Abstract
This study examined mechanical and thermal hypersensitivity in the rat hind paw during cast immobilization of the hind limbs for 4 or 8 weeks and following cast removal. Blood flow, skin temperature, and volume of the rat hind paw were assessed in order to determine peripheral circulation of the hind limbs. Sensitization was analyzed by measuring the expression of the calcitonin gene-related peptide (CGRP) in the spinal dorsal horn following cast immobilization. Two weeks post immobilization, mechanical and thermal sensitivities increased significantly in all rats; however, peripheral circulation was not affected by immobilization. Cast immobilization for 8 weeks induced more serious hypersensitivity compared to cast immobilization for 4 weeks. Moreover, CGRP expression in the deeper lamina layer of the spinal dorsal horn increased in the rats immobilized for 8 weeks but not in those immobilized for 4 weeks. These findings suggest that immobilization-induced hypersensitivity develops during the immobilization period without affecting peripheral circulation. Our results also highlight the possibility that prolonged immobilization induces central sensitization in the spinal cord.
Keywords: Immobilization, Hypersensitivity, CGRP, Spinal cord, Rat
Introduction
It is well known that joint immobilization of cats aids the recuperation of biological and mechanical properties following injury; however, cast immobilization can also result in joint contracture and muscle atrophy [1]. In addition, recently it has been suggested that cast immobilization may cause thermal and mechanical hypersensitivity. For example, Terkelsen et al. [2] conducted an experiment on human subjects who had their forearms immobilized in a cast for 4 weeks; they subsequently observed transient movement-provoked pain and increased skin temperature, along with mechanical and cold hypersensitivity in the distal parts of the immobilized extremity. The mechanism governing this phenomenon is likely to be central neuronal changes, as suggested by several previous observations. Indeed, Ushida and Willis [3] reported that immobilization of the rat forearm for 4 weeks induces an increase in the number of wide-dynamic-range neurons and neurons responding to movement, and they demonstrated that these changes induce mechanical allodynia. In a recent study, it was reported that cast immobilization for 5 weeks induces phenotypic changes associated with alterations in the calcitonin gene-related peptide (CGRP)-positive neuron size in the dorsal root ganglion, as well as increased CGRP expression in the spinal dorsal horn of rats [4]. These changes are similar to those observed in an inflammatory and neuropathic model of pain [5, 6]. It has been suggested that CGRP plays a role in the processing of nociceptive information in primary afferents and the spinal cord, and, furthermore, that the release of CGRP into the spinal dorsal horn induces mechanical hyperalgesia and spinal sensitization via the enhanced release of substance P and other neuropeptides [7–10].
With regards to the mechanism underlying immobilization-induced hypersensitivity and spinal sensitization, Ohmichi et al. [11] suggested that cast immobilization induces ischemia and reperfusion injury in the hind limb, which results in the production of oxygen free radicals following removal of the cast and subsequent mechanical hypersensitivity. It is well known that disruption of peripheral circulation can be induced during or following cast immobilization, which may in turn induce hypersensitivity [12]; however, in the studies mentioned above, behavioral tests for sensitivity, as well as evaluation of peripheral circulation, were not performed during the immobilization period. Thus, it is unclear whether immobilization-induced hypersensitivity develops during immobilization or after cast removal.
The aims of this study were to investigate changes in sensitivity during the immobilization period and to estimate the degree of sensitization in primary afferents and the spinal cord of rats with immobilized ankle joints.
Materials and methods
Animals
The Ethics Review Committee for Animal Experimentation of Nagasaki University approved all experiments. Wistar rats (n = 60, aged 8 weeks) were obtained from Kudo Laboratories (Tokyo, Japan) and were randomly divided into 3 groups: immobilization for 4 weeks (Im-4 weeks, n = 20); immobilization for 8 weeks (Im-8 weeks, n = 15); and age-matched controls (n = 25). The rats were housed in plastic cages at an ambient temperature of 24 °C and maintained on a 12-h light/dark cycle. Water and food were available ad libitum. Rats in the Im-4 weeks and Im-8 weeks groups were anesthetized with diethyl ether. Plaster casts were used to immobilize their right hind limbs, leaving them with full plantar flexion of the ipsilateral ankle joint. Left ankle joints were not immobilized (contralateral hind limb). The plaster cast was positioned from above the knee joint to the distal foot. The cast was wrapped carefully to prevent disruption to peripheral circulation and edema.
Behavior tests
Behavior tests for mechanical sensitivity were performed on 10 rats from each group before the immobilization period and once every 3 days following application of the cast. The tests were performed using a homemade restrainer made of cloth since the Im-4 weeks and Im-8 weeks rats could not walk on their hind limbs due to the ankle joint contracture. The restrainer allowed the animal to dangle safely and to position their legs freely without the burden of their weight [13]. Rats were placed individually in the restrainer after their casts were removed and allowed to acclimate for 20 min in a quiet room that was maintained at 24 °C. The bilateral skin of the hind paw was probed 10 times with a von Frey filament (15 g; North Coast Medical, Morgan Hill, California) at intervals of 10 s. The lifting or pulling back of a paw was considered the “paw withdrawal response” (PWR), and this was assessed by a single experimenter. The bilateral threshold temperature for PWR was measured using a pain thermometer (UDH-104, Unique Medical Inc., Tokyo, Japan) in order to evaluate thermal hypersensitivity. The heating probe (diameter: 2 cm) was manually attached to the dorsal surface of the hind paw and imparted a pressure of 50 g. The temperature of the heating probe, which was initially set to 42 °C, was increased by 0.25 °C/s. The test was repeated 5 times on the same hind paw at 10-min intervals. The cut-off temperature was set at 55 °C to avoid any tissue damage. At the end of each test day, Im-4 weeks and Im-8 weeks rats were re-immobilized. Following both immobilization periods, the same 10 rats from each group were released from cast immobilization. These rats were maintained in a normal breeding condition, and the behavioral tests were performed for a further 4 weeks in order to assess mechanical sensitivity following cast removal.
Evaluation of peripheral circulation
To evaluate the peripheral circulation of the immobilized hind paw, blood flow and temperature of the hind paw were evaluated every week during the immobilization period, as according to a previous study [14]. In the Im-4 weeks group, 5 rats that did not undergo any behavioral testing were used for this evaluation. The rats were anesthetized with pentobarbital sodium (40 mg/kg), administered intraperitoneally, and they were subsequently placed in the prone position on a heating pad in order to maintain a rectal temperature of 37.0 ± 1.0 °C. The probe of the laser Doppler flowmeter (ALF21 N, ADVANCE Co. Ltd, Tokyo, Japan) was positioned at the center of the right (ipsilateral) hind paw. The probe was then carefully moved over the hind paw in order to determine maximal basal skin blood flow. The measurement was taken for 10 min, and the average mean temperature was calculated. Subsequently, skin temperature was measured using an infrared thermometer (TN006, OHM Electric Inc., Saitama, Japan) placed at the center of the ipsilateral hind paw; the measurement was repeated 3 times. Paw volume measurement was calculated by wrapping a piece of thread around the circumference of the hind paw, which enabled us to evaluate the induction of edema. At the end of the 4-week immobilization period, a small quantity of venous blood (less than 0.01 ml) was obtained from the hind paw using a needle. Subsequently, blood pH and lactate blood concentration were measured using a microminiature needle with a pH meter (CMN-141, Chemical Instruments Co. Ltd., Tokyo, Japan) and a blood lactate test meter (Lactate Pro2, ARKRAY, Inc, Shiga, Japan), respectively. Measurements for blood pH and lactate concentration were also taken in the age-matched control rats (n = 5).
Analysis of calcitonin gene-related peptide in the spinal dorsal horn
At the end of the immobilization period (4 and 8 weeks), 5 rats from each group that did not undergo the behavioral testing or evaluation of peripheral circulation were anesthetized with sodium pentobarbital (40 mg/kg). The spinal cord (L4-5) of these rats were removed following transcardial perfusion with saline and 4 % paraformaldehyde dissolved in 0.1 M phosphate-buffer (PB; pH 7.4). The tissue was soaked for 24 h in 10 % sucrose dissolved in PB, followed by 24 h in 20 % sucrose dissolved in 0.01 M phosphate-buffered saline (PBS; pH 7.4). Spinal cord frozen sections (10 μm) were cut with a microtome. To inhibit endogenous peroxidases, the sections were incubated for 30 min at room temperature with 0.3 % H2O2 dissolved in methanol. Next, sections were blocked for 20 min with 5 % bovine albumin dissolved in PBS, followed by incubation with an anti-CGRP polyclonal antibody (1:500 rabbit; ImmunoStar Inc. Hudson, WI, USA) overnight at 4 °C. Sections underwent three 5-min washes in PBS. Subsequently, they were incubated with goat anti-rabbit IgG conjugated to Texas Red® (1:600, Vector Labs, CA, USA) diluted in PBS for 1 h at room temperature. Quantitative evaluation of CGRP expression in the ipsilateral and contralateral dorsal horn was performed using an image analysis software (NIS-Element Ver. 3, Nikon Instruments Inc., NY, USA). The spinal dorsal horn was divided into the superficial layer (lamina I-II) and the deeper layer (lamina III-VI), according to previously described criteria [15]. The intensity of CGRP expression reflected the quantity of fluorescence observed in the superficial (lamina I-II) and deeper (lamina III-VI) layers of the spinal dorsal horn in 5 sections per tissue.
Statistical analysis
All data are presented as the mean ± SE. Differences between groups were assessed utilizing the Mann–Whitney U test and one-way analysis of variance (ANOVA), followed by the Fisher’s PLSD post hoc test. P values <0.05 were considered significant.
Results
Mechanical sensitivity
The PWR on the ipsilateral hind paw, as measured by 15-g von Frey filaments, increased significantly 2 weeks following immobilization in both the Im-4 weeks and Im-8 weeks groups but not in the control group. This PWR increase in the Im-4 weeks and Im-8 weeks groups persisted for the duration of immobilization. However, PWR in the Im-4 weeks group gradually decreased following cast removal, such that by 4 weeks post immobilization, there was no significant difference in PWR in the Im-4 weeks group compared to the control group. The increased PWR in the Im-8 weeks group also slowly decreased following cast removal; however, it maintained a significant difference with the control group 4 weeks after cast removal (Fig. 1a). Indeed, it was not until 14 weeks after cast removal that the PWR in the Im-8 weeks group reached control levels (data not shown). The PWR of the contralateral hind paw in both the Im-4 weeks and Im-8 weeks groups was not significantly different from that of controls during and after the immobilization period (Fig. 1b).
Fig. 1.
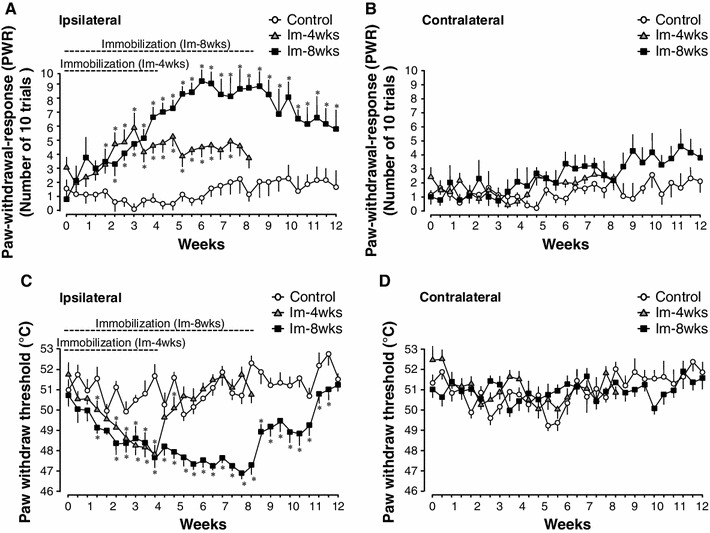
Time course of changes in mechanical and thermal sensitivities in the hind paw. a and b: mechanical sensitivity. The skin of the ipsilateral (a) and contralateral (b) hind paw was probed 10 times with a von Frey filament (15 g) and the number of positive PWR was counted. c and d: thermal sensitivity. The threshold temperatures of PWR in the ipsilateral (c) and contralateral (d) hind paw were recorded. Behavioral tests were extended for 4 weeks after cast removal. Mechanical and thermal hypersensitivity was observed during cast immobilization. Values are given as the mean ± SE. *P < 0.05, significantly different compared to the control group
Thermal sensitivity
In both the Im-4 weeks and Im-8 weeks groups, the threshold temperature of PWR on the ipsilateral hind paw significantly decreased 2 weeks after immobilization. The decrease in threshold temperature in the Im-4 weeks and Im-8 weeks groups persisted for the duration of immobilization. Following cast removal, the threshold temperature in the Im-4 weeks group recovered within 3 days. The threshold temperature gradually recovered in the Im-8 weeks group; however, it still displayed significant differences compared to the control group more than 3 weeks after cast removal (Fig. 1c). Four weeks after cast removal, PWR between the control and Im-8 weeks groups exhibited no statistical difference. No significant changes were observed in the contralateral paw in the Im-4 weeks and Im-8 weeks groups (Fig. 1d).
Peripheral circulation during immobilization
Blood flow (Fig. 2a), skin temperature (Fig. 2b), and circumference of the hind paw (Fig. 2c) in the ipsilateral hind limb were not affected by immobilization; these values did not change during immobilization for 4 weeks. At the end of the 4-week immobilization period, there was no difference in the blood pH (Fig. 3a) or lactate blood concentration (Fig. 3b) in the ipsilateral hind paw of Im-4 weeks rats and age-matched controls.
Fig. 2.
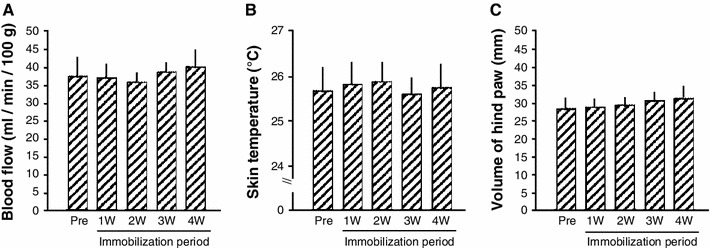
Blood flow, skin temperature, and volume of rat hind paw in the Im-4 weeks group. No changes were observed in blood flow (a), skin temperature (b), and volume of hind paw (c) during the immobilization period (4 weeks). Values are given as mean ± SE
Fig. 3.
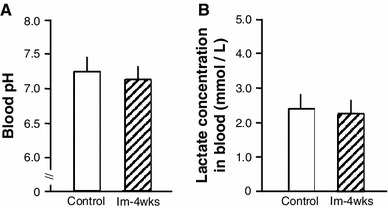
Blood pH and lactate concentration of peripheral blood in the hind paw at end of immobilization period (4 weeks). Blood pH (a) and lactate concentration (b) in the Im-4 weeks group was not different compared to age-matched control group. Values are given as mean ± SE
Calcitonin gene-related peptide expression intensity in the spinal dorsal horn
The CGRP immune response in the ipsilateral dorsal horn of Im-4 weeks and Im-8 weeks rats was greater than that of controls. In particular, CGRP-positive neural fibers were clearly observed in the deep layer of the spinal dorsal horn in the Im-8 weeks group (Fig. 4a). The CGRP expression intensity analyses showed higher values in the superficial layer (laminae I-II) of the dorsal horn in the Im-4 weeks and Im-8 weeks groups compared to the age-matched control group; the Im-8 weeks presented the strongest intensity (Fig. 4b). In the deep layer (laminae III-VI) of the dorsal horn, the intensity value of CGRP expression in the Im-8 weeks group was significantly increased compared to the control and Im-4 weeks groups (Fig. 4c). Contrastingly, no significant difference in CGRP expression intensity was observed in the contralateral superficial and deep layer of the dorsal horn in the Im-4 weeks and Im-8 weeks groups compared to the age-matched control group (Fig. 4d, e).
Fig. 4.
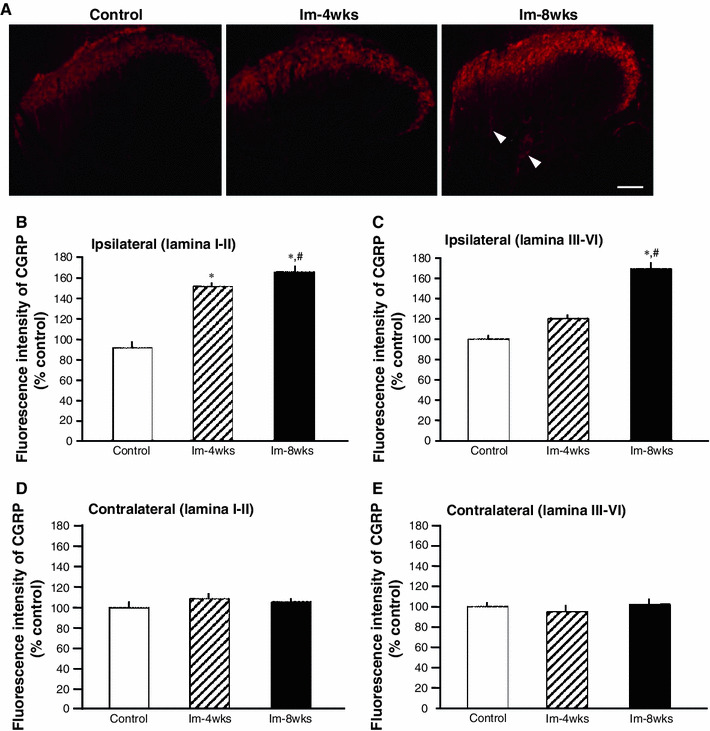
Intensity of calcitonin gene-related peptide (CGRP) expression in the ipsilateral dorsal horn of the spinal cord. Representative photomicrographs of CGRP immunohistochemistry in the ipsilateral dorsal horn from the Im-4 weeks, Im-8 weeks, and the control groups (age-matched with the Im-4 weeks group) are shown (a). The CGRP-positive neural fibers were clearly observed in the deep layer of the dorsal horn only in the Im-8 weeks group (arrowheads). Percentage control of fluorescence intensity of CGRP expression in the superficial layer (laminae I-II) (b, d) and deep layers (laminae III-VI) were calculated (c, e) in the ipsilateral and contralateral dorsal horn. *P < 0.05, significantly different compared to the age-matched control group. # P < 0.05, significantly different compared to the Im-4 weeks group. Scale bar = 100 μm
Discussion
This study examined changes in mechanical and thermal sensitivities in rats during and after cast immobilization of the hind limb. The present findings clearly demonstrate that immobilization produces mechanical and thermal hypersensitivities. Several previous reports have indicated that immobilization decreases the pain threshold [11, 13, 16], and we observed similar results, notably an increase in the onset of mechanical and thermal sensitivities. Ohmichi et al. [11] reported that cast immobilization induces ischemia/reperfusion injury in the hind limb and that the resultant production of oxygen free radicals in rats is one of the mechanisms underlying mechanical hypersensitivity. In their study, the cast was not changed during the immobilization period, and the ischemia/reperfusion injury occurred following the cast removal at the end of the 2-week immobilization period. It is known that ischemia/reperfusion injury induces a decrease in blood pH and an increase in blood lactate concentration [17], which may in turn trigger hypersensitivity [18, 19]. Contrastingly, in this study, the cast was changed every 3 days in order to prevent disruption to the peripheral circulation and ischemia/reperfusion injury. As a result, blood flow, as well as the volume and temperature of the hind paw skin, were not affected by the 4-week immobilization period. Additionally, exacerbation of the immobilization-induced hypersensitivity was not observed following cast removal in the Im-4 weeks and Im-8 weeks groups, which might indicate that ischemia/reperfusion injury did not occur in the hind limbs of these rats. This finding perhaps indicates that immobilization-induced hypersensitivity was not triggered by disruption to peripheral circulation in this study; however, it should be noted that the laser Doppler flowmetry probe was reattached at each time point and is only valid for evaluating relative changes in perfusion rather than absolute values. Thus, it is possible that undetected changes in the absolute values for blood flow occurred during the immobilization period.
Ushida and Willis [3] reported that immobilization of the rat forearm for 4 weeks induced mechanical allodynia and led to plastic changes in dorsal horn neurons, such as an elevated number of wide-dynamic-range neurons and neurons responding to movement. Nishigami et al. [4], employing a forearm rat immobilization model for 5 weeks, noted that spinal lamina of the ipsilateral side displayed significantly higher CGRP expression relative to the contralateral side. These findings from previous studies indicate that immobilization-induced hypersensitivity may depend upon the development of spinal sensitization. The current results demonstrate that extended immobilization leads to greater elevation of mechanical and thermal sensitivities. Furthermore, we observed that immobilization-induced hypersensitivity was long lasting following cast removal in rats immobilized for 8 weeks (Im-8 weeks), whereas these conditions were transient in rats immobilized for 4 weeks (Im-4 weeks). It has previously been demonstrated that CGRP release into the superficial layer (laminae I-II) of the spinal dorsal horn induces hypersensitivity via the increased release of substance P and other neuropeptides [7], and this mechanism has been implicated in central sensitization induced by acute inflammation [8, 9, 20]. In this study, CGRP expression intensity was significantly increased in the superficial layer (laminae I-II) of the dorsal horn in the Im-8 weeks group compared to the Im-4 weeks group. The Im-8 weeks group also exhibited significantly increased CGRP expression in the deep layer (laminae III-VI) of the spinal dorsal horn compared to the Im-4 weeks group. Wide dynamic range (WDR) neurons were distributed in the deep layers. Electrophysiological studies have shown that WDR neurons respond to noxious as well as innocuous stimuli [3], and a rat model of allodynia has demonstrated a significant decrease in the proportion of low-threshold neurons and an increase in the proportion of WDR neurons [21]. Moreover, electrophysiological recordings from WDR neurons in the spinal dorsal horn of hyperalgesic rats has revealed significantly increased spontaneous activity and noxious mechanical stimuli [22]. Additionally, it has been reported that CGRP increases the discharge frequency of WDR neurons in the dorsal horn, which is blocked by the CGRP receptor antagonist, CGRP8-37 [23, 24]. Increased CGRP expression in the spinal deep layers, as observed in the Im-8 weeks group, could modulate nociception through WDR neuronal activation. These findings suggest that the degree of central sensitization was more severe in the Im-8 weeks group compared to Im-4 weeks group, which leads us to speculate that the differences between the 2 groups in terms of recovery after cast removal might be explained by the initial degree of central sensitization. However, the CGRP result does not explain why the recovery period was different for mechanical and thermal hypersensitivity. The thermal hypersensitivity induced by 4 weeks immobilization was recovered to the control level within 1 week in the Im-4 weeks group, while it took 4 weeks to meet control levels in the Im-8 weeks group. In contrast, the recovery period from mechanical hypersensitivity was substantially longer, such that it took 4 weeks for the Im-4 weeks group and 14 weeks for the Im-8 weeks group to reach control levels. A longer recovery period for mechanical hypersensitivity compared to thermal hypersensitivity was also observed in a previous study on inflammation-induced hypersensitivity [25]; however, the reason was not indicated.
This study did not determine the underlying trigger for immobilization-induced hypersensitivity and central sensitization, though it is our estimation that peripheral tissue changes influenced the mechanical and thermal sensitivity. The CGRP is expressed by C- and Aδ-fiber primary afferents, the majority of which express the ATP receptor (P2X family) channel and the transient receptor potential channels (TRP family) [26]. Especially, P2X3 and TRPV1 are important receptors for mechanical and thermal nociception, respectively [27], and it is known that release of CGRP is related to the expression and function of these receptors [28, 29]. Thus, in the Im-4 weeks and Im-8 weeks groups, it is possible that receptors for mechanical and thermal nociception of primary afferents were activated at nerve endings in the skin and other peripheral tissues. Additionally, we have previously demonstrated that epidermal thinning and an increase in peripheral nerve profiles occurs in the skin tissue of immobilized rats [13]. The up-regulation of neurocutaneous inflammatory signaling has been reported in a rat model of tibial fracture; after the fracture and cast immobilization, keratinocytes of the hind paw skin expressed increased NK1 receptors, tumor necrosis factor-α, interleukin 1β and NGF [30]. This cutaneous change might be partly responsible for immobilization-induced hypersensitivity. On the other hand, it is widely known that acute or chronic stress under severe conditions, such as restraint stress, which can also be caused by immobilization, triggers mechanical hypersensitivity via changes in neural systems [31, 32]. This stress-induced hypersensitivity develops bilaterally in limbs via changes in multiple neural systems in the brain [33]; however, cast immobilization in the present study induced mechanical hypersensitivity of the hind paw in the ipsilateral paw but not in the contralateral paw. Since the immobilization-induced hypersensitivity was unilateral in the immobilization group, this change could not be attributed to changes in neural systems caused by restraint stress.
In summary, cast immobilization causes mechanical and thermal hypersensitivity associated with central sensitization in the spinal cord and without disruption to peripheral circulation during the immobilization period. Interestingly, it has been reported that nearly half of patients with complex regional pain syndrome (CRPS) are initially immobilized with casting or splinting, which in turn might contribute to the development of CRPS [34]. A phenomenon similar to immobilization-induced hypersensitivity may influence the induction of pain in patients. The current results should be considered exploratory and used to guide future research.
Acknowledgments
This research was supported in part by a Dean’s Fund Grant from the School of Health Sciences, Nagasaki University and a Grant-in-aid for scientific research (No. 23500587) from the Japan Society for the Promotion of Science.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Okita M, Yoshimura T, Nakano J, Motomura M, Eguchi K. Effects of reduced joint mobility on sarcomere length, collagen fibril arrangement in the endomysium, and hyaluronan in rat soleus muscle. J Muscle Res Cell Motil. 2004;25:159–166. doi: 10.1023/B:JURE.0000035851.12800.39. [DOI] [PubMed] [Google Scholar]
- 2.Terkelsen AJ, Bach FW, Jensen TS. Experimental forearm immobilization in humans induces cold and mechanical hyperalgesia. Anesthesiology. 2008;109:297–307. doi: 10.1097/ALN.0b013e31817f4c9d. [DOI] [PubMed] [Google Scholar]
- 3.Ushida T, Willis WD. Changes in dorsal horn neuronal responses in an experimental wrist contracture model. J Orthop Sci. 2001;6:46–52. doi: 10.1007/s007760170024. [DOI] [PubMed] [Google Scholar]
- 4.Nishigami T, Osako Y, Tanaka K, Yuri K, Kawasaki M, Ikemoto T, McLaughlin M, Ishida K, Tani T, Ushida T. Changes in calcitonin gene-related peptide expression following joint immobilization in rats. Neurosci Lett. 2009;454:97–100. doi: 10.1016/j.neulet.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 5.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. 1996;384:360–364. doi: 10.1038/384360a0. [DOI] [PubMed] [Google Scholar]
- 6.Nitzan-Luques A, Devor M, Tal M. Genotype-selective phenotypic switch in primary afferent neurons contributes to neuropathic pain. Pain. 2011;152:2413–2426. doi: 10.1016/j.pain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 7.Bennett AD, Chastain KM, Hulsebosch CE. Alleviation of mechanical and thermal allodynia by CGRP (8–37) in a rodent model of chronic central pain. Pain. 2000;86:163–175. doi: 10.1016/S0304-3959(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 8.Sun RQ, Lawand NB, Willis WD. The role of calcitonin gene-related peptide (CGRP) in the generation and maintenance of mechanical allodynia and hyperalgesia in rats after intradermal injection of capsaicin. Pain. 2003;104:201–208. doi: 10.1016/S0304-3959(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 9.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 10.Kangrga I, Randic M. Tachykinins and calcitonin gene-related peptide enhance release of endogenous glutamate and aspartate from the rat spinal dorsal horn slice. J Neurosci. 1990;10:2026–2038. doi: 10.1523/JNEUROSCI.10-06-02026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohmichi Y, Sato J, Ohmichi M, Sakurai H, Yoshimoto T, Morimoto A, Hashimoto T, Eguchi K, Nishihara M, Arai YC, Ohishi H, Asamoto K, Ushida T, Nakano T, Kumazawa T. Two-week cast immobilization induced chronic widespread hyperalgesia in rats. Eur J Pain. 2011;16:338–348. doi: 10.1002/j.1532-2149.2011.00026.x. [DOI] [PubMed] [Google Scholar]
- 12.Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- 13.Nakano J, Sekino Y, Hamaue Y, Sakamoto J, Yoshimura T, Origuchi T, Okita M. Changes in hind paw epidermal thickness, peripheral nerve distribution and mechanical sensitivity after immobilization in rats. Physiol Res. 2012;61:643–647. doi: 10.33549/physiolres.932362. [DOI] [PubMed] [Google Scholar]
- 14.Koganezawa T, Ishikawa T, Fujita Y, Yamashita T, Tajima T, Honda M, Nakayama K. Local regulation of skin blood flow during cooling involving presynaptic P2 purinoceptors in rats. Br J Pharmacol. 2006;148:579–586. doi: 10.1038/sj.bjp.0706765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molander C, Xu Q, Grant G. The cytoarchitectonic organization of the spinal cord in the rat. I. The lower thoracic and lumbosacral cord. J Comp Neurol. 1984;230:133–141. doi: 10.1002/cne.902300112. [DOI] [PubMed] [Google Scholar]
- 16.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108:95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Petrat F, Ronn T, de Groot H. Protection by pyruvate infusion in a rat model of severe intestinal ischemia–reperfusion injury. J Surg Res. 2011;167:e93–e101. doi: 10.1016/j.jss.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Coderre TJ, Bennett GJ. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology. Pain Med. 2010;11:1224–1238. doi: 10.1111/j.1526-4637.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Birklein F, Weber M, Neundorfer B. Increased skin lactate in complex regional pain syndrome: evidence for tissue hypoxia? Neurology. 2000;55:1213–1215. doi: 10.1212/WNL.55.8.1213. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Wang P, Zou X, Li D, Fang L, Lin Q. Increases in transient receptor potential vanilloid-1 mRNA and protein in primary afferent neurons stimulated by protein kinase C and their possible role in neurogenic inflammation. J Neurosci Res. 2009;87:482–494. doi: 10.1002/jnr.21844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hao JX, Kupers RC, Xu XJ. Response characteristics of spinal cord dorsal horn neurons in chronic allodynic rats after spinal cord injury. J Neurophysiol. 2004;92:1391–1399. doi: 10.1152/jn.00121.2004. [DOI] [PubMed] [Google Scholar]
- 22.Weng HR, Cordella JV, Dougherty PM. Changes in sensory processing in the spinal dorsal horn accompany vincristine-induced hyperalgesia and allodynia. Pain. 2003;103:131–138. doi: 10.1016/S0304-3959(02)00445-1. [DOI] [PubMed] [Google Scholar]
- 23.Yu Y, Lundeberg T, Yu LC. Role of calcitonin gene-related peptide and its antagonist on the evoked discharge frequency of wide dynamic range neurons in the dorsal horn of the spinal cord in rats. Regul Pept. 2002;103:23–27. doi: 10.1016/S0167-0115(01)00326-3. [DOI] [PubMed] [Google Scholar]
- 24.Yan Y, Yu LC. Involvement of opioid receptors in the CGRP8-37-induced inhibition of the activity of wide-dynamic-range neurons in the spinal dorsal horn of rats. J Neurosci Res. 2004;77:148–152. doi: 10.1002/jnr.20111. [DOI] [PubMed] [Google Scholar]
- 25.Casey GP, Paul D, Gould HJ., 3rd Insulin is essential for the recovery from allodynia induced by complete Freund’s adjuvant. Pain Med. 2010;11:1401–1410. doi: 10.1111/j.1526-4637.2010.00936.x. [DOI] [PubMed] [Google Scholar]
- 26.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, Willcockson HH, Valtschanoff JG. Increased expression of CGRP in sensory afferents of arthritic mice—effect of genetic deletion of the vanilloid receptor TRPV1. Neuropeptides. 2008;42:551–556. doi: 10.1016/j.npep.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388:75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Hullugundi SK, Ferrari MD, van den Maagdenberg AM, Nistri A. The mechanism of functional up-regulation of P2X3 receptors of trigeminal sensory neurons in a genetic mouse model of familial hemiplegic migraine type 1 (FHM-1) PLoS One. 2013;8:e60677. doi: 10.1371/journal.pone.0060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kingery WS. Role of neuropeptide, cytokine, and growth factor signaling in complex regional pain syndrome. Pain Med. 2010;11:1239–1250. doi: 10.1111/j.1526-4637.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 31.Imbe H, Murakami S, Okamoto K, Iwai-Liao Y, Senba E. The effects of acute and chronic restraint stress on activation of ERK in the rostral ventromedial medulla and locus coeruleus. Pain. 2004;112:361–371. doi: 10.1016/j.pain.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Quintero L, Cuesta MC, Silva JA, Arcaya JL, Pinerua-Suhaibar L, Maixner W, Suarez-Roca H. Repeated swim stress increases pain-induced expression of c-Fos in the rat lumbar cord. Brain Res. 2003;965:259–268. doi: 10.1016/S0006-8993(02)04224-5. [DOI] [PubMed] [Google Scholar]
- 33.Nasu T, Taguchi T, Mizumura K. Persistent deep mechanical hyperalgesia induced by repeated cold stress in rats. Eur J Pain. 2009;14:236–244. doi: 10.1016/j.ejpain.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Allen G, Galer BS, Schwartz L. Epidemiology of complex regional pain syndrome: a retrospective chart review of 134 patients. Pain. 1999;80:539–544. doi: 10.1016/S0304-3959(98)00246-2. [DOI] [PubMed] [Google Scholar]


