Abstract
Neuronal loss is reported to be an important pathological process in Alzheimer’s disease (AD). Neurogenesis is a process of generation of new neurons to fill the neuronal loss. Xanthoceraside has been shown to attenuate the cognitive deficits in several AD animal models. However, little is known about the effect of xanthoceraside on neurogenesis in APP/PS1 transgenic mice. Thus, in this study, we investigated whether xanthoceraside can ameliorate learning and memory impairment by promoting NSCs proliferation and neuronal differentiation. The results suggested that xanthoceraside significantly ameliorated the cognitive impairment and induced NSCs proliferation and neuronal differentiation in APP/PS1 transgenic mice. Meanwhile, in vitro study revealed that xanthoceraside increased the size of NSCs and induced NSCs differentiation into neurons compared with amyloid beta-peptide (25–35) (Aβ25–35) treatment. Furthermore, we found that xanthoceraside significantly increased the expression of Wnt3a and p-GSK3β, decreased the expression of p-β-catenin, and induced nuclear translocation of β-catenin in APP/PS1 transgenic mice. Furthermore, in vitro study found that the effect of xanthoceraside on inducing NSCs proliferation and neuronal differentiation were inhibited by Wnt pathway inhibitor Dickkopf-1 (Dkk-1). Our data demonstrated that xanthoceraside may promote the proliferation and differentiation of NSCs into neurons by up-regulating the Wnt/β-catenin pathway to fill the neuronal loss, thereby improving learning and memory impairment in APP/PS1 transgenic mice.
Keywords: Alzheimer’s disease, Xanthoceraside, Neurogenesis, Learning and memory, Wnt/β-catenin pathway
Introduction
Alzheimer’s disease (AD), as the most common form of senile dementia, affects more than 30 million people worldwide [1]. Growing evidence supports that amyloid-beta (Aβ), which is derived from β-amyloid precursor protein (APP), plays a crucial role in triggering a complex pathological cascade that leads to the neurodegenerative conditions, loss of neurons, and cognitive impairment observed in AD mice [2]. As reported, in APP/PS1 transgenic mice, spatial memory impairment appears at 3 months of age [3] and amyloid deposition visibly at 5 months of age [4]. The APP/PS1 transgenic mouse model of AD shows significant inhibition of neurogenesis at 7 and 10 months of age [5, 6]. Neurogenesis generates newly born cells to help brain repair in neurological diseases [7]. The human brain generates most of its newly born cells during the embryonic and post-natal periods of development. However, recent research has shown that the brain is capable of regenerating neurons throughout the adult lifespan in two main neurogenic regions of the brain including the subventricular zone (SVZ), which lines the lateral ventricles, and the dentate gyrus (DG) of the hippocampal formation [8]. The hippocampus is considered to be an important area for the functions of learning and memory, demonstrated by one of the reasons for the increasing survival of newborn neurons in learning and memory tasks [9]. It is known that adult hippocampal cells proliferate and differentiate into neurons, which play an important role in learning and memory processes [10]. There is increasing interest in the relationship between AD and hippocampal NSCs.
Neurogenesis during the adult lifespan is modulated by many variables, such as positive factors (including exercise [11], learning [12], and an enriched environment [13]), and negative factors (including aging [14], stress [15], sleep deprivation [16], and inflammation [17]). It is reported that Wnt/β-catenin signaling is mainly involved in neurogenesis in the hippocampal DG and SVZ during several extrinsic and intrinsic cellular programs [18] and regulates neurodevelopmental processes [19, 20]. The Wnt/β-catenin pathway, which mainly includes the proteins of wnt3a, secreted frizzled-related protein 3, glycogen synthase kinase-3β (GSK3β) and β-catenin. The Wnt/β-catenin pathway stimulated by Wnt ligands can elicit the inactivation of phosphorylation of GSK-3β at Ser9 (pSer9-GSK3β), leading to decreased levels of p-β-catenin, which can restrain β-catenin degradation through proteasomal degradation and induce the translocation of β-catenin into the nucleus to promote neurogenesis [21]. Defects in the Wnt/β-catenin signaling pathway have been considered to contribute to the pathogenesis of AD [22].
Xanthoceraside (Fig. 1a), a triterpenoid saponin monomer compound extracted from the husks of Xanthoceras sorbifolia Bunge, is used as a traditional treatment of rheumatism in Chinese folk medicine. Xanthoceraside exhibits protective effects on impairments of spatial memory, oxidative stress, and inflammatory reactions induced by the intracerebroventricular injection of the aggregated Aβ1–42 in mice [23]. Moreover, xanthoceraside might be associated with decreasing Aβ deposition [24] to protect cognitive functioning in AD animals. However, there is little known about the effect of xanthoceraside on neurogenesis. Thus, in this study, we investigated whether xanthoceraside can promote NSCs proliferation and neuronal differentiation to ameliorate learning and memory impairment in APP/PS1 transgenic mice; we also examined the potential related signaling pathways.
Fig. 1.
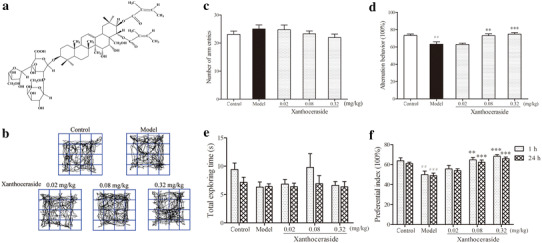
Effects of xanthoceraside on learning and memory impairment in APP/PS1 transgenic mice. a Chemical structure of xanthoceraside. b The pathway traveled by the mice during the open field test. c, d Total number of arm entries and spontaneous alternation behavior (%) in the Y-maze test. e, f Total exploration time and preferential index at 1 and 24 h during the testing phase. Values are expressed as the mean ± SEM (n = 11–12). ## p < 0.01 and ### p < 0.001 vs. control; **p < 0.01 and ***p < 0.001 vs. model
Materials and methods
Materials
The xanthoceraside provided by the Shenyang Institute of Applied Ecology, Chinese Academy of Sciences (Shenyang, China) was dissolved in double-distilled water (0.1% DMSO). Amyloid beta-peptide (25–35) (Aβ25–35) and 5-bromo-20-deoxyuridine (BrdU) obtained from Sigma-Aldrich (St. Louis, MO, USA) were dissolved in double-distilled water (0.1% DMSO) and 0.9% NaCl, respectively. Dickkopf-1 (Dkk-1) obtained from Peprotech (USA) was dissolved in double-distilled water.
Animals and experimental design
Male APPswe/PS1 deltaE9 double transgenic mice (APP/PS1) on a C57BL/6J background (3 months of age; n = 59) expressing an identical mouse/human APP Swedish mutations (K595N/M596L) and the exon-9-deleted variant of human presenilin 1 (PS1-deltaE9) were purchased from the Beijing HFK Bioscience Co. Ltd in these experiments.
APP/PS1 transgenic mice were randomly divided into model and xanthoceraside (0.02, 0.08, or 0.32 mg/kg) treatment groups. C57BL/6J mice were used as wild-type controls. Mice were orally administered xanthoceraside or vehicle once daily at 3 months of age until the mice were sacrificed at 8 months of age (n = 11–12 for each group). Open field test, Y-maze test, and novel object recognition test were started at the age of 7 months. The animals were handled daily for 1 week prior to initiating the experiment. Every test was conducted during the light cycle.
Animals were maintained on a 12/12 light–dark cycle in a SPF level laboratory under standard housing conditions (room temperature 22.5 °C ± 1 and humidity 50 ± 5%). Food and water were administered ad libitum. All surgery was performed under 0.6% pentobarbital sodium and all efforts were made to minimize suffering.
Open-field test
All animals were tested for spontaneous locomotor using an open-field test [25]. Mice were individually placed in the center area of an open-field apparatus (25 × 25 × 50 cm) with white walls. All movements were traced and the data were collected by video for 5 min without any disturbance.
Y-maze test
To evaluate spatial working memory performance, a Y-maze was used to record the spontaneous alternation behavior [26]. A custom-made Y-maze made of wood with three identical arms (40 × 10 × 12 cm, 120° apart) was used. This behavioral test was performed under dim lighting conditions, and each mouse was placed at the end of one fixed arm facing the center and allowed to move freely through the maze for 5 min. The total number of arm entries (N) and the sequence of entries were recorded. Successful alternation was defined as consecutive entry into all three arms. Percent alternation was calculated as the number of successful alternations/(N−2) × 100%.
Novel object recognition test
The novel object recognition test as a cognitive behavioral assay is based on the trait of the animals to spontaneously explore the environment, which is mainly dependent on the activity of their hippocampus [27]. Two days prior to the experiments, the mice received a habituation of 5 min, twice per day, in an empty open-field area (45 × 45 × 15 cm) with wood walls. For the duration of the training phase, two identical objects, A1 and A2, were placed parallel to and approximately 15 cm away from the back wall. The mice were placed alone in the open field to explore both objects freely for 5 min. During the testing phase 1 h later, a different object (object B), instead of A2, was used as a novel object, and the amount of time mice spent exploring each object was recorded, with the assumption that they will spend more time exploring a novel object than a familiar one [28]. After a 24-h retention interval, the mice were placed again in the same box with another novel object (C), replacing B, and allowed to explore freely for 5 min.
Object exploration was operationally defined as the duration of time that mice spent physically contacting the object with their nose at a distance of less than 2 cm or touching the object (e.g., sniffing or scratching). The time(s) spent exploring each object in the test phase was recorded and calculated as a preferential index (PI): [time spent investigating novel object/total exploration time × 100].
During all behavioral tests, the experiment equipment was cleaned with 10% ethyl alcohol to remove the odors left behind after each individual animal.
Tissue preparations and immunohistochemistry staining
After anesthesia with 0.6% pentobarbital sodium, brain tissues were removed from the animals and post-fixed in 4% paraformaldehyde overnight at 4 °C. After being successively dehydrated in 0.1 M phosphate buffer containing 20 and 30% sucrose separately until they had sunk, the tissues were cut into coronal sections of 15 μm thicknesses. The level of generation of new cells was determined by a single intraperitoneal injection of BrdU at a concentration of 100 mg/kg/day for three consecutive days and is incorporated during the S-phase into the cell and thus labels the proliferating cells. The mice were sacrificed 24 h after the last injection of BrdU.
The immunohistochemistry test of the hippocampus was prepared as previously described [29] with few modifications. For BrdU immunohistochemistry staining, brain tissue sections were incubated in HCl (2 N) for 30 min at 37 °C to break open the DNA structure of the labeled cells and rinsed in borate buffer (0.1 M) for 10 min. The sections were placed in blocking solution at 37 °C for 30 min. Then, incubation with rat-anti-BrdU (OBT0030, Accurate Chemical and Scientific) diluted in PBS was overnight at 4 °C. After incubation with the primary antibody, these sections were treated with goat anti-rat IgG-R secondary antibody (sc-2093, Santa Cruz) at 37 °C for 1 h. For NeuN and DCX staining, sections were blocked and treated with rabbit-anti-NeuN (ab177487, Abcam) and rabbit-anti-Doublecortin (ab18723, Abcam) overnight at 4 °C. After washing in PBS, the sections were then incubated in goat anti-rabbit IgG-FITC secondary antibodies (sc-2012, Santa Cruz). Cells were further counter-stained using DAPI (10236276001, Roche) for nuclei staining.
Isolation and culture of hippocampal NSCs
A hippocampus-derived NSCs culture was performed as previously described [30]. In brief, the brains of rats born within 24 h were dissected to remove the hippocampal zone, which contains a pool of NSCs and readily forms neurospheres in culture. The hippocampal zone was cut into small pieces and mechanically triturated to a single cell suspension, then plated at an initial concentration of 5 × 105 cells/ml using neurobasal medium containing 20 ng/ml bFGF (400-29, Peprotech), 20 ng/ml EGF (400-25, Peprotech), and 2% B-27 (17504-044, Gibco) supplement. Neurosphere formation begins after 5–6 days of culture, and all experiments were performed during the second passage.
Neurosphere proliferation assay
To investigate the effect of xanthoceraside on NSCs proliferation and neurosphere formation, we detected neurosphere proliferation as previously described [31]. Hippocampus-derived signal cell suspension was plated in a 12-well plate at a density of 5 × 104 cells/well. The xanthoceraside (0.1 μM) was treated with NSCs 24 h after incubating with aggregated Aβ25–35 (20 μM) for 3 h. The number and size of neurospheres was analyzed.
Immunofluorescence staining
To differentiate NSCs cultures, cells were transferred into complete proliferation or differentiation medium with the drugs [32]. The cells were divided into five groups, including xanthoceraside (0.1 μM), Aβ25–35 (20 μM), Aβ25–35 (20 μM) + xanthoceraside (0.1 μM), Aβ25–35 (20 μM) + xanthoceraside (0.1 μM) + Dkk-1 (100 ng/ml), and Dkk-1 (100 ng/ml) groups. In brief, cells were incubated with blocking solution for 2 h at room temperature, followed by incubation in primary antibodies of rat anti-BrdU (OBT0030, Accurate Chemical and Scientific), mouse anti-GFAP (60190-1, Proteintech), and rabbit anti-MAP-2 (sc-20172, Santa Cruz) for 12 h at 4 °C.
Western blot
To determine the levels of total proteins and nuclear proteins involved in neurogenesis, separation and extraction of proteins were performed using kits from Beyotime as previously described [33]. Protein (30 μg) was separated on a 10% gradient SDS-PAGE gel. After blocking with 5% skim milk for 2 h at room temperature, the membranes were incubated overnight at 4 °C with primary antibodies specific for rabbit anti-wnt3a (WL0199a, Wanleibio), rabbit anti-GSK3β (12456, CST), rabbit anti-p-GSK3β (5558, CST), rabbit anti-β-catenin (WL01160, Wanleibio), rabbit anti-p-β-catenin (9561, CST), rabbit anti-NeuN (ab177487, Abcam) and mouse anti-β-actin (sc-47778, Santa Cruz). After washing, the membranes were incubated with the secondary antibodies anti-mouse IgG and anti-rabbit IgG for 2 h at room temperature and washed again. Protein bands were finally visualized with an ECL Western blotting kit (Kangwei Biotechnology, China).
Statistical analysis
For all experiments, GraphPad Prism version 5.0 (San Diego, CA, USA) was used to analyze the data. All quantitative data were analyzed using a one-way analysis of variance (ANOVA) with SPSS software (Chicago, IL, USA) followed by LSD test. Confocal images were taken using a Nikon C2 Plus confocal microscope. Data were expressed as the mean ± SEM. A value of p < 0.05 was considered to be statistically significant.
Results
Xanthoceraside ameliorated the impairment of learning and memory in APP/PS1 transgenic mice
We first detected the spontaneous locomotion of animals using an open-field box that consisted of a square white box. Spontaneous trails of each group were shown in Fig. 1b and no significant differences were found among the groups in the total distances traveled, average velocity spent, or activity time (data not shown). Next, we tested the effect of xanthoceraside on spatial working memory deficit in the Y-maze test. Spontaneous alternations and total arm entries were calculated. There was no significant change in total arm entries, suggesting that treatment had no effect on general motor activity (F 4, 54 = 0.900, p = 0.470; Fig. 1c). Compared with the control group, APP/PS1 transgenic mice exhibited a reduced spontaneous alternation behavior (F 4, 54 = 8.794, p < 0.001, post hoc, p < 0.001), and xanthoceraside (0.08 and 0.32 mg/kg) dose-dependently attenuated the impairment of spontaneous alternation behavior in the Y-maze (0.08 mg/kg: p < 0.01; 0.32 mg/kg: p < 0.001; Fig. 1d). Novel object recognition test was used to evaluate the efficacy of xanthoceraside on reversing the impairment of visual recognition. The total exploring time had no significant difference in either group at 1 or 24 h (1 h: F 4, 54 = 1.583, p = 0.192; 24 h: F 4, 54 = 0.158, p = 0.959; Fig. 1e). The level of exploratory preferences for the novel objects B (1 h) and C (24 h) (both the PI and DI were calculated, DI not shown) in the model group was significantly decreased compared with the control group (1 h: F 4, 54 = 6.770, p < 0.001, post hoc, p < 0.01 24 h: F 4, 54 = 12.355, p < 0.001, post hoc, p < 0.001; Fig. 1f). The xanthoceraside (0.08 and 0.32 mg/kg)-treated mice showed more interest in the novel object, and spent more time exploring the novel object than the previously studied object compared with APP/PS1 transgenic mice at 1 and 24 h (0.08 mg/kg: 1 h: p < 0.01, 24 h: p < 0.001; 0.32 mg/kg: p < 0.001 for both 1 and 24 h; Fig. 1f).
Xanthoceraside rescued neuronal loss in the hippocampal DG and CA1 of APP/PS1 transgenic mice
To evaluate the effects of xanthoceraside on neuronal density in hippocampal CA1 and DG regions, we performed immunohistochemistry staining against NeuN, a specific neuronal marker [34]. Xanthoceraside treatment increased conspicuous neurons compared with the model group in hippocampal DG and CA1 regions (Fig. 2a). The result was confirmed by the quantitative analysis of the area and number of neurons per field in the hippocampal DG and CA1 regions (DG: F 4, 25 = 5.080, p < 0.01, post hoc, p < 0.05; CA1: F 4, 25 = 3.499, p < 0.05, post hoc, p < 0.05; Fig. 2b). Moreover, we found that xanthoceraside significantly increased the protein level of NeuN in hippocampus compared with the model group (F 4, 24 = 11.324, p < 0.001; Fig. 2c).
Fig. 2.
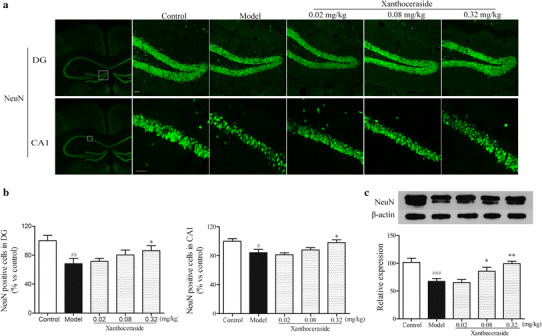
Effects of xanthoceraside on neurons in hippocampal DG and CA1 of APP/PS1 transgenic mice. a Immunostaining against the NeuN in hippocampal DG and CA1 regions. b Quantitative analysis of neurons in hippocampal DG and CA1 regions. Values are expressed as the mean ± SEM (n = 6). c Western blots showing the protein level of NeuN in the hippocampus. Scale bar = 20 μm. Values are expressed as the mean ± SEM (n = 5–6). # p < 0.05, ## p < 0.01 and ### p < 0.001 vs. control; *p < 0.05 and **p < 0.01 vs. model
Xanthoceraside enhanced the proliferation and neuronal differentiation in the hippocampal DG and SVZ regions of APP/PS1 transgenic mice
Neurogenesis is the generation of new neurons from NSCs, which includes proliferation, differentiation, cell maturation and survival, and integration of newborn cells into functional neuronal circuits [35]. To assess the effect of xanthoceraside (0.02, 0.08, and 0.32 mg/kg) on the proliferation and viability of NSCs in the hippocampal DG and SVZ, we performed BrdU immunohistochemistry staining (Fig. 3a). It showed that compared with the control group, the number of BrdU-positive cells both in DG (F 4, 25 = 4.162, p < 0.05, post hoc, p < 0.01; Fig. 3b) and SVZ (F 4, 25 = 3.032, p < 0.05, post hoc, p < 0.05; Fig. 3c) decreased significantly in APP/PS1 transgenic mice. Xanthoceraside at a dose of 0.32 mg/kg significantly increased the BrdU-positive cells in DG (p < 0.05; Fig. 3b) and SVZ (p < 0.05; Fig. 3c) of APP/PS1 transgenic mice. Next, in order to investigate whether xanthoceraside can induce an increase of the number of immature neurons, we used DCX-immunohistochemistry staining (DCX: a marker of newly generated immature neurons) in the hippocampal DG of APP/PS1 transgenic mice (Fig. 3d). Results revealed that the number of DCX-positive cells was also significantly higher in the xanthoceraside-treated group (0.32 mg/kg) than that in APP/PS1 transgenic mice (F 4, 25 = 7.225, p < 0.001, post hoc, p < 0.01; Fig. 3e).
Fig. 3.
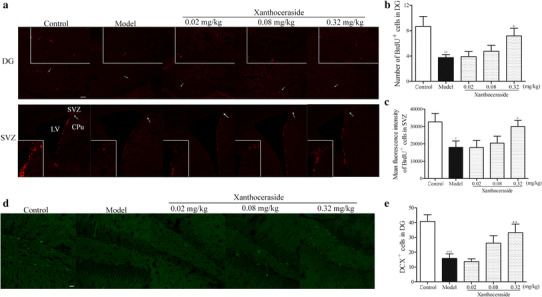
Effects of xanthoceraside on proliferation and differentiation of NSCs in hippocampal DG and SVZ regions. a Confocal photomicrographs showing BrdU+ cells in hippocampal DG and SVZ regions. b, c Bar diagram showing the quantitative analysis of BrdU+ cells in hippocampus DG and SVZ regions. Scale bar = 100 μm. d, e Images and quantitative analysis of DCX+ cells in hippocampal DG region. Scale bar = 20 μm. Values are expressed as the mean ± SEM (n = 6). # p < 0.05, ## p < 0.01 and ### p < 0.001 vs. control; *p < 0.05 and **p < 0.01 vs. model
Xanthoceraside induced proliferation and differentiation of NSCs in hippocampus-derived neurospheres in vitro
To assess the effect of xanthoceraside on the hippocampal multipotent NSCs, we identified the NSCs by nestin immunofluorescence (Fig. 4a). We found that Aβ25–35 damaged the gross morphology (Fig. 4b), size, and number of neurospheres (size: F 2, 6 = 17.714, p < 0.01, post hoc, p < 0.01; number: F 2, 6 = 11.774, p < 0.01, post hoc, p < 0.01; Fig. 4c). Xanthoceraside significantly increased the size of neurospheres compared with the Aβ25–35 group (p < 0.05; Fig. 4c), but the number of neurospheres had no significant difference between the xanthoceraside group and the model group (p = 0.773; Fig. 4c). These results suggested that xanthoceraside treatment induced NSCs proliferation against Aβ25–35. New proliferating cells of NSCs in the hippocampus may have different fates, where they undergo neuronal or glial differentiation. To investigate the effect of xanthoceraside treatment on NSCs differentiation, we detected the neuronal and glial differentiation of NSCs in hippocampus-derived neurospheres treated with xanthoceraside in vitro using double immuno-co-labeling with MAP-2 and GFAP (Fig. 4d). We found that xanthoceraside significantly increased the number of neurons, but had no effect on astrocytes (MAP-2: F 2, 96 = 11.174, p < 0.001, post hoc, p < 0.001; GFAP: F 2, 96 = 0.990, p = 0.375; Fig. 4e).
Fig. 4.
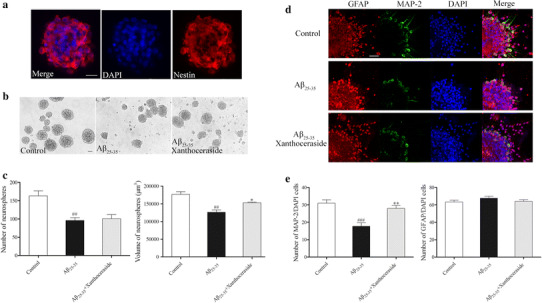
Effects of xanthoceraside on proliferation and differentiation of the hippocampus-derived NSCs in vitro. a Identification of neurospheres that were fixed and stained with NSCs marker nestin (red) and then counterstained with DAPI (blue) as the nuclear marker. b Neurosphere growth kinetics in the control, Aβ25–35 (20 μM), and Aβ25–35 (20 μM) combined with the xanthoceraside (0.1 μM)-treated group were studied by phase-contrast photomicrograph. c Bar diagram showing the number and volume of NSCs-derived neurospheres (neurospheres with a diameter ≥30 μm were used for calculating). d Images showing the effect of xanthoceraside on the differentiation potential of NSCs, as assessed by immuno-labeling with a neuronal marker (MAP-2, green) and an astrocyte marker (GFAP, red) and then counterstained with DAPI (blue). e A number of MAP-2-labeled and GFAP-labeled cells were calculated. Scale bar = 20 μm. Values are expressed as the mean ± SEM (n = 3). ## p < 0.01 and ### p < 0.001 vs. control; *p < 0.05 and **p < 0.01 vs. Aβ25–35 group
Xanthoceraside regulated Wnt/β-catenin signaling pathway in the hippocampus of APP/PS1 transgenic mice
Wnt/β-catenin signaling is believed to be the key factor in regulating the proliferation and differentiation of adult NSCs. Therefore, we examined the effects of xanthoceraside on the expression of the Wnt3a, GSK3β, and β-catenin in the hippocampus (Fig. 5a). The results showed that treatment with xanthoceraside (0.32 mg/kg) significantly increased the expression of Wnt3a (F 4, 24 = 6.648, p < 0.001, post hoc, p < 0.05; Fig. 5b) and p-GSK3β (F 4, 24 = 4.622, p < 0.01, post hoc, p < 0.01; Fig. 5c) and decreased the expression of p-β-catenin (F 4, 24 = 22.681, p < 0.001, post hoc, p < 0.001; Fig. 5d) in APP/PS1 transgenic mice. Meanwhile, we found that xanthoceraside significantly induced nuclear translocation of β-catenin to activate neurogenesis in APP/PS1 transgenic mice (F 4, 24 = 15.014, p < 0.001, post hoc, p < 0.05; Fig. 5e). In order to investigate whether xanthoceraside affects NSCs proliferation and neuronal differentiation via the Wnt/β-catenin pathway, hippocampus-derived NSCs were treated with Wnt/β-catenin pathway inhibitor Dkk-1 (inhibits interaction of Wnt with LRP-5/6) before xanthoceraside treatment (Fig. 6a, c). We found that treatment with xanthoceraside increased proliferation (F 4, 10 = 53.503, p < 0.001, post hoc, p < 0.05; Fig. 6b) and neuronal differentiation (F 4, 10 = 14.155, p < 0.001, post hoc, p < 0.05; Fig. 6d) of NSC compared with Aβ25–35 group in culture, while the effect of xanthoceraside on inducing NSC proliferation and neuronal differentiation were significantly blocked by Dkk-1 treatment (p < 0.05; Fig. 6b, d). These findings indicated that the Wnt/β-catenin pathway activation is involved in xanthoceraside-mediated increase of proliferation and neuronal differentiation of NSCs.
Fig. 5.
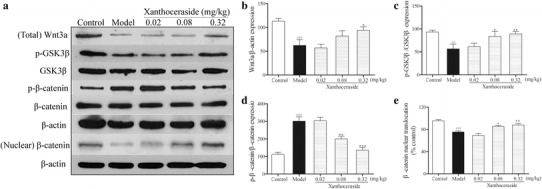
Effects of xanthoceraside on the Wnt/β-catenin signaling pathway in the hippocampus of APP/PS1 transgenic mice. a Representative image of immunoblots for Wnt3a, GSK3β, p-GSK3β, β-catenin, and p-β-catenin in the hippocampus. b–d Quantitative analysis of Wnt3a, p-GSK3β, and p-β-catenin protein expression by Western blot. e Quantitative analysis of nuclear translocation of β-catenin by Western blot. Values are expressed as the mean ± SEM (n = 5–6). ## p < 0.01 and ### p < 0.001 vs. control; *p < 0.05, **p < 0.01 and ***p < 0.001 vs. model
Fig. 6.
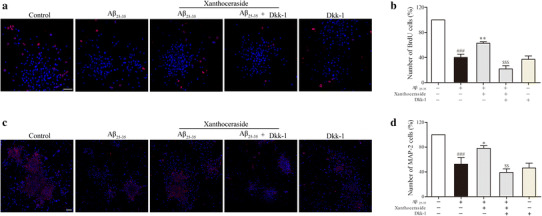
Inhibition of the Wnt pathway affects xanthoceraside-mediated stimulatory effects on NSCs proliferation and neuronal differentiation in culture. a, c Representative immunofluorescent images showed that NSCs culture was treated with xanthoceraside in the presence and absence of the Wnt pathway inhibitor Dkk-1 in order to understand the effects on proliferation and differentiation. Scale bar = 20 μm. b, d Quantitative analysis of the proliferation and differentiation of NSCs. Scale bar = 50 μm. Values are expressed as the mean ± SEM (n = 3). ### p < 0.001 vs. control; *p < 0.05 and **p < 0.01 vs. Aβ25–35; $$ p < 0.01 and $$$ p < 0.001 vs. Aβ25–35 + xanthoceraside
Discussion
AD is a progressive neurodegenerative disorder characterized by Aβ deposits, neurofibrillary tangles, and neuronal death, leading to learning and memory impairment [36]. Decreasing neurons in the hippocampus is tied to the loss of memory performance in both aged rats [36] and AD mice [37]. Herein, we found that APP/PS1 transgenic mice treated with xanthoceraside from 3 to 8 months of age could significantly enhance the working memory and visual recognition memory in the Y-maze and novel object recognition test compared with the model group, respectively, while open-field test results showed that the activity of mice between each group did not have a significant difference. This suggested that xanthoceraside improved the learning and memory ability without affecting cortical excitability and athletic ability. Moreover, xanthoceraside increased neurons in the hippocampal DG and CA1 regions of APP/PS1 transgenic mice. These results revealed that xanthoceraside alleviated learning and memory impairment might be through rescuing of neuronal loss.
Adult neurogenesis plays an essential role in learning and memory function [38]. New neurons continuously regenerated from NSCs throughout the life can fill the neuronal loss in hippocampus [35, 38]. Some studies have reported that cell proliferation in the SVZ and DG is increased in AD and cerebral ischemia patients [39, 40], which may be a compensatory mechanism for its impairment. However, more extensive studies have suggested that animal models of AD-like pathology [41, 42] and human brains with AD [43] are consistently identified with an exacerbating decline of hippocampal neurogenesis. APP/PS1 transgenic mice showed significantly decreased BrdU- and DCX-positive cells, suggesting that NSCs proliferation and neuronal differentiation were inhibited compared with non-transgenic mice aged 9 months [44]. It is suggested that inducing neurogenesis may be one of the main strategies for the treatment of AD. We found that APP/PS1 transgenic mice used in this study showed excessive impairment of NSCs proliferation and neuronal differentiation. Previous studies in our lab have shown that xanthoceraside could significantly increase the expression of growth-associated protein 43 (GAP43) in both the hippocampus and the cortex of AD model animals. GAP43 is an activity-dependent phosphoprotein neurogenesis marker that mediates neurite outgrowth in developing neurons [45]. Therefore, we detected the effect of xanthoceraside on neurogenesis. We found that xanthoceraside significantly increased the number of BrdU- and DCX-positive cells in the hippocampal DG and SVZ regions compared with the model group of APP/PS1 transgenic mice, which suggests that xanthoceraside can promote NSCs proliferation and neuronal differentiation.
To further confirm the effect of xanthoceraside on neurogenesis, NSCs were primary cultured and Aβ25–35 was used as a model drug. Aβ25–35, as a convenient alternative in AD investigations, being with the smaller 11-amino acid fragment of the full-length peptide, such as Aβ1–42 and Aβ1–40, can retain most of the toxicological properties of Aβ1–42 and Aβ1–40 [46]. Previous studies reported that Aβ25–35 oligomer can inhibit the proliferation of NSCs in vitro and inhibit neurogenesis in hippocampal DG in vivo [47, 48]. Herein, we found that xanthoceraside treatment for 24 h significantly increased the size of NSCs and induced NSCs differentiation into neurons compared with Aβ25–35 group. These results suggested that xanthoceraside rescued neuronal loss possibly through inducing NSCs proliferation and neuronal differentiation. In addition, previous studies showed that xanthoceraside could reduce Aβ deposition, inhibit neuron apoptosis, and inhibit the release of the inflammatory factors to protect neurons against death [24, 49, 50]. These effects may also be related to the increase in the number of hippocampal neurons with treatment of xanthoceraside.
Wnt/β-catenin signaling mediates cell survival, the proliferation of NSCs, and differentiation into neurons in the hippocampus and SVZ [51, 52]. Wnt3a, a key ligand of the canonical Wnt/β-catenin pathway, induces nuclear transcription of β-catenin to activate neurogenesis [53]. It is well known that over-expression of wnt3a can enhance neurogenesis [54], whereas wnt3a-deficient mice exhibit a degenerated hippocampus [55]. Herein, we also found that treatment with xanthoceraside caused upregulation of wnt3a protein levels significantly in APP/PS1 transgenic mice. Wnt3a can regulate downstream protein expression of pSer9-GSK-3β, further inhibit β-catenin degradation, and activate the Wnt/β-catenin pathway. In this study, we found that xanthoceraside could upregulate the ratio of pSer9-GSK-3β/GSK-3β, reduce the ratio of p-β-catenin/β-catenin, and induce nuclear transcription of β-catenin to activate neurogenesis in APP/PS1 transgenic mice. Wnt/β-catenin pathway inhibitor Dkk-1 was used to investigate the relationship between proliferation and neuronal differentiation of NSCs induced by xanthoceraside and the Wnt/β-catenin pathway. It showed that xanthoceraside-mediated increase of proliferation and differentiation of NSCs was blocked by Dkk-1 treatment. These results suggest that chronic oral treatment with xanthoceraside from 3 to 8 months of age promoted NSCs proliferation and neuronal differentiation and rescued neuronal loss in the hippocampus, which might be through the upregulation of the Wnt/β-catenin pathway in APP/PS1 transgenic mice.
In summary, we firstly demonstrated that xanthoceraside rescued neuronal loss to ameliorate learning and memory deficits, which might be related to the enhancement of neurogenesis through upregulating the Wnt/β-catenin pathway in APP/PS1 transgenic mice.
Abbreviations
- Aβ
Amyloid-beta
- AD
Alzheimer’s disease
- APP
Amyloid precursor protein
- BrdU
5-Bromo-20-deoxyuridine
- DG
Dentate gyrus
- Dickkopf-1
Dkk-1
- NSCs
Neural stem cells
- SVZ
Subventricular zone
Author contributions
L. Zhu conceived the experiments, contributed to research data, and drafted the manuscript; X. Zhao and L. Yang contributed to technical assistance; S. Song and Q. Lu participated in raising animals; X. Ji, T. Chi, and P. Liu revised the manuscript; L. Wang provided the xanthoceraside; L. Zou revised the manuscript and supervised the analysis.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
All animal procedures were performed in strict accordance with the guidelines for the care and use of laboratory animals of the Institute for Experimental Animals at Shenyang Pharmaceutical University and with the laws of China. The protocols were approved by the Committee on the Ethics of Animal Experiments of the Shenyang Pharmaceutical University (SYPU-IACUC-S20140317-04).
Funding
This study was supported by the Research Fund for the National Natural Science Foundation of China (No. 81373992) and the Career Development Program for Young Teachers in Shenyang Pharmaceutical University (No. ZQN2015028).
References
- 1.Xia M, Yang L, Sun G, Qi S, Li B. Mechanism of depression as a risk factor in the development of Alzheimer’s disease: the function of AQP4 and the glymphatic system. Psychopharmacology (Berl) 2016;234(3):365–379. doi: 10.1007/s00213-016-4473-9. [DOI] [PubMed] [Google Scholar]
- 2.Dong H, Csernansky CA, Martin MV, Bertchume A, Vallera D, Csernansky JG. Acetylcholinesterase inhibitors ameliorate behavioral deficits in the Tg2576 mouse model of Alzheimer’s disease. Psychopharmacology. 2005;181(1):145–152. doi: 10.1007/s00213-005-2230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li XY, Men WW, Zhu H, Lei JF, Zuo FX, Wang ZJ, et al. Age- and brain region-specific changes of glucose metabolic disorder, learning, and memory dysfunction in early Alzheimer’s disease assessed in APP/PS1 transgenic mice using 18F-FDG-PET. Int J Mol Sci. 2016;17(10):1707. doi: 10.3390/ijms17101707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jankowsky JL, Fadale DJ, Anderson J, Xu GM, Gonzales V, Jenkins NA, et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo: evidence for augmentation of a 42-specific gamma secretase. Hum Mol Genet. 2004;13(2):159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- 5.Parthsarathy V, McClean PL, Holscher C, Taylor M, Tinker C, Jones G, et al. A novel retro-inverso peptide inhibitor reduces amyloid deposition, oxidation and inflammation and stimulates neurogenesis in the APPswe/PS1DeltaE9 mouse model of Alzheimer’s disease. PLoS One. 2013;8(1):e54769. doi: 10.1371/journal.pone.0054769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verret L, Jankowsky JL, Xu GM, Borchelt DR, Rampon C. Alzheimer’s-type amyloidosis in transgenic mice impairs survival of newborn neurons derived from adult hippocampal neurogenesis. J Neurosci. 2007;27(25):6771–6780. doi: 10.1523/JNEUROSCI.5564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- 8.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 9.Aimone JB, Wiles J, Gage FH. Potential role for adult neurogenesis in the encoding of time in new memories. Nat Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 10.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 11.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7(8):841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 13.Monteiro BM, Moreira FA, Massensini AR, Moraes MF, Pereira GS. Enriched environment increases neurogenesis and improves social memory persistence in socially isolated adult mice. Hippocampus. 2014;24(2):239–248. doi: 10.1002/hipo.22218. [DOI] [PubMed] [Google Scholar]
- 14.Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2(10):894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- 15.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller AD, Meerlo P, McGinty D, Mistlberger RE. Sleep and adult neurogenesis: implications for cognition and mood. Curr Top Behav Neurosci. 2015;25:151–181. doi: 10.1007/7854_2013_251. [DOI] [PubMed] [Google Scholar]
- 17.Esposito G, Scuderi C, Valenza M, Togna GI, Latina V, De Filippis D, et al. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS One. 2011;6(12):e28668. doi: 10.1371/journal.pone.0028668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830(2):2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohata S, Nakatani J, Herranz-Perez V, Cheng J, Belinson H, Inubushi T, et al. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83(3):558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari SK, Agarwal S, Seth B, Yadav A, Ray RS, Mishra VN, et al. Inhibitory effects of bisphenol-A on neural stem cells proliferation and differentiation in the rat brain are dependent on Wnt/beta-catenin pathway. Mol Neurobiol. 2015;52(3):1735–1757. doi: 10.1007/s12035-014-8940-1. [DOI] [PubMed] [Google Scholar]
- 22.Mudher A, Lovestone S. Alzheimer’s disease–do Tauists and Baptists finally shake hands? Trends Neurosci. 2002;25(1):22–26. doi: 10.1016/S0166-2236(00)02031-2. [DOI] [PubMed] [Google Scholar]
- 23.Lu P, Mamiya T, Lu L, Mouri A, Ikejima T, Kim HC, et al. Xanthoceraside attenuates amyloid beta peptide25–35-induced learning and memory impairments in mice. Psychopharmacology. 2012;219(1):181–190. doi: 10.1007/s00213-011-2386-1. [DOI] [PubMed] [Google Scholar]
- 24.Jin G, Wang LH, Ji XF, Chi TY, Qi Y, Jiao Q, et al. Xanthoceraside rescues learning and memory deficits through attenuating beta-amyloid deposition and tau hyperphosphorylation in APP mice. Neurosci Lett. 2014;573:58–63. doi: 10.1016/j.neulet.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 25.Kumar A, Ekavali J, Mishra K, Chopra K, Dhull DK. Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacology. 2016;233(1):137–152. doi: 10.1007/s00213-015-4095-7. [DOI] [PubMed] [Google Scholar]
- 26.Suryavanshi PS, Ugale RR, Yilmazer-Hanke D, Stairs DJ, Dravid SM. GluN2C/GluN2D subunit-selective NMDA receptor potentiator CIQ reverses MK-801-induced impairment in prepulse inhibition and working memory in Y-maze test in mice. Br J Pharmacol. 2014;171(3):799–809. doi: 10.1111/bph.12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Win-Shwe TT, Fujimaki H, Fujitani Y, Hirano S. Novel object recognition ability in female mice following exposure to nanoparticle-rich diesel exhaust. Toxicol Appl Pharmacol. 2012;262(3):355–362. doi: 10.1016/j.taap.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Frick KM, Gresack JE. Sex differences in the behavioral response to spatial and object novelty in adult C57BL/6 mice. Behav Neurosci. 2003;117(6):1283–1291. doi: 10.1037/0735-7044.117.6.1283. [DOI] [PubMed] [Google Scholar]
- 29.Valero J, Espana J, Parra-Damas A, Martin E, Rodriguez-Alvarez J, Saura CA. Short-term environmental enrichment rescues adult neurogenesis and memory deficits in APPSw,Ind transgenic mice. PLoS One. 2011;6(2):e16832. doi: 10.1371/journal.pone.0016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari SK, Agarwal S, Seth B, Yadav A, Nair S, Bhatnagar P, et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano. 2014;8(1):76–103. doi: 10.1021/nn405077y. [DOI] [PubMed] [Google Scholar]
- 31.Collins SJ, Tumpach C, Li QX, Lewis V, Ryan TM, Roberts B, et al. The prion protein regulates beta-amyloid-mediated self-renewal of neural stem cells in vitro. Stem Cell Res Ther. 2015;6:60. doi: 10.1186/s13287-015-0067-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu WX, Wang J, Xie ZM, Xu N, Zhang GF, Jia M, et al. Regulation of glutamate transporter 1 via BDNF-TrkB signaling plays a role in the anti-apoptotic and antidepressant effects of ketamine in chronic unpredictable stress model of depression. Psychopharmacology. 2016;233(3):405–415. doi: 10.1007/s00213-015-4128-2. [DOI] [PubMed] [Google Scholar]
- 33.Fan YT, Yin GJ, Xiao WQ, Qiu L, Yu G, Hu YL, et al. Rosmarinic acid attenuates sodium taurocholate-induced acute pancreatitis in rats by inhibiting nuclear factor-kappaB activation. Am J Chin Med. 2015;43(6):1117–1135. doi: 10.1142/S0192415X15500640. [DOI] [PubMed] [Google Scholar]
- 34.Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116(1):201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132(4):645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bruel-Jungerman E, Davis S, Rampon C, Laroche S. Long-term potentiation enhances neurogenesis in the adult dentate gyrus. J Neurosci. 2006;26(22):5888–5893. doi: 10.1523/JNEUROSCI.0782-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, et al. Increased hippocampal neurogenesis in Alzheimer’s disease. Proc Natl Acad Sci U S A. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtenwalner RJ, Parent JM. Adult neurogenesis and the ischemic forebrain. J Cereb Blood Flow Metab. 2006;26(1):1–20. doi: 10.1038/sj.jcbfm.9600170. [DOI] [PubMed] [Google Scholar]
- 41.Faure A, Verret L, Bozon B, El Tannir N, El Tayara M, Ly F, Kober F, et al. Impaired neurogenesis, neuronal loss, and brain functional deficits in the APPxPS1-Ki mouse model of Alzheimer’s disease. Neurobiol Aging. 2011;32(3):407–418. doi: 10.1016/j.neurobiolaging.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Fiorentini A, Rosi MC, Grossi C, Luccarini I, Casamenti F. Lithium improves hippocampal neurogenesis, neuropathology and cognitive functions in APP mutant mice. PLoS One. 2010;5(12):e14382. doi: 10.1371/journal.pone.0014382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boekhoorn K, Joels M, Lucassen PJ. Increased proliferation reflects glial and vascular-associated changes, but not neurogenesis in the presenile Alzheimer hippocampus. Neurobiol Dis. 2006;24(1):1–14. doi: 10.1016/j.nbd.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, He J, Zhang Y, Luo H, Zhu S, Yang Y, et al. Increased hippocampal neurogenesis in the progressive stage of Alzheimer’s disease phenotype in an APP/PS1 double transgenic mouse model. Hippocampus. 2009;19(12):1247–1253. doi: 10.1002/hipo.20587. [DOI] [PubMed] [Google Scholar]
- 45.Benowitz LI, Routtenberg A. GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20(2):84–91. doi: 10.1016/S0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 46.Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer’s Aβ (1–42) and Aβ (25–35) J Am Chem Soc. 2001;123(24):5625–5631. doi: 10.1021/ja010452r. [DOI] [PubMed] [Google Scholar]
- 47.Choi H, Park HH, Lee KY, Choi NY, Yu HJ, Lee YJ, et al. Coenzyme Q10 restores amyloid beta-inhibited proliferation of neural stem cells by activating the PI3K pathway. Stem Cells Dev. 2013;22(15):2112–2120. doi: 10.1089/scd.2012.0604. [DOI] [PubMed] [Google Scholar]
- 48.Wang C, Chen T, Li G, Zhou L, Sha S, Chen L. Simvastatin prevents β-amyloid 25–35-impaired neurogenesis in hippocampal dentate gyrus through α7nAChR-dependent cascading PI3K-Akt and increasing BDNF via reduction of farnesyl pyrophosphate. Neuropharmacology. 2015;97:122–132. doi: 10.1016/j.neuropharm.2015.05.020. [DOI] [PubMed] [Google Scholar]
- 49.Qi Y, Zou LB, Wang LH, Jin G, Pan JJ, Chi TY, et al. Xanthoceraside inhibits pro-inflammatory cytokine expression in Aβ25-35/IFN-gamma-stimulated microglia through the TLR2 receptor, MyD88, nuclear factor-kappaB, and mitogen-activated protein kinase signaling pathways. J Pharmacol Sci. 2013;122(4):305–317. doi: 10.1254/jphs.13031FP. [DOI] [PubMed] [Google Scholar]
- 50.Chi TY, Wang LH, Ji XF, Shen L, Zou LB. Protective effect of xanthoceraside against beta-amyloid-induced neurotoxicity in neuroblastoma SH-SY5Y cells. J Asian Nat Prod Res. 2013;15(9):1013–1022. doi: 10.1080/10286020.2013.821982. [DOI] [PubMed] [Google Scholar]
- 51.Inestrosa NC, Arenas E. Emerging roles of Wnts in the adult nervous system. Nat Rev Neurosci. 2010;11(2):77–86. doi: 10.1038/nrn2755. [DOI] [PubMed] [Google Scholar]
- 52.Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25(11):2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 53.Tiwari SK, Seth B, Agarwal S, Yadav A, Karmakar M, Gupta SK, et al. Ethosuximide induces hippocampal neurogenesis and reverses cognitive deficits in an amyloid-beta toxin-induced Alzheimer rat model via the phosphatidylinositol 3-kinase (PI3K)/Akt/Wnt/beta-catenin pathway. J Biol Chem. 2015;290(47):28540–28558. doi: 10.1074/jbc.M115.652586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129(9):2087–2098. doi: 10.1242/dev.129.9.2087. [DOI] [PubMed] [Google Scholar]
- 55.Lee SM, Tole S, Grove E, McMahon AP. A local Wnt-3a signal is required for development of the mammalian hippocampus. Development. 2000;127(3):457–467. doi: 10.1242/dev.127.3.457. [DOI] [PubMed] [Google Scholar]


