Abstract
Our aim was to compare the basal levels of plasma brain-derived neurotrophic factor (BDNF) and global histone H4 acetylation in peripheral blood mononuclear cells (PBMCs) of healthy amateur runners (EXE group) with sedentary individuals (SED group) as well as to investigate the acute effect of a running race on these markers in the EXE group. Five days before the race, all participants were submitted to a basal blood collection. On the race day, two blood samples were collected in the EXE group before the running started and immediately at the end. In the basal period, a significant increase of plasma BDNF levels in the EXE individuals when compared to the SED group (p = 0.036) was demonstrated, while no difference in global histone H4 acetylation levels was observed. These parameters were unaltered in the EXE group after the race. The increased levels of BDNF might be linked to healthy middle-aged runners’ phenotype.
Keywords: Exercise, Epigenetic, Neurotrophins, Adult men, Plasma, Peripheral blood mononuclear cells (PBMCs)
Introduction
Brain-derived neurotrophic factor (BDNF) is a member of the neurotrophins family found throughout the brain. It is known to induce synaptic plasticity, neurogenesis and neuronal survival, growth and maturation [1]. Furthermore, BDNF exert a pivotal role in learning and memory abilities [2]. It is important to highlight that BDNF is able to cross the blood–brain barrier in a bi-directional manner via the high capacity of the saturable transport system. Indeed, the peripheral levels of this neurotrophin seem to present a strong correlation with the cerebrospinal fluid levels [3]. Therefore, peripheral BDNF levels have been used as a biomarker in several clinical studies [4, 5].
In this context, a large body of evidence conducted with human individuals at different stages of development has shown that a variety of exercise protocols and modalities are able to increase BDNF concentration and expression in serum and plasma [4, 6–9]. In addition, it was also demonstrated that a daily endurance training program for 3 months also enhances BDNF release from the human brain in healthy sedentary young males [10]. Altogether, these studies support the hypotheses that BDNF is a key mediator related to the positive effects of exercise in brain function. However, these findings are focused on analyzing the impact of exercise on BDNF levels in sedentary individuals while less attention has been devoted to endurance-trained populations.
Among the different categories of physical activity available, running is probably the oldest performed by humans. In ancient times, it was performed for survival, but nowadays is practiced for many reasons, such as health, good shape, or just to have a challenge. Running has become very popular due to ease of access, low cost, and contact with nature [11]. Despite these findings, the molecular mechanism by which running enhances BDNF levels in trained individuals has not yet been elucidated. Experimental studies point out that this phenomenon occurs via epigenetic pathways [12–15].
Epigenetic machinery modulates gene expression patterns without affecting the DNA primary sequence and is typically divided into two categories: DNA methylation and histone post-translational modifications [16]. DNA methylation is a biochemical modification catalyzed by DNA methyltransferases (DNMTs), which is typically associated with gene repression [17]. Histone acetylation is the most extensively studied post-translational modification, regulated by histone acetyltransferases (HATs) and histone deacetylases (HDACs). HATs produce a larger chromatin structure, a characteristic that allows transcriptional activation, whereas HDACs exert the opposite effect [18].
Clinical studies have also been suggesting exercise as an epigenetic modulator in peripheral tissues [19–22]. However, it should be emphasized that these reports are conducted with elderly and patient populations, whereas the effects in middle-aged healthy individuals has been poorly investigated. Indeed, they aimed to evaluate DNA methylation parameters, while the modulation of acetylation parameters in response to exercise are rare.
Interestingly, experimental data revealed that treadmill running increases global histone H4 acetylation levels in rat brain [23]. Moreover, other authors have pointed out that histone acetylation status regulates neurotrophins [24, 25]. In fact, a relationship between histone H4 acetylation levels and BDNF modulation in different tissues was recently described [26–29]. Taken together, these reports indicate an important cross-talk between histone H4 acetylation levels and regulation of BDNF signaling. Then, we hypothesize that this epigenetic signal might be affected by the regular practice of running in healthy trained subjects.
In view of these considerations, the aim of this study was to compare the basal levels of plasma BDNF and global histone H4 acetylation in the peripheral blood mononuclear cells (PBMCs) of middle-aged healthy amateur runners (EXE group) with sedentary individuals (SED group). Furthermore, we also investigated the acute effect of a running race on these markers in the EXE group.
Methods
Participants
Twenty-six healthy males (aged 30–50 years) participated in this study. They were allocated in the exercised (EXE) and sedentary (SED) groups, composed by amateur runners and individuals that do not practice regular physical activity, respectively. Inclusion criteria for the EXE group consisted in been practicing running for at least 2 years, have participated in two long-distance running races in the last 6 months and be properly registered in a 10-km running race. The SED group included individuals that have not performed regular physical activity in the past 6 months. Exclusion criteria for both groups were the presence of chronic diseases, the use of drug-containing HDAC inhibitors, and smoking. The participants were oriented not to use anti-inflammatory and antibiotic medicines 72 h before all blood collections.
This study was approved by the Research Ethics Committee of the Centro Universitário Metodista do IPA (number 918.889) and followed all the principles of the Helsinki Declaration. Informed consent was obtained from all individual participants included in the study.
Procedure and blood collection
Initially, the effect of the regular practice of running on plasma levels of BDNF and global histone H4 acetylation in PBMCs was evaluated. Then, 5 days before the race, all participants (SED and EXE groups) entered the Laboratory of Exercise Physiology at the Centro Universitário Metodista do IPA after 12-h of fasting and a blood sample (15 ml) was obtained from an antecubital vein (basal period).
In order to evaluate the acute effect of running on BDNF and global histone H4 acetylation levels, two blood samples (15 ml) were collected in the EXE group 30 min before starting the race (pre-period) and immediately after the race (post-period). The blood sample was collected immediately after the race. This time point was chosen based on our previous experimental results demonstrating that the modulation of histone acetylation status following treadmill training occurs in a short-term period, immediately up to 1 h, without any delayed effects [12, 15, 23].
The performance time obtained by each participant in the race was also recorded. It is important to point out that the situation at which the experimental data was collected was at a sporting event and a good performance is one of the main training motivations of the volunteers. Furthermore, the EXE group was encouraged and received verbal motivation to perform the race as soon as possible. Then, it could be supposed that the individuals have performed the trial at maximum intensity.
For the biochemical assays, the PBMCs were extracted by Ficoll gradient. Histopaque® (Sigma-Aldrich 1077) was added into the collected sample in a 1:1 proportion in a conical tube and then centrifugation was held at 1500 rpm for 30 min at room temperature. After that, the “buffer coat” was removed from the portion between plasma and Histopaque®. The “buffer coat” was washed five times with phosphate buffered saline solution (PBS—pH 7.4) and then centrifugation was held at 1800 rpm for 10 min at room temperature. The formed pellet was collected and used for evaluation of acetylation levels of histone H4. The remaining plasma was collected and stored in conical tubes (1.5 ml) at −80 °C for later determination of BDNF levels.
Determination of plasma BDNF levels
Plasma BDNF levels were determined with the ELISA method, from Sigma–Aldrich commercial kit (catalog number RAB0026) according to the manufacturer’s instructions. Briefly, the sample and BDNF-specific standards were added to an ELISA microplate and incubated for 2.5 h at room temperature. Subsequently, the solutions were discarded and the same plate was washed four times with wash buffer (PBS, Tween 20 0.01 %). After washing, the secondary antibody bound to biotin was added and incubated for 1 h at room temperature with gentle agitation. The plate was again washed with wash buffer and streptavidin solution was added and the plate was incubated at room temperature for 45 min with gentle agitation. The solution was discarded and the plate passed through the washing process. Tetramethylbenzidine (TMB) was added, and then it was incubated for 30 min at room temperature, in light deprivation, with gentle agitation. The stop solution was added and the plate was read in a spectrophotometer at a wavelength of 450 nm. The plasma BDNF levels were expressed as ng/ml.
Global histone H4 acetylation levels in PBMCs measurement
The global histone H4 acetylation levels in PBMCs were determined using the Global Histone H4 Acetylation Assay Kit (Colorimetric Detection, catalog number P-4009, EpiQuik USA) according to the manufacturer’s instructions. The samples were incubated with the capture antibody followed by incubation with detection antibody. After, were incubated with developing solution followed by the addition of the Stop Solution. The absorbance was measured on a spectrophotometer at a wavelength of 450 nm. The global histone H4 acetylation levels in PBMCs were expressed as ng/mg protein. The protein concentration of each sample was measured by the Coomassie Blue method using bovine serum albumin as standard [30].
Statistical analysis
The sample characterization was described through brief descriptive analysis by position averages (average or median), dispersion (standard deviation or first and third quartiles), and frequency (absolute and relative). Data normality was verified by the Shapiro–Wilk test.
To assess the differences in normally distributed variables (sample characterization and basal plasma BDNF levels in SED and EXE groups), the t test was used. To analyze the non-parametric variables (basal levels of global histone H4 acetylation levels in PBMCs in SED and EXE groups as well the acute effect of the running race on global histone H4 acetylation and BDNF levels in the EXE group), the Mann–Whitney and Friedman tests were used. Correlations were evaluated using the Spearman test. All analyses were performed using SPSS 20.0 software (Chicago, IL, USA) and used the significance level of 5 % (p ≤ 0.05) for all tests.
Results
The sample consisted of 26 participants, eight from the SED group and 18 from the EXE group. The groups proved to be homogenous in all basal characteristics, as described in Table 1. The characterization of the EXE group regarding training aspects are highlighted in Table 2.
Table 1.
Sample characterization (EXE and SED individuals)
| Variables | EXE group | SED group |
|---|---|---|
| Age (years) | 38.61 ± 6.36 | 32.54 ± 5.32 |
| Height (cm) | 174 ± 24 | 167 ± 17 |
| Weight (kg) | 72.4 ± 6.20 | 74.3 ± 2.16 |
| BMI (kg/m2) | 23.95 ± 2.88 | 25.45 ± 2.36 |
| Fat mass (%) | 23.99 ± 4,92 | 25.24 ± 1.65 |
| Lean body mass (%) | 56.68 ± 7.22 | 53.98 ± 2.87 |
Data expressed as average ± standard deviation
BMI body mass index
Table 2.
Training characteristics of EXE group
| Variables | EXE group |
|---|---|
| Weekly training (times) | |
| One | 1 (5.6) |
| Two | 2 (11.1) |
| Three | 10 (55.6) |
| Four | 3 (16.7) |
| Five | 2 (11.1) |
| Training session duration (min) | |
| 30 | 1 (5.6) |
| 60 | 17 (94.4) |
| Training time (years) | |
| Two | 10 (55.6) |
| Three | 2 (11.1) |
| Four | 2 (11.1) |
| Five years or more | 4 (22.2) |
| Sleep (h) | |
| Six | 7 (38.9) |
| Seven | 9 (50.0) |
| Eight | 1 (5.6) |
| Nine | 1 (5.6) |
Data expressed as absolute frequency (%)
In the basal period, the plasmatic levels of BDNF were significantly increased in the EXE individuals when compared to the SED group (0.73 ± 0.17 vs. 0.58 ± 0.12, respectively; p = 0.036), as highlighted in Fig. 1a. On the other hand, the global histone H4 acetylation levels in PBMC did not differ between groups (p = 0.601; Fig. 1b).
Fig. 1.
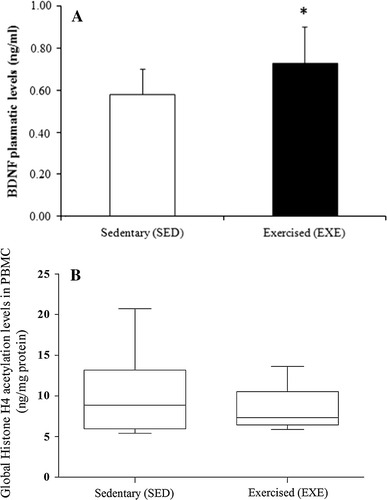
a Plasma BDNF levels in the SED (n = 8) and EXE (n = 18) groups in the basal period. Results are expressed as average ± standard deviation; t test (p = 0.036) *statistically different from the SED group. b Global histone H4 acetylation levels in PBMCs from SED (n = 8) and EXE (n = 18) groups in the basal period. Mann–Whitney test (p = 0.601)
On the race day, the EXE group was encouraged to exercise to their limit. The time spent for the individuals was 48:23 ± 5:13 min. No acute effect of running was observed in either of the evaluated parameters (plasma BDNF and global histone H4 acetylation levels in PMBCs) (p = 0.420 and p = 0.882, respectively) as illustrated in Fig. 2a, b.
Fig. 2.
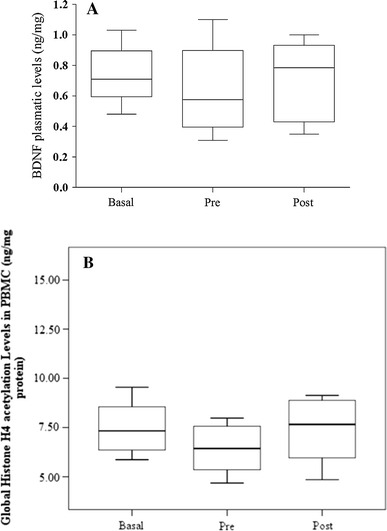
a Plasma BDNF levels in the basal, pre- and post-running race periods in the EXE group (n = 18); Friedman test (p = 0.420). b Global histone H4 acetylation levels in PBMCs from EXE group in the basal, pre- and post-running race periods (n = 18); Friedman test (p = 0.882)
There was a positive correlation between BDNF and global histone H4 acetylation levels in the pre- running race period in the EXE group (r = 0.893; p = 0.007). Also, the weekly frequency of training was positively correlated with BDNF levels after running in the EXE group (r = 0.492; p = 0.053).
Discussion
The current study showed that exercise, specifically the running practice, is an important strategy for enhancing BDNF levels in plasma of trained healthy middle-aged men. Our data could be related to those obtained by Zoladz and colleagues demonstrating that young well-trained athletes including sprinters, jumpers, and runners showed higher basal plasma BDNF concentration than sedentary subjects [6]. Taken together, it seems reasonable to surmise that enhanced plasma BDNF levels may be an important mark of endurance phenotype in both young and middle-aged healthy trained individuals.
BDNF have been described as an important regulator of energy metabolism related to endurance exercise [31]. Specifically, increased BDNF levels observed in response to exercise seems to mediate, at least in part, the enhanced insulin sensitivity following endurance training. In addition, a role of BDNF as a mediator of cardiovascular adaptations to endurance running have also been suggested. Finally, it was postulated that BDNF acts as an integrator of many of the major systems that regulate neural, endocrine, and cardiovascular adaptations to and for endurance exercise, such as running [32].
When we analyzed the acute effect of running on plasma BDNF levels in the EXE group, no significant changes were found. Conversely, a moderate-intensity endurance cycling training (for 5 weeks, 4 sessions/week) conducted with sedentary healthy young men acutely elevated plasma BDNF concentration [6]. In addition, plasma BDNF concentration after training was remarkably more elevated than before training, suggesting both acute and chronic effects of exercise on BDNF enhancement [6]. This divergence could be related, at least in part, to aspects such as the training status of the individuals as well as to the exercise protocol used. Specifically, endurance training seems to exert a bimodal influence on BDNF, increasing its plasmatic levels acutely and chronically in sedentary individuals. On the other hand, well-trained runners, our studied subjects, seem to have an adaptive response to exercise, with no changes on plasma BDNF levels immediately after a regular training/races.
In the post-test period, plasma BDNF levels had a positive correlation with the weekly exercise frequency. Our finding can be related to those obtained by Abel and colleagues [33], which demonstrated that hippocampal BDNF levels were positively correlated with the amount of wheel running in mice. In addition, this correlation was also observed in cerebellum, a brain area strictly related to motor skills [33]. Therefore, we may postulate that factors such as the training frequency and amount of exercise might influence BDNF release and/or expression. In this sense, it is known that runners are likely motivated by a set of attention and control areas of the brain that are used to support demands that are needed during effortful performance with a changing environment [34].
As mentioned above, the molecular proprieties of BDNF allow this neurotrophin to be assessed in the periphery. Then, the exercise impact on BDNF levels/concentration in clinical studies has been evaluated in both serum and plasma. Importantly, few reports indicate a substantial variation in BDNF content in these blood components, including in response to exercise [35, 36]. However, the comparison of the acute and delayed effects of the running practice on BDNF levels in plasma and serum was not the aim of this study. It was previously suggested that the plasma BDNF increases post-exercise is secreted primarily from the brain [36], as well as modifications in plasma BDNF concentration are considered to reflect its changes in the brain [37]. Considering these arguments, we performed our analyses in this blood component.
To the best of our knowledge, this is the first evidence analyzing the impact of exercise on histone acetylation markers in trained healthy individuals. Whilst no significant differences in global histone H4 acetylation levels in PBMCs between SED and EXE groups were found, a positive correlation between BDNF and global histone H4 acetylation levels in the EXE group in pre-running race period was found. This finding suggests that BDNF upregulation in response to regular running practice might engage the modulation of histone H4 acetylation status. Moreover, we cannot discard that BDNF enhancement could activate other epigenetic pathways. Regarding this question, increased histone H3 acetylation levels in hippocampi of exercised rats was demonstrated after a voluntary running-wheel exercise protocol for 1 week [14]. Therefore, we may postulate that histone H3 could also be involved in BDNF regulation in middle-aged healthy individuals. Our preliminarily data encourage future studies to investigate and elucidate these questions.
No changes in global histone H4 acetylation levels were observed after the race in the EXE group, indicating that this epigenetic marker is unchanged in response to a single bout of exercise in trained individuals. Similar to our results, it was recently demonstrated that healthy endurance-trained males showed unaltered levels of global DNA methylation in PMBC immediately and up to 24 h following a bout of prolonged exercise compared to the basal periods [38]. On the other hand, studies conducted with healthy sedentary young people reported opposite effects. McGee and colleagues [39] demonstrated that a single bout of 60 min of cycling was able to increase the H3 acetylation levels in skeletal muscle by 64 %. In addition, decreased levels in whole genome methylation in skeletal muscle biopsies of sedentary men and women after an acute session on cycle ergometer were also observed [40]. Altogether, these findings led us to hypothesize that epigenetic machinery is remarkably responsive to acute exercise in sedentary people, while the regular practice of physical activity may induce an adaptation in the epigenome of the trained population.
These clinical findings may be related to an experimental study conducted by Elsner and colleagues [12], who showed that a single exercise session on a treadmill induced a hyperacetylation status immediately and 1 h after training in hippocampus from young adult rats, whereas no change in acetylation markers was observed in response to the chronic protocol. Although it is impossible to establish at this moment why this phenomenon occurs, we might suggest that it is a protective mechanism to maintain the homeostasis of the transcriptional machinery, avoiding maladaptive conditions.
As described above, there are several cases of evidence in the literature reporting the epigenetic modulation in response to both chronic interventions as well single bouts of exercise in sedentary individuals [19–22, 41, 42]. However, the impact of exercise in these markers in well-trained subjects has been poorly investigated. Then, our study was focused in this context, measuring the chronic impact and acute response of exercise in middle-aged healthy amateur runners. In this sense, the SED group was included in the study with the unique propose to be compared with the EXE group in the basal period in order to verify the long-term outcomes of exercise on global histone H4 acetylation levels, justifying why the SED group not submitted to exercise.
Conclusions
In summary, our study supports the idea that healthy, trained runners present increased plasma levels of BDNF compared to sedentary individuals, suggesting that this neurotrophin exerts a stable profile in response to long-term running practice. Furthermore, global histone H4 acetylation levels did not differ between groups and in response to the running race in the EXE individuals, which might be linked to epigenome adaptation in response to chronic exercise exposure.
Notably, the results of the present study should be considered within the context of its limitations. First, we did not analyze the polymorphism of BDNF, an important topic that can be performed by future researchers. Moreover, we measured only one epigenetic mark: global histone H4 acetylation levels. Thus, it is suggested that additional studies should consider the modulation of other parameters that could epigenetically respond to exercise, such as histone H3 acetylation levels, modifications in histone methylation status, and miRNA regulation as well as the expression of specific genes. These findings could contribute to elucidating whether and how the regular practice of running shapes the human epigenome in middle-aged trained healthy individuals.
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq/Brazil; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)/Brazil and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS)/Brazil.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Poo MM. Neurotrophins as synaptic modulators. Nat Rev Neurosci. 2001;2(1):24–32. doi: 10.1038/35049004. [DOI] [PubMed] [Google Scholar]
- 2.Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22(3):123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood–brain barrier. Neuropharmacology. 1998;37(12):1553–1561. doi: 10.1016/S0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 4.Schuch FB, da Silveira LE, de Zeni TC, da Silva DP, Wollenhaupt-Aguiar B, Ferrari P, Reischak-Oliveira A, Kapczinski F. Effects of a single bout of maximal aerobic exercise on BDNF in bipolar disorder: a gender-based response. Psychiatry Res. 2015;229(1–2):57–62. doi: 10.1016/j.psychres.2015.07.072. [DOI] [PubMed] [Google Scholar]
- 5.Erickson KI, Miller DL, Roecklein KA. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 2012;18(1):82–97. doi: 10.1177/1073858410397054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;7:119–132. [PubMed] [Google Scholar]
- 7.Zoladz JA, Pilc A. The effect of physical activity on the brain derived neurotrophic factor: from animal to human studies. J Physiol Pharmacol. 2010;61(5):533–541. [PubMed] [Google Scholar]
- 8.Jeon YK, Ha CH. Expression of brain-derived neurotrophic factor, IGF-1 and cortisol elicited by regular aerobic exercise in adolescents. J Phys Ther Sci. 2015;27(3):737–741. doi: 10.1589/jpts.27.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babaei P, Azali Alamdari K, Soltani Tehrani B, Damirchi A. Effect of six weeks of endurance exercise and following detraining on serum brain-derived neurotrophic factor and memory performance in middle-aged males with metabolic syndrome. J Sports Med Phys Fit. 2013;53(4):437–743. [PubMed] [Google Scholar]
- 10.Seifert T, Brassard P, Wissenberg M, Rasmussen P, Nordby P, Stallknecht B, Adser H, Jakobsen AH, Pilegaard H, Nielsen HB, Secher NH. Endurance training enhances BDNF release from the human brain. Am J Physiol Regul Integr Comp Physiol. 2010;298(2):R372–R377. doi: 10.1152/ajpregu.00525.2009. [DOI] [PubMed] [Google Scholar]
- 11.Rosa JP, de Souza AA, de Lima GH, Rodrigues DF, de Aquino Lemos V, da Silva Alves E, Tufik S, de Mello MT. Motivational and evolutionary aspects of a physical exercise training program: a longitudinal study. Front Psychol. 2015;6:648. doi: 10.3389/fpsyg.2015.00648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsner VR, Lovatel GA, Bertoldi K, Vanzella C, Santos FM, Spindler C, de Almeida EF, Nardin P, Siqueira IR. Effect of different exercise protocols on histone acetyltransferases and histone deacetylases activities in rat hippocampus. Neurosci. 2011;192:580–587. doi: 10.1016/j.neuroscience.2011.06.066. [DOI] [PubMed] [Google Scholar]
- 13.Elsner VR, Lovatel GA, Moyses F, Bertoldi K, Spindler C, Cechinel LR, Muotri AR, Siqueira IR. Exercise induces age-dependent changes on epigenetic parameters in rat hippocampus: a preliminary study. Exp Gerontol. 2013;48(2):136–139. doi: 10.1016/j.exger.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33(3):383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spindler C, Cechinel LR, Basso C, Moyses F, Bertoldi K, Roesler R, Lovatel GA, Rostirola Elsner V, Siqueira IR. Treadmill exercise alters histone acetyltransferases and histone deacetylases activities in frontal cortices from Wistar rats. Cell Mol Neurobiol. 2014;34(8):1097–1101. doi: 10.1007/s10571-014-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci. 2007;8(5):355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 17.Sultan FA, Day JJ. Epigenetic mechanisms in memory and synaptic function. Epigenomics. 2011;3(2):157–181. doi: 10.2217/epi.11.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Hashimoto S, Fujii C, Hida S, Ito K, Matsumura T, Sakaizawa T, Morikawa M, Masuki S, Nose H, Higuchi K, Nakajima K, Taniguchi S. NFkappaB2 gene as a novel candidate that epigenetically responds to interval walking training. Int J Sports Med. 2015;36(9):769–775. doi: 10.1055/s-0035-1547221. [DOI] [PubMed] [Google Scholar]
- 20.Zimmer P, Baumann FT, Bloch W, Schenk A, Koliamitra C, Jensen P, Mierau A, Hulsdunker T, Reinart N, Hallek M, Elter T. Impact of exercise on pro inflammatory cytokine levels and epigenetic modulations of tumor-competitive lymphocytes in non-Hodgkin-lymphoma patients-randomized controlled trial. Eur J Haematol. 2014;93(6):527–532. doi: 10.1111/ejh.12395. [DOI] [PubMed] [Google Scholar]
- 21.Zimmer P, Bloch W, Schenk A, Zopf EM, Hildebrandt U, Streckmann F, Beulertz J, Koliamitra C, Schollmayer F, Baumann F. Exercise-induced natural killer cell activation is driven by epigenetic modifications. Int J Sports Med. 2015;36(6):510–515. doi: 10.1055/s-0034-1398531. [DOI] [PubMed] [Google Scholar]
- 22.Dorneles GP, da Silva IRV, Korb A, Bertoldi K, Siqueira IR, Elsner VR, Romão PRT, Peres A. High-intensity interval exercise enhances the global HDAC activity in PBMC and anti-inflammatory cytokines of overweight-obese subjects. Obes Med. 2016;2:25–30. doi: 10.1016/j.obmed.2016.05.004. [DOI] [Google Scholar]
- 23.Lovatel GA, Elsner VR, Bertoldi K, Vanzella C, Moysés Fdos S, Vizuete A, Spindler C, Cechinel LR, Netto CA, Muotri AR, Siqueira IR. Treadmill exercise induces age-related changes in aversive memory, neuroinflammatory and epigenetic processes in the rat hippocampus. Neurobiol Learn Mem. 2013;101:94–102. doi: 10.1016/j.nlm.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Barrichello T, Generoso JS, Simões LR, Faller CJ, Ceretta RA, Petronilho F, Lopes-Borges J, Valvassori SS, Quevedo J. Sodium butyrate prevents memory impairment by re-establishing BDNF and GDNF expression in experimental pneumococcal meningitis. Mol Neurobiol. 2015;52(1):734–740. doi: 10.1007/s12035-014-8914-3. [DOI] [PubMed] [Google Scholar]
- 25.Wu X, Chen PS, Dallas S, Wilson B, Block ML, Wang CC, Kinyamu H, Lu N, Gao X, Leng Y, Chuang DM, Zhang W, Lu RB, Hong JS. Histone deacetylase inhibitors up-regulate astrocyte GDNF and BDNF gene transcription and protect dopaminergic neurons. Int J Neuropsychopharmacol. 2008;11(8):1123–1134. doi: 10.1017/S1461145708009024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Li Q, Chang R, Yang D, Song Z, Guo Q, Huang C. Curcumin alleviates neuropathic pain by inhibiting 300/CBP histone acetyltransferase activity-regulated expression of BDNF and cox-2 in a rat model. PLoS One. 2014;9(3):e91303. doi: 10.1371/journal.pone.0091303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chandwani S, Keilani S, Ortiz-Virumbrales M, Morant A, Bezdecny S, Ehrlich ME. Induction of DARPP-32 by brain-derived neurotrophic factor in striatal neurons in vitro is modified by histone deacetylase inhibitors and Nab2. PLoS One. 2013;8(10):e76842. doi: 10.1371/journal.pone.0076842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida H, Matsushita Y, Ueda H. Epigenetic regulation of BDNF expression in the primary sensory neurons after peripheral nerve injury: implications in the development of neuropathic pain. Neurosci. 2013;14(240):147–154. doi: 10.1016/j.neuroscience.2013.02.053. [DOI] [PubMed] [Google Scholar]
- 29.Jia M, Liu WX, Sun HL, Chang YQ, Yang JJ, Ji MH, Yang JJ, Feng CZ. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, attenuates postoperative cognitive dysfunction in aging mice. Front Mol Neurosci. 2015;23(8):52. doi: 10.3389/fnmol.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 31.Noble EE, Billington CJ, Kotz CM, Wang C. The lighter side of BDNF. Am J Physiol Regul Integr Comp Physiol. 2011;300(5):R1053–R1069. doi: 10.1152/ajpregu.00776.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mattson MP. Evolutionary aspects of human exercise–born to run purposefully. Ageing Res Rev. 2012;11(3):347–352. doi: 10.1016/j.arr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abel JL, Rissman EF. Running-induced epigenetic and gene expression changes in the adolescent brain. Int J Dev Neurosci. 2013;31(6):382–390. doi: 10.1016/j.ijdevneu.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci USA. 1998;95(3):853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med Sci Sports Exerc. 2007;39(4):728–734. doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 36.Gilder M, Ramsbottom R, Currie J, Sheridan B, Nevill AM. Effect of fat free mass on serum and plasma BDNF concentrations during exercise and recovery in healthy young men. Neurosci Lett. 2014;7(560):137–141. doi: 10.1016/j.neulet.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 37.Rasmussen P, Brassard P, Adser H, Pedersen MV, Leick L, Hart E, Secher NH, Pedersen BK, Pilegaard H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp Physiol. 2009;94(10):1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 38.Robson-Ansley PJ, Saini A, Toms C, Ansley L, Walshe IH, Nimmo MA, Curtin JA. Dynamic changes in DNA methylation status in peripheral blood mononuclear cells following an acute bout of exercise: potential impact of exercise-induced elevations in interleukin-6 concentration. J Biol Regul Homeost Agents. 2014;28(3):407–417. [PubMed] [Google Scholar]
- 39.McGee SL, Fairlie E, Garnham AP, Hargreaves M. Exercise-induced histone modifications in human skeletal muscle. J Physiol. 2009;587(Pt 24):5951–5958. doi: 10.1113/jphysiol.2009.181065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, Caidahl K, Krook A, O’Gorman DJ, Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 41.Denham J, O’Brien BJ, Marques FZ, Charchar FJ. Changes in the leukocyte methylome and its effect on cardiovascular-related genes after exercise. J Appl Physiol. 2015;118(4):475–488. doi: 10.1152/japplphysiol.00878.2014. [DOI] [PubMed] [Google Scholar]
- 42.Rönn T, Volkov P, Davegårdh C, Dayeh T, Hall E, Olsson AH, Nilsson E, Tornberg A, Dekker Nitert M, Eriksson KF, Jones HA, Groop L, Ling C. A six months exercise intervention influences the genome-wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013;6:e1003572. doi: 10.1371/journal.pgen.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]


