Abstract
In behavior methods to quantify neuropathic pain, visual observations of limb-withdrawal reflexes to stimuli are not always clear-cut, so this method is partly subjective. Our current data suggest that measurement of electrophysiological EMG magnitudes enables more reliable and objective assessment for quantifying nocifensive behaviors related to neuropathic pain.
Keywords: Neuropathic pain, Electromyography, Quantitative method
Introduction
Neuropathic pain can be caused by peripheral injury and inflammation and is characterized by mechanical, cold, and heat hyperalgesia, or allodynia with spontaneous pain [1]. Several independent mechanisms in the peripheral and central nervous systems are responsible for the specific sensory symptoms. Thus, a thorough analysis of sensory symptoms using mechanical and thermal stimuli may help to find underlying pathophysiological mechanisms that are mainly active in a particular neuropathic patient [2]. Animal models, which mimic human peripheral neuropathic pain, involve partial sciatic nerve injury (PSI), chronic constriction injury (CCI), and spinal nerve ligation (SNL) in rats and have a common feature of partial nerve injury [3]. The abnormal sensory symptoms in these models are analyzed by visual observation of hindlimb withdrawal in response to graded mechanical, heat, or cold stimuli [4]. However, this visual observation method in an all-or-none manner is not always clear-cut and interpretation may vary between investigators, and so may be influenced by subjectivity.
Electromyography (EMG) is generally used to quantify muscle functions and has been frequently used to quantify sleep latency after peripheral nerve injury [5] and visceromotor responses to visceral mechanical stimuli [6]. This may be used as a new technique for quantifying hyperalgesia in neuropathic rats, which will provide more objective and reasonable assessments than visual observations. There are few reports showing the feasibility of using EMG magnitude for hindlimb-withdrawal responses in neuropathic pain rats. In this brief report, we describe a simple EMG method for quantifying hindlimb-withdrawal responses in rat neuropathic pain behaviors.
Materials and methods
Animals
Adult male Sprague–Dawley rats (n = 24, 350–400 g) were used. They were housed one animal per cage with free access to water and food. The animals were kept under a 12-h light/dark cycle at 22°C. All procedures involving the use of animals conformed with the guidelines of the International Association for the Study of Pain and the National Institutes of Health and were approved by the Catholic University Institutional Animal Care and Use Committee.
Electrode implantation and neuropathic pain surgery
The rats were divided into normal (n = 11) and neuropathic (n = 11) groups. In the normal group, only the EMG electrodes were implanted, without nerve injury. In the neuropathic group, electrode implantation and neuropathy surgery were performed at the same time. The surgery for partial sciatic nerve injury (PSI)-induced neuropathy was carried out on the left hindlimb as described previously [7]. Briefly, under isoflurane anesthesia, a segment of the sciatic nerve was exposed at the mid-thigh level, and the tibial and sural nerves were cut, whereas the common peroneal nerve was left intact. During the operation, EMG electrodes were implanted. The EMG electrodes were prepared as follows; a stainless-steel needle (0.15 mm diameter, 6 mm length, shaft of acupuncture needle) was inserted about 3 mm into a Teflon-coated multistrand wire (0.45 mm diameter) and fixed with acrylic adhesive (Fig. 1a). A pair of electrodes was implanted into the biceps femoris muscle 10 mm from the left hindlimb. The electrodes were fixed by suturing on to the muscle surface at the site of implantation using 6-0 Nylon to prevent them from being dislodged. A subcutaneous tunnel was made for exit of the electrode at the back of the animal’s neck. The wound was closed (Fig. 1b). All implanted electrodes were well retained during all experiment periods (about one month) without any side effects.
Fig. 1.
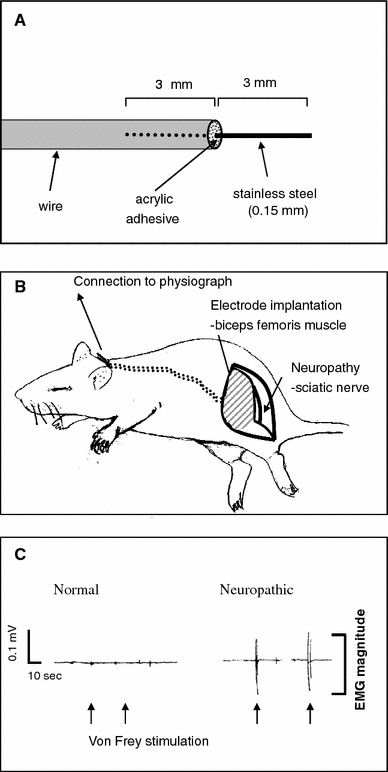
Electrode implantation and neuropathic pain surgery. a EMG electrode. The electrode was constructed simply by inserting a stainless-steel needle (shaft of a stainless-steel acupuncture needle) into a Teflon-coated multistrand wire and then bonded with acrylic adhesive. b Surgery for electrode implantation and neuropathy. For neuropathy, the tibial and sural nerves were cut, whereas the common peroneal nerve was left intact. During the neuropathy operation, one pair of electrodes was implanted into the biceps femoris muscle. c Representative EMGs. The neuropathic rat (right) showed high EMG magnitudes to von Frey stimulation with 0.6 g force (arrows), compared with normal rat (left)
Electromyographic recording
Behavioral testing with EMG recording was performed 14 days after surgery for electrode implantation and/or neuropathy. The rat was placed on a metal mesh floor under a custom-made transparent plastic dome (8 × 8 × 18 cm). Before beginning the behavioral test, the EMG electrode was connected to a polygraph (model 79, Grass, USA). The EMG signals were amplified and filtered (3 Hz–3 kHz). The rats were acclimated in the experimental chamber for about 20–30 min. The EMGs were recorded during the hindlimb responses to the applied mechanical, thermal, or cold (acetone) stimuli. The mean EMG magnitude (in mV) of the hindlimb withdrawal was used for the numerical analysis (Fig. 1c).
Mechanical, thermal, and cold stimulation
For mechanical stimuli, the medial area of the hind paw was mechanically stimulated from below with an ascending bending force series of von Frey filaments (bending forces 0.2, 0.4, 0.6, 0.8, 1.0 and 2.0 g, respectively). Each filament was used to stimulate the hind paw ten times at a rate of once every 3–4 min. Thermal stimulation was performed using an ascending series of warm water at 30, 40, or 50°C. One milliliter warm water from a water bath was gently sprayed on to the paw with a syringe connected to a 21-gauge needle. The time intervals between stimulations were at least 5 min. The water temperature in the water bath (Buchi Waterbath, Korea) was well controlled thermostatically within the preset range. For cold stimulation, acetone (99%, Sigma) was applied five times (once every 5 min) to the same area of the hind paw.
Statistical analysis
All values are expressed as mean ± SEM (standard error of the mean). Statistical significance was analyzed by one-way analysis of variance (ANOVA) or by use of paired t-tests. P values <0.05 were regarded as statistically significant.
Results and discussion
In this study, we examined the feasibility of using EMG measurements for withdrawal responses under neuropathic conditions. This is first report that EMG tests in response to mechanical, thermal, and cold stimuli after partial sciatic nerve injury.
A major feature of the neuropathic model was the marked hypersensitivity in response to normally innocuous mechanical stimuli. The neuropathic rats showed abrupt limb withdrawals (which is interpreted as pain response) to innocuous von Frey filaments (0.6–2.0 g force), whereas normal rats showed passive avoidance responses (e.g., slow movements in both lifting and lowering of the limb and slight paw re-positioning; no pain response) to these von Frey filaments (Fig. 2a). With visual observation, EMG magnitudes for abrupt hindlimb-withdrawal responses (pain response) in neuropathic rats were over 0.1 mV whereas passive responses to innocuous stimuli in normal rats were less than 0.1 mV in EMG magnitudes. Taken together, these data suggest that EMG analysis can be used to differentiate hyperalgesic (pain) responses from passive avoidance responses to innocuous von Frey stimuli.
Fig. 2.
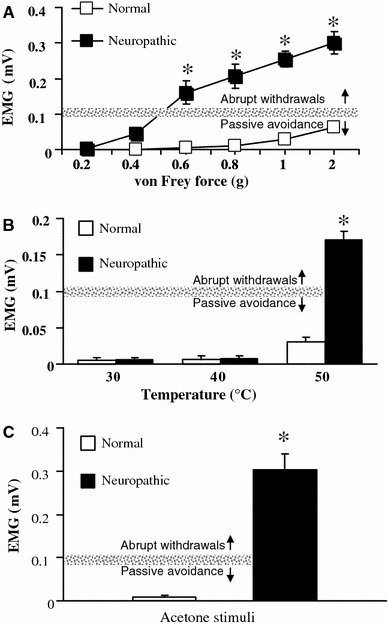
EMG analysis of limb-withdrawal responses to mechanical, thermal, and cold stimuli in neuropathic rat. a The EMG magnitude of the limb-withdrawal reflexes and the intensity of the von Frey filament in normal and neuropathic rats. b The EMG magnitude of the hindlimb-withdrawal responses following thermal stimuli in normal and neuropathic rats. c The EMG magnitude of the hindlimb-withdrawal responses to acetone (cold stimulation). The gray line in each figure represents the minimum EMG magnitude value (0.1 mV) that showed abrupt hindlimb-withdrawal reflexes (painful response) to stimuli. Data are mean ± SEM. *P < 0.05 versus the normal group
In previous studies, the 50% paw withdrawal thresholds for the most sensitive areas in the neuropathic model were less than 2 g [3]. Consistent with our results, the least bending force of the von Frey filaments showing significant increase in EMG magnitude values in the neuropathic group was 0.6 g. However the same bending force did not cause any changes in EMG magnitudes in normal rats. These data suggest that the electrophysiological method using EMG magnitude can help determine more accurate mechanical threshold measurements than conventional visual observations in neuropathic pain rats.
In addition, in our thermal stimulation experiments, neither neuropathic nor normal rats showed any hindlimb-withdrawal reflexes to the innocuous heat stimuli of 30 and 40°C. The EMG magnitudes were close to baseline values (0) (Fig. 2b). However, when 50°C thermal stimulation was applied, the neuropathic rats displayed thermal hyperalgesia, manifested by exaggerated paw-withdrawal reflexes to stimulation, which are different behaviors from simple lifting-avoidance in normal rats. The different withdrawal in response to 50°C stimulation was well expressed as much higher EMG magnitude in the neuropathic group than in the normal group. The 50°C stimulus to elicit limb-withdrawal reflexes in this study has been used in a previous neuropathy study [8]. Although their behavioral patterns of withdrawal responses to 50°C stimulation in normal and neuropathic rats were manifested as simple lifting-avoidance or exaggerated paw-withdrawal reflexes, respectively, results from visual observation in an all-or-none manner might be variable among investigators, indicating a limitation of conventional visual observation methods. An electrophysiological method measuring EMG magnitudes may enable more reliable and objective assessment for quantifying thermal hyperalgesia.
Acetone application to the hind paw is widely used to evaluate cold hyperalgesia in neuropathic rats [8]. In this study, neuropathic rats displayed large responses to acetone stimulation. Although this is a stimulus that normally evokes no response, or at most a brief response, in normal rats, it was recorded as markedly high magnitudes in electromyography of neuropathic rats compared with normal rats (Fig. 2c), indicating it can be used as an indicator of cold hyperalgesia.
To further confirm whether the increase of EMG values in response to stimuli in neuropathic rats was because of pain, we injected an analgesic, morphine (2 mg/kg), subcutaneously in neuropathic (n = 3) and normal (n = 3) rats and measured EMG magnitudes. The EMG values magnitudes evoked by mechanical (0.6 g force), heat (50°C) or, cold (acetone) stimulation in neuropathic rats were reduced to the baseline values of normal rats (less than 0.01 mV) 20 min after injection (data not shown). This confirmed that the EMG magnitudes evoked by stimuli in neuropathic rats indicate pain response.
In addition, EMG measurement of hindlimb withdrawal may help to differentiate supraspinal responses from nociceptive withdrawal response. In visual observation, lifting of the hindlimb accompanied by supraspinal responses (body repositioning and locomotion) during and/or at the end of mechanical or thermal stimuli could not be differentiated from nociceptive withdrawal reflex. On the other hand, the EMG activity during nociceptive hindlimb response showed a distinct sharp burst with high voltages (Fig. 1c), compared with prolonged, low-level electric activity during supraspinal response. Despite these advantages, it should be noted that EMG recordings have several disadvantages, for example individual housing of each rat, extra equipment, cable connection prior to study of behaviors, and risk of infections.
In conclusion, partial sciatic nerve injury-induced neuropathic rats displayed mechanical, thermal, and cold hyperalgesia, and all of these responses were well expressed by the EMG measurements. This brief study showed that electrophysiological EMG magnitude measurements may provide more reliable and objective assessments for quantifying nocifensive behaviors related to neuropathic pain.
Acknowledgment
This work was supported by the SRC program of KOSEF (R11-2005-014), Republic of Korea.
References
- 1.Maag R, Baron R. Neuropathic pain: translational research and impact for patient care. Curr Pain Headache Rep. 2006;10:191–198. doi: 10.1007/s11916-006-0045-8. [DOI] [PubMed] [Google Scholar]
- 2.Baron R. Mechanisms of disease: neuropathic pain—a clinical perspective. Nat Clin Pract Neurol. 2006;2:95–106. doi: 10.1038/ncpneuro0113. [DOI] [PubMed] [Google Scholar]
- 3.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 4.Kim KJ, Yoon YW, Chung JM. Comparison of three rodent neuropathic pain models. Exp Brain Res. 1997;113:200–206. doi: 10.1007/BF02450318. [DOI] [PubMed] [Google Scholar]
- 5.Takeda Y, Ishida T, Tsutsui R, Toide K, Tanimoto-Mori S, Watanabe S, Kanai Y, Kamei C. Studies on somnolence in the daytime caused by drugs used for neuropathic pain. J Pharmacol Sci. 2008;107:246–250. doi: 10.1254/jphs.08059FP. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Dong L, Sun B, Guillon MA, Burbach LR, Nunn PA, Liu X, Vilenski O, Ford AP, Zhong Y, Rong W. The role of metabotropic glutamate receptor mGlu5 in control of micturition and bladder nociception. Neurosci Lett. 2009;450:12–17. doi: 10.1016/j.neulet.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Lee BH, Won R, Baik EJ, Lee SH, Moon CH. An animal model of neuropathic pain employing injury to the sciatic nerve branches. Neuroreport. 2000;11:657–661. doi: 10.1097/00001756-200003200-00002. [DOI] [PubMed] [Google Scholar]
- 8.Datta S, Waghray T, Torres M, Glusman S. Amiodarone decreases heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Anesth Analg. 2004;98:178–184. doi: 10.1213/01.ANE.0000093223.35824.23. [DOI] [PubMed] [Google Scholar]


