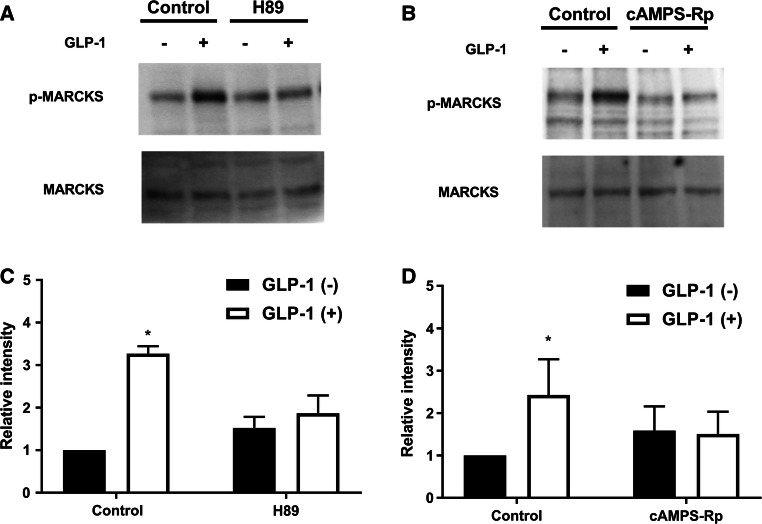
Fig. 3.
Inhibition of GLP-1-induced MARCKS phosphorylation by PKA inhibitors. a, b MARCKS and p-MARCKS in lysates (20 μg protein) were detected by Western blotting analysis using anti-MARCKS and anti-p-MARCKS antibodies, respectively. After pretreatment with H89 (10 μM) for 10 min (a) and cAMPS-Rp (15 μM) for 15 min (b), acini were stimulated with GLP-1 (25 nM) for 15 min. c, d The Western blotting data for p-MARCKS were normalized against the absence of GLP-1 and inhibitor. Values show the means ± SE from three independent experiments. The asterisks indicate a significant difference (P < 0.05) compared with the control in absence of GLP-1
Fig. 4.
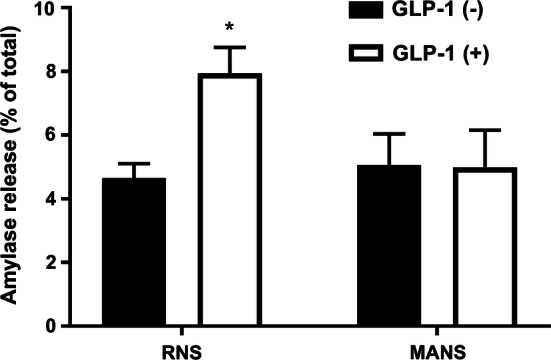
Inhibition of GLP-1-induced amylase release by MARCKS-related peptide (MANS). After pretreatment with RNS (50 μM) or MANS (50 μM) for 15 min, acini were incubated with or without GLP-1 (25 nM) for 15 min. Amylase release was expressed as the percentage of the total amylase activity. Values show the means ± SE from three independent experiments. The asterisk indicates a significant difference (P < 0.05) compared with the control in absence of GLP-1
Results
GLP-1-induced amylase release
In order to assess whether GLP-1 induces amylase release in pancreatic acini, the acini were stimulated with 25 nM GLP-1, which was reported to show the maximal effect on amylase release [21], for 5, 10, and 15 min. As shown in Fig. 1a, GLP-1 induced amylase release from pancreatic acini in a time-dependent manner. A cell-permeable cAMP analog, 8-bromo-cAMP, also induced amylase release (Fig. 1b). These results suggest that GLP-1 induces amylase release and is involved in the cAMP-pathway in acini isolated from the pancreas. Next, we examined the effects of two PKA inhibitors, H89 and cAMPS-Rp, on GLP-1-induced amylase release. Pancreatic acini were preincubated with H89 (10 μM) for 10 min or cAMPS-Rp (15 μM) for 15 min, and then the acini were incubated with GLP-1 (25 nM) for 15 min. As seen in Fig. 1c, d, both inhibitors inhibited GLP-1-induced amylase release, but had no effect on amylase release in the absence of GLP-1. These results suggest that PKA contributes to the GLP-1-induced amylase release in isolated pancreatic acini.
GLP-1-induced MARCKS phosphorylation
We next assessed MARCKS phosphorylation in pancreatic acini that were stimulated with GLP-1 (25 nM) for 5, 10, and 15 min. The results of Western blotting using anti-MARCKS and anti-p-MARCKS antibodies clearly showed that GLP-1 induced MARCKS phosphorylation in a time-dependent manner but did not affect the amount of MARCKS (Fig. 2a, c). 8-bromo-cAMP also induced MARCKS phosphorylation (Fig. 2b, d). These results suggest that GLP-1 induces MARCKS phosphorylation and is involved in the cAMP-pathway in the isolated pancreatic acini. We next investigated the effects of PKA inhibitors on MARCKS phosphorylation. Pancreatic acini were preincubated with H89 (10 μM) for 10 min or cAMPS-Rp (15 μM) for 15 min, and then the acini were stimulated with GLP-1 (25 nM) for 15 min. As shown in Fig. 3, both inhibitors inhibited GLP-1-induced MARCKS phosphorylation, but had no effect on the phosphorylation in the absence of GLP-1. In addition, the inhibitors did not affect the total amount of MARCKS. These results suggest that GLP-1-induced MARCKS phosphorylation is evoked by PKA activation.
Effects of MARCKS-related peptide on GLP-1-induced amylase release
MANS peptide is a useful inhibitor of the function of MARCKS because it suppresses secretory function in intact cells [11, 12], cultured cells [14], and in vivo [15]. Therefore, we employed the MANS peptide to examine whether inhibition of the function of MARCKS would suppress the GLP-1-induced pancreatic amylase release. Pancreatic acini were preincubated with the MANS peptide (50 μM) for 15 min and then stimulated with GLP-1 (25 nM) for 15 min. As shown in Fig. 4, the MANS peptide suppressed the GLP-1-induced amylase release. In contrast, the control peptide, RNS (50 μM), had no effect on the release. These peptides had no effect on amylase release in the absence of GLP-1. These results suggest that MARCKS is involved in the GLP-1-induced amylase release in isolated pancreatic acini.
Discussion
In this study we demonstrated that (1) GLP-1 induces amylase release and MARCKS phosphorylation, (2) these effects of GLP-1 are inhibited by PKA inhibitors, and (3) MANS peptide inhibits GLP-1-induced amylase release. Consequently, it is likely that MARCKS is involved in cAMP-dependent amylase release in acini isolated from rat pancreas.
GLP-1R was expressed in rodent pancreatic acinar cells [6, 8, 21]. Here, we demonstrated that GLP-1 induces amylase release in rat pancreatic acini (Fig. 1a). Furthermore, that release was inhibited by PKA inhibitors (Fig. 1c, d). GLP-1 binds to GLP-1R, leading to G protein-mediated elevation of intracellular cAMP levels and activation of PKA [2]. In mouse parotid acinar cells, intracellular cAMP levels were increased by GLP-1 [8, 21]. Moreover, Hou et al. demonstrated that GLP-1 induces amylase release in mouse pancreatic acinar cells [21]. Our results are in line with those earlier observations. On the other hand, Wewer Albrechtsen et al. demonstrated that GLP-1, not directly, but indirectly induced pancreatic amylase release in dispersed acinar cells using an isolation system capable of sustaining long-term culture for more than 1 week [8]. This system includes a cooling step in which cells are put on ice [23]. In contrast, in our present study, cells are maintained at a constant 37 °C during isolation, suggesting that the condition of isolated pancreatic acinar cells may differ between the two systems. In fact, exocytosis occurs in PC12 cells when the temperature is lowered from 37 to 4 °C [24]. Furthermore, cold temperature induced mucin hypersecretion via MARCKS function in NHBE cells [25]. Therefore, in isolated pancreatic acini, it is likely that GLP-1 induces amylase release through PKA activation. However, pancreatic amylase release is induced mainly via a Ca2+-pathway, rather than a cAMP-pathway [9]. CCK-induced signal transduction, which has been most studied, involves coupling of CCK receptors with heterotrimeric G proteins of the Gq family to activate phospholipase C, leading to increased inositol trisphosphate and Ca2+ levels [9]. Subsequently, an increase in diacylglycerol (DAG) activates PKC, and then this pathway stimulates amylase release [22]. We previously reported CCK-induced amylase release via PKC activation in rat pancreatic acini [11]. Thus, the signal transduction of amylase release might consist of crosstalk between the Ca2+- and cAMP-pathways.
We also reported that CCK induced MARCKS phosphorylation via PKC activation in rat pancreatic acini [11]. In the present study, we demonstrated that GLP-1 induced MARCKS phosphorylation in rat pancreatic acini (Fig. 2a). Interestingly, that protein phosphorylation was inhibited by PKA inhibitors (Fig. 3). In rat parotid acinar cells, MARCKS phosphorylation was inhibited by both PKA and PKC inhibitors [12]. Thus, it is likely that MARCKS can be phosphorylated by PKC via both Ca2+- and cAMP-dependent pathways. We hypothesize that an indirect mechanism may underlie PKC activation by PKA. In a rat insulinoma cell line (INS-1 cells), GLP-1 induced MARCKS phosphorylation via PKC activation [26]. An adenylate cyclase activator activated PKC by elevating DAG. Phospholipase D (PLD), a regulator of DAG production, was activated via a Ca2+-independent pathway in rat parotid acinar cells [27]. It was reported that PLD activation was involved in the cAMP-dependent pathway in rat parotid acinar cells [28, 29]. Furthermore, PLD activation was suppressed by PKA inhibitors in a rat mast cell line (RBL-2H3 cells) [30]. Taken together, PKC appears to be activated by DAG, which is induced by PLD via PKA activation. In a separate experiment, we found that a PLD inhibitor suppressed GLP-1-induced amylase release in rat pancreatic acini [31]. That observation strongly supports our above hypothesis.
The MANS peptide is a myristoylated, cell-permeable peptide corresponding to the first 24 amino acids of MARCKS, which inhibit MARCKS function [14]. We demonstrated that GLP-1-induced amylase release was inhibited in isolated pancreatic acini that had been preincubated with the MANS peptide (Fig. 4). The peptide had no effect on non-stimulated secretion, suggesting that MARCKS is involved in GLP-1-induced amylase release. We previously reported that MARCKS is involved in PKA-dependent and PKC-dependent amylase release in rat parotid [12] and pancreatic [11] acinar cells, respectively. p-MARCKS is translocated from the cell membrane to the cytosol [13]. We previously showed that, in rat pancreatic acini, PKC-mediated MARCKS phosphorylation induces MARCKS translocation from lipid rafts of the cell membrane to the cytosol [11]. Taken together, MARCKS translocation is involved in membrane trafficking, such as exocytosis. These observations suggest that MARCKS plays a role in GLP-1-induced exocytotic amylase release in isolated pancreatic acini.
In conclusion, these findings suggest that MARCKS is involved in GLP-1-induced amylase release via activation of PKA in isolated pancreatic acini.
Acknowledgements
The authors thank Dr. Noriko Koyama (Asahi University School of Dentistry) for helpful advice.
Author contributions
KS conception and design of research; KS, MO, and AM performed experiments; KS, MO, and AM analyzed data; KS, MO, and MK interpreted results of experiments; KS prepared figures; KS drafted manuscript; KS, MO, AM, and MK edited and revised the manuscript; KS, MO, AM, and MK approved final version of manuscript.
Funding
This work was supported by JSPS KAKENHI Grant numbers 15K21322 and 15K11060.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
In accordance with the established related guidelines, the experimental design of the study was approved by the Animal Offices of Asahi University (Approval number: 17-024) and Dokkyo Medical University (Approval numbers: 0876 and 1046).
References
- 1.Drucker DJ. Glucagon-like peptides. Diabetes. 1998;47:159–169. doi: 10.2337/diab.47.2.159. [DOI] [PubMed] [Google Scholar]
- 2.Doyle ME, Egan JM. Mechanisms of action of glucagon-like peptide 1 in the pancreas. Pharmacol Ther. 2007;113:546–593. doi: 10.1016/j.pharmthera.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hadjiyanni I, Siminovitch KA, Danska JS, Drucker DJ. Glucagon-like peptide-1 receptor signalling selectively regulates murine lymphocyte proliferation and maintenance of peripheral regulatory T cells. Diabetologia. 2010;53:730–740. doi: 10.1007/s00125-009-1643-x. [DOI] [PubMed] [Google Scholar]
- 4.Hsieh J, Longuet C, Baker CL, Qin B, Federico LM, Drucker DJ, Adeli K. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia. 2010;53:552–561. doi: 10.1007/s00125-009-1611-5. [DOI] [PubMed] [Google Scholar]
- 5.Kim M, Platt MJ, Shibasaki T, Quaqqin SE, Backx PH, Seino S, Simpson JA, Drucker DJ. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19:567–575. doi: 10.1038/nm.3128. [DOI] [PubMed] [Google Scholar]
- 6.Raufman JP, Singh L, Singh G, Eng J. Truncated glucagon-like peptide-1 interacts with exendin receptors on dispersed acini from guinea pig pancreas. Identification of a mammalian analogue of the reptilian peptide exendin-4. J Biol Chem. 1992;267:21432–21437. [PubMed] [Google Scholar]
- 7.Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-like-peptide-1 receptor expression in normal and diseased human thyroid and pancreas. Mod Pathol. 2015;28:391–402. doi: 10.1038/modpathol.2014.113. [DOI] [PubMed] [Google Scholar]
- 8.Wewer Albrechtsen NJ, Albrechtsen R, Bremholm L, Svendsen B, Kuhre RE, Poulsen SS, Christiansen CB, Jensen EP, Janus C, Hilsted L, Deacon CF, Hartmann B, Holst JJ. Glucagon-like peptide 1 receptor signaling in acinar cells causes growth-dependent release of pancreatic enzymes. Cell Rep. 2016;17:2845–2856. doi: 10.1016/j.celrep.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Williams JA. Intracellular signaling mechanisms activated by cholecystokinin-regulating synthesis and secretion of digestive enzymes in pancreatic acinar cells. Annu Rev Physiol. 2001;63:77–97. doi: 10.1146/annurev.physiol.63.1.77. [DOI] [PubMed] [Google Scholar]
- 10.Stumpo DJ, Graff JM, Albert KA, Greengard P, Blackshear PJ. Molecular cloning, characterization, and expression of a cDNA encoding the “80- to 87-kDa” myristoylated alanine-rich C kinase substrate: a major cellular substrate for portion kinase C. Proc Natl Acad Sci U S A. 1989;86:4012–4016. doi: 10.1073/pnas.86.11.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Satoh K, Narita T, Katsumata-Kato O, Sugiya H, Seo Y. Involvement of myristoylated alanine-rich C kinase substrate phosphorylation and translocation in cholecystokinin-induced amylase release in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol. 2016;310:G399–G409. doi: 10.1152/ajpgi.00198.2015. [DOI] [PubMed] [Google Scholar]
- 12.Satoh K, Matsuki-Fukushima M, Qi B, Guo MY, Narita T, Fujita-Yoshigaki J, Sugiya H. Phosphorylation of myristoylated alanine-rich C kinase substrate is involved in the cAMP-dependent amylase release in parotid acinar cells. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1382–G1390. doi: 10.1152/ajpgi.90536.2008. [DOI] [PubMed] [Google Scholar]
- 13.Aderem A. The MARCKS brothers: a family of protein kinase C substrates. Cell. 1992;71:713–716. doi: 10.1016/0092-8674(92)90546-O. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Martin LD, Spizz G, Adler KB. MARCKS protein is a key molecule regulating mucin secretion by human airway epithelial cells in vitro. J Biol Chem. 2001;276:40982–40990. doi: 10.1074/jbc.M105614200. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, Adler KB. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nature Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 16.Salli U, Saito N, Stormshak F. Spatiotemporal interactions of myristoylated alanine-rich C kinase substrate (MARCKS) protein with the actin cytoskeleton and exocytosis of oxytocin upon prostaglandin F2α stimulation of bovine luteal cells. Biol Reprod. 2003;69:2053–2058. doi: 10.1095/biolreprod.103.017640. [DOI] [PubMed] [Google Scholar]
- 17.Rosé SD, Lejen T, Zhang L, Trifaró JM. Chromaffin cell F-actin disassembly and potentiation of catecholamine release in response to protein kinase C activation by phorbol esters is mediated through myristoylated alanine-rich C kinase substrate. J Biol Chem. 2001;276:36757–36763. doi: 10.1074/jbc.M006518200. [DOI] [PubMed] [Google Scholar]
- 18.Morita A, Ouchi M, Terada M, Kon H, Kishimoto S, Satoh K, Otani N, Hayashi K, Fujita T, Inoue KI, Anzai N. Reproducible insulin secretion from isolated rat pancreas preparations using an organ bath. Exp Anim. 2017;67:15–22. doi: 10.1538/expanim.17-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Bernfeld P. Amylase α and β. Methods Enzymol. 1955;1:149–158. doi: 10.1016/0076-6879(55)01021-5. [DOI] [Google Scholar]
- 21.Hou Y, Ernst SA, Heidenreich K, Williams JA. Glucagon-like peptide-1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cAMP. Am J Physiol Gastrointest Liver Physiol. 2016;310:G26–G33. doi: 10.1152/ajpgi.00293.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matozaki T, Williams JA. Multiple sources of 1,2-diacylglycerol in isolated rat pancreatic acini stimulated by cholecystokinin. J Biol Chem. 1989;264:14729–14734. [PubMed] [Google Scholar]
- 23.Gout J, Pommier RM, Vincent DF, Kaniewski B, Martel S, Valcourt U, Bartholin L. Isolation and culture of mouse primary pancreatic acinar cells. J Vis Exp. 2013 doi: 10.3791/50514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshimura R, Nakagawa M, Endo Y. Cold stimulation evokes exocytotic vesicle release from PC12 cells. Biomed Res. 2016;37:381–383. doi: 10.2220/biomedres.37.381. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Li Q, Yang G, Kolosov VP, Perelman JM, Zhou XD. Cold temperature induces mucin hypersecretion from normal human bronchial epithelial cells in vitro through a transient receptor potential melastatin 8 (TRPM8)-mediated mechanism. J Allergy Clin Immunol. 2011;128(626–634):e5. doi: 10.1016/j.jaci.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki Y, Zhang H, Saito N, Kojima I, Urano T, Mogami H. Glucagon-like peptide 1 activates protein kinase C through Ca2+-dependent activation of phospholipase C in insulin-secreting cells. J Biol Chem. 2006;281:28499–28507. doi: 10.1074/jbc.M604291200. [DOI] [PubMed] [Google Scholar]
- 27.Guillemain I, Rossignol B. Receptor- and phorbol ester-mediated phospholipase D activation in rat parotid involves two different pathways. Am J Physiol. 1994;266:C692–C699. doi: 10.1152/ajpcell.1994.266.3.C692. [DOI] [PubMed] [Google Scholar]
- 28.Dohke Y, Fujita-Yoshigaki J, Sugiya H, Furuyama S, Hara-Yokoyama M. Involvement of phospholipase D in the cAMP-regulated exocytosis of rat parotid acinar cells. Biochem Biophys Res Commun. 2002;299:663–668. doi: 10.1016/S0006-291X(02)02713-4. [DOI] [PubMed] [Google Scholar]
- 29.Satoh K, Kashimata M, Sugiya H. Involvement of PLD in β-agonist-induced amylase release via MARCKS phosphorylation in rat parotid acinar cells. J Physiol Sci. 2017;67(Suppl):S96. [Google Scholar]
- 30.Choi WS, Chahdi A, Kim YM, Fraundorfer PF, Beaven MA. Regulation of phospholipase D and secretion in mast cells by protein kinase A and other protein kinases. Ann N Y Acad Sci. 2002;968:198–212. doi: 10.1111/j.1749-6632.2002.tb04336.x. [DOI] [PubMed] [Google Scholar]
- 31.Satoh K, Kashimata M. Involvement of MARCKS and PLD in parotid and pancreatic amylase release. J Physiol Sci. 2018;68(Suppl):S115. [Google Scholar]