Abstract
Hemodynamics are usually evaluated in the supine position at rest. This is only a snapshot of an individual’s daily activities. This study describes circulatory adaptation, as assessed by magnetic resonance imaging, to changes in position and exercise. Phase contrast magnetic resonance imaging of blood flow within systemic and pulmonary arteries and veins was performed in 24 healthy volunteers at rest in the prone and supine position and with bicycle exercise in the supine position. No change was seen in systemic blood flow when moving from prone to supine. Exercise resulted in an increased percentage of cardiac output towards the lower body. Changes in position resulted in a redistribution of blood flow within the left lung—supine positioning resulted in decreased blood flow to the left lower pulmonary vein. With exercise, both the right and left lower lobes received increased blood flow, while the upper lobes received less.
Keywords: Magnetic resonance imaging, Phase contrast, Exercise, Blood flow
Introduction
When subjects are evaluated with regards to their hemodynamics in the clinical setting, they are traditionally assessed in the supine position at rest. However, this condition is only a snapshot of an individual’s daily activities. Routine exercise physiology studies typically focus on measures of global cardiorespiratory reserve (i.e. oxygen consumption) [1–9]. Both invasive (right heart catheterization) and non-invasive (echocardiography) techniques have been used, but they only describe changes in gross measures of cardiac performance in response to exercise (i.e. stroke volume and cardiac output) [3–7, 10]. Little is known about how cardiac output is distributed to specific organ territories within the systemic and pulmonary vasculature, or how regional blood flow distributions are affected by changes in position and with exercise.
Phase contrast magnetic resonance imaging (PCMRI) is the gold standard for measurements of flow and cardiac output, and is not limited by acoustic windows. This technique is, therefore, able to measure blood flow in any vessel.
Typically, flow assessments by MRI studies are performed in the supine position, and at rest. Very few MRI based reports are available measuring cardiac output in different body positions, and at exercise. Furthermore, most studies concentrate on systemic blood flow and not pulmonary blood flow [11, 12]. The aim of this study was to examine how body position and exercise change the distribution of blood flow throughout the systemic and pulmonary vasculature in healthy adult individuals.
Materials and methods
Volunteer population
Following approval by the institutional research ethics board, and written informed consent, healthy volunteers who participated in the study were screened for any history of underlying acute or chronic cardiac or respiratory conditions and for contraindications to MRI—including metallic implants, pacemakers, or internal cardiac defibrillators. Those with known cardiac or respiratory abnormalities were excluded from the study. Individuals with more than four pulmonary veins draining back to the left atrium were excluded, due to the variability in the pulmonary venous drainage patterns seen in subjects with more than four pulmonary veins.
MRI protocol
All examinations were performed with a 1.5-Tesla MR scanner (Avanto; Siemens Medical Solutions, Erlangen, Germany). The first part of the examination was carried out with the subject in the prone position. Following the initial scout images, ECG-gated balanced steady-state free precession localizer images were obtained in axial, sagittal, and coronal orientations during free breathing.
Using these ECG-gated reference images, PCMRI measurements were acquired during free breathing, using the following settings: inplane spatial resolution of 1.0 × 1.0 mm, slice thickness 5.0 mm, minimal echo and repetition times, 2 averages, bandwidth 391 Hz/Px, and 25 true phases per cardiac cycle. The velocity encoding limit (VENC) was initially set to 150 cm/s for arteries and 120 cm/s for veins. If aliasing was noted, the sequence was repeated with an increased VENC. The PCMRI sequences were performed with retrospective ECG gating.
The following vessels were imaged: ascending aorta (AAO) at the level of the right pulmonary artery, superior vena cava (SVC) at the level of the right pulmonary artery, descending aorta at the level of the diaphragm (DAO), both proximal pulmonary arteries, and all pulmonary veins. For the pulmonary veins, the imaging plane was prescribed such that neither the left atrium nor any of the branch pulmonary veins were included in the imaging plane (Fig. 1). Upon completion of the study in the prone position, the volunteers assumed a supine position, and the same sequences were then applied in the supine position at rest.
Fig. 1.
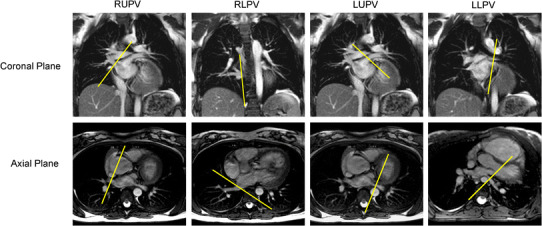
Balanced steady state free precession imaging in the coronal and axial planes with the yellow line outlining the prescriptions for the various phase contrast imaging planes of the right upper pulmonary vein (RUPV), right lower pulmonary vein (RLPV), left upper pulmonary vein (LUPV) and left lower pulmonary vein (LLPV) (color figure online)
Once the acquisitions at rest were completed, the volunteers were asked to exercise in the supine position, using an MRI compatible bicycle ergometer (MRI cardiac ergometer; Lode, Groningen, The Netherlands) [12–17]. The ergometer was mounted onto the MRI table and the torque controlled from the MRI control room. Thigh lengths, position of the table, and scanner bore diameter did not permit cycling with the subject’s thorax at the isocenter. Therefore, the participants were outside the bore of the magnet while cycling, and were moved into the bore only for scanning. Volunteers were asked to cycle against a variable resistance to achieve a submaximal heart rate that was at least 60 % of their target aerobic heart rate [(220 − subject age) × 0.60]. This level of exercise was chosen to enable the volunteers to cycle intermittently until all vessel measurements (a total of seven acquisitions) were achieved and in order to decrease the amount of heart rate decline after exercise. The latter would have been greater with higher target heart rates. Upon achieving the desired heart rate, volunteers were asked to stop cycling and to remove their feet from the pedals while the table was moved to the isocenter of the bore. Without further delay, PCMRI was performed for each of the above-mentioned vessels during free breathing. In order to maintain the heart rate at approximately 60 % of their target aerobic heart rate, volunteers cycled intermittently between PCMRI sequences, with imaging commencing once 60 % of their target heart rate was once again achieved.
In order to complete the PCMRI acquisition after exercise as rapidly as possible, parallel imaging with a factor of 2, and decreased spatial resolution without violating the minimum spatial resolution needed for accurate flow measurements, were used [18].
Post processing
Flow analysis was performed in the usual fashion, using commercially available software (QFlow v.5.1; MEDIS Medical Imaging Systems, Leiden, The Netherlands). Regions of interest were drawn around the vessel of interest. Contours were drawn off the velocity-encoded portion of the PCMRI sequence. During periods of no flow, the magnitude portion of the PCMRI was used to produce the contours for the region of interest. Flows in each vessel were indexed to body surface area (mL/min/m2).
Statistical analysis
Statistical analysis was performed using InStat (Graphpad Software, La Jolla, USA) and R v.2.15.1 (R Core Team, Vienna, Austria). The descriptive data for continuous variables are presented as means (±standard deviations) or medians and ranges if applicable. To allow comparisons of blood flow (actual values or percentages of total) across the three conditions (i.e. supine, prone, or exercise) in which each subject was measured, a mixed effects model was used, with a random intercept for each subject and fixed effects for the conditions being compared. The overall effect of condition on blood flow in a vessel was evaluated with an F test and differences between flow in pairs of conditions (e.g., prone vs. exercise) were calculated and tested using linear contrasts. The lme4 library in R was used and p values of less than 0.05 were considered significant.
Results
Twenty-four healthy volunteers participated in the study. Two individuals had a separate right middle pulmonary vein to the left atrium and were excluded. In the 22 remaining volunteers (9 males and 13 females), the mean age was 35.2 ± 11.4 years (range 17–59 years), with a mean weight of 72 ± 14 kg (range 50–106 kg) and height of 168.2 ± 8.5 cm (range 152–185 cm). The mean body surface area was 1.81 ± 0.2 m2 (range 1.47–2.13 m2).
Of the 22 volunteers, 18 were able to complete the entire protocol. Four subjects were unable to complete the exercise portion of the protocol, but were able to complete the resting examinations in prone and supine positions. Their data were included for this part of the analysis. Reasons for not being able to complete the exercise portion included leg fatigue, resulting in an inability to complete the cycling in three participants, and claustrophobia in the bore immediately after completing the first step of exercise in one.
Systemic blood vessels
The phase contrast data for the systemic blood vessels—AAO, SVC, and DAO—are shown in Table 1 under all three conditions.
Table 1.
Systemic blood vessel phase contrast data
| Prone ± SD (mL/min/m2) | Supine ± SD (mL/min/m2) | Percentage change between prone and supine | Exercise ± SD (mL/min/m2) | Percentage change between exercise and supine at rest | |
|---|---|---|---|---|---|
| AAO | 3506 ± 580 | 3208 ± 500 | −9.3 (p = 0.16) | 6005 ± 773 | 85.7 (p < 0.0001) |
| SVC | 1234 ± 279 | 1106 ± 189 | −11.6 (p = 0.10) | 1548 ± 336 | 39.6 (p < 0.0001) |
| DAO | 2524 ± 403 | 2294 ± 443 | −10.0 (p = 0.30) | 5027 ± 805 | 117.7 (p < 0.0001) |
Blood flow indexed to body surface area under the three conditions—supine at rest, prone at rest, and supine under exercise
AAO ascending aorta, SVC superior vena cava, DAO mid-thoracic descending aorta
When changing from prone to supine, there was no significant change in the blood flow to the systemic blood vessels (AAO, DAO, and SVC). With exercise, there was an expected increase in AAO, SVC, and DAO flows, when compared to at rest in either the supine or prone conditions. However, the blood flow in the SVC did not increase to the same degree as it did in the AAO and DAO. The SVC only had an additional 39.6 % increase in blood flow compared with the AAO (85.7 % increase in blood flow, p = 0.003) and the DAO (117.7 % increase in blood flow, p < 0.0001). In other words, there was a significant difference in the distribution of the total cardiac output (as measured by AAO flow) between the upper (as measured by SVC flow) and lower body (as measured by DAO flow) between the rest and exercise conditions (Table 3). With exercise, there was a redistribution of blood flow to the lower body, with an increased percentage of the total cardiac output through the DAO and a concomitant decrease in SVC flow.
Table 3.
Percentage of cardiac output to the various vessels
| Prone % ± SD | Supine % ± SD | Significant difference between prone and supine (p) | Exercise % ± SD | Significant difference between exercise and supine (p) | |
|---|---|---|---|---|---|
| SVC | 25.2 ± 5.9 | 24.7 ± 4.8 | 0.71 | 16.3 ± 5.5 | <0.001 |
| DAO | 72.2 ± 4.6 | 71.3 ± 5.2 | 0.61 | 83.7 ± 7.9 | <0.001 |
| RPA | 52.4 ± 3.6 | 53.1 ± 12.4 | 0.47 | 50.0 ± 12.7 | 0.63 |
| LPA | 47.6 ± 3.7 | 46.8 ± 12.4 | 0.47 | 50.0 ± 12.7 | 0.63 |
The percentage of blood flow, expressed as a percentage of the ascending aortic blood flow, that is distributed to the various systemic blood vessels
SVC superior vena cava, DAO mid-thoracic descending aorta
Pulmonary blood vessels
The phase contrast data for the pulmonary arteries and veins under all three conditions are shown in Table 2.
Table 2.
Pulmonary blood vessel phase contrast data
| Prone ± SD (mL/min/m2) | Supine ± SD (mL/min/m2) | Percentage change between prone and supine (p) | Exercise ± SD (mL/min/m2) | Percentage change between exercise and supine at rest (p) | |
|---|---|---|---|---|---|
| RPA | 1855 ± 304 | 1,996 ± 274 | 6.8 (0.37) | 3,691 ± 554 | 84.1 (<0.0001) |
| LPA | 1,687 ± 283 | 1,592 ± 266 | −6.0 (0.43) | 3,248 ± 463 | 102.4 (<0.0001) |
| RUPV | 968 ± 211 | 932 ± 185 | −3.8 (0.83) | 1,516 ± 326 | 61.6 (<0.0001) |
| RLPV | 1,071 ± 217 | 917 ± 196 | −16.8 (0.08) | 2,157 ± 394 | 133.9 (<0.0001) |
| LUPV | 868 ± 188 | 1,089 ± 153 | 20.3 (0.002) | 1,842 ± 394 | 68.6 (<0.0001) |
| LLPV | 970 ± 203 | 973 ± 315 | 0.003 (1.00) | 2,094 ± 340 | 115 (<0.0001) |
Blood flow indexed to body surface area under the three conditions—supine at rest, prone at rest, and supine under exercise. For individuals who had a separate insertion of the right middle pulmonary vein (RMPV) to the left atrium, the flow volume calculated was incorporated into the total volume of the RLPV
RPA right pulmonary artery, LPA left pulmonary artery, RUPV right upper pulmonary vein, RLPV right lower pulmonary vein, LUPV left upper pulmonary vein, LLPV left lower pulmonary vein
Between the different conditions at rest and at exercise, the distribution of blood flow between the right and left lung, based on pulmonary artery flow remained constant with both the right and left pulmonary arteries receiving nearly equal amounts of blood flow (Table 3). Both the RPA and LPA showed a significant increase in blood flow with exercise (Table 2).
Table 4 displays the percentage of total pulmonary blood flow that each pulmonary vein carried, in comparison to the total amount of blood received by the ipsilateral lung [i.e. right upper pulmonary vein (RUPV) flow versus total right lung flow]. There was a near equal distribution of pulmonary blood flow between the RUPV and right lower pulmonary vein (RLPV) in both the prone and supine positions at rest. With exercise, there was a redistribution of blood flow, with an increase in blood flow to the right lower lobe (from 50.7 % at rest to 58.1 % at exercise, p < 0.0001) and resultant decrease in relative blood flow to the right upper lobe (from 49.3 to 41.9 %). In contrast, when looking at left pulmonary venous flow, when moving from the prone to supine position, there was also redistribution of blood to the upper lobes with the left upper pulmonary vein (LUPV) receiving 54.3 % (up from 46.7 % in the prone position, p = 0.002) and the left lower pulmonary vein (LLPV) receiving 43.7 % (down from 53.3 %) of total left lung blood flow. With exercise, a response similar to that noted in the right lung was seen—with increased blood flow from the LLPV (from 45.7 % at rest to 53.3 % at exercise) and decreased flow from the LUPV (from 54.3 to 46.7 %, p = 0.0005).
Table 4.
Percentage of blood flow carried by each pulmonary vein as a percentage of ipsilateral pulmonary flow
| Prone % ± SD | Supine % ± SD | Significant difference between prone and supine (p) | Exercise % ± SD | Significant difference between exercise and supine (p) | |
|---|---|---|---|---|---|
| RUPV | 48.3 ± 5.5 | 49.3 ± 6.3 | 0.526 | 41.9 ± 5.5 | ≤0.0001 |
| RLPV | 51.6 ± 5.5 | 50.7 ± 6.3 | 0.526 | 58.1 ± 5.5 | ≤0.0001 |
| LUPV | 46.7 ± 6.7 | 54.3 ± 6.9 | 0.002 | 46.7 ± 6.0 | 0.0005 |
| LLPV | 53.3 ± 6.7 | 45.7 ± 6.9 | 0.002 | 53.3 ± 6.0 | 0.0005 |
The percentage of blood flow carried from each pulmonary vein in comparison to the total amount of blood flow to the ipsilateral lung
RUPV right upper pulmonary vein, RLPV right lower pulmonary vein, LUPV left upper pulmonary vein, LLPV left lower pulmonary vein
Discussion
Routinely, hemodynamic evaluations are performed in the supine position, at rest. Arguably, when resting in the MRI magnet, or within the cardiac catheterization or echocardiography laboratory, the circulation is under the least amount of strain and least likely to reveal abnormalities that may be unmasked by exercise. This state mirrors the physiology only during a portion of the subject’s daily routine and is not representative of the activity during most of the waking hours. Limited information is available about the body’s circulatory adaptation to changes in body position and in response to exercise.
Previous MRI-based studies of normal volunteers and in patients with congenital heart disease focused on changes in stroke volume and ventricular function [12, 14, 15, 19–21]. PCMRI is a technique that directly measures blood flow volume within specific vessels and, as such, allows one not only to measure cardiac output but also to assess regional blood flow distribution [10, 22–24]. It has been well validated both in terms of accuracy and reproducibility for the assessment of blood flow, including the pulmonary veins [10, 22, 25–29].
Effects of exercise on systemic blood flow
In this cohort of healthy adult volunteers, we found that, within the systemic vessels (i.e. AAO, SVC, DAO), there was no change in flow between the supine and prone position. With exercise, there was a 1.9-fold increase in cardiac output, resulting in a 1.5-fold increase in perfusion to the upper body (as seen by SVC flow) compared to a 2.3-fold increase in flow to the lower body (as seen by DAO flow).
Three other studies have utilized PCMRI during exercise in healthy volunteers, all focusing on biventricular cardiac output and/or systemic blood flows [11, 20, 21]. Weber et al. [20] examined the changes in flow volume, velocity, and flow patterns within the aorta and main pulmonary artery of normal subjects during physical exertion. Corresponding to our findings, they found a 1.7-fold increase in cardiac output with exercise.
Cheng et al. [11] examined regional changes in blood flow distribution between rest and exercise in ten healthy 10- to 14-year-old children, using a 0.5-T open bore magnet with an MRI compatible ergometer in the upright position. Comparable to our results, the authors found a threefold increase in lower body flow.
With exercise, relative lower body perfusion increased from 71 % of total cardiac output at rest to 84 % with exercise. The disproportionate increase in blood to the lower body during exercise, which was also observed by Cheng et al., is thought to be related to the decrease in systemic vascular resistance within the exercising muscle group. Systemic vasodilatation is a well-described mechanism to promote blood flow [30–33]. This hypothesis is supported by a study by Steeden et al. who examined 20 healthy volunteers during exercise, measuring blood flow with real-time spiral PCMRI. They used blood pressure measurements to calculate systemic vascular resistance. The authors found that, with increasing exercise, there was a decrease in systemic vascular resistance [21]. The cellular molecular mechanisms for local vasodilatation are unclear, but it has been suggested that local mediators (i.e. nitric oxide, prostaglandins, and nucleotides like adenosine tri-phosphate), signals from the sympathetic nervous system, as well as muscular contraction and expansion, all combine to facilitate blood flow to exercising muscle [30–33].
Effects of position on pulmonary blood flow
In the past, most studies on the distribution of pulmonary blood flow relied on nuclear medicine imaging [34–36]. These studies focused on the distribution of pulmonary blood flow examining regional differences, either semi-quantitatively or in terms of lung perfusion in milliliters per gram of lung tissue. However, due to their use of a radio-labeled tracer, studying the same subject under different conditions, particularly with exercise, was not feasible, at least not in humans. MRI allows for the same subject to be tested sequentially under different conditions within the same setting.
The distribution of pulmonary arterial blood flow to the right and left lung did not differ between prone, supine at rest, and supine during exercise (Table 3), similar to previous reports [11].
When shifting from the prone to the supine position, the proportion of right pulmonary flow received by the right pulmonary veins remained constant with near equal amounts of blood passing through the RUPV and RLPV. However, there was an increase in the proportion of left pulmonary blood flow received by the LUPV, and a resultant decrease in relative blood flow through the LLPV. The upper pulmonary veins drain a larger portion of the ventral lung fields, while the lower pulmonary veins mainly drain the dorsal lung segments. We hypothesize that the changes in left pulmonary blood flow distribution are related to the position of the heart within the left hemithorax—in the supine position, the heart exerts a mild mass effect on the lower segments of the left lung. This mass effect may cause decreased aeration of the lower segments and a resultant decrease in flow. In addition, while in the supine position, the heart may also mildly compress the left lower pulmonary vein as the vessel passes between the descending aorta and the left atrium (see Fig. 1). In contrast, while in the prone position, the heart “falls” anteriorly and away from the lung and the left lower pulmonary vein. While well tolerated by adult volunteers with normal cardiac anatomy, this mechanism of pulmonary venous compression may be significant in patients with cardiomegaly, as is occasionally observed in clinical practice. In fact, pseudostenosis of the pulmonary vein and compression of the pulmonary veins between the left atrium and descending aorta are recognized phenomena [37]. This study is the first that we are aware of that has shown a possible functional implication of this pseudostenosis in healthy volunteers. Many studies have shown the appearance of anatomical narrowing of the left lower pulmonary vein using computerized tomographic (CT) imaging or MRI. Yamaji et al. [38] found that, in 11 out of 116 patients undergoing pulmonary vein isolation therapy for atrial fibrillation, CT imaging demonstrated a greater than 50 % reduction in left lower pulmonary vein diameter in the supine position. However, when imaged in the prone position, none of the 11 patients appeared to have left lower pulmonary vein stenosis. However, we feel that, in order to diagnose pulmonary vein stenosis, pulmonary vein size may not be a robust measure of functional stenosis. Based on echocardiographic data, we know that pulmonary vein flow occurs throughout the entire cardiac cycle. As a result, the pulmonary vein cross-sectional area changes throughout the cardiac cycle, and this leads to uncertainty concerning which point in the cardiac cycle a cross-sectional measurement should be made to assess for narrowing. We feel that total amounts of flow measured throughout the cardiac cycle would be a more robust measure. Our study is the first to show how this pseudostenosis functionally impacts normal volunteers. The relief of this mechanism of compression may also contribute to the not infrequently improved hemodynamic situation in critically ill patients when they are positioned prone [38].
Pulmonary blood flow distribution with exercise
Compared to the resting state, the relative perfusion of the upper lobes decreased during exercise, with a resultant increase in relative blood flow to the lower lobes. We believe that this observation may be a function of the changes in blood flow within the lung to maintain ventilation–perfusion (VQ) matching. At rest in the supine position, the dependent portions of the lung (primarily right and left lower lungs) are recruited for gas exchange to a lesser extent. With exercise, tidal volume increases, and those dependent segments of lung become ventilated and expand, recruiting additional alveolae for gas exchange. We speculate that the increased ventilation to the more dependent portions results in a redistribution of blood flow to these now better ventilated segments, maintaining optimal VQ matching via the Euler–Liljestrand reflex [39]. As a result, the proportion of blood flow to these segments of the lung will be increased with a resulting relative decrease in flow from the other segments.
These findings highlight that hemodynamic information on regional pulmonary blood flow acquired at rest and in the supine position cannot necessarily be extrapolated to a scenario during which the subject is vertical and active.
Limitations
As the bore diameter and ergometer geometry did not permit cycling while scanning, PCMRI measurements were obtained immediately after cessation of pedaling. Between that time point and the end of the acquisition (<20 s in duration), the heart rate typically dropped between 10 and 20 % of what had been achieved during the exercise. However, this study was not designed to investigate the physiology at maximal or even submaximal exercise. Rather, we were interested in the changes of blood flow distribution between different body positions and exertional states.
Changes in heart rate also affect the absolute flow measured by the PCMRI sequences, as minute flow volume is the product of heart rate and stroke volume. Consequently, physiological variations in heart rate between sequences may well have led to differences between pulmonary arterial and pulmonary venous flow. As a result, there was incomplete agreement between the venous blood flow and the arterial blood flow for either the total amount of pulmonary blood flow, or the amount of blood to a particular lung (right or left), particularly during exercise. Additionally, bronchial arterial blood flow, which constitutes approximately 5 % of pulmonary venous flow but is not included in pulmonary arterial flow, is also a potential source for the discrepancy.
Finally, while we hypothesized that the resulting changes in lung perfusion during exercise were related to the physiological response of optimizing VQ matching, and that changes in lung perfusion related to changes in position were related to the position of the heart within the left hemithorax, we were unable to test this as MR does not provide delineation of lung segments. We would assert that further experimentation is needed to confirm our findings.
Conclusions
The increase in blood flow to activated muscle groups during exercise is accomplished not only by an increase in stroke volume and heart rate but also by a redistribution of blood flow, presumably through decrease in vascular resistance within exercising muscle groups. Within the pulmonary vasculature, regional pulmonary perfusion is dependent on subject position. When assessing the hemodynamics in a clinical setting, it must be considered that blood flow distribution data acquired supine and at rest are not representative of the subject’s physiology when upright and active. Differences in aeration are thought to result in a physiologic response to maintain an optimal ventilation/perfusion match through redistribution of pulmonary blood flow.
References
- 1.Nottin S, Vinet A, Stecken F, Nguyen LD, Ounissi F, Lecoq AM, Obert P. Central and peripheral cardiovascular adaptations during a maximal cycle exercise in boys and men. Med Sci Sports Exerc. 2002;3:456–463. doi: 10.1097/00005768-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Vinet A, Nottin S, Lecoq AM, Obert P. Cardiovascular responses to progressive cycle exercise in healthy children and adults. Int J Sports Med. 2002;4:242–246. doi: 10.1055/s-2002-29076. [DOI] [PubMed] [Google Scholar]
- 3.Rowland T. Echocardiography and circulatory response to progressive endurance exercise. Sports Med. 2008;7:541–551. doi: 10.2165/00007256-200838070-00002. [DOI] [PubMed] [Google Scholar]
- 4.Rowland T, Obert P. Doppler echocardiography for the estimation of cardiac output with exercise. Sports Med. 2002;15:973–986. doi: 10.2165/00007256-200232150-00002. [DOI] [PubMed] [Google Scholar]
- 5.Rowland T, Garrison A, Delulio A. Circulatory responses to progressive exercise: insights from positional differences. Int J Sports Med. 2003;7:512–517. doi: 10.1055/s-2003-42016. [DOI] [PubMed] [Google Scholar]
- 6.Rowland T, Potts J, Potts T, Sandor G, Goff D, Ferrone L. Cardiac responses to progressive exercise in normal children: a synthesis. Med Sci Sports Exerc. 2000;2:253–259. doi: 10.1097/00005768-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Rowland T, Popowski B, Ferrone L. Cardiac responses to maximal upright cycle exercise in healthy boys and men. Med Sci Sports Exerc. 1997;9:1146–1151. doi: 10.1097/00005768-199709000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Sundstedt M, Hedberg P, Jonason T, Ringqvist I, Brodin LA, Henriksen E. Left ventricular volumes during exercise in endurance athletes assessed by contrast echocardiography. Acta Physiol Scand. 2004;1:45–51. doi: 10.1111/j.1365-201X.2004.01304.x. [DOI] [PubMed] [Google Scholar]
- 9.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;2:281–291. doi: 10.1161/01.RES.58.2.281. [DOI] [PubMed] [Google Scholar]
- 10.Hundley WG, Li HF, Hillis LD, Meshack BM, Lange RA, Willard JE, Landau C, Peshock RM. Quantitation of cardiac output with velocity-encoded, phase-difference magnetic resonance imaging. Am J Cardiol. 1995;17:1250–1255. doi: 10.1016/S0002-9149(99)80772-3. [DOI] [PubMed] [Google Scholar]
- 11.Cheng CP, Herfkens RJ, Lightner AL, Taylor CA, Feinstein JA. Blood flow conditions in the proximal pulmonary arteries and vena cavae: healthy children during upright cycling exercise. Am J Physiol Heart Circ Physiol. 2004;2:H921–H926. doi: 10.1152/ajpheart.00022.2004. [DOI] [PubMed] [Google Scholar]
- 12.Roest AA, Lamb HJ, van der Wall EE, Vliegen HW, van den Aardweg JG, Kunz P, de Roos A, Helbing WA. Cardiovascular response to physical exercise in adult patients after atrial correction for transposition of the great arteries assessed with magnetic resonance imaging. Heart. 2004;6:678–684. doi: 10.1136/hrt.2003.023499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roest AA, Kunz P, Lamb HJ, Helbing WA, van der Wall EE, de Roos A. Biventricular response to supine physical exercise in young adults assessed with ultrafast magnetic resonance imaging. Am J Cardiol. 2001;5:601–605. doi: 10.1016/S0002-9149(00)01438-7. [DOI] [PubMed] [Google Scholar]
- 14.Roest AA, de Roos A, Lamb HJ, Helbing WA, van den Aardweg JG, Doornbos J, van der Wall EE, Kunz P. Tetralogy of Fallot: postoperative delayed recovery of left ventricular stroke volume after physical exercise assessment with fast MR imaging. Radiology. 2003;1:278–284. doi: 10.1148/radiol.2261011164. [DOI] [PubMed] [Google Scholar]
- 15.Roest AA, Helbing WA, Kunz P, van den Aardweg JG, Lamb HJ, Vliegen HW, van der Wall EE, de Roos A. Exercise MR imaging in the assessment of pulmonary regurgitation and biventricular function in patients after tetralogy of fallot repair. Radiology. 2002;1:204–211. doi: 10.1148/radiol.2231010924. [DOI] [PubMed] [Google Scholar]
- 16.Sundareswaran KS, Pekkan K, Dasi LP, Whitehead K, Sharma S, Kanter KR, Fogel MA, Yoganathan AP. The total cavopulmonary connection resistance: a significant impact on single ventricle hemodynamics at rest and exercise. Am J Physiol Heart Circ Physiol. 2008;6:H2427–H2435. doi: 10.1152/ajpheart.00628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehead KK, Pekkan K, Kitajima HD, Paridon SM, Yoganathan AP, Fogel MA. Nonlinear power loss during exercise in single-ventricle patients after the Fontan: insights from computational fluid dynamics. Circulation. 2007;11(Suppl):I165–I171. doi: 10.1161/CIRCULATIONAHA.106.680827. [DOI] [PubMed] [Google Scholar]
- 18.Hofman MB, Visser FC, van Rossum AC, Vink QM, Sprenger M, Westerhof N. In vivo validation of magnetic resonance blood volume flow measurements with limited spatial resolution in small vessels. Magn Reson Med. 1995;6:778–784. doi: 10.1002/mrm.1910330606. [DOI] [PubMed] [Google Scholar]
- 19.Tops LF, Roest AA, Lamb HJ, Vliegen HW, Helbing WA, van der Wall EE, de Roos A. Intraatrial repair of transposition of the great arteries: use of MR imaging after exercise to evaluate regional systemic right ventricular function. Radiology. 2005;3:861–867. doi: 10.1148/radiol.2373041347. [DOI] [PubMed] [Google Scholar]
- 20.Weber TF, von Tengg-Kobligk H, Kopp-Schneider A, Ley-Zaporozhan J, Kauczor HU, Ley S. High-resolution phase-contrast MRI of aortic and pulmonary blood flow during rest and physical exercise using a MRI compatible bicycle ergometer. Eur J Radiol. 2011;1:103–108. doi: 10.1016/j.ejrad.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 21.Steeden JA, Atkinson D, Taylor AM, Muthurangu V. Assessing vascular response to exercise using a combination of real-time spiral phase contrast MR and noninvasive blood pressure measurements. J Magn Reson Imaging. 2010;4:997–1003. doi: 10.1002/jmri.22105. [DOI] [PubMed] [Google Scholar]
- 22.Frayne R, Steinman DA, Ethier CR, Rutt BK. Accuracy of MR phase contrast velocity measurements for unsteady flow. J Magn Reson Imaging. 1995;4:428–431. doi: 10.1002/jmri.1880050410. [DOI] [PubMed] [Google Scholar]
- 23.Powell AJ, Maier SE, Chung T, Geva T. Phase-velocity cine magnetic resonance imaging measurement of pulsatile blood flow in children and young adults: in vitro and in vivo validation. Pediatr Cardiol. 2000;2:104–110. doi: 10.1007/s002469910014. [DOI] [PubMed] [Google Scholar]
- 24.Prakash A, Garg R, Marcus EN, Reynolds G, Geva T, Powell AJ. Faster flow quantification using sensitivity encoding for velocity-encoded cine magnetic resonance imaging: in vitro and in vivo validation. J Magn Reson Imaging. 2006;3:676–682. doi: 10.1002/jmri.20654. [DOI] [PubMed] [Google Scholar]
- 25.Stahlberg F, Mogelvang J, Thomsen C, Nordell B, Stubgaard M, Ericsson A, Sperber G, Greitz D, Larsson H, Henriksen O. A method for MR quantification of flow velocities in blood and CSF using interleaved gradient-echo pulse sequences. Magn Reson Imaging. 1989;6:655–667. doi: 10.1016/0730-725X(89)90535-3. [DOI] [PubMed] [Google Scholar]
- 26.Evans AJ, Iwai F, Grist TA, Sostman HD, Hedlund LW, Spritzer CE, Negro-Vilar R, Beam CA, Pelc NJ. Magnetic resonance imaging of blood flow with a phase subtraction technique. In vitro and in vivo validation. Invest Radiol. 1993;2:109–115. doi: 10.1097/00004424-199302000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Goo HW, Al-Otay A, Grosse-Wortmann L, Wu S, Macgowan CK, Yoo SJ. Phase-contrast magnetic resonance quantification of normal pulmonary venous return. J Magn Reson Imaging. 2009;3:588–594. doi: 10.1002/jmri.21691. [DOI] [PubMed] [Google Scholar]
- 28.Grosse-Wortmann L, Al-Otay A, Goo HW, Macgowan CK, Coles JG, Benson LN, Redington AN, Yoo SJ. Anatomical and functional evaluation of pulmonary veins in children by magnetic resonance imaging. J Am Coll Cardiol. 2007;9:993–1002. doi: 10.1016/j.jacc.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 29.Valsangiacomo ER, Barrea C, Macgowan CK, Smallhorn JF, Coles JG, Yoo SJ. Phase-contrast MR assessment of pulmonary venous blood flow in children with surgically repaired pulmonary veins. Pediatr Radiol. 2003;9:607–613. doi: 10.1007/s00247-003-0983-9. [DOI] [PubMed] [Google Scholar]
- 30.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;1:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 31.Hughson RL. Regulation of blood flow at the onset of exercise by feed forward and feedback mechanisms. Can J Appl Physiol. 2003;5:774–787. doi: 10.1139/h03-058. [DOI] [PubMed] [Google Scholar]
- 32.Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;4:315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- 33.Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol. 1996;4(Pt 2):H1697–H1701. doi: 10.1152/ajpheart.1996.271.4.H1697. [DOI] [PubMed] [Google Scholar]
- 34.Petersson J, Rohdin M, Sanchez-Crespo A, Nyren S, Jacobsson H, Larsson SA, Lindahl SG, Linnarsson D, Neradilek B, Polissar NL, Glenny RW, Mure M. Regional lung blood flow and ventilation in upright humans studied with quantitative SPECT. Respir Physiol Neurobiol. 2009;1:54–60. doi: 10.1016/j.resp.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 35.Petersson J, Sanchez-Crespo A, Rohdin M, Montmerle S, Nyren S, Jacobsson H, Larsson SA, Lindahl SG, Linnarsson D, Glenny RW, Mure M. Physiological evaluation of a new quantitative SPECT method measuring regional ventilation and perfusion. J Appl Physiol. 2004;3:1127–1136. doi: 10.1152/japplphysiol.00092.2003. [DOI] [PubMed] [Google Scholar]
- 36.Petersson J, Rohdin M, Sanchez-Crespo A, Nyren S, Jacobsson H, Larsson SA, Lindahl SG, Linnarsson D, Neradilek B, Polissar NL, Glenny RW, Mure M. Posture primarily affects lung tissue distribution with minor effect on blood flow and ventilation. Respir Physiol Neurobiol. 2007;3:293–303. doi: 10.1016/j.resp.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell CP, Lock JE, Powell AJ, Perry SB. Compression of pulmonary veins between the left atrium and the descending aorta. Am J Cardiol. 2003;2:248–251. doi: 10.1016/S0002-9149(02)03120-X. [DOI] [PubMed] [Google Scholar]
- 38.Yamaji H, Hina K, Kawamura H, Murakami T, Murakami M, Yamamoto K, Hirohata A, Miyoshi T, Hirohata S, Kusachi S. Prone position is essential for detection of pulmonary vein pseudostenosis by enhanced multidetector computed tomography in patients who undergo pulmonary vein isolation. Circ J. 2008;9:1460–1464. doi: 10.1253/circj.CJ-08-0055. [DOI] [PubMed] [Google Scholar]
- 39.Sylvester JT, Shimoda LA, Aaronson PI, Ward JP. Hypoxic pulmonary vasoconstriction. Physiol Rev. 2012;1:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


