Abstract
Diabetes is characterized by absolute or relative insulin deficiency complicated with microangiopathy, whereas obesity stems from insulin resistance. A psychosomatic approach to obesity and diabetes has been highlighted, including the brain-oriented obesity control system (BOOCS). Impaired deformability of erythrocytes in obese or diabetic patients is closely linked to disturbed microcirculation, and improvement of abnormal erythrocyte rheology is a prerequisite for the prevention and treatment of microangiopathy. Therefore, erythrocyte filterability, whole cell deformability defined as flow rate of erythrocyte suspension relative to that of saline, was assessed by the nickel-mesh-filtration technique. Subjects included healthy controls (group A, n = 14), diabetic, non-obese participants (group B, n = 29), and non-diabetic, obese participants (group C, n = 32) in the 6-month BOOCS program, and most patients in groups B and C (86.9 %) completed this program. Baseline mean erythrocyte filterabilities were 89.4 ± 1.7 % in group A, 82.8 ± 5.2 % in group B, and 84.1 ± 5.6 % in group C, showing significant intergroup differences (p < 0.001). This program significantly improved (p < 0.001) the impaired erythrocyte filterability in groups B (87.9 ± 4.4 %) and C (88.5 ± 3.7 %). Declines in HbA1c (p = 0.387) and body mass index (p = 0.479) were not correlated to this improvement. These findings indicate that the mechanisms of BOOCS-induced improvement of diabetic or obese patients’ erythrocyte deformability are multifactorial, and that the BOOCS program for these patients is a holistic, cost-effective, and highly compliant approach possibly ameliorating microcirculation.
Keywords: BOOCS, Deformability, Diabetes, Erythrocytes, Obesity
Introduction
It is well known that microcirculation is impaired in diabetes mellitus, which is characterized by absolute or relative insulin deficiency causing hyperglycemia, glucosuria, and various diabetic complications including microangiopathy. This diabetic environment has a harmful rheological impact on circulating erythrocytes. Human erythrocytes have a diameter greater than the smallest capillary network, and hence intact erythrocytes cannot pass through the microvascular network without any physiologic deformation. Therefore, deformability of erythrocytes plays a key role in fluent microcirculation [1]. We have investigated erythrocyte filterability, a surrogate of whole cell deformability, using the highly sensitive and reproducible nickel-mesh-filtration technique, which is useful for assessing the physiological bending deformation of intact circulating erythrocytes [2–4].
Our recent study clarified the impaired filterability of diabetic rat and human erythrocytes quantitatively [4]. This impairment is closely associated with diabetic microangiopathy in that undeformable erythrocytes may increase the shear stress, elevate vascular resistance, and cause cardiac overload and renal dysfunction. In our study, the impairment of diabetic erythrocyte filterability was closely linked to glycemic stress on erythrocytes quantified by glycated hemoglobin (HbA1c). Therefore, we anticipated that reduction of HbA1c may contribute to improvement of the impaired diabetic erythrocyte filterability. For glycemic control in diabetic patients, self-promoting management supported by healthcare professionals is important, and positive healthy emotion protects patients from distress and depression observed in long-term anti-diabetic treatment [5].
Diabetes is often associated with obesity which is characterized by insulin resistance. Obesity is a target of current psychosomatic approaches [6, 7], one of which stems from the theory that stress-induced so-called ‘brain-fatigue’ leads to taste resetting and dysfunction, which lead to abnormal eating behaviors such as overeating and eating of oily, pungent food. Therefore, psychosocial stress reduction causes natural taste recovery and body weight reduction without any calorie restriction. This kind of holistic medicine actually provided a better understanding of the psychobiological aspects of obesity and reliable weight reduction among participants. This program was termed the brain-oriented obesity control system (BOOCS) [8]. Therefore, we hypothesized that this BOOCS program may be helpful not only for diabetic subjects but also for obese patients, and may improve the impaired erythrocyte filterability in these subjects. The present study was designed to test this hypothesis.
Methods
Subjects
This study design was approved by the internal ethics committee of The Institute of Rheological Function of Foods Co. Ltd. (Hisayama, Fukuoka, Japan), and performed according to the Declaration of Helsinki (2001), that is, signed informed consent was obtained from subjects prior to enrollment on the BOOCS program and, then, to this study design. Subjects included healthy volunteers (group A, n = 14, mean age 46.7 ± 9.4 years), non-obese, diabetic participants (group B, n = 29, mean age 47.2 ± 8.8 years), and obese, non-diabetic participants (group C, n = 32, mean age 49.6 ± 9.5 years) for the 6-month BOOCS program. In our recent study, diabetes and obesity synergistically deranged erythrocyte filterability [4]. Therefore, patients with concomitant diabetes and obesity were excluded from this study to evaluate the individual rheological effects of these two common diseases. Diabetes mellitus was defined as HbA1c (NGDP) >6.5 % and/or ongoing anti-diabetic medication, whereas obesity was defined as body mass index (BMI) >25.
BOOCS program
Because the BOOCS program is not based on the balance between calorie intake and energy expenditure, calorie-calculation, carbo-counting, and exercise energy consumption are not scheduled. Instead, the basic principle consists of two paradoxical items as follows; (1) prohibition of hard practices that the participant does not like, even if they are favorable to a healthy life, and (2) promotion of pleasant practices that the participant likes, even if they are believed unfavorable to a healthy life [8]. After explaining the basic principle and practical methodologies of this program, outpatients participated in this program voluntarily. These participants were followed up monthly in the BOOCS clinics (Fukuoka, Japan). Prescriptions of these participants were not altered during the study period. Smoking, drinking, and physical activity in daily life were not regulated according to the above principle.
Erythrocyte suspensions
Erythrocyte suspensions were prepared as described elsewhere [4]. In detail, about 10 ml of venous blood was drawn from the antecubital vein of subjects, using 21-gauge needles and disposable syringes (Terumo Japan, Tokyo, Japan) filled with 1/10 volume of 3.8 % trisodium citrate as an anticoagulant. Venous blood sampling was performed in the morning after an overnight fast. Blood cell counting and hematocrit measurements were carried out using a hemocytometer (Ace Counter, FLC-240A; Fukuda Denshi, Tokyo, Japan). Concurrently, HbA1c was estimated using a routine autoanalyzer (Sysmex model K-4500; Sysmex, Tokyo, Japan). After centrifugation of venous blood at 1,300g for 10 min, supernatant was carefully aspirated to replace buffy coat and plasma with saline buffered with N-(2-hydroxyethyl)-piperazine-N′-2-ethanesulfonic acid (HEPES) sodium salt (HEPES-Na). The composition of HEPES-Na-buffered saline (HBS) was NaCl 141 mM and HEPES-Na 10 mM. Osmolality and pH of the HBS were 287 mOsm/kg H2O and 7.4, respectively. The osmolality of the HBS was measured using a freezing point depression-type osmometer (Fiske Mark 3 Osmometer; Fiske Associates, MA, USA). Erythrocytes were then washed three times by repeated resuspension with HBS and centrifugation at 800g, 600g, and 500g for 10 min, respectively. The final hematocrit of human erythrocyte suspension was adjusted to 3.0 %. These procedures were performed within 2 h after blood sampling for subsequent filtration experiments.
Nickel-mesh-filter
Figure 1a shows an electron microscopic photograph of a nickel mesh filter that was produced in accordance with our specifications by a photofabrication technique (Dainippon Printing, Tokyo, Japan). We specified that this filter should have an outer diameter of 13 mm, a filtration area 8 mm in diameter, 11 μm thick, with an interpore distance of 35 μm (Tsukasa Sokken, Tokyo, Japan). The vertical and cylindrical pores were distributed regularly across the filter without coincidence or branching. The pore entrances exhibited round and smooth transition into the pore interior. Pore diameters were all exactly identical in a specific nickel-mesh-filter. Filters with a specific pore diameter ranging from 3 to 6 μm were available to be selected depending on the suspension materials. After repeating preliminary experiments to choose an appropriate pore size, a nickel-mesh-filter with a pore diameter of 4.94 μm was chosen for human erythrocyte suspensions.
Fig. 1.
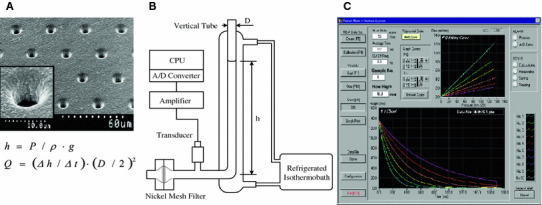
a Scanning electron microscopic photograph of a nickel-mesh-filter. Magnification of a single pore in nickel-mesh shows the smooth transition into the pore interior (inset). b Schematic illustration of gravity-based, nickel-mesh-filtration system. Height of the meniscus within the vertical tube (h) was obtained by continuous reduction of filtration pressure (P), specific gravity (ρ), and acceleration of gravity (g). Flow rate (Q) was calculated automatically by the first time derivatives of h (dh/dt) and internal cross-sectional area of the vertical tube. D internal diameter of vertical tube. c Photograph of computer screen showing P–Q (upper right) and h–t (lower) relationships
Erythrocyte filterability
Filtration experiments were performed blindly using a gravity-based nickel mesh filtration apparatus (Model NOBU-II; Tsukasa Sokken) as shown in Fig. 1b. In brief, the relationship between hydrostatic pressure (P; mmH2O) and time (t; s) was obtained during continuous filtration by gravity using a pressure transducer. P was transformed to the height of meniscus in vertical tube (h; mm). The tangent of the h–t curve determined by drawing points corresponding to different heights gives the rate of fall of the meniscus (dh/dt). Thereafter, by multiplying the rate of fall by the internal cross-sectional area of the vertical tube, the complete set of flow rates (Q; ml/min) and corresponding P, the P–Q relationship, was obtained. This procedure was automatically performed by measurement software installed in a personal computer (Dell Latitude CS; Dell, Round Rock, TX, USA) and monitored on the main window of the computer screen (Fig. 1c). Together with the start of data acquisition, the measurement software displays the h–t curve continuously during the filtration process. When the filtration has been completed, the software displays the P–Q curve. The h–t and P–Q curves are shown on the computer screen and stored simultaneously in Microsoft Office Excel 2003 for Windows XP (Microsoft, Tokyo, Japan). Temperature of the specimens was kept at 25 °C by circulating isothermal water through a water jacket surrounding the vertical tube (Fig. 1b). The percentage of the flow rate of erythrocyte suspension to that of HBS at 100 mmH2O was used as an index of erythrocyte filterability.
Erythrocyte shape
An aliquot of the erythrocyte suspension was fixed with isotonic 1.0 % glutaraldehyde solution containing 24.5 mM NaCl and 50 mM phosphate buffer (pH 7.4). The shape of erythrocytes was then examined using a differential interference contrast microscope (Diaphoto 300; Nikon;, Tokyo, Japan) at ×400 magnification. These experiments were performed at room temperature (22 ± 3 °C).
Data analyses
All data are expressed as mean ± SD. Because the distributions of HbA1c (p < 0.001) and erythrocyte filterability (p < 0.001) assessed by Kolmogorov–Smirnov test were not normal, comparison of these variables between two groups was conducted with Mann–Whitney test, and that among more than three groups was performed using Kruskal–Wallis test. The assumptions used for power calculations required a sample size of more than 27 patients in groups B and C, assuming a drop-out rate of 15 %, to provide 90 % power to detect a 5.0 % difference of erythrocyte filterability [4], with a 5 % type I error rate for a two-sided test. Practical computation was performed using Predictive Analytics Software (PASW) 18.0 version for Windows (SPSS, Chicago, IL, USA). Because missing values during the data acquisition are completely random (missing rate of 7.4 %), no specific treatment according to the SPSS Missing Value Analysis™ was attempted. Differences with a two-sided p < 0.050 were considered significant.
Results
BOOCS program
The incentive of the participants in the 6-month BOOCS program was obviously weight reduction and possible diabetic control. Totals of 27 non-obese, diabetic patients (group B) and 26 obese, non-diabetic patients (group C) completed this program. Final levels of compliance with this program were 93.1 and 81.3 % in groups B and C, respectively. Overall compliance was 86.9 % and, hence, the drop-out rate was 13.1 %. Interviews to the compliant participants (n = 53) revealed that fundamental life-style was not changed in that they continued smoking and drinking which may have affected on erythrocyte filterability if they liked, and that they did not controlled physical activity in daily life. Importantly, many of them confessed that they preferred to low-calorie, natural food rather than to oily pungent food according to the progression of this program.
Outcome of diabetes
HbA1c in diabetic patients prior to the enrollment was 7.20 ± 1.40 %, whereas HbA1c after the completion of this program was 6.87 ± 1.41 %. There was a significant (p = 0.007) reduction of HbA1c obtained by the fulfillment of the BOOCS program.
Outcome of obesity
As in our prior intervention with the BOOCS program for obese subjects [8], mean body weight was decreased significantly (p < 0.001) from 74.1 ± 9.6 to 69.0 ± 8.8 kg after completion of this program. Similarly, mean BMI was reduced significantly (p < 0.001) from 27.4 ± 2.6 to 25.6 ± 2.5 kg/m2.
Erythrocyte filterability
Mean erythrocyte filterability in the subjects of group A (n = 14) was 89.4 ± 1.7 %. Before starting BOOCS program, the filterabilities in the groups B (n = 27) and C (n = 26) were 82.8 ± 5.2 and 84.1 ± 5.6 %, respectively. Baseline filterability showed a significant difference among the three groups (p < 0.001). After the completion of the 6-month BOOCS program, the filterabilities in groups B and C were 87.9 ± 4.4 and 88.5 ± 3.7 %. Significant improvement of erythrocyte filterability was observed in both groups B and C by the fulfillment of the BOOCS program (p < 0.001). These results are summarized in Fig. 2, and the improvements of individual erythrocyte filterability in groups B and C are detailed in Figs. 3 and 4.
Fig. 2.
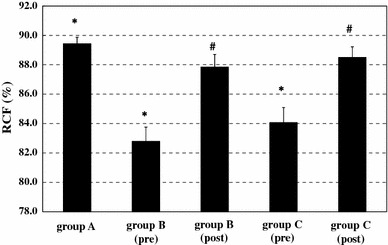
Mean erythrocyte filterability in groups A (control subjects, n = 14), B (non-obese, diabetic patients, n = 29), and C (obese, non-diabetic patients, n = 32). Baseline filterability showed a significant (p < 0.001) difference among the groups of A, B (pre), and C (pre). Erythrocyte filterability was significantly improved in group B (p < 0.001) and group C (p < 0.001) after the fulfillment of the BOOCS program. Columns and bars indicate means and standard errors, respectively. RCF red cell filterability (%). *p < 0.001 indicates a significant difference in baseline RCF among the three groups, and # p < 0.001 denotes a significant improvement of RCF within a group
Fig. 3.
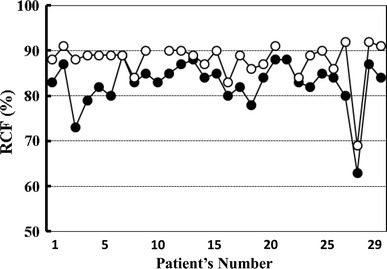
Individual improvement of erythrocyte filterability in group B (non-obese, diabetic patients). Closed symbols indicate erythrocyte filterability (%) prior to the participation in the BOOCS program, and open symbols mean the filterability after the completion of this program. RCF red cell filterability (%)
Fig. 4.
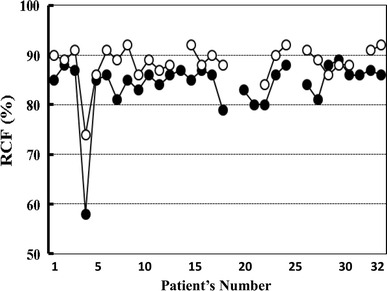
Individual improvement of erythrocyte filterability in group C (obese, non-diabetic patients). Closed symbols indicate erythrocyte filterability (%) prior to the participation in the BOOCS program, and open symbols mean the filterability after the completion of this program. RCF red cell filterability (%)
The association of HbA1c (y) with erythrocyte filterability (x) was investigated in pooled data of groups B and C. As shown in Fig. 5, linear regression analysis with the least square method was applied, and the linear equation was y = 0.022x + 5.178 (r = 0.083), yielding no statistical significance (p = 0.387). Likewise, the relationship between BMI and erythrocyte filterability was not significant (p = 0.479).
Fig. 5.
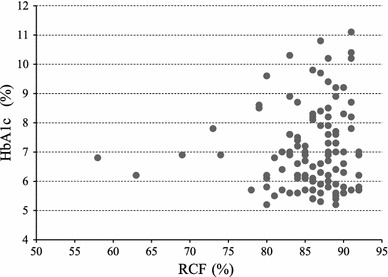
Relationship between HbA1c (y) and erythrocyte filterability (x) showing linear equation of y = 0.022 x + 5.178 (r = 0.083), but yielding no significance (p = 0.387). RCF red cell filterability (%)
Erythrocyte shape
Erythrocyte morphology in the control subjects (group A) did not demonstrate any outstanding abnormalities. Likewise, erythrocytes obtained from participants of the BOOCS program (groups B and C) showed no discernible shape changes at the stage before starting the program. The same was true at the stage of completing this program.
Follow-up study
Most diabetic (n = 25 in group B) or obese (n = 23 in group C) patients who completed the BOOCS program continued this program for the purpose of weight or diabetic control after the study periods. Re-exacerbation of diabetes in group B and body weight cycling (so-called ‘rebound’ phenomenon) in group C were not observed, at least in these participants (n = 48) during the follow-up period ranging from 1 to 6 years (2.8 ± 1.0 years).
Discussion
The main finding of this study is an improvement of obese or diabetic human erythrocyte filterability assessed by the nickel-mesh-filtration technique after completion of the 6-month BOOCS program. Circulating intact human erythrocytes show physiological deformability to maintain fluent microcirculation. Erythrocyte filterability is considered to reflect whole cell deformability, and evaluation of filterability strictly depends on the filter property. The nickel-mesh-filtration technique is highly sensitive, quantitative, and reproducible by assessing the physiological bending deformation of intact erythrocytes passing by gravity through the uniform, smooth, and regularly distributed pores in the nickel-mesh-filter without erythrocyte trapping or stagnation [9–11]. This technique recently clarified the impaired diabetic erythrocyte filterability [4], which was difficult in filtration experiments using conventional filters [12].
An increasing prevalence of obesity in the modern world is an urgent healthcare problem. The BOOCS program for the treatment of obesity is based on the ‘brain-fatigue’ hypothesis. Psychosocial stress in industrialized countries causes human taste resetting and resultant oily, pungent food preference and overeating, which leads to obesity and possibly diabetes. To prevent this sequence, the BOOCS program affirms patients’ own native appetite and denies the patients’ stoicism for eating. On the other hand, this program promotes healthy activities that the participant can do and likes. Therefore, this program is a ‘low-tech’ psychosomatic approach without any drug or device interventions. Consequently, this program attenuates psychosocial stress, restores natural taste sensing, and reduces body weight without any calorie restriction. This may be attributed to preference to the natural, low-calorie food after the disappearance of taste resetting and abnormal eating behaviors. High compliance of this program obtained by this study (86.9 %) is considered to be due to its simple and cost-effective nature that is suitable for Japanese [8].
Microcirculatory dysfunction is already present in non-diabetic obese subjects [13]. Impaired diabetic erythrocyte deformability is closely linked to the disturbed microcirculation [14], and underlies diabetic microangiopathy [15], such as retinopathy [16], nephropathy [17], and neuropathy [18]. Although the present study demonstrated the improvement of the impaired obese or diabetic human erythrocyte deformability by completion of the BOOCS program (Figs. 2, 3, 4), the cause of this amelioration remains unknown. However, some possibilities can be postulated. From the hemorheological viewpoint, erythrocyte deformability is regulated by three factors, namely, internal viscosity, geometry, and membrane property of intact circulating erythrocytes [1, 10]. Internal viscosity of erythrocytes is evaluated by mean corpuscular hemoglobin concentration and strictly regulated in physiological range. Microscopic observation showed no discernible changes of erythrocyte shape (geometry). Furthermore, impaired filterability showed no direct correlation with glycation of hemoglobin (Fig. 5). Therefore, this rheological effect may have been mediated by miscellaneous factors which were improved by BOOCS program but not evaluated by this study design, i.e., psychosomatic stress reduction alters neuroendocrine system that influences lipid profile [19], feeding behavior, energy consumption [20], and blood pressure regulation [21].
Circulating erythrocytes as physiological oxygen carriers are the main target of oxidant stress. Oxidant stress is introduced by hyperglycemia and obesity [22, 23], and deranges erythrocyte membrane integrity by lipid peroxidation and protein degradation, reduces the membrane fluidity, and impairs deformability profoundly, which was confirmed by this nickel-mesh-filtration technique [24–26]. Human redox status depends on high-calorie intake, and, importantly, feeding behavior is altered by completion of the BOOCS program, that is, participants of this program prefer natural, low-calorie Japanese food rather than Western food spontaneously [8]. This trend may contribute to attenuate the systemic oxidant stress and, hence, to improve the erythrocyte filterability.
In conclusion, although this single-center, small-sample study did not evaluate the subjects’ lipid profile, nutritional background, or redox status, it indicates that diabetic or obese human erythrocyte deformability is impaired, that the BOOCS program, a psychosomatic, cost-effective approach to lifestyle per se, ameliorates the impaired erythrocyte deformability in these patients, and that the nickel-mesh-filtration technique is useful to evaluate subtle but significant alterations of human erythrocyte deformability.
Acknowledgments
The authors would like to thank staff of the BOOCS clinic (Fukuoka, Japan) for clinical assistance, and staff of the Institute of Rheological Function of Foods Co. Ltd. (Hisayama, Fukuoka) for technical assistance. We lost our collaborator, Dr. Nobuhiro Uyesaka (Department of Physiology, Nippon Medical University), during the preparation of this manuscript, and dedicate this work to honor his memory.
Conflict of interest
The authors declare that there are no conflicts of interest in relation to this manuscript.
References
- 1.Mohandas N, Chasis JA. Red cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 2.Ejima J, Ijichi T, Ohnishi Y, Maruyama T, Kaji Y, Kanaya S, et al. Relationship of high-density lipoprotein cholesterol and red blood cell filterability: cross-sectional study of healthy subjects. Clin Hemorheol Microcirc. 2000;22:1–7. [PubMed] [Google Scholar]
- 3.Ariyoshi K, Maruyama T, Odashiro K, Akashi K, Fujino T, Uyesaka N. Impaired erythrocyte filterability of spontaneously hypertensive rats: investigation by nickel mesh filtration technique. Circ J. 2010;74:129–136. doi: 10.1253/circj.CJ-09-0252. [DOI] [PubMed] [Google Scholar]
- 4.Saito K, Kokawa Y, Fukata M, Odashiro K, Maruyama T, Akashi K, Fujino T. Impaired deformability of erythrocytes in diabetic rat and human: investigation by the nickel-mesh-filtration technique. J Biorheol. 2011;25:18–26. doi: 10.1007/s12573-011-0032-5. [DOI] [Google Scholar]
- 5.Robertson SM, Stanley MA, Cully JA, Naik AD. Positive emotional health and diabetes care: concepts, measurement, and clinical implications. Psychosomatics. 2012;53:1–12. doi: 10.1016/j.psym.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Brown J, Wimpenny P. Developing a holistic approach to obesity management. Int J Nurs Pract. 2011;17:9–18. doi: 10.1111/j.1440-172X.2010.01899.x. [DOI] [PubMed] [Google Scholar]
- 7.Kitzmann KM, Dalton WT, Stanley CM, Beech BM, Reeves TP, Buscemi J, Egli CJ, Gamble HL, Midgett EL. Lifestyle interventions for youth who are overweight: a meta-analytic review. Health Psychol. 2010;29:91–101. doi: 10.1037/a0017437. [DOI] [PubMed] [Google Scholar]
- 8.Fujino T. Proposal of a new hypothesis for the psychosomatic treatment of obesity and its application. Fukuoka Acta Med. 1999;90:353–364. [PubMed] [Google Scholar]
- 9.Rodgers GP, Dover GJ, Uyesaka N, Noguchi CT, Schechter AN, Nienhuis AW. Augmentation by erythropoietin of the fetal-hemoglobin response to hydroxyurea in sickle cell disease. N Engl J Med. 1993;328:73–80. doi: 10.1056/NEJM199301143280201. [DOI] [PubMed] [Google Scholar]
- 10.Hiruma H, Noguchi CT, Uyesaka N, Schechter AN, Rodgers GP. Contributions of sickle hemoglobin polymer and sickle cell membranes to impaired filterability. Am J Physiol. 1995;268:H2003–H2008. doi: 10.1152/ajpheart.1995.268.5.H2003. [DOI] [PubMed] [Google Scholar]
- 11.Oonishi T, Sakashita K, Uyesaka N. Regulation of red blood cell filterability by Ca2+ influx and cAMP-mediated signaling pathways. Am J Physiol. 1997;273:C1828–C1834. doi: 10.1152/ajpcell.1997.273.6.C1828. [DOI] [PubMed] [Google Scholar]
- 12.Caimi G, Presti RL. Techniques to evaluate erythrocyte deformability in diabetes mellitus. Acta Diabetol. 2004;41:99–103. doi: 10.1007/s00592-004-0151-1. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer-Aguiar LG, Laflor CM, Bouskela E. Skin microcirculatory dysfunction is already present in normoglycemic subjects with metabolic syndrome. Metabolism. 2008;57:1740–1746. doi: 10.1016/j.metabol.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 14.Diamantopoulos EJ, Kittas C, Charitos D, Grigoriadou M, Ifanti G, Raptis SA. Impaired erythrocyte deformability precedes vascular changes in experimental diabetes mellitus. Horm Metab Res. 2004;36:142–147. doi: 10.1055/s-2004-814337. [DOI] [PubMed] [Google Scholar]
- 15.Shin S, Ku YH, Ho JX, Kim YK, Suh JS, Singh M. Progressive impairment of erythrocyte deformability as indicator of microangiopathy in type 2 diabetes mellitus. Clin Hemorheol Microcirc. 2007;36:253–261. [PubMed] [Google Scholar]
- 16.Huang SY, Jeng C, Kao SC, Yu JJ, Liu DZ. Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin Nutr. 2004;23:615–621. doi: 10.1016/j.clnu.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Brown CD, Ghali HS, Zhao Z, Thomas LL, Friedman EA. Association of reduced red blood cell deformability and diabetic nephropathy. Kidney Int. 2005;67:295–300. doi: 10.1111/j.1523-1755.2005.00082.x. [DOI] [PubMed] [Google Scholar]
- 18.Young MJ, Bennett JL, Liderth SA, Veves A, Boulton AJ, Douglas JT. Rheological and microvascular parameters in diabetic peripheral neuropathy. Clin Sci (Lond) 1996;90:183–187. doi: 10.1042/cs0900183. [DOI] [PubMed] [Google Scholar]
- 19.Perez-Tilve D, Habbeger KM, Tschop MH, Hofmann SM. Neural regulation of cholesterol metabolism. Curr Opin Lipidol. 2011;22:283–287. doi: 10.1097/MOL.0b013e328348a459. [DOI] [PubMed] [Google Scholar]
- 20.Cha SH, Hu Z, Chohnan S, Lane MD. Inhibition of hypothalamic fatty acid synthase triggers rapid activation of fatty acid oxidation in skeletal muscle. Proc Natl Acad Sci USA. 2005;102:14557–14562. doi: 10.1073/pnas.0507300102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harshfield GA, Dong Y, Kapuku GK, Zhu H, Hanevold CD. Stress-induced sodium retention and hypertension: a review and hypothesis. Curr Hypertens Rep. 2009;11:29–34. doi: 10.1007/s11906-009-0007-8. [DOI] [PubMed] [Google Scholar]
- 22.Vincent HK, Innes KE, Vincent KR. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes Obes Metab. 2007;9:813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 23.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovasc disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 24.Uyesaka N, Hasegawa S, Ishioka N, Ishioka R, Shio H, Schechter AN. Effects of superoxide anions on red cell deformability and membrane proteins. Biorheology. 1992;29:217–229. doi: 10.3233/bir-1992-292-303. [DOI] [PubMed] [Google Scholar]
- 25.Iwata H, Ukeda H, Maruyama T, Fujino T, Sawamura M. Effect of carbonyl compounds on red blood cells deformability. Biochem Biophys Res Comm. 2004;321:700–706. doi: 10.1016/j.bbrc.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto K, Maruyama T, Kaji Y, Harada M, Mawatari S, Fujino T, et al. Verapamil prevents impairment in filterability of human erythrocytes exposed to oxidative stress. Jpn J Physiol. 2004;54:39–46. doi: 10.2170/jjphysiol.54.39. [DOI] [PubMed] [Google Scholar]


