Abstract
Recent studies on the association between particular single nucleotide polymorphisms of serine–threonine kinase with no lysine (K) 4 gene (WNK4) and essential hypertension have yielded controversial results. Here, frequencies of Ala589Ser polymorphism within exon 8 of the WNK4 gene were assessed among 259 unrelated ethnic Chinese patients with essential hypertension and 235 strictly matched normotensive controls. All subjects were derived from a relatively isolated population identified in the Kerqin desert region in Zhangwu county of Liaoning, northeastern China, which features a dry climate and the people having a high dietary salt intake, in addition to a significantly higher prevalence (~35%) of essential hypertension. Genotypes were verified with polymerase chain reaction-restriction fragment length polymorphism and confirmed by direct sequencing. Expression pattern and regulatory mechanisms of the WNK4 gene were also explored using Northern blotting and in vitro hormone stimulation assays. Strong associations between the Ala589Ser polymorphism and both raised systolic and diastolic blood pressures were identified. In addition to the kidneys, WNK4 gene expression was also found in many other organs. Several cis-acting elements had been discovered in the promoter region of the gene. As revealed by preliminary experiment, various hormones can down-regulate the expression of WNK4, among which glucocorticoid hormone seems to act in a dose-dependent manner. The WNK4 gene probably plays an important role in the pathogenesis of essential hypertension. As a missense mutation, the Ala589Ser polymorphism may bring changes to the enzyme’s function(s), resulting in increased susceptibility to the disease.
Keywords: Association, Essential hypertension, WNK4, SNP
Introduction
The newly cloned serine–threonine kinase with no lysine (K) 4 gene (WNK4) has been mapped at 17q12-21, a hot locus for blood pressure regulation [1, 2]. Mutations in WNK4 can cause a Mendelian trait featuring pseudohypoaldosteronism type II (PHAII; Online Mendelian Inheritance in Man No. 145260) [3], an autosomal dominant disorder characterized by severe hypertension, hyperkalemia and renal tubular acidosis caused by impaired K+ and H+ secretion. WNK4 is predominantly expressed in the distal convoluted tubule, connecting tubule and cortical collecting duct of the kidneys, which are crucial areas for regulation of salt and water reabsorption [3, 4].
In 1999, our group started to investigate an isolated population identified in the Kerqin desert region in Zhangwu county of Liaoning, northeastern China, where a high prevalence of essential hypertension was discovered. The region also features a dry climate and the people having a high dietary salt intake. The standardized morbidity from essential hypertension in this region reaches 35%, much higher than other areas in China [5].
This group has provided an ideal population for genetic epidemiological research on essential hypertension. In recent years, we have systematically searched among the population for particular single nucleotide polymorphisms (SNPs) within candidate genes, including endothelial channel beta-subunit (ENAC), G-protein β3 subunit (GNB3) and β-adrenergic receptor (β-AR) family, whose roles in hypertension have already been confirmed [6, 7].
Following the discovery of WNK4 gene, a number of studies have been conducted to investigate the association between particular SNPs within the gene and the onset of hypertension. So far, however, results of such studies have been controversial [8–13]. By sequencing the entire coding regions of WNK4, Kokubo et al. [14] identified 21 polymorphisms among 771 hypertensive subjects and 1,047 controls randomly sampled in Suita city in Japan. Their results indicated that systolic blood pressure in men with the CT + TT genotype for WNK4 C14717T was 3.1 mmHg higher than those with the CC genotype (P = 0.042). In addition, three missense mutations of the WNK4 gene, clustered into a small region within exon 7 but distant from the catalytic kinase domain, were identified in PHA II families [3]. Any of these can lead to a functional reduction of WNK4, whose normal function is to suppress the expression of Na–Cl co-transporter (NCCT) on the cell surface, therefore reducing the reabsorption of salt and water [15, 16]. In our previous work, we screened WNK4 exon 7 and found only one G/A polymorphism (nucleotide position: 1155547 in sequence NT_010840.8) for which the frequency of A allele was significantly higher in the hypertensive group [17]. However, this turned out to be a synonymous polymorphism. Here, we have tested a G/T polymorphism (Ala589Ser) in exon 8 of WNK4 (nucleotide position: 1155942 in sequence NT_010840.8) by case-control study and assessed the relevance of this SNP to the clinical phenotypes. In addition, the expression pattern and regulatory mechanisms of the WNK4 gene were also explored using Northern blotting and in vitro hormone stimulation assays.
Materials and methods
Subjects
A total of 259 unrelated patients with essential hypertension and 235 unrelated normal controls were recruited. All subjects were from Zhangwu County and diagnosed with the criteria that systolic blood pressure (SBP) was above 140 mmHg and/or diastolic blood pressure (DBP) was above 90 mmHg, or usage of antihypertensive agents. Blood pressure was measured three times on the right arm of seated participants and averaged after at least 5 min resting. All subjects had routine laboratory tests for plasma electrolytes, glucose, total cholesterol (T-chol), low-density lipoprotein cholesterol (LDL-chol), high-density lipoprotein cholesterol (HDL-chol) and triglyceride (TG). Ethical approval and informed consent were obtained from the local ethics committee as well as from all subjects.
Genotyping
Genomic DNA was extracted from peripheral blood by samples phenol and chloroform. The targeted polymorphism of WNK4, Ala589Ser, was detected by polymerase chain reaction (PCR)-based restriction fragment length polymorphism (RFLP). PCRs were performed with forward primer 5′-TGGAAACCCATTTTCCCCTGG-3′ and reverse primer 5′-AGGTGGTGAGGCCTAGAAAGT-3′ at the annealing temperature of 62°C. Ten microliters of PCR product was digested overnight at 37°C with 5 units of restriction endonuclease AlwNI (New England Biolab, Ipswich, MA). Digested products were run on 1.2% agarose gel and stained with ethidium bromide to check their patterns. Wild-type (A589) was cut into 180 and 112 bp, whereas variant type (S589) was uncut (Fig. 1). Genotypes were confirmed by direct sequencing (Shenggong Inc., Shanghai, China).
Fig. 1.
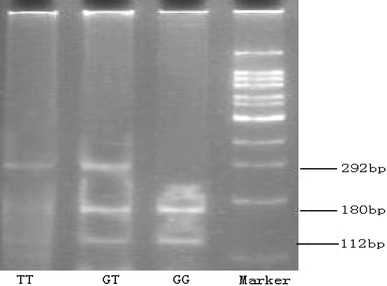
Detection of Ala589Ser polymorphism of WNK4 gene using PCR-RFLP. PCR products were digested with AlwNI. Wild-type (A589) was cut into 180 and 112 bp, whereas variant type (S589) was uncut
Statistical analysis
All statistical analyses were performed with SPSS version 11.5 for Windows. χ2 test was used to examine whether the genotype distributions differed from expected with Hardy–Weinberg equilibrium. Subjects were compared with respect to plasma glucose, T-chol, TG, LDL-chol and HDL-chol using the independent sample t-test. Statistical significance for differences in genotypes and allele frequencies between patients and controls and between different populations was assessed using the χ2 test. Association between genotype and hypertension was evaluated by multiple linear regression analysis, where SBP and DBP were regarded as dependent variables, and other parameters were sequentially entered as independent variables. Ala589Ser carriers were grouped together because of low TT prevalence. P values less than 0.05 were considered to be statistically significant.
Northern blotting
Total RNA was extracted from various human tissues (with informed consent obtained) using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA (20 mg) was fractionated in a 1.2% agarose-formaldehyde gel and then transferred onto a nylon membrane Hybond-N (Amersham Biosciences, Piscataway, NJ). A 321-bp antisense cRNA from hWNK4 cDNA was labeled, hybridized and detected by CDP-Star using DIG Northern starter kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions. Signals of hybridization bands were detected on X-ray film and quantified by densitometric analysis.
Influence of various hormones on the expression of hWNK4
COS-7 cells derived from African green monkey SV40-transferred kidney fibroblast were maintained in Dulbecco’s Modified Eagle’s Medium (Gibco/BRL, Bethesda, MD) with 10% fetal bovine serum, 100 unit/ml penicillin and 100 mg/ml streptomycin at 37.8°C in a humidified atmosphere containing 5% CO2. To assess the influence of various hormones on the expression of WNK4 gene, cells cultures were switched to serum-free media and then exposed to particular hormones for 24 h (detailed dosages please refer to Fig. 3). To assess the influence of various dosages of dexamethasone on the expression of WNK4, COS-7 cells were transfected with a pCAT-WNK4 promotor and stimulated with 1 and 10 nM of dexamethasone. Twenty-four hours later, absorbance at 405 nm was measured using an ELISA plate reader; the ratios between the sample protein and total protein concentrations were then calculated.
Fig. 3.
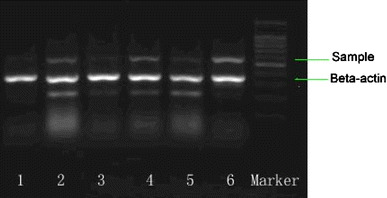
Down-regulating effect of various hormones on the expression of WNK4 gene. COS-7 cells were treated with the above hormones [lane 1 estrogen (final concentration 1 μmol/l), 2 insulin (100 nmol/l), 3 dexamethasone (1 nmol/l), 4 angiotensin II (5 μmol/l), 5 growth hormone (6 nmol/l), 6 blank control] for 24 h. RNA of the cells were then extracted with the Trizol method and quantified with RT-PCR
Results
Clinical characteristics of subjects
Characteristics of hypertensive patients and normotensive controls are summarized in Table 1. Compared with the control group, hypertensive groups had significantly higher levels of blood glucose, T-chol, TG, LDL-chol and BMI in addition to raised systolic and diastolic blood pressures.
Table 1.
Characteristics of subjects in the hypertensive and control groups
| Variable | Hypertensives | Controls | P |
|---|---|---|---|
| Number of subjects | 259 | 235 | |
| Male:female | 118:141 | 99:136 | 0.443 |
| Age (years) | 51.45 ± 12.09 | 49.50 ± 13.97 | 0.097 |
| SBP (mmHg) | 156.12 ± 25.56 | 113.23 ± 12.73 | <0.0001 |
| DBP (mmHg) | 96.88 ± 11.92 | 73.78 ± 8.99 | <0.0001 |
| T-chol (mmol/l) | 5.09 ± 1.02 | 4.39 ± 1.08 | <0.0001 |
| TG (mmol/l) | 1.68 ± 1.36 | 1.06 ± 0.86 | <0.0001 |
| HDL-chol (mmol/l) | 1.59 ± 0.40 | 1.58 ± 0.37 | 0.929 |
| LDL-chol (mmol/l) | 3.02 ± 0.80 | 2.57 ± 0.80 | <0.0001 |
| Blood glucose (mmol/l) | 4.72 ± 1.58 | 4.59 ± 0.89 | 0.048 |
| Serum ferrum (μmol/l) | 16.35 ± 6.81 | 16.56 ± 6.97 | 0.735 |
| Serum calcium (mmol/l) | 2.40 ± 0.09 | 2.38 ± 0.19 | 0.095 |
| Serum natrium (mmol/l) | 142.67 ± 13.22 | 141.72 ± 10.14 | 0.375 |
| Serum potassium (mmol/l) | 4.07 ± 0.53 | 4.04 ± 0.44 | 0.542 |
| BMI (kg/m2) | 25.37 ± 6.54 | 22.02 ± 3,57 | <0.0001 |
Variables are presented as mean ± SD
SBP Systolic blood pressure, DBP diastolic blood pressure (DBS), T-chol total cholesterol, LDL-chol low-density lipoprotein cholesterol, HDL-chol high-density lipoprotein cholesterol, TG triglyceride
Frequencies of Ala589Ser polymorphisms of WNK4
For the 494 subjects in the two populations, the genotype and allele frequencies of WNK4 gene did not deviate from Hardy–Weinberg equilibrium (χ2 = 2.73, P = 0.10), and the overall frequencies were similar to those of African Americans [9] (P > 0.05) (Table 2). However, the frequency of T allele in the hypertensive group was significantly higher than that of controls (25.9 vs. 20.2%, P = 0.035). OR for hypertension to carry T allele was 1.38 (95% CI 1.02–1.86). GT and TT genotypes were closely associated with both raised systolic and diastolic blood pressures (Table 3).
Table 2.
Frequency of Ala589Ser polymorphism in Chinese and African American populations
| Cases | Genotype frequency, n (%) | Allele frequency, n (%) | ||||
|---|---|---|---|---|---|---|
| GG | GT | TT | G | T | ||
| Chinese | 494 | 285 (57.69) | 189 (38.26) | 20 (4.05) | 759 (76.82) | 229 (23.18) |
| African American | 172 | 103 (59.88) | 61 (35.47) | 8 (4.65) | 267 (77.62) | 77 (22.38) |
Table 3.
Comparison of WNK4 gene Ala589Ser polymorphism between hypertension and control groups
| Cases | Genotype frequency, n (%) | Allele frequency, n (%) | ||||
|---|---|---|---|---|---|---|
| GG | GT | TT | G | T | ||
| Hypertensives | 259 | 136 (52.51) | 112 (43.24) | 11 (4.25) | 384 (74.13) | 134 (25.87) |
| Controls | 235 | 149 (63.40) | 77 (32.77) | 9 (3.83) | 375 (79.79) | 95 (20.21) |
| χ2 = 4.43 | P = 0.035 | |||||
Association of Ala589Ser polymorphism with particular clinical characteristics
In addition to the significant differences in both systolic and diastolic blood pressures between subjects with different genotypes (Table 4), stepwise regression analysis, in which plasma electrolytes, glucose, T-chol, TG, LDL-chol, HDL-chol, age, body mass index (BMI) and genotype (0 = GG, 1 = GT + TT) were considered as independent variables, also suggested TG, LDL-chol and genotype to be significantly associated with SBP and DBP in all subjects (Table 5).
Table 4.
Characteristics of subjects with different genotypes of WNK4 Ala589Ser polymorphism
| Clinical phenotype | GG | GT + TT | P |
|---|---|---|---|
| SBP (mmHg) | 133.39 ± 28.44 | 138.89 ± 30.99 | 0.041 |
| DBP (mmHg) | 84.59 ± 14.81 | 87.66 ± 16.68 | 0.032 |
| T-chol (mmol/l) | 4.77 ± 1.11 | 4.74 ± 1.10 | 0.725 |
| TG (mmol/l) | 1.36 ± 1.07 | 1.41 ± 1.33 | 0.620 |
| HDL-chol (mmol/l) | 1.57 ± 0.38 | 1.60 ± 0.39 | 0.372 |
| LDL-chol (mmol/l) | 2.85 ± 0.83 | 2.76 ± 0.83 | 0.233 |
| Blood glucose (mmol/l) | 4.77 ± 1.48 | 4.62 ± 1.01 | 0.196 |
| Serum ferrum (μmol/l) | 16.47 ± 7.30 | 16.43 ± 6.28 | 0.956 |
| Serum calcium (mmol/l) | 2.39 ± 0.18 | 2.39 ± 0.10 | 0.585 |
| Serum natrium (mmol/l) | 141.87 ± 12.69 | 142.70 ± 10.62 | 0.441 |
| Serum potassium (mmol/l) | 4.06 ± 0.50 | 4.05 ± 0.50 | 0.772 |
Variables are presented as mean ± SD
SBP Systolic blood pressure, DBP diastolic blood pressure (DBS), T-chol total cholesterol, LDL-chol low-density lipoprotein cholesterol, HDL-chol high-density lipoprotein cholesterol, TG triglyceride
Table 5.
Multiple regression analysis of blood pressure
| β-Coefficient | P | |
|---|---|---|
| SBP | ||
| Constant | 67.034 | <0.0001 |
| TG | 4.526 | <0.0001 |
| LDL | 10.215 | <0.0001 |
| Natrium | 0.427 | 0.002 |
| Genotype | 5.308 | 0.039 |
| R 2 = 0.135 | ||
| DBP | ||
| Constant | 64.889 | <0.0001 |
| TG | 2.424 | <0.0001 |
| LDL | 4.676 | <0.0001 |
| Genotype | 3.328 | 0.015 |
| R 2 = 0.124 | ||
Dependent variables: SBP, DBP
SBP Systolic blood pressure, DBP diastolic blood pressure (DBS), LDL low-density lipoprotein, TG triglyceride
Expression pattern of the WNK4 gene and its potential regulatory mechanisms
As revealed by Northern blotting, the WNK4 gene was also found to express in many other organs besides the kidneys (Fig. 2). Using software including TRANSFAC 4.0 (available at http://transfac.gbf.de/TRANSFAC/), TSSG/TSSH (available at http://www.cbs.dtu.dk/services/Promoter/) and NSITE (available at http://www.softberry.com/berry.phtml), a number of cis-acting elements, e.g., AP1, SP1, GRE and GATA, were identified upstream (0 to −600 bp) of the gene. As indicated with a RT-PCR assay, expression of WNK4 may be down-regulated by various hormones (Fig. 3). Among these, glucocorticoid hormones, e.g., dexamethasone, seem to act in a dose-dependent manner (Table 6).
Fig. 2.
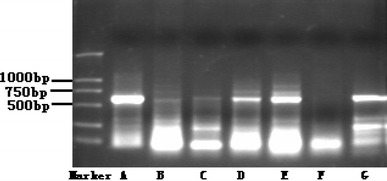
Expression of WNK4 gene in selected human tissues except the liver. Lane A kidney, B heart, C brain, D small intestine, E spleen, F liver, G lungs
Table 6.
Influence of dexamethasone on the transcription activity of WNK4 gene
| Blank control | Dexmethasone (1 nM) | Dexmethasone (10 nM) | |
|---|---|---|---|
| Total protein in cell lyse OD595 | 0.567 | 0.691 | 0.689 |
| Total protein (mg/ml) | 14.28 | 18.55 | 18.48 |
| CAT value OD405 | 0.143 | 0.116 | 0.177 |
| Target protein concentration (mg/ml) | 2.043 | 2.020 | 2.072 |
| Target protein/total protein | 0.143 | 0.109 | 0.112 |
Discussion
A great discrepancy seems to exist between our findings and the results by Erlich et al. [9], which rejected the association between the Ala589Ser polymorphism and essential hypertension in American populations. A similar discrepancy was reported by Speirs et al. [11], who failed to detect the association between an intron 10 polymorphism and hypertension. Notably, the relatively smaller size and greater genetic heterogeneity in their subjects may have contributed to the bias. The population in our study derives from a relatively isolated region featuring a high prevalence of hypertension. The dry climate, high dietary salt intake, low migration and high morbidity of hypertension have made it a typical and ideal subject for genetic epidemiological research on the disease. Our previous studies on the same population have identified an association between polymorphisms of β1-adrenergic receptor gene and essential hypertension, but excluded those with β2-adrenergic receptor, β3-adrenergic receptor or G-protein β3 subunit genes [6, 7].
The frequency of S598 polymorphism of WNK4 was found to be elevated in hypertensives in this study, which also found support from subsequent multiple linear regression analysis. We also analyzed other blood pressure-related characteristics; however, besides TG, LDL-chol and the Ala589Ser polymorphism in WNK4 exon 8, no other parameters could be associated with increased risk for the disease. How much of the effect is attributable to the polymorphism? Which parameters fell out of the analysis? Smoking? Obesity? Gender?
As a missense mutation, the Ala589Ser polymorphism can result in substitution of a nonpolar residue alanine by a polar residue serine. The location of this polymorphism is close to three mutations clustered to the first putative coil domain of the WNK4 and thereby may cause PHA II by diminishing the function(s) of its product. It seems that this change in polarity can trigger a spatial conformation change for the functional domain of WNK4 kinase, which may affect its activity or interaction(s) with downstream protein(s).
Taken together, our results have suggested that various hormones may influence the expression of WNK4 gene in the kidneys. As a missense mutation, the Ala589Ser polymorphism may play an important role in the increased susceptibility for essential hypertension.
Acknowledgements
This study was sponsored by a grant from the Natural Scientific Foundation of China (no. 30300204) and a Doctoral Degree Starting Grant from Liaoning Province (no. 20051041). We are grateful to all patients and their families for their support.
References
- 1.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, Cupples LA, Myers RH. Evidence for a gene influencing blood pressure on chromosome 17. Hypertension. 2000;36:477–483. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Baima J, Nicolaou M, Schwartz F, DeStefano AL, Manolis A, Gavras I, Laffer C, Elijovich F, Farrer L, Baldwin CT, Gavras H. Evidence for linkage between essential hypertension and a putative locus on human chromosome 17. Hypertension. 1999;34:4–7. doi: 10.1161/01.hyp.34.1.4. [DOI] [PubMed] [Google Scholar]
- 3.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard J-M, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel A, Jeunemaitre X, Lifton RP. Human hypertension causes by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 4.Lifton RP, Gharavi AG, Geller DS. Molecular mechanism of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/S0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 5.Shi JP, Wang HL, Li H, Dong W, Fu LY, Qi GX, Jia ZM, Yang HY, Gong W, Kang H, Gao XG, Wang WL, Jiang YS, Li JG. The epidemiological survey of prevalence rate of hypertension in the countryside of Zhangwu county, Liaoning province. Chin J Epidemiol. 2003;24:547–550. [PubMed] [Google Scholar]
- 6.Dai SP, Shi JP, Ding Q, Wang HL, Dong LY, Sun D, Fang K, Zhao YY. Polymorphism analysis of 825C/T of the G-protein beta 3 subunit in high risk population of hypertension in the northeast China. Acta Genet Sin. 2002;29:294–298. [PubMed] [Google Scholar]
- 7.Liang Y, Zhao YY, Liu H, Shi JP. The genotype analysis of beta adrenergic receptor gene family in high risk population of hypertension in northeast China. Chin J Med Genet. 2004;21:124–127. [PubMed] [Google Scholar]
- 8.Monti J, Zimdahl H, Schulz H, Plehm R, Ganten D, Hubner N. The role of WNK4 in polygenic hypertension: a candidate gene analysis on rat chromosome 10. Hypertension. 2003;41:938–942. doi: 10.1161/01.HYP.0000063147.92433.7D. [DOI] [PubMed] [Google Scholar]
- 9.Erlich PM, Cui J, Chazaro I, Farrer LA, Baldwin CT, Gavras H, DeStefano AL. Genetic variants of WNK4 in whites and African Americans with hypertension. Hypertension. 2003;41:1191–1195. doi: 10.1161/01.HYP.0000070025.30572.91. [DOI] [PubMed] [Google Scholar]
- 10.Benjafield AV, Katyk K, Morris BJ. Association of EDNRA, but not WNK4 or FKBP1B, polymorphisms with essential hypertension. Clin Genet. 2003;64:433–438. doi: 10.1034/j.1399-0004.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- 11.Speirs HJ, Morris BJ. WNK4 intron 10 polymorphism is not associated with hypertension. Hypertension. 2004;43:766–768. doi: 10.1161/01.HYP.0000120121.43524.cd. [DOI] [PubMed] [Google Scholar]
- 12.Kamide K, Takiuchi S, Tanaka C, Miwa Y, Yoshii M, Horio T, Mannami T, Kokubo Y, Tomoike H, Kawano Y, Miyata T. Three novel missense mutations of WNK4, a kinase mutated in inherited hypertension, in Japanese hypertensives: implication of clinical phenotypes. Am J Hypertens. 2004;17:446–449. doi: 10.1016/j.amjhyper.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 13.Turner ST, Schwartz GL, Chapman AB, Boerwinkle E. WNK1 kinase polymorphism and blood pressure response to a thiazide diuretic. Hypertension. 2005;46:758–765. doi: 10.1161/01.HYP.0000186240.81996.57. [DOI] [PubMed] [Google Scholar]
- 14.Kokubo Y, Kamide K, Inamoto N, Tanaka C, Banno M, Takiuchi S, Kawano Y, Tomoike H, Miyata T. Identification of 108 SNPs in TSC, WNK1, and WNK4 and their association with hypertension in a Japanese general population. J Hum Genet. 2004;49(9):507–515. doi: 10.1007/s10038-004-0181-0. [DOI] [PubMed] [Google Scholar]
- 15.Wilson FH, Kahle KT, Ernesto Sabath, Maria DL, Rapson AK, Hoover RS, Hebert SC, Gamba G, Lifton RP. Molecular pathogenesis of inherited hypertension with hyperkalemia: the Na–Cl cotransporter is inhibited by wild-type but not mutant WNK4 . Proc Natl Acad Sci USA. 2003;100:680–684. doi: 10.1073/pnas.242735399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang CL, Angell J, Mitchell R, Ellison DH. WNK kinases regulate thiazide-sensitive Na–Cl cotransport. J Clin Invest. 2003;111:1039–1045. doi: 10.1172/JCI17443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun ZJ, Wang XN, Lu JY, Ding Q, Dong LY, Zhao YY. Correlation analysis between WNK4 gene and essential hypertension. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2003;25:145–148. [PubMed] [Google Scholar]


