Abstract
Hibiscus sabdariffa (HS) has been traditionally used as a herbal medicine in Nigeria mainly because of its antihypertensive action. In view of the recent increase in the prevalence of renal failure, we have investigated the effect of HS consumption on renal function in Nigerians with mild to moderate hypertension. A total of 78 newly diagnosed but untreated subjects with mild to moderate hypertension attending the medical outpatients unit of Enugu State University Teaching Hospital (Enugu, Nigeria) were recruited for the study. These subjects were randomly divided into three equally sized groups that received HS or lisinopril (treatment groups) or placebo (control group), once daily for 4 weeks. Indices of renal function (urine volume and creatinine clearance) were measured at baseline and weekly throughout the study period. HS and lisinopril significantly increased (P < 0.001) urine volume compared to placebo, and HS significantly (P < 0.001) increased urine volume more than lisinopril. HS significantly increased (P < 0.001) creatinine clearance compared to placebo whereas lisinopril did not. These results indicate that HS consumption improved indices of renal function in our study population of Nigerians with mild to moderate hypertension.
Keywords: Hibiscus sabdariffa, Lisinopril, Urine volume, Creatinine clearance, Essential hypertension
Introduction
Hibiscus sabdariffa (HS) Linn (Family Malvaceae) has been traditionally used as a herbal medicine in Nigeria. The calyx of the plant is used to produce a popular beverage called ‘Zobo’ among Nigerians [1]. Zobo has been used for the treatment of various diseases, such as high blood pressure (BP), liver diseases, anemia, cancer and fever [2, 3] urinary bladder and kidney stones [4]. Infusion made from calyces of HS are also useful to treat bilious conditions and heart and nerve diseases [5].
The antihypertensive properties of HS have been widely reported in both animal [6–8] and human [9–11] studies. Different mechanisms for the BP-lowering effect of HS have been suggested, including vasodilation via endothelium-dependent and -independent mechanisms [6] and vasodilatory action mediated through cholinergic and/or histaminergic mechanisms [7]. Other researchers have reported that its antihypertensive action is mediated via inhibition of angiotensin-converting enzyme (ACE) [9, 12]. The aqueous extract of HS has been found to have a diuretic effect in experimental animals [8, 13]. A significant uricosuric action was observed in rats given a decoction of dried calyces of HS [14]. Human studies have also shown that HS exerts effects on the kidney, with reports of ACE inhibition [9] and reduced plasma aldosterone level [12] in subjects with mild to moderate hypertension and altered chemical composition of urine and prevention of kidney stone formation in healthy young adults [15].
The kidney plays an important and central role in the long-term control of BP, mainly through the renin-angiotensin-aldosterone system (RAAS). The kidney is one of the target organs of hypertension, and many drugs, including antihypertensive drugs, are eliminated partially or totally through the kidney. Essential hypertension produces a significant reduction in renal function in some patients [16]; consequently, estimates of renal function are a useful tool in such patients [17]. Glomerular filtration rate (GFR) is a direct measure of renal function. and creatinine clearance (Ccr) is used as a clinical marker to measure GFR [18]. To date, renal function in hypertensive animals/subjects treated with HS has not be investigated. We have therefore studied the effects of HS on indices of renal function, namely, urine output and Ccr, in subjects with mild to moderate hypertension and compared these subjects with those receiving a standard antihypertensive drug (lisinopril) that exerts its action on the kidney. The results of this comparison will assist in determining the suitability or otherwise of using HS to treat patients with mild to moderate hypertension.
Materials and methods
Study setting
The study was carried out in Enugu, the capital city of the Nigerian state of Enugu, located in the South-East Zone of Nigeria. Enugu is a cosmopolitan city with an estimated population of over 1 million people. Most of the inhabitants are civil servants, traders and artisans. The study period lasted for 4 weeks.
Study subjects
A total of 78 (45 men, 33 women; age range 35–68 years) subjects with mild to moderate hypertension who were attending the medical outpatient (MOP) clinic of Enugu State University Teaching Hospital, Parklane, Enugu, were recruited for this study. These subjects were randomly divided into three groups (placebo, HS and lisinopril) of 26 subjects per group. However, only 75 subjects completed the study; two subjects in the lisinopril group developed cough and their medication was changed, and one subject withdrew from the HS group for non-medical reasons.
The inclusion criteria were: (1) newly diagnosed but untreated mild to moderate hypertension [19]; (2) adequate understanding of the study and provision of informed consent. Individuals were excluded from entry into the study if they had diabetes, nephropathy, cardiopathy, hepatic disease or cancer, were pregnant, showed signs of secondary hypertension, were chronic smokers or suffered from alcoholism or habitually drank ‘zobo'. In addition, all data pertaining to those who prematurely left the study were excluded.
Study protocol
All participants were prohibited from participating in other clinical studies and from consuming alcohol during the study period. Those individuals who met the inclusion criteria were randomly divided into three groups—placebo (control group), HS and lisinopril (both treatment groups), respectively. Lisinopril is an ACE inhibitor which is commonly prescribed in Nigeria and was chosen as the positive control since HS has been reported to have an inhibitory effect on ACE. Subjects randomized to the placebo group received 150 mg/kg of placebo orally once daily before breakfast for 4 weeks; those randomized to the HS group (group B) were given an HS infusion (150 mg/kg) orally once daily before breakfast for 4 weeks; those randomized to the lisinopril group (group C) took 10 mg lisinopril (Zestril®; Reals Pharmaceuticals; Lagos, Nigeria) orally once daily before breakfast for 4 weeks.
Both the subjects and the researchers/physicians taking the measurements were blinded to the treatment for each group. All newly recruited subjects were given 2 days before the commencement of treatment to become familiar with the procedure. This allowed sufficient time for the collection of baseline 24-h urine samples.
All participants in the study were given weekly appointments and a 1-week long supply of infusion/medication. Serum creatinine level, 24-h urine volume, urine creatinine level and BP were measured at baseline and at weekly intervals during the 4-week-long study. Clinical evaluation and control of treatment adherence were also conducted weekly.
The study was carried out in accordance with the guidelines of the Helsinki Declaration for human studies (as amended) and approved by the ethical committee of our institution (ESUTTH/EC/11002).
Hibiscus sabdariffa
Plant collection
Dried calyces of H. sabdariffa were purchased from the Ogbete Main Market, Enugu. They were subsequently authenticated by A. Ozioko of the herbarium section of the Botany Department, University of Nigeria, Nsukka, and specimen voucher number UNH/314b was assigned to them.
Preparation of HS decoction
We used the method of Herrera-Arellano et al. [9] as modified by Nwachukwu et al. [11] to prepare the HS decoction. In brief, 20 g of dry calyces were weighed and ground with an electric mill to obtain particles of <2 mm. We then added 1 L of boiling clean bottled water (Aquafina; Pepsi Nigeria. Ltd., Lagos, Nigeria) to these particles and allowed the mixture to stand for 30 before filtering it through Whatman’s no.1 filter paper (Whatman International Ltd., Maidstone, UK). The filtrates were stored in clean plastic containers at room temperature and given to subjects on weekly basis.
Calculation of HS dosage
Daily dose = 150 mg/kg;
1 kg = 150 mg;
Weight of patient = W kg.
W kg = 150 × W mg = 0.15 × W g.
As 20 g calyces was dissolved in 1 L of water, then
20 g = 1 L, and
0.15 × W g = (0.15 × W/ 20 × 1)L = 0.0075 × W/L.
To calculate the volume of HS decoction given to a subject, the weight was multiplied by a constant, 0.0075.
The amount of 150 mg/kg was chosen because it is far below the median lethal dose of HS (>5000 mg/kg) and produced approximately the same color as the locally brewed ‘Zobo’ drink.
Preparation of placebo
Blackcurrant (GlaxoSmithKline, Brentford, UK) was used as placebo. It was first diluted with clean bottled water (Aquafina, Pepsi Nigeria Ltd.) to obtain approximately the same color as the HS infusion. Blackcurrant was chosen among other drinks because its color is similar to that of the locally prepared “Zobo” drink, and a preliminary investigation of 20 healthy subjects given an equivalent dose of it for 2 weeks showed that it has no effect on BP. Subjects in the placebo group were monitored by telephone calls every 2 days to ensure that there were no adverse symptoms.
Quantification of anthocyanin in HS
The quantity of anthocyanins in HS was estimated by colorimetry as described by Fuleki and Francis [20]. This method is based on the ability of anthocyanin pigments (present in HS) to undergo a reversible structural transformation with changes in the pH that can be visualized strikingly different absorbance spectra. Anthocyanins produce a color at pH 1.0 that disappears at pH 4.5. This special characteristic is produced by a pH-dependent structural transformation of anthocyanin chromophore where the colored oxonium ion pre-dominates at pH 1.0, while the non-colored hemiketal is present at pH 4.5. This measurement method allows a rapid and accurate determination of total anthocyanins, even in the presence of polymerized degraded pigments and other interfering compounds. This procedure was performed with 1 ml of the HS solution (20 g dried HS calyx extracted in 1 L water). Two samples were modified to a 5-ml solution at pH 1.0 and 4.5, respectively. These solutions were filtered through a 0.45-mm membrane (Gelman Acrodisk®; Pall Corp., New Washington, NY) and analyzed with a spectrophotometer at 510 and 700 nm. The concentration of total anthocyanins was determined using the formula:
where A is the absorbance of the diluted sample, MW is the molecular weight of anthocyanin, FD is the dilution factor and ε is molar absorptivity).
Based on this calculation, the total amount of anthocyanin contained in 20 g of HS dissolved in 1 L was 10.04 mg.
Measurements
BP measurement
Sitting BP was measured using an Accoson® mercury sphygmomanometer (AC Cossor & Son Surgical Ltd, Harlow, UK). The systolic blood pressure (SBP) was measured as the first appearance of Korotkov sounds, and the diastolic blood pressure (DBP) was measured as the point of disappearance of the sounds (Phase V). Two consecutive readings were taken for each subject at a 5-min interval between measurements, and the average of these was taken as the mean BP reading. All measurements were taken between 0800 hours and 1000 hours. Any constrictive clothing on the arm was removed before the measurement was taken.
Pulse pressure is the difference between the SBP and DBP readings, i.e. PP = SBP − DBP., and represents the force that the heart generates each time it contracts.
Weight and height measurements
Weight (to the nearest 0.1 kg) and height (to the nearest 0.1 cm) were measured using a stadiometer (Seca Ltd., Birminghan, UK), and the body mass index (BMI) was calculated using the formula: BMI = weight (kg)/height (m2).
24-h Urine collection and analysis
The subjects were taught the proper way to collect a 24-h urine sample under supervision, mostly by relatives. A 4-L plastic container treated with HCl was given to each subject for urine collection; each female subject was also given a plastic funnel to facilitate urine collection. The subjects were instructed to empty their bladder at 0800 hours at the start of collection; all subsequent urine was passed into the container until the morning of the next day which was their appointment day. They were told to pass the final urine collection into the container at 0800 hours and the importance of carefully collecting all urine during the period was emphasized. The volume of urine was measured using a measuring cylinder.
Measurement of urine creatinine
Urine creatinine concentration was determined using Jaffe’s method [21]. A 0.5-ml sample of the collected urine of each subject was put into a test tube (test tube A) and 0.5 ml of the standard was measured out and placed in test tube B. We then added 3 ml of diluent into tube A, followed by 1 ml each of Na tungstate and 2/3 N H2SO4 to precipitate chromogenes. Tube A was then shaken and then left to stand for 10 min. The mixture in tube A was then centrifuged at 4000 rpm for 2 min, following which 3 ml of the supernatant was removed and added to 1 ml of 0.004 N picric acid and 1 ml of 0.25 N sodium hydroxide. This solution was then shaken and allowed to stand at room temperature for 15 min, following which 12 ml of diluent was added to the standard in tube B. The solutions from A and B were read on a colorimeter (spectrophotometer) at 500 nm. The concentration of urine creatinine was then calculated by simple proportion knowing the value of the standard: for example, the concentration of creatinine in urine (mg/dl) = (A sample/B standard) × 2.
Measurement of serum creatinine
Venous blood (2 ml) was withdrawn from medial cubital vein into a vacutainer and allowed to stand undisturbed for 25 min. Samples were collected between 0800 hours and 1000 hours at baseline and on the day of the appointment. The clot formed was removed by centrifugation at 2000 rpm for 10 min. The resulting supernatant (serum) was transferred to a clean polypropylene tube using Pasteur pipette. Creatinine concentration was determined using Jaffe’s method as described in the preceding section [21].
Calculation of Ccr
The Ccr value was calculated using the formula:
Data analysis
All results were presented as mean ± standard error of mean. Data were analyzed using SPSS version 20 (IBM Corp., Armonk, NY). One-way analysis of variance with Bonferroni posttest using the Prism 5.0 software package (GraphPad Software Inc. La Jolla, CA) was used to compare differences between groups. P ≤ 0.05 was considered to be statistically significant.
Results
Changes in BP
At the end of the 4-week treatment period, subjects receiving HS showed the greatest decrease in SBP (−17.08 ± 2.01 mmHg), DBP (−12.12 ± 1.04 mmHg) and MAP (−13.68 ± 1.82 mmHg), followed by those receiving lisinopril, with decreases in SBP, DBP and MAP of −12.60 ± 1.15, −9.20 ± 1.10 and −10.31 ± 1.14 mmHg, respectively (Table 1). The decrease in SBP and DBP in the HS and lisinopril treatment groups during the study period is shown in Figs. 1 and 3, respectively. Comparison of the SBP values shown in Fig. 1 with the baseline values revealed that the changes in SBP in the HS group compared to the SBP in the placebo group were significant (P < 0.001) at each weekly measurement point during the 4 weeks of the study, while the changes in SBP in the lisinopril group compared to the SBP in the placebo group were significant (P < 0.001) at the measurement points for weeks 2–4 (Fig. 2). The changes in DBP followed a similar pattern: comparison of the DBP values in Fig. 3 with the baseline values revealed that the changes in DBP in the HS group were significant compared to the DBP in the placebo group at each weekly measurement point during weeks 1–4, while the changes in DBP in the lisinopril group compared to the DBP in the placebo group were significant at the measurement points for weeks 2–4 (Fig. 4). When the reductions in SBP and DBP caused by HS and lisinopril were compared, the reduction in SBP in the HS group was significantly greater than that in the lisinopril group at weeks 2 (P < 0.01), 3 and 4 (both P < 0.001) (Fig. 2); the reduction in DBP in the HS group was also significantly greater than that in the lisinopril group at weeks 2 (P < 0.05), 3 and 4 (both P < 0.001) (Fig. 4).
Table 1.
Basic clinical characteristics of hypertensive subjects according to groups
| Parameter | Placebo | Hibiscus sabdariffa | Lisinopril |
|---|---|---|---|
| Age (years) | 48.90 ± 5.06 | 49.92 ± 3.40 | 53.25 ± 3.25 |
| Body mass index (kg/m2) | 27.27 ± 1.50 | 28.10 ± 2.48 | 27.87 ± 2.45 |
| Change in systolic blood pressure after 4 weeks (mmHg) | −1.10 ± 0.05 | −17.08 ± 2.01*α | −12.60 ± 1.15* |
| Change in diastolic blood pressure after 4 weeks (mmHg) | −0.40 ± 0.02 | −12.12 ± 1.04* | −9.20 ± 1.10* |
| Change in Mean arterial pressure after 4 weeks (mmHg) | −0.95 ± 0.20 | −13.68 ± 1.82* | −10.31 ± 1.14* |
*Significant at P < 0.001 with respect to placebo; αSignificant at P < 0.05 with respect to lisinopril
Results are presented as the mean ± standard error of the mean. Two-way analysis of variance was used to compare groups
Fig. 1.
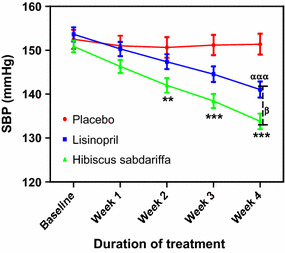
Effect of treatments on systolic blood pressure (SBP) in subjects with mild to moderate hypertension. Each point on the graph represents the average of at least 25 independent measurements. Error bars Standard error of mean (SEM). **P < 0.01, ***P < 0.001 (placebo vs. Hibiscus sabdariffa), ααα P < 0.001 (placebo vs. lisinopril); β P < 0.05 (H. sabdariffa vs. lisinopril). All analyses were performed with two-way analysis of variance (ANOVA) with the Bonferroni posttest using the GraphPad Prism 5.0 analysis program
Fig. 3.
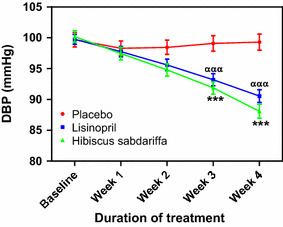
Effect of treatments on diastolic blood pressure (DBP). Each point on the graph represents the average of at least 25 independent measurements. Error bars SEM. ***P < 0.001 (placebo vs. H. sabdariffa); ααα P < 0.001 (placebo vs. lisinopril). All analyses were performed with two-way ANOVA with the Bonferroni posttest using the GraphPad Prism 5.0 analysis program
Fig. 2.
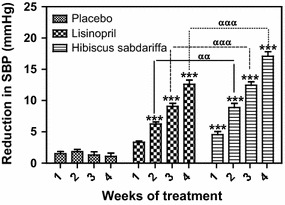
Reduction in SBP in subjects with mild to moderate hypertension, derived from a comparison of the BP values in Fig. 1 with the baseline values. Each point on the graph represents the average of at least 25 independent derivations. Error bars SEM. ***P < 0.001 (H. sabdariffa and lisinopril vs.placebo); αα P < 0.01, ααα P < 0.001 (H. sabdariffa vs. lisinopril). All analyses were performed with two-way ANOVA with the Bonferroni posttest using the GraphPad Prism 5.0 analysis program
Fig. 4.
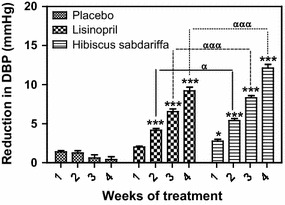
Reduction in DBP in subjects with mild to moderate hypertension, derived from a comparison of the BP values in Fig. 2 with the baseline values. Each point on the graph represents the average of at least 25 independent derivations. Error bars SEM. *P < 0.05, ***P < 0.001 (H. sabdariffa and lisinopril vs. placebo); α P < 0.01, ααα P < 0.001 (H. sabdariffa vs. lisinopril). All analyses were performed with two-way ANOVA with the Bonferroni posttest using the GraphPad Prism 5.0 analysis program
Changes in urine volume
There was a significant increase in urine volume (P < 0.001) in the Hs treatment group compared to the placebo group at each measurement point during the entire study period; in comparison, the increase in urine volume in the lisinopril group compared to the placebo group was significant (P < 0.001) only at the measurement points in weeks 3 and 4 (Fig. 5). When both treatment groups were compared to each other, the increase in urine volume in the HS group was significantly higher than that in the lisinopril group (P < 0.001) at all measurement points during the weeks 1–4 (Fig. 5).
Fig. 5.
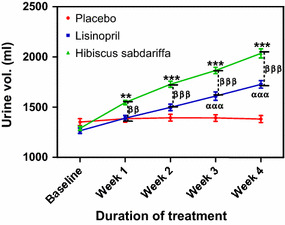
Urine volume following the administration of placebo (control), lisinopril and H. sabdariffa in subjects with mild to moderate hypertension. Each point on the graph represents the average of at least 25 independent measurements. Error bars SEM. **P < 0.01 ***P < 0.001(H. sabdariffa vs. placebo); ααα P < 0.001 (lisinopril vs. placebo); ββ P < 0.01,βββ P < 0.001(H. sabdariffa vs.lisinopril. All analyses were performed with two-way ANOVA with the Bonferroni posttest using the GraphPad Prism 5.0 analysis program
Creatinine clearance
There was a significant increase in Ccr in the HS group compared to the placebo group at weeks 3 (P < 0.01) and 4 (P < 0.001); in comparison, Ccr in the lisinopril group was not significantly different from that in the placebo group throughout the study period (Fig. 6). The increase in Ccr in the HS group was not significantly different from that in the lisinopril group (Fig. 6).
Fig. 6.
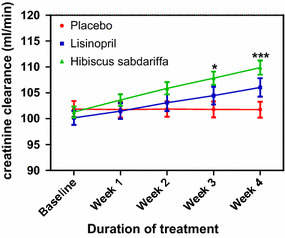
Creatinine clearance following the administration of placebo (control), Lisinopril and Hibiscus sabdariffa in mild to moderate hypertensive subjects. Each point on the graph represents the average of at least 25 independent measurements. Error bars SEM. *P < 0.01, ***P < 0.001(Hibiscus sabdariffa vs. placeb. All analyses were performed with two-way ANOVA with the Bonferroni posttest using the GraphPad Prism 5.0 analysis program
Discussion
The clearance of many drugs and their metabolites depends on adequate renal function, and the monitoring of this function is vital in terms of the appropriate prescription of drugs in many disease conditions, such as hypertension [18]. The results of our study show that treatment with HS improved both indices of renal function (urine output and Ccr) better than lisinopril. The significantly higher volume of urine produced by the patients receiving HS signifies that HS has a better diuretic activity than lisinopril and that this diuretic activity may be responsible for the greater antihypertensive activity observed in HS group. Diuretics are well-known antihypertensive agents; thus, the diuretic activity of HS may be one of its important antihypertensive mechanisms of action. This effect of HS on the kidney may be due to the action of anthocyanins, which are predominant chemical compounds in HS. Human studies have reported that HS possesses ACE inhibitory activity [9, 22] and can reduce the plasma aldosterone level [12]. Both an inhibition of ACE and a reduced plasma aldosterone level will reduce reabsorption in the kidney and increase urine output. Our results therefore agree with those from these studies. ACE inhibitors are also known to cause vasodilation by reducing angiotensin II formation [23]. Renal vasodilation will reduce reabsorption at the distal convoluted tubules and collecting ducts of the kidney thereby promoting diuresis [24]. Quantification of the amount of anthocyanins in the HS decoction given to our HS subjects revealed that the amount was similar to that reported by Herrera-Arellano et al. in a study carried out in Mexico [9], although we observed a greater reduction in both SBP and DBP. These authors obtained 9.6 mg of total anthocyanins from their sample, while we obtained 10.04 mg from a similar sample. SBP was reduced by −14.15 ± 11.28 mmHg in the Mexican study and by −17.08 ± 2.01 mmHg in our study. Similarly, DBP reduced by −11.18 ± 6.91 mmHg in the Mexican study and by 12.12 ± 1.04 mmHg in our study. Herrera-Arellano et al. [9] compared the effectiveness of HS as an antihypertensive with that of captopril (an ACE inhibitor) and reported that captopril (50 mg daily) produced a better effect; in contrast, in our study we found that HS produced better antihypertensive and renal effects than lisinopril (10 mg daily). The difference in dosage, anthocyanin content of HS and racial factors may be responsible for this disparity in the results of the two studies. Taken as a whole, our results validate the ethnomedicinal use of HS as a diuretic in a number of countries, such as Egypt and Sudan [25].
Another important action of HS on the kidney that has also been attributed to anthocyanins is its angiotensin receptor blocking activity [26]. Angiotensin receptor blockers are drugs that antagonize the action of angiotensin II in a highly selective manner at the angiotensin AT1-receptor. AT1-receptors mediate all of the classical effects of angiotensin II; thus, blocking the receptors will reduce systemic vascular resistance that results from the inhibition of angiotensin II-mediated vasoconstriction, reduced sympathetic nervous system activity and reduced extracellular volume [26]. Thus, this effect might have contributed to the diuretic and antihypertensive activity observed in the HS group.
Another possible diuretic mechanism of HS may be due to its ability to cause nitric oxide release [6], which in turn produces renal vasodilation via inhibition of Ca2+ influx. The ability of HS to inhibit Ca2+ influx is mediated by quercetin and eugenol present in HS extract [27] and has been demonstrated in previous studies [6, 24]. In these studies, inhibition of Ca2+ influx was achieved via endothelium-dependent and -independent mechanisms, with the endothelium-dependent vasodilator effect being due to activation of the endothelium-derived nitric oxide/cGMP-relaxant pathway and the endothelium-independent effect being possibly due to the inhibition of Ca2+ influx by the action of quercetin and eugenol.
Our results on diuresis are not in agreement with those of Odigie et al. [28] who observed no change in urine volume in hypertensive rats after treatment with HS infusion. One possible explanation for this difference is that Odigie et al. [28] used rats with renovascular (secondary) hypertension where the RAAS was highly stimulated whereas we used humans with essential (primary) hypertension.
Creatinine clearance is a direct measure of GFR, and GFR is an index of renal function [17]. The increased Ccr observed in the HS group indicates improved renal function, which can be largely attributed to the action of anthocyanins in increasing the GFR via inhibition of angiotensin II production. The vasodilatory actions of other constituents of HS, such as eugenol and quercetin, might have also contributed to the increase in Ccr by increasing renal blood flow and thus GFR. The increase in Ccr was higher in the HS group than in the lisinopril group despite lisinopril being a drug whose antihypertensive effectiveness has been reported in mild to moderate hypertensive patients [12, 29]. Our results also show that both HS and lisinopril exerted nephroprotective effects as renal function was improved in both groups. This result is in agreement with that of Cinotti and Zucchelli [30] who demonstrated that lisinopril possesses specific nephroprotective actions in addition to BP control and strengthens the hypothesis proposed by these authors that ACE inhibitors are effective in slowing down the progression of renal insufficiency. Lisinopril has also been shown to be effective in moderate to severe hypertensive patients with impaired renal function [31], an action supported by our study results which indicate that lisinopril improved renal function and significantly reduced BP in our subjects with mild to moderate hypertension. We observed that the HS treatment improved renal function and reduced BP better than the lisinopril treatment, suggesting that the former may be a better antihypertensive agent this patient population.
Conclusion
Consumption of HS with a standardized amount of 10.04 mg anthocyanins improved renal function in patients with mild to moderate hypertension, with better effects than lisinopril.
Recommendation
We recommend that consumption of HS should be controlled by appropriate government regulatory agencies, such as the National Agency for Drug Administration and Control (NAFDAC). Further research is required to explore the possibility of utilizing our important finding and integrating it into our National Health Care Program since HS is easily available and affordable in Nigeria.
Acknowledgments
We wish to express our gratitude to the management of Enugu State University Teaching for allowing us use their facility for this study. We also express our gratitude to Mr. John Ugwuona and Mr. Stanley Ugwubujo for their assistance during the preparation of the HS infusion.
Compliance with ethical standards
Funding
This was a self-sponsored study with no external funding.
Conflict of interest
The authors declare that they have no conflict of interest regarding this study.
References
- 1.Gbile ZO, Adesina SK. Nigeria flora and its pharmaceutical potentials. J Ethnopharmacol. 1986;19:1–16. doi: 10.1016/0378-8741(87)90135-8. [DOI] [PubMed] [Google Scholar]
- 2.Sharaf A. The pharmacological characteristics of Hibiscus sabdariffa . Planta Med. 1962;10:48–52. doi: 10.1055/s-0028-1100273. [DOI] [Google Scholar]
- 3.Dalziel JM. The useful plants of west Tropical Africa. London: The Crown Agents; 1973. pp. 314–315. [Google Scholar]
- 4.Farnsworth NR, Bunyaprephatsara N (1992) Thai medicinal plants recommended for primary health care system. Mahidol University, Bangkok
- 5.Chopra RN, Nayar SL, Vanna BS. Supplement to glassOIY of Indian medicinal plants. Publications and information directorate. New Delhi: Council of Scientific and Industrial Research; 1969. p. 119. [Google Scholar]
- 6.Obiefuna PCM, Owlabi OA, Adegunloye BJ. The petal extract of Hibiscus sabdariffa produces relaxationof isolared rat aorta. Int J Pharmacog. 1994;32:69–74. doi: 10.3109/13880209409082975. [DOI] [Google Scholar]
- 7.Adegunloye BJ, Omoniyi JO, Ajabonna OP, Sofola OA, Coker HAB. Mechanisms of the blood pressure lowering effects of the calyx extract of Hibiscus sabdariffa in rats. Afr J Med Med Sci. 1996;25:235–238. [PubMed] [Google Scholar]
- 8.Onyenekwe PC, Ajani EO, Ameh DA, Gamaniel KS. Antihypertensive effect of roselle (Hibiscus sabariffa) calyx infusion in spontaneously hypertensive rats and a comparison of its toxicity with that in Wistar rats. Cell Biochem Funct. 1999;17:199–206. doi: 10.1002/(SICI)1099-0844(199909)17:3<199::AID-CBF829>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Herrera-Arellano A, Flores-Romero S, Chavez-Soto MA, Tortoriello J. Effectiveness and tolerability of a standardize extract from Hibiscus Sabdariffa in patients with mild to moderate hypertension: a controlled and randomized clinical trial. Phytomedicine. 2004;11:375–382. doi: 10.1016/j.phymed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 10.McKay DL, Chen CY, Saltzman E, Blumberg JB. Hibiscus sabdariffa L. tea (tisane) lowers blood pressure in prehypertensive and mildly hypertensive adults. J Nutr. 2010;140:298–303. doi: 10.3945/jn.109.115097. [DOI] [PubMed] [Google Scholar]
- 11.Nwachukwu DC, Aneke E, Obika LFO, Nwachukwu NZ. Investigation of antihypertensive effectiveness and tolerability of Hibiscus sabdariffa in mild to moderate hypertensive subjects in Enugu, south-east, Nigeria. Am J Phytomed Clin Ther. 2015;3:339–345. [Google Scholar]
- 12.Nwachukwu DC, Aneke EI, Obika LF, Nwachukwu NZ. Effects of aqueous extract of Hibiscus sabdariffa on the renin-angiotensin aldosterone system of Nigerians with mild to moderate essential hypertension: a comparative study with lisinopril. Indian J Pharmacol. 2015;47:540–545. doi: 10.4103/0253-7613.165194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mojiminiyi FBO, Adegunloye BJ, Egbeniyi YA, Okolo RU. An investigation of the diuretic effect of an aqueous extract of Hibiscus sabdariffa. J Med Med Sci. 2000;2:77–80. [Google Scholar]
- 14.Caceres A, Giron LM, Martinez AM. Diuretic activity of plants used for treatment of urinary ailments in Guatemala. J Ethnopharmacol. 1987;19:233–245. doi: 10.1016/0378-8741(87)90001-8. [DOI] [PubMed] [Google Scholar]
- 15.Kirdpon S, Narkon SN, Kirdpon W. Changes in urinary chemical composition in healthy volunteers after consuming roselle (Hibiscus sabdariffa Linn) juice. J Med Assoc Thail. 1994;76(6):314–321. [PubMed] [Google Scholar]
- 16.Bigazzi R, Bianchi S, Campese VM, Baldari G. Prevalence of mocroalbuminuria in a large population of patients with mild to moderate essential hypertension. Nephron. 1992;61:94–97. doi: 10.1159/000186842. [DOI] [PubMed] [Google Scholar]
- 17.Nankivell BJ. Creatinine clearance and assessment of renal function. Aust Prescr. 2001;24:15–17. doi: 10.18773/austprescr.2001.009. [DOI] [Google Scholar]
- 18.Walter FB, Emile LB (2009) Medical physiology: a cellular and molecular approach. Elsevier, London
- 19.Guidelines Subcommittee (1999) World Health Organization–International Society of Hypertension guidelines for the management of hypertension. J Hypertens 17:151–183 [PubMed]
- 20.Fuleki T, Francis FJ. Quantitative methods of anthocyanins. 2. Determination of total anthocyanin and degradation index for cranberry juice. J Food Sci. 1968;33:78–83. doi: 10.1111/j.1365-2621.1968.tb00888.x. [DOI] [Google Scholar]
- 21.Pesce AJ, Kaplan LA. Creatinine clearance. In: Pesce AJ, Kaplan LA, editors. Methods in clinical chemistry. St. Louis: Mosby; 1987. pp. 13–17. [Google Scholar]
- 22.Jonadet M, Bastide J, Boyer B, Carnat AP, Lamaison JL (1990) In vitro enzyme inhibitory and in vivo cardioprotective activities of Hibiscus (Hibiscus sabdariffa L.). Pharm Belg 45(2):120–124 [PubMed]
- 23.Caballero-George C, Vanderheyden PML, De Bruyne T, Shahat AA, Van den Heuvel H, Solis PN, et al. In vitro inhibition of angiotensin II binding on human AT1 recptors by proanthocyanidins from Guazuma ulmifolia bark. Planta Med. 2002;68:1066–1071. doi: 10.1055/s-2002-36344. [DOI] [PubMed] [Google Scholar]
- 24.Ajay M, Chai HJ, Mustafa AM, Gilani AH, Mustafa MR. Mechanisms of the anti-hypertensive effect of Hibiscus sabdariffa L. calyces. J Ethnopharmacol. 2007;109:388–393. doi: 10.1016/j.jep.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Leung A, Foster S. Encyclopedia of common natural ingredients used in food, drugs, and cosmetics. 2. New York: Wiley; 1996. [Google Scholar]
- 26.Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent) 2003;16:123–126. doi: 10.1080/08998280.2003.11927893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salah AM, Gathumbi J, Vierling W. Inhibition of intestinal motility by methanolic extracts of Hibiscus sabdariffa L. (Malvaceae) in rats. Phytother Res. 2002;16:283–285. doi: 10.1002/ptr.846. [DOI] [PubMed] [Google Scholar]
- 28.Odigie IP, Ettarh RR, Adigun SA. Chronic administration of aqueous extract of Hibiscus sabdariffa attenuates hypertension and reverses cardiac hypertrophy in 2 K-1 C hypertensive rats. J Ethnopharmacol. 2003;86:181–185. doi: 10.1016/S0378-8741(03)00078-3. [DOI] [PubMed] [Google Scholar]
- 29.Jalal S, Sofi FA, Abass SM, Alai MS, Bhat MA, Rather HA, Lone NA, Siddiqi MA. Effect of amlodipine and lisinopril on microalbuminuria in patients with essential hypertension: a prospective study. Indian J Nephrol. 2010;20(1):15–20. doi: 10.4103/0971-4065.62090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cinotti GA, Zucchelli PC. Effect of lisinopril on the progression of renal insufficiency in mild proteinuric non-diabetic nephropathies. Nephrol Dial Transplant. 2001;16:961–966. doi: 10.1093/ndt/16.5.961. [DOI] [PubMed] [Google Scholar]
- 31.De Jong PE, Apperloo AJ, Heeg JE, de Zeeuw D. Lisinopril in hypertensive patients with renal function impairment. Nephron. 1990;55:43–48. doi: 10.1159/000186034. [DOI] [PubMed] [Google Scholar]


