Abstract
This study investigated the relationship between fetal movements and acute maternal emotional changes during pregnancy. Two empirically validated feature film clips were used for the external generation of two subjectively and facially well-characterized target emotions: happiness and sadness. We simultaneously monitored separate fetal arm, leg, and trunk movements by means of two ultrasound apparatuses while maternal emotions were manipulated by film clip presentation. The number of fetal arm movements, but not the duration, was increased when pregnant women were being shown a happy film. Both the number and the duration of fetal arm movements decreased with the sad film presentation. Neither the presentation of happiness nor the presentation of sadness affected fetal leg or trunk movements. These findings suggest that induced emotions in pregnant women primarily affect arm movements of their fetuses, and that positive and negative emotions have the opposite effects on fetus movement.
Keywords: Maternal emotions, Film-induced positive and negative emotions, Fetal movement, Ultrasonography, Pregnancy
Introduction
The association between maternal psychological well-being during pregnancy and fetal welfare has recently attracted increasing attention. Chronic stress exposure for pregnant women affects fetal development, resulting in preterm birth and low birth weight [1–4]. In addition to stress, persistent negative maternal emotions during pregnancy such as anxiety, depression, and anger also exert an influence on fetal and later development of a child [5–9].
Increased maternal anxiety during pregnancy has been shown to be associated with preterm birth, higher incidence of low birth weight [10], and increased neonatal risk indicated by a low Apgar score [11, 12]. The Apgar score is commonly used to evaluate neonatal well-being immediately after birth [13]. It has also been reported that newborn infants of anxious women cry significantly more frequently than infants of less anxious women [5]. In depressed pregnant women, obstetric complication, shorter gestation, and spontaneous preterm labor are observed more frequently [14, 15]. Newborn infants of depressed women have shown poor performance on the Brazelton Neonatal Behavior Assessment Scale and such excessive crying that they were difficult to soothe [16–20]. Furthermore, the newborns of high-anger women show disorganized sleep patterns and less optimal performance on the Brazelton Neonatal Behavior Assessment Scale [9].
Persistent effects of maternal negative emotions on prenatal and postnatal development of children have been researched extensively, but there is remarkably little understanding of fetal responsiveness in situ to acute maternal emotional changes. Furthermore, no research has been reported on the effects of positive emotions of pregnant women on fetal behaviors. In the present study, we investigated whether fetuses respond to acute maternal positive and negative emotional changes, defined as happiness and sadness.
Previous ultrasonographic studies have assessed fetal activities by counting the total number of fetal limb and trunk movements without discriminating individual body parts [8, 21–23]. To fully understand fetal behaviors, movements of individual body parts should be measured separately. In the present study, we applied two ultrasounds to simultaneously observe fetal arm, leg, and trunk movements separately, enabling us to discriminatively count the numbers of the three different body part movements.
Methods
Participants
Forty-eight pregnant women with singleton fetuses ranging in gestational age from 28 to 36 weeks were recruited at Nagasaki University and Ushimaru Obstetric Clinic. Thirteen women were excluded from participation because they smoked or drank alcohol (n = 2), obstetrical complications were noted (e.g., maternal diabetes, hypertension, or placenta previa) (n = 9), or they suffered from mental disorders (n = 2). Among 35 mothers, 6 participants were excluded from the final sample for the following reasons: examination interrupted (n = 3) or fetal movements not recorded because of technical problems (n = 3). We monitored fetal heart rate (FHR) to determine fetal arousal levels to control for fetal condition. The FHR tracings were judged visually and divided into four heart rate patterns (HRP): A through D [24]. It has been reported that HRP-B (active sleep) is the most frequently observed FHR pattern during the observation time [24–26]. Twenty-four fetuses showed HRP-B, which has a wider oscillation bandwidth with frequent accelerations during movements; three fetuses showed HRP-A, quiet sleep, which has stable heart rate with a narrow oscillation bandwidth; and two fetuses showed both HRP-A and HRP-B during the observation time. Thus, we performed the following experiments only for 24 pregnant mothers with HRP-B out of the 29 participant mothers.
The pertinent characteristics of the participants are given in Table 1. The mean age of final participants (n = 24) was 30.6 years (SD = 5.9, ranging from 20 to 39 years); all of them were Japanese. Twelve women were nulliparous, and the remaining twelve women had one or two children. The average gestational age of the 24 fetuses at the time of testing was 31.7 weeks (SD = 3.6 weeks, ranging from 28 to 36 weeks). All participating women were followed up until delivery. All fetuses were delivered after 37 weeks (mean ± SD = 39.3 ± 3.6 weeks, ranging from 37 to 41 weeks), and none of them were small-for-date. The average weight of the neonates was 3,076 g at birth (SD = 430, ranging from 2,460 to 4,420 g); 12 of them were male and 12 female. All newborns had an Apgar score >7 at 5 min and did well after birth. The study was approved by the Ethics Committee of Nagasaki University, and informed consent was obtained from each subject. We determined gestational age by a combination of last menstrual period and sonogram.
Table 1.
Maternal and infant characteristics for each group
| Happiness group (n = 10) | Sadness group (n = 14) | Total (n = 24) | |
|---|---|---|---|
| Pregnant mother | |||
| Age (years) | 30.9 (6.7) | 30.3 (4.9) | 30.6 (5.9) |
| Primiparae/multiparae | 5/5 | 7/7 | 12/12 |
| Gestational age (weeks) | 32.5 (3.4) | 31.2 (3.8) | 31.7 (3.6) |
| Infant | |||
| Sex | |||
| Male | 7 | 5 | 12 |
| Female | 3 | 9 | 12 |
| Birth weight (g) | 3,162 (225) | 3,036 (501) | 3,076 (430) |
| Gestational age at delivery (weeks) | 39.8 (1.4) | 39.1 (1.0) | 39.3 (3.6) |
| Apgar score (1 min) | 8.8 (0.4) | 8.5 (0.8) | 8.6 (0.7) |
| Apgar score (5 min) | 9.0 (0) | 8.9 (4.5) | 8.9 (0.4) |
Data are presented as means (SD)
Stimulus films
Westermann et al. [27] demonstrated that the presentation of a film clip is most effective in inducing human positive and negative emotions in the laboratory. Thus two empirically validated feature film clips were used for the external generation of two subjectively and facially well-characterized target emotions, happiness and sadness. The happiness-inducing film clip was taken from “The Sound of Music” and depicted a scene of a female teacher smiling and singing for children. The sadness-inducing film clip was from “The Champ”, in which a boy cries at the death of his father. This video clip was validated by Gross and Levenson [28] and has been extensively used [29]. An emotionally neutral film clip was used to control for potentially confounding features of the emotion-generating film task, such as emotionally irrelevant visual stimulation and eye movement. This clip consisted of many figures slowly appearing, disappearing, or moving around.
We examined the effects of the two film clips on emotions of 22 pregnant women at gestational age 28–36 weeks in the same experimental conditions as following experimental conditions where effects of maternal emotion on the fetal movement were investigated. Participants were randomly assigned to the one of two independent groups (happy film group: n = 11 or sad film group: n = 11) in order to avoid any interactions between the effect of emotions elicited by the happy film and the effect of emotions elicited by the sad film. During the examinations, the pregnant women rested in a semi-supine position in a 30 lux lit room. Film clips were shown on a 21 inch monitor placed on a table in front of the women at 1 m distance. The women listened to the sound of the film through headphones. We presented the films in the following order: neutral and happy for the happy film group, or neutral and sad for sad film group. After the each film was presented, they were asked to rate their emotional state with the visual analog scale (VAS [30]) on the two emotional dimensions of happiness and sadness (see Table 2). VAS is a horizontal line 10 cm long with a statement at one end that the emotion is stronger and at the other, that it is completely absent. After watching the film clips, subjects made a mark somewhere along its length to indicate their impression of the strength of those emotions on a 10-point scale. To assess the effect of the film clips on their emotional states, paired t test was conducted. In the happy film group, happiness was increased after happy film presentation compared with that after neutral film presentation [t (10) = 3.30, p < 0.01], but sadness was not changed [t (10) = 0.72]. In the sad film group, sadness was increased after the sad film presentation compared with that after neutral film presentation [t (10) = 3.42, p < 0.01] and happy was decreased after sad film presentation compared with that after neutral film exposure [t (10) = 3.33, p < 0.01]. Taken together, these results showed that film clips of “The Sound of Music” and “The Champ” elicited the targeted emotions in pregnant women in the following experimental conditions.
Table 2.
The effects of the film clips on their emotion states
| After neutral film presentation (0–10) | After emotional film presentation (0–10) | p value | |
|---|---|---|---|
| Happy film group (n = 11) | |||
| Happiness | 2.82 (3.07) | 6.91 (2.80) | <0.01 |
| Sadness | 0.64 (1.64) | 0.82 (1.31) | 0.72 |
| Sad film group (n = 11) | |||
| Happiness | 3.81 (2.85) | 2.09 (2.20) | 0.01 |
| Sadness | 0.59 (1.80) | 4.05 (3.09) | 0.01 |
Data are presented as mean (SD)
Procedures
Ultrasound observations of fetal activity and FHR monitoring were carried out for 30 min between 2:00 and 4:00 pm in a quiet room. The women were asked to refrain from food and coffee for at least 2 h prior to their visit.
Participant mothers were randomly assigned to the following two groups: (1) the happiness group (n = 10), pregnant mothers who were presented the happy film; and (2) the sadness group (n = 14), pregnant mothers who were presented the sad film. These two independent groups were homogeneous for maternal age, t (22) = 0.22, p = 0.83; primiparous/multiparous, χ 2 = 0, p = 0.66; gestational age at the time of testing, t (22) = 0.86, p = 0.40; birth gestational age, t (22) = 0.86, p = 0.40; sex of neonate, χ 2 = 2.74, p = 0.11; birth weight of neonate, t (22) = 1.27, p = 0.22; and Apgar score (5 min), t (22) = 0.38, p = 0.70 (see Table 1).
During the examinations, the pregnant women rested in a semi-supine position in a 30 lux lit room. After brief ultrasound examination to determine fetal position for monitoring, film clips were shown on a 21 inch monitor placed on a table in front of the women at 1 m distance. The women listened to the sound of the film through headphones so the fetuses would not directly respond to the sound of films coming from outside the abdomen. We presented the films in the following order: neutral, happy, neutral, or neutral, sad, neutral for the happiness and sadness groups, respectively.
Measures
Ultrasound observations of fetal activity were made with the aid of two 5.0 MHz real-time linear array ultrasound scanners (ALOKA model ProSound SSD-5000SV and ALOKA model ProSound SSD-5500SV). Examinations were carried out by two operators. One operator held one transducer stationary on the maternal abdomen to observe isolated fetal arm movements. The other operator used another transducer to observe isolated fetal leg movements and fetal trunk movements. The ultrasound screen was placed out of sight of the pregnant women. Another technician, knowledgeable about the kind of film being shown, monitored FHR for 30 min using a cardiotocograph (Atom Corometrics 116, JAPAN; paper speed 3 cm/min) to analyze the FHR patterns.
Data analysis
Fetal body movements were recorded on DVD for off-line analysis. Women with fetuses that did not show HRP-B were excluded from this study, as described previously. We counted the number of fetal movements of individual body parts per 5 min and the duration time from the beginning to the end of each movement. Fetal movement recordings occurring within 2 s of each other were considered to belong to the same movement [25, 31]. However, there was a case that could not be observed because the movements were intense and shifted from the screen. Inter-rater reliability, calculated on one-third of the sample for two observers, yielded the following kappa values: isolated fetal arm movements = 0.87, isolated fetal leg movements = 0.86, fetal trunk movement = 1.0. Our method was, therefore, found to be valuable [32]. Then, we calculated the mean values for each of the 5 min periods (neutral, happy or sad, neutral).
Statistics
Data are presented as mean ± standard error (SE). To assess the differential effect of film stimuli on fetal movements, one-way repeated measures ANOVAs followed by Fisher’s PLSD post-hoc test were computed. All analyses were done with Stat View 5.0J.
Results
Number and duration of arm movements
The numbers of fetal arm movements are shown in Fig. 1. There were significant differences in the number of arm movements in the happiness group [F (2, 14) = 5.9, p < 0.05]. While pregnant women were viewing the happy film, the number of fetal arm movements increased compared with movements during the neutral film presentation (p < 0.05). Afterward, the number of arm movements returned to basal level during the next neutral film presentation (p < 0.01) (Fig. 1a). In the case of the sadness group, significant differences were observed in the number of arm movements [F (2, 16) = 6.9, p < 0.01]. The number of arm movements decreased during the sad film presentation (p < 0.05), but did not return to basal level during the next neutral film exposure (p < 0.005) (Fig. 1b).
Fig. 1.
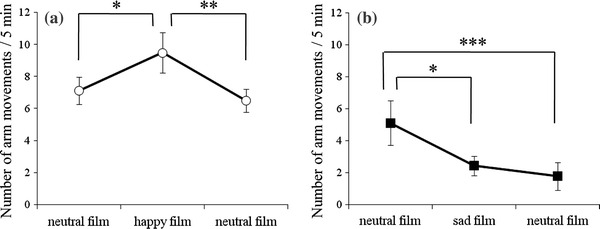
The number of fetal arm movements for a the happiness group (n = 8) and b the sadness group (n = 9). Asterisks indicate a significant difference from baseline (*p < 0.05, **p < 0.01, ***p < 0.005)
There were no differences in the duration of fetal arm movements in the happiness group (Fig. 2a). In contrast, significant differences were observed in the duration of arm movements in the sadness group [F (2, 16) = 4.0, p < 0.05]. Presentation of sadness did not change the duration of fetal arm movements significantly, but during the next neutral film presentation, a significant decrease in duration was observed (p < 0.05) compared with that during the first neutral film (Fig. 2b).
Fig. 2.
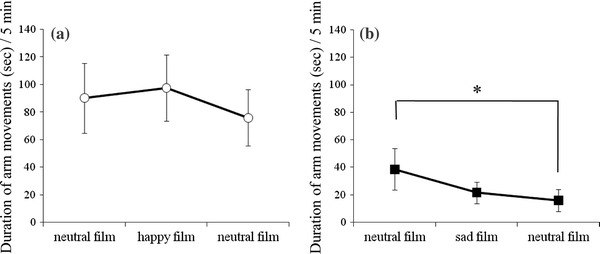
The duration of fetal arm movements for a the happiness group (n = 8) and b the sadness group (n = 9). The asterisk indicates a significant difference from baseline (*p < 0.05)
Number and duration of leg movements
The number and duration of leg movements were not affected by film presentations in either the happiness group or the sadness group (Figs. 3, 4).
Fig. 3.
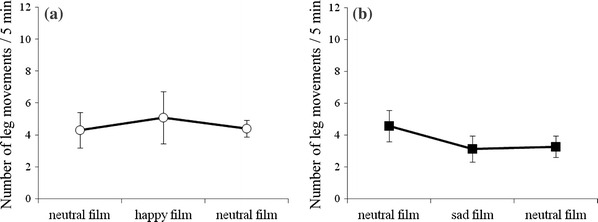
The number of fetal leg movements for a the happiness group (n = 10) and b the sadness group (n = 14)
Fig. 4.
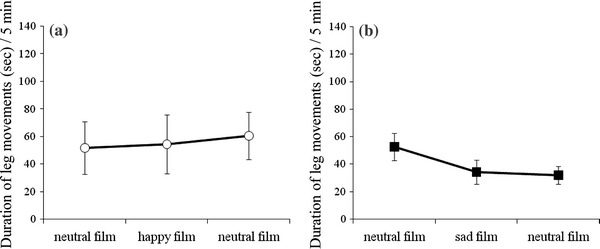
The duration of fetal leg movements for a the happiness group (n = 10) and b the sadness group (n = 14)
Number and duration of trunk movements
The number and duration of trunk movements were not affected by film presentations in either the happiness group or the sadness group (Figs. 5, 6).
Fig. 5.
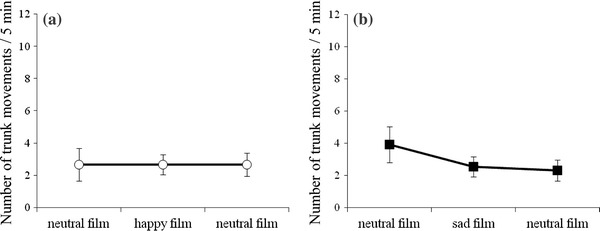
The number of fetal trunk movements for a the happiness group (n = 6) and b the sadness group (n = 13)
Fig. 6.
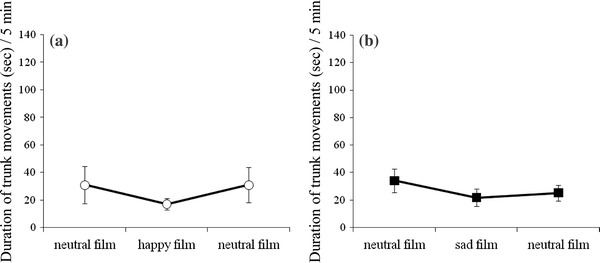
The duration of fetal trunk movements for a the happiness group (n = 6) and b the sadness group (n = 13)
Discussion
In the present study, we simultaneously monitored fetal movements of arm, leg, and trunk separately by means of two ultrasound apparatuses while maternal emotions were manipulated by film clip presentation. The ultrasonographic observation revealed that the number of fetal arm movements increased when pregnant women were being shown a happy film. However, fetal arm movements decreased during presentation of a sad film. These findings suggest that induced emotions in pregnant women affect arm movements of their fetuses, and the effects of positive and negative emotions on fetus movements are opposite.
The fetal leg movements were not affected by maternal happiness or sadness and the fetal trunk movements were also not affected. In this case, the effects of maternal emotions could not be detected by counting the number of leg or trunk movements possibly because trunks and legs were too big to easily move inside the uterus. Thus it is suggested that the number of fetal arm movements could be a sensitive parameter in assessing the fetal response to induced maternal emotions.
There is a substantial body of knowledge concerning the effects of long-lasting maternal emotional changes on fetal activity, that is, the association between maternal dispositional attributes and fetal behaviors. Ultrasound observations have revealed that more frequent movements were observed in the fetuses of mothers with relatively high anxiety [8, 17] with a significant positive correlation between the level of maternal anxiety and fetal movement [33]. The fetuses of high-anger women were also reported to be more active [9], suggesting that long-lasting maternal negative feelings accelerate neonatal body movements. However, it should be noted that fetuses of anxious mothers exhibited less body movement during active sleep [34].
On the other hand, there have been few reports on the nature of fetal response in situ to acute maternal emotional changes. When mental stresses, possibly including negative emotions, were induced in pregnant women using the Stroop Color and Word Test, fetal activity decreased [35], consistent with the finding observed in the present study that the negative emotion of sadness decreased fetal activity. However, suppression of arm movement by sad film presentation did not return to the base line level during the next neutral film presentation. In the present study, we could not clarify that mechanism. Indeed the effectiveness of acute emotional induction is higher for negative than for positive mood [27]. It is possible that this may be due to the fact that subjects usually enter the experiment in a rather positive mood and that such a positively biased mood is harder to enhance than to negate.
Our findings are in contrast with a previous study reporting an increase in fetal activity associated with induced maternal anxiety [36]. However, in that study researchers observed fetal behavior in mothers who experienced panic after an earthquake, a severe anxiety that does not occur in daily life. Furthermore, potential confounding effects of maternal smoking, caffeine ingestion, and meal intake were not controlled for in their study. In addition, we did not elicit both happy and sad emotions in the same subject to avoid any interactions between the effects of emotion elicited by the happy film and the sad film. Therefore, a direct comparison between that study and the present study is difficult to make.
Here, we demonstrated that positive and negative emotions exert opposite effects on fetal movements. However, what are the implications and consequences of the fetal arm movements? With regard to fetus development, fetal movement is one of five items used to assess fetal well-being [37]. Decreased fetal movements predict oligoamnios, intrapartum fetal distress, low Apgar score, and meconium in the amniotic fluid [38]. Meanwhile, with regard to maternal well-being or health, it has been reported that fetal movements enhanced the maternal-fetal attachment process [39]. It is worth noting that fetuses responded to maternal emotional changes that can occur in daily life, such as watching TV programs and movies.
In the present study, we could not clarify the mechanism underlying fetal movement associated with induced maternal emotions. However, previous literature suggests three possible mechanisms.
First, it is possible that induced maternal emotions enhance sympathetic nervous system activity, which affects fetal activity by contracting the uterus; uterine contractions have been shown to decrease fetal movement [40]. However, this may be unlikely because at the end of pregnancy the numbers of both myometrial and perivascular adrenergic nerves are decreased in humans [41, 42].
Second, increases in fetal cortisol, resulting from maternal cortisol response to stress [43, 44], might activate fetuses because the peak of diurnal rhythms in fetal movement characteristically occurs at the nadir of diurnal rhythms in maternal cortisol concentration. However, a strong correlation between maternal and fetal cortisol levels may not explain an immediate link between maternal mood and fetal behavior because it takes about 15 min for plasma cortisol to start to increase, but fetal movements changed within 5 min of film presentation in the present study.
A third possible mechanism is reduced blood flow through the uterine arteries due to the increased catecholamines resulting from maternal negative emotions. Increases in plasma noradrenaline concentrations were observed in pregnant women with high scores for anxiety [45]. Also, infusion of noradrenaline reduced uterine blood flow, both in pregnant sheep [46] and pregnant guinea pigs [47]. Recently, abnormal uterine blood flow has been shown to be closely associated with maternal anxiety [48].
Until now, many researchers have typically counted the total number of fetal movements of trunk and limbs [8, 9, 23, 31] and have not discriminated individual body movements. Because we introduced two ultrasounds to assess individual body parts separately as isolated limbs and trunk, we were able to observe that the acute emotional changes in pregnant women that may occur in daily life can influence fetal behavior.
Zimmer et al. [49] used real-time ultrasound scanning and reported a significant decrease in fetal breathing movements and a trend toward an increase in body movements while mothers were listening to their preferred type of music. However, they did not find any effect on fetal motor activity or behavioral state organization as a result of maternal emotions induced by watching a film of a normal delivery. It has been shown that massage therapy during pregnancy can reduce depression and its associated elevated stress hormones, modulated by brain neurotransmitters, resulting in more optimal neonatal outcomes [21].
If the emotion of sadness is stressful, it appears that to help the growth and development of fetuses, to reduce negative feelings or stress and to increase positive feelings in pregnant women would be beneficial.
Acknowledgments
This research was supported financially by the Research Institute of Science and Technology for Society (RISTEX), Japan Science and Technology Agency (JST). We are grateful to the medical staff of the clinic and hospital for their technical help. We are also grateful to Sumihisa Honda for providing statistical processing help.
References
- 1.Sandman CA, Wadhwa PD, Dunkel-Schetter C, Chicz-DeMet A, Belman J, Porto M, Murata Y, Garite TJ, Crinella FM. Psychobiological influences of stress and HPA regulation on the human fetus and infant birth outcomes. Ann N Y Acad Sci. 1994;739:198–210. doi: 10.1111/j.1749-6632.1994.tb19822.x. [DOI] [PubMed] [Google Scholar]
- 2.McCubbin JA, Lawson EJ, Cox S, Sherman JJ, Norton J, Read JA. Prenatal maternal blood pressure response to stress predicts birth weight and gestational age: a preliminary study. Am J Obstet Gynecol. 1996;175:706–712. doi: 10.1053/ob.1996.v175.a74286. [DOI] [PubMed] [Google Scholar]
- 3.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- 4.Wadhwa PD, Porto M, Garite TJ, Chicz-DeMet A, Sandman CA. Maternal corticotropin-releasing hormone levels in the early third trimester predict length of gestation in human pregnancy. Am J Obstet Gynecol. 1998;179:1079–1085. doi: 10.1016/S0002-9378(98)70219-4. [DOI] [PubMed] [Google Scholar]
- 5.Ottinger DR, Simmons JE. Behavior of human neonates and prenatal maternal anxiety. Psychol Rep. 1964;14:391–394. [Google Scholar]
- 6.Steer RA, School TO, Hediger M, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol. 1992;45:1093–1099. doi: 10.1016/0895-4356(92)90149-H. [DOI] [PubMed] [Google Scholar]
- 7.Glover V, Teixeira J, Gitau R, Fisk NM. Mechanisms by which maternal mood in pregnancy may affect the fetus. Contemp Rev Obstet Gynecol. 1999;11:155–160. [Google Scholar]
- 8.Dieter J, Field T, Hernandez-Reif M, Jones NA, Lecanuet JP, Salman FA, Redzepl M. Maternal depression and increased fetal activity. J Obstet Gynecol. 2001;21:468–473. doi: 10.1080/01443610120072009. [DOI] [PubMed] [Google Scholar]
- 9.Field T, Diego M, Hernandez-Reif M, Salman F, Schanberg S, Kuhn C, Regina Y, Bendell D. Prenatal anger effects on the fetus and neonate. J Obstet Gynecol. 2002;22:260–266. doi: 10.1080/01443610220130526. [DOI] [PubMed] [Google Scholar]
- 10.Davids A, DeVault S. Maternal anxiety during pregnancy and childbirth abnormalities. Psychosom Med. 1962;24:464–470. doi: 10.1097/00006842-196209000-00004. [DOI] [PubMed] [Google Scholar]
- 11.McDonald R, Gynther MD, Christakos AC. Relations between maternal anxiety and obstetric complications. Psychosom Med. 1963;25:357–363. [Google Scholar]
- 12.Crandon AJ. Maternal anxiety and neonatal well being. J Psychosom Res. 1979;23:113–115. doi: 10.1016/0022-3999(79)90015-1. [DOI] [PubMed] [Google Scholar]
- 13.Apgar V. Proposal for new method of evaluation of new-born infant. Curr Res Anesth Analg. 1953;32:260–267. [PubMed] [Google Scholar]
- 14.Dayan J, Creveuil C, Herlicoviez M, Herbel C, Baranger E, Savoye C, Thouin A. Role of anxiety and depression in the onset of spontaneous preterm labor. Am J Epidemiol. 2002;155:293–301. doi: 10.1093/aje/155.4.293. [DOI] [PubMed] [Google Scholar]
- 15.Larsson C, Sydsjo G, Josefsson A. Health, sociodemographic data, and pregnancy outcome in women with antepartum depressive symptoms. Obstet Gynecol. 2004;104:459–466. doi: 10.1097/01.AOG.0000136087.46864.e4. [DOI] [PubMed] [Google Scholar]
- 16.Abram SM, Field T, Scafidi F, Prodromidis M. Newborns of depressed mothers. Infant Ment Health J. 1995;16:233–239. doi: 10.1002/1097-0355(199523)16:3<233::AID-IMHJ2280160309>3.0.CO;2-1. [DOI] [Google Scholar]
- 17.Field T, Sandberg D, Quetel TA, Garcia R, Rosario M. Effects of ultrasound feedback on pregnancy anxiety, fetal activity, and neonatal outcome. Obstet Gynecol. 1985;66:525–528. [PubMed] [Google Scholar]
- 18.Field T. Infants of depressed mothers. Infant Behav Dev. 1995;18:1–13. doi: 10.1016/0163-6383(95)90003-9. [DOI] [PubMed] [Google Scholar]
- 19.Nishitani S, Miyamura T, Tagawa M, Sumi M, Takase R, Doi H, Moriuchi H, Shinohara K. The calming effect of a maternal breast milk odor on the human newborn infant. Neurosci Res. 2009;63:66–71. doi: 10.1016/j.neures.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Zuckerman B, Bauchner H, Parker S, Cabral H. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11:190–194. doi: 10.1097/00004703-199008000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn Cynthia K, Yando R, Bendell D. Depressed withdrawn and intrusive mothers’ effects on their fetuses and neonates. Infant Behav Dev. 2001;24:27–39. doi: 10.1016/S0163-6383(01)00066-2. [DOI] [Google Scholar]
- 22.Gotoh H, Masuzaki H, Yoshida A, Yoshimura S, Miyamura T, Ishimaru T. Predicting incomplete uterine rupture with vaginal sonography during the late second trimester in women with prior cesarean. Obstet Gynecol. 2000;95:596–600. doi: 10.1016/S0029-7844(99)00620-1. [DOI] [PubMed] [Google Scholar]
- 23.Sjostrom K, Valentin L, Thelin T, Marsal K. Maternal anxiety in late pregnancy: effect on fetal movements and fetal heart rate. Early Hum Dev. 2002;67:87–100. doi: 10.1016/S0378-3782(01)00256-0. [DOI] [PubMed] [Google Scholar]
- 24.Mulder EJH, Visser GHA, Bekedam DJ, Prechtl HF. Emergence of behavioral states in fetuses of type-1 diabetic women. Early Hum Dev. 1987;15:231–252. doi: 10.1016/0378-3782(87)90082-X. [DOI] [PubMed] [Google Scholar]
- 25.Robles de Medina PG, Visser GHA, Huizink AC, Buitelaar JK, Mulder EJH. Fetal behavior does not differ between boys and girls. Early Hum Dev. 2003;73:17–26. doi: 10.1016/S0378-3782(03)00047-1. [DOI] [PubMed] [Google Scholar]
- 26.Sjostrom K, Thelin T, Marsal K, Valentin L. Effects of maternal anxiety on perception of fetal movements in late pregnancy. Early Hum Dev. 2003;72:111–122. doi: 10.1016/S0378-3782(03)00031-8. [DOI] [PubMed] [Google Scholar]
- 27.Westermann R, Spies K, Stahl G, Hesse F. Relative effectiveness and validity of mood induction procedures: a meta-analysis. Eur J Soc Psychol. 1996;26:557–580. doi: 10.1002/(SICI)1099-0992(199607)26:4<557::AID-EJSP769>3.0.CO;2-4. [DOI] [Google Scholar]
- 28.Gross JJ, Levenson R. Emotion elicitation using films. Cognit Emot. 1995;9:87–108. doi: 10.1080/02699939508408966. [DOI] [Google Scholar]
- 29.Goldin PR, Hutcherson CAC, Ochsner KN, Glover GH, Gabrieli GDE, Gross JJ. The neural bases of amusement and sadness: a comparison of block contrast and subject-specific emotion intensity regression approaches. NeuroImage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell C. Sensitivity and accuracy of the visual analogue scale: a psycho-physical classroom experimenrt. Br J Clin Pharmacol. 1978;6:15–24. doi: 10.1111/j.1365-2125.1978.tb01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ten Hof J, Nijhui IJM, Nijhuis JG, Narayan H, Taylor DJ, Visser GH, Mulder EJ. Quantitative analysis of fetal general movements: methodological considerations. Early Hum Dev. 1999;56:57–73. doi: 10.1016/S0378-3782(99)00035-3. [DOI] [PubMed] [Google Scholar]
- 32.Diego MA, Dieter JN, Field T, Lecanuet JP, Hernandez-Reif M, Beutler J, Largie S, Redzepi M, Salman FA. Fetal activity following stimulation of the mother’s abdomen, feet, and hands. Dev Psychobiol. 2002;41(4):396–406. doi: 10.1002/dev.10071. [DOI] [PubMed] [Google Scholar]
- 33.Van den Bergh BRH, Mulder EJH, Visser GHA, Poelmann-Weesjes G, Bekedam DJ, Prechtl HFR. The effect of (induced) maternal emotions on fetal behavior: a controlled study. Early Hum Dev. 1989;19:9–19. doi: 10.1016/0378-3782(89)90100-X. [DOI] [PubMed] [Google Scholar]
- 34.Groome L, Swiber M, Bentz LS, Holland SB, Atterbury JL. Maternal anxiety during pregnancy: effect on fetal behavior at 38 to 40 weeks of gestation. J Dev Behav Pediatr. 1995;16:391–396. doi: 10.1097/00004703-199512000-00001. [DOI] [PubMed] [Google Scholar]
- 35.DiPietr JA, Costigan KA, Gurewitsch ED. Fetal response to induced maternal stress. Early Hum Dev. 2003;74:125–138. doi: 10.1016/j.earlhumdev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Ianniruberto A, Tajani E. Ultrasonographic study of fetal movements. Semin Perinatol. 1981;5:175–181. [PubMed] [Google Scholar]
- 37.Manning FA, Platt LD, Sipos L. Antepartum fetal evaluation: development of a fetal biophysical profile. Am J Obstet Gynecol. 1980;136(6):787–795. doi: 10.1016/0002-9378(80)90457-3. [DOI] [PubMed] [Google Scholar]
- 38.Berbey R, Manduley A, Vigil-De Gracia P. Counting fetal movements as a universal test for fetal wellbeing. Int J Gynaecol Obstet. 2001;74(3):293–295. doi: 10.1016/S0020-7292(01)00438-6. [DOI] [PubMed] [Google Scholar]
- 39.Mikhail MS, Freda MC, Merkatz RB, Polizzotto R, Mazloom E, Merkatz IR. The effect of fetal movement counting on maternal attachment to fetus. Am J Obstet Gynecol. 1991;165(4Pt 1):988–991. doi: 10.1016/0002-9378(91)90455-z. [DOI] [PubMed] [Google Scholar]
- 40.Natale R, Clewlow F, Dawes GS. Measurement of forelimb movements in the lamb in utero. Am J Obstet Gynecol. 1981;140:545–551. doi: 10.1016/0002-9378(81)90231-3. [DOI] [PubMed] [Google Scholar]
- 41.Wikland M, Lindblom B, Dahlstrom A, Haglid KG. Structual and functional evidence for the denervation of human myometrium during pregnancy. Obstet Gynecol. 1984;64:503–509. [PubMed] [Google Scholar]
- 42.Morizaki N, Morizaki J, Hayashi RH, Garfield R. A functional and structural study of the innervation of the human uterus. Am J Obstet Gynecol. 1989;160:218–228. doi: 10.1016/0002-9378(89)90126-9. [DOI] [PubMed] [Google Scholar]
- 43.Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- 44.Gitau R, Fisk NM, Teixeria JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–109. doi: 10.1210/jc.86.1.104. [DOI] [PubMed] [Google Scholar]
- 45.Starkman MN, Cameron OG, Nesse RM, Zelnik T. Peripheral catecholamine levels and symptoms of anxiety: studies in patients with and without pheochromocytoma. Psychosom Med. 1990;52:129–142. doi: 10.1097/00006842-199003000-00001. [DOI] [PubMed] [Google Scholar]
- 46.Rosenfield CR, West J. Circulatory response to systemic infusion of norepinephrine in the pregnant ewe. Am J Obstet Gynecol. 1977;127:376–383. doi: 10.1016/0002-9378(77)90493-8. [DOI] [PubMed] [Google Scholar]
- 47.Fried G, Thoresen M. Effects of neuropeptide Y and noradrenaline on uterine artery blood pressure and blood flow velocity in the pregnant guinea pig. Regul Pept. 1990;28:1–9. doi: 10.1016/0167-0115(90)90059-6. [DOI] [PubMed] [Google Scholar]
- 48.Teixeira JM, Fisk N, Glover V. Association between maternal anxiety in pregnancy and increased uterine artery resistance index: cohort based study. BMJ. 1999;318:153–157. doi: 10.1136/bmj.318.7177.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zimmer EZ, Divon MY, Vilensky A. Maternal exposure to music and fetal activity. Eur J Obstet Gynecol Reprod Biol. 1982;13:209–213. doi: 10.1016/0028-2243(82)90101-0. [DOI] [PubMed] [Google Scholar]


