Abstract
Adaptation of morphological, metabolic, and contractile properties of skeletal muscles to inhibition of antigravity activities by exposure to a microgravity environment or by simulation models, such as chronic bedrest in humans or hindlimb suspension in rodents, has been well reported. Such physiological adaptations are generally detrimental in daily life on earth. Since the development of suitable countermeasure(s) is essential to prevent or inhibit these adaptations, effects of neural, mechanical, and metabolic factors on these properties in both humans and animals were reviewed. Special attention was paid to the roles of the motoneurons (both efferent and afferent neurograms) and electromyogram activities as the neural factors, force development, and/or length of sarcomeres as the mechanical factors and mitochondrial bioenergetics as the metabolic factors.
Keywords: Gravitational unloading; Responses of skeletal muscles; Morphological, metabolic, and contractile properties; Neural, mechanical, and metabolic factors
Introduction
Exposure to microgravity environments has been well reported to induce various prominent changes in the properties of skeletal muscles responsible for maintenance of posture and ground support, i.e., muscles composed predominantly of slow-twitch fibers [1–12]. Atrophy associated with a shift of fiber phenotype toward a faster type has been a consistent finding in response to chronic gravitational unloading. Here we briefly review the mechanism responsible for the muscular adaptation to gravitational unloading. The morphological, metabolic, and contractile properties of skeletal muscles are influenced by neural [6–8, 13–17], mechanical [4, 6, 10, 15, 16, 18–20], and metabolic activities [18, 21–26]. Therefore, we will also cite the data obtained from various ground-based studies that reported on the muscular responses to altered levels of these activities.
Morphological properties
It has been reported that gravitational unloading by exposure to weightlessness causes atrophy mainly in slow anti-gravity muscles and/or muscle fibers [4–9, 12, 27–38]. Modest or no atrophy was generally seen in fast-twitch muscles and/or muscle fibers of rats [9, 39] and mice [30, 34, 35, 37]. However, some recent studies reported comparable atrophy in the mouse soleus, which is composed mainly of fast-twitch fibers, as well as fast-twitch plantaris and gastrocnemius, following unloading [40, 41]. It was also reported that 16–36 % fiber atrophy in the fast-twitch vastus lateralis muscle of astronauts was observed after 11 days of spaceflight, with the relative effects being greater atrophy in fast- than slow-twitch fibers [42]. Possible mechanisms responsible for such adverse effects are discussed in the following Sect. “Phenotype-specific responses.” Muscle atrophy is also induced by ground-based simulation models, such as hindlimb suspension [9, 43–49], denervation [50], deafferentation [50, 51], spinal cord transection [52], tenotomy [15, 53, 54], joint immobilization [55–57] in animals, and/or human bedrest [58–60].
Since the atrophy and hypertrophy of fibers are influenced by a dynamic equilibrium between muscle protein synthesis and breakdown, responses of the distribution of myonuclei and satellite cells in single muscle fibers were studied [6, 20]. In response to 16 days of hindlimb suspension of adult Wistar Hannover rats, the mean numbers of myonuclei and mitotic active/quiescent satellite cells in a single fiber, sampled from tendon to tendon, decreased by 49 and 70/63 % from those of the pre-experimental levels in the soleus [20]. Growth-associated increases of the number of myonuclei and satellite cells, as well as the fiber cross-sectional area (CSA), in soleus were also inhibited by hindlimb suspension from day 4 after birth [61, 62]. These results clearly suggest that gravitational unloading inhibits the protein synthesis in antigravity muscles.
Roles of neuromuscular activation
One of the causes for the unloading-related changes in muscle properties is often attributed to a decrease in neuromuscular activation [13–17]. Electromyogram (EMG) activity of the soleus [63] and adductor longus [77] in rats and humans (unpublished observation) decreased when they were exposed to μ-G during the parabolic flight of a jet airplane. Both soleus EMG and afferent neurogram levels, recorded at the 5th lumbar segmental level of spinal cord in rats, were increased in response to the elevation of gravity (1.5- to 2-G) during the ascending phase [63]. These activities were suddenly dropped in the μ-G environment, increased again during the descending phase (1.5-G), and normalized toward the 1-G level. However, the responses of efferent neurogram levels were minor.
The daily activation levels, analyzed by chronic recording of EMG in ankle plantarflexors [13, 15, 17], not dorsiflexors, and adductor longus muscle [6] also decrease in response to hindlimb suspension. Soleus EMG (~80 %) and both afferent (~30–40 %) and efferent neurograms (~20 %) decreased in response to acute hindlimb suspension relative to those recorded during quadrupedal prone rest on the floor [15]. Although the EMG levels were stable during the first 3–4 days of continuous suspension, these activities were gradually increased thereafter. After 14 days, they reached the baseline levels recorded at rest on the floor before the initiation of suspension. Similar responses were also noted in the neurograms. The afferent neurograms were kept low during the first 1 week, then gradually increased and reached levels even above the baseline. Similar responses of efferent neurograms were also seen. Their levels between 10 and 14 days after suspension were even greater than the baseline on the floor.
The properties of slow and fast muscles are altered in response to cross innervation [64]. Therefore, it was thought that the properties of muscles are regulated by motoneurons. However, recent studies suggested that the properties of skeletal muscles are regulated by the neural and mechanical activities of muscle itself through the afferent-input-associated positive or negative feedback [6, 15], since the activities and patterns of afferent neurograms are closely related to the muscular activities.
Responses of EMGs in the soleus, plantaris, lateral portion of gastrocnemius, and tibialis anterior to 9-week continuous suspension were studied [17]. Suspension-related decreases and gradual recoveries of EMG levels, which are described above, were also seen in the ankle plantarflexors during 1–2 weeks. However, the EMG activities of these muscles gradually decreased, and the lowest activities were seen at the end of suspension. On the contrary, the activities of ankle dorsiflexors, tibialis anterior, even increased in response to hindlimb suspension first. Although they decreased gradually, the mean activity levels were maintained above those of the cage controls (p > 0.05). The EMG activities of all muscles immediately increased in response to reloading on the floor. A prominent increase was noted especially in the tibialis anterior. These activities generally returned to the cage control levels within 1 week.
The recoveries of soleus EMGs and neurograms were closely related to the reorganization of sarcomeres. Due to the plantarflexion-related passive shortening of muscle fibers and sarcomeres (Fig. 1B) in response to hindlimb suspension, tension development was inhibited (Fig. 1A) [15]. Since the number of sarcomeres was decreased (Fig. 1C) and the interval of each sarcomere was elongated after 2 weeks of continuous suspension, tension development at the plantarflexed position (120° and 140°) became possible to some degree because of this reorganization of sarcomeres (Fig. 1A). However, the soleus muscle continued to atrophy in spite of the return to normal activation levels [17, 49], clearly indicating that the direct cause of atrophy was not simply a decrease in the activation level of the muscle. Furthermore, it is unclear why EMG levels of plantarflexors decreased again during the continuous chronic suspension.
Fig. 1.
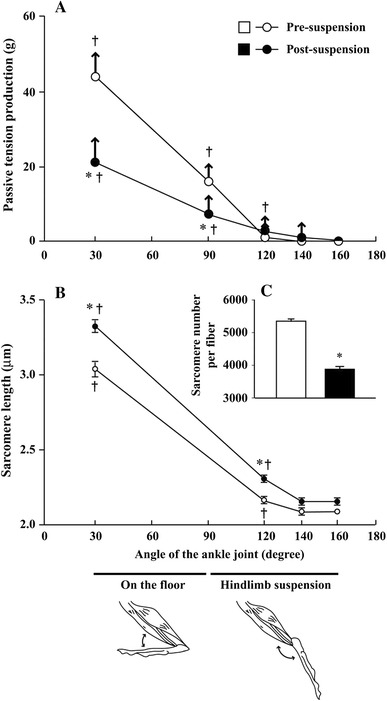
Relationship between the anterior angle of the ankle joint and passive (open and closed circles) and active (arrows) tension development (A: n = 5 for each group) and the mean in vivo length of sarcomeres (B; 10 rats for each group and 5 muscles or 5 × 30 fibers for each angle) before and after 14-day hindlimb suspension. The total sarcomere number per fiber is also illustrated (C; 5 muscles or 5 × 60 fibers for each group). Mean ± SEM. * and †: p < 0.05 vs. pre-suspension control and the levels at a 160° ankle joint angle, respectively. Cited from [15]
The unloading-related fiber atrophy and shift of phenotype to fast characteristics in slow-twitch adductor longus muscle, which are shown below, were more prominent in the caudal than in the rostral region [6]. The EMG activity in the caudal region, with higher distribution of fibers expressing slow-type myosin heavy chains (MHCs) and of myonuclei and satellite cells, at rest on the floor was tonic. But the integrated EMG activities in the caudal region decreased and the patterns became phasic in response to unloading.
Roles of mechanical stress
In addition to the unloading-related changes in EMG levels, there are also decreases in active and passive force development. During unloading, the ankle joints are chronically extended, resulting in a passive shortening of the ankle plantarflexors [10, 15, 16]. In this “slack” position, force production is minimized further, even if the muscles are activated. For example, the tension development at the distal tendon of the soleus muscle was minimal because of the plantarflexion of ankle joints, maintained at ~140° to 160°, during hindlimb suspension of rats (Fig. 1) [15]. Thus, both the active and passive tension levels of the plantarflexors would be expected to be relatively low, particularly during the early phases (up to 2 weeks) after the initiation of unloading.
Wang et al. [20] reported that the distribution of satellite cells was significantly lowered in the central region of a single muscle fiber in atrophied soleus muscle following hindlimb suspension. The mean length of each sarcomere in the central region was ~2.0 μm in the muscle fibers of hindlimb-suspended rats vs. 2.6 μm in muscle fibers sampled from quadrupedal resting rats on the floor. No tension was developed when the sarcomere length in soleus muscle was shortened more than 2.1 vs. 3.0 μm during rest on the floor (Fig. 1) [15]. Thus, it is suggested that the loss of satellite cells in the central region of muscle fibers [20] may be influenced by the decrease in the mechanical stress. A decrease in myonuclear number was also associated with atrophy in these muscle fibers, indicating that protein synthesis was inhibited in response to lowered mechanical stress.
Region-specific responses of various parameters in adductor longus muscle to 16 days of hindlimb unloading and reloading were reported [6]. Prominent unloading-related fiber atrophy in adductor longus muscle was noted in the caudal compared to rostral region. Atrophy of type I fibers in the caudal, not rostral, region was induced after unloading, but recovered toward the control level after 16-day ambulation (Fig. 2). The growth-associated increase of the myonuclear number seen in the caudal region of control rats was inhibited by unloading. The number of mitotic active satellite cells decreased after unloading only in the caudal region. Sarcomere length in the caudal region was passively shortened during unloading (2.35 ± 0.29 vs. 2.84 ± 0.24 μm on the floor), but that in the rostral region was unchanged or even stretched slightly. The EMG activity in the caudal region decreased after acute unloading, but that in the rostral region was even elevated during continuous unloading. The EMG levels in the caudal region gradually increased up to the 6th day, but decreased again. The responses of fiber properties in adductor longus muscle to unloading and reloading were indicated to be closely related to the region-specific mechanical as well as neural activities, the caudal region being more responsive.
Fig. 2.
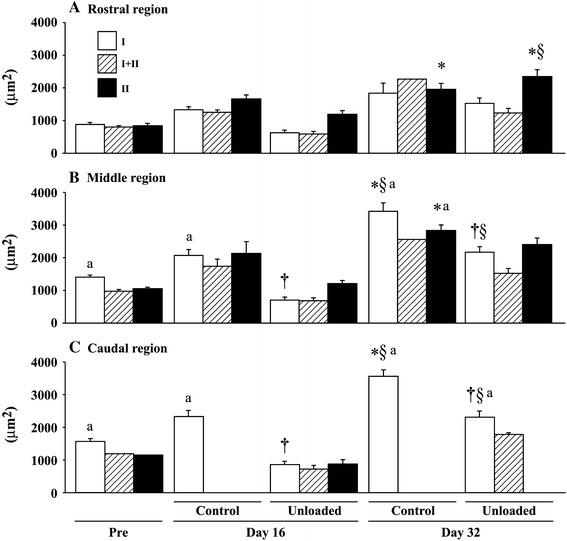
Region-specific responses of fiber cross-sectional area in adductor longus muscle to 16 days of hindlimb suspension and ambulation recovery in Wistar Hannover rats. Mean ± SEM; n = 5 in each group/stage. Pre, day 16, and day 32: before hindlimb unloading, immediately after termination of 16-day unloading or cage housing, and 16 days after ambulation on the floor or 32 days of cage housing, respectively. I, II, and I+II: fibers expressing pure type I (slow) and II (fast), and coexpressing both type I and II myosin heavy chain. *, †, and §: p < 0.05 vs. pre, age-matched control, and respective group at day 16, respectively. a: p < 0.05 vs. the rostral region. Cited from [6]
Effects of 14 days of hindlimb unloading or overloading caused by ablation of synergists, with or without deafferentation, on the fiber CSA, myonuclear number, size, and domain, the number of nucleoli in a single myonucleus, and the levels in the phosphorylation of the ribosomal protein S6 (S6) and 27 kDa heat shock protein (HSP27) were studied in rat soleus muscle [51]. Hypertrophy of fibers (+24 %, Table 1), associated with increased nucleolar number (from 1–2 to 3–5) within a myonucleus and myonuclear domain (+27 %) compared to the pre-experimental level, was induced by ablation of synergists. Such phenomena were associated with increased levels of phosphorylated S6 (+84 %, Table 2) and HSP27 (+28 %). Fiber atrophy (−52 %), associated with a decreased number (−31 %) and domain size (−28 %) of myonuclei and phosphorylation of S6 (−98 %) and HSP27 (−63 %), and with increased myonuclear size (+19 %) and ubiquitination of MHC (+33 %, p > 0.05), was observed after unloading, which inhibited the mechanical load.
Table 1.
Responses of fiber properties in rat soleus to functional overloading (FO), hindlimb unloading (HU), and deafferentation with (DA+FO) or without FO (DA) vs. normal cage controls
| CSA | Myonuc. No./mm |
Myonuc. domain | Myonuc. CSA | Nucleoli No./myonuc. |
|
|---|---|---|---|---|---|
| FO | ↑↑ | → | ↑↑ | → | ↑↑ |
| HU | ↓↓↓ | ↓↓ | ↓ | ↑↑ | → |
| DA | ↓ | → | ↓ | → | → |
| DA+FO | → | → | → | ↑ | ↑ |
Functional overloading was performed by ablation of the distal gastrocnemius and plantaris tendons. Deafferentation was performed at the fifth level of the spinal cord. Cited from [51]
CSA, cross-sectional area; myonuc, myonuclei or myonucleus
Table 2.
Responses of muscle mass and phosphorylation of ribosomal protein S6 (p-S6) and 27-kDa heat shock protein (p-HSP27) in rat soleus muscle to functional overloading (FO), hindlimb unloading (HU), and deafferentation with (DA+FO) or without FO (DA) vs. normal cage controls
| Mass | p-S6 | Total HSP27 | p-HSP27 | |
|---|---|---|---|---|
| FO | ↑↑ | ↑↑↑ | → or ↑ | ↑↑ |
| HU | ↓↓↓ | ↓↓↓ | → | ↓↓↓ |
| DA | ↓ | ↓↓ | → | ↓ |
| DA+FO | → | → or ↑ | → | → |
Responses to deafferentation, which inhibited the EMG level (−47 %), were generally similar to those caused by hindlimb unloading, although the magnitudes were minor (Tables 1, 2) [51]. The deafferentation-related responses were prevented and the nucleolar number even increased (+18 %) by the addition of synergist ablation, even though the integrated EMG level was still 30 % less than that of controls. It is suggested that the load-dependent maintenance or upregulation of the nucleolar number and/or phosphorylation of S6 and HSP27 plays an important role(s) in the regulation of muscle mass. All of these data strongly indicate that the muscle adaptations induced by gravitational unloading are directly associated with the levels of mechanical loading. It was also indicated that such regulation was not necessarily associated with the neural activity.
Effects of metabolic factor(s)
Responses to heat stress
Effects of metabolic factors, other than the mechanical and/or neural factors, on the morphological properties of skeletal muscle are also reported. Naito et al. [65] reported that preconditioning by application of heat stress in order to increase the heat shock protein 72 (HSP72) expression attenuated unloading-related muscle atrophy. HSP72, one of the stress proteins, in skeletal muscle is also upregulated by exercise training [66, 67]. Even though the specific effect of heat stress itself or upregulation of HSPs on the response of muscle is still unclear, the exposure to heat causes an increase in the muscular protein content via the stimulation of protein synthesis and/or depression of protein degradation. Goto et al. [68] also reported beneficial effects of heat stress on the recovery of atrophied muscle.
Heat stress was also reported to enhance the proliferative potential of skeletal muscle and induce muscle hypertrophy [69]. An increase in the number of muscle satellite cells in skeletal muscle was observed in response to heat stress [70]. Heat stress has also been suggested to stimulate p70 S6 kinase [69] and calcineurin [71] in skeletal muscle. The hypertrophic effects of heat stress on human skeletal muscles [72, 73] and cultured muscle cells [74] were also evaluated.
Responses to mitochondrial bioenergetics
Mitochondrial bioenergetics is closely related to the contents of high-energy phosphates and/or metabolic rate. Chronic exposure to cold stimulated the mitochondrial bioenergetics, associated with lowered high-energy phosphate content and increased mitochondrial enzyme activities in skeletal muscle [75]. Similar phenomena were also induced in rat skeletal muscles by feeding with creatine analog β-guanidinopropionic acid [26, 76].
Hip joints of rats were abducted and extended backward during exposure to microgravity [77] and hindlimb suspension [6]. Such phenomena may cause the loss of muscle temperature to ambient air. Lower temperatures in the calf, foot, rectal and tail regions were reported in rats during 10 days of hindlimb suspension [78]. Decreases in blood flow [79] and water content in hindlimb muscles [16] during gravitational unloading of rats were also reported. These results suggest that one of the causes of muscle atrophy induced in response to gravitational unloading might be related to the lowered muscular temperature in addition to the inhibited contractile activity indicated by reduced EMG activities [6, 13, 15, 17]. However, it is obvious that lowered temperature may not be the factor causing the inhibition of mitochondrial metabolism, indicated by the lowered enzyme activities shown below, due to gravitational unloading.
Phenotype-specific responses
Greater atrophy of slow fibers vs. fast fibers was induced in soleus muscle of rats after spaceflight and hindlimb suspension [9]. However, the size of fibers, irrespective of type, was unaffected in the medial portion of the gastrocnemius and tibialis anterior sampled from the same rats [39]. These results indicated that the antigravity effect on slow-twitch muscle or muscle fiber atrophy was more than on fast-twitch muscles and muscle fibers, and on ankle extensors more than flexors. Furthermore, fibers in the caudal region atrophied more than those in the rostral region of slow-twitch adductor longus muscles of the same Wistar Hannover rats in response to unloading [6]. This region-specific response was related to the different effects of neural (EMG) and mechanical stress (sarcomere length) induced by the altered dimensional structure of muscle. An unloading-related decrease in the EMG activity, with a shift from slow-type tonic to fast-type phasic patterns, and passive shortening of sarcomeres, which inhibits the mechanical stress and then mitochondrial bioenergetics, were prominent in the caudal region.
However, it is still unclear why fibers expressing a specific type of MHC are more susceptible to unloading, even within the same muscle. Further, greater atrophy of soleus muscle is induced in Wistar Hannover than Wistar and Sprague-Dawley rats following the same kind of hindlimb unloading [6, 20]. This is why it is speculated that gene expression, specific to the types of fibers and/or animal species, may play a critical role in the induction of these adverse responses.
Metabolic properties
Oxidative enzymes
The specific activities of mitochondrial enzymes (per unit weight or protein), such as succinate dehydrogenase (SDH) [16], β-hydroxyacyl CoA dehydrogenase [16, 19, 43], citrate synthase [19, 43, 79–81], and cytochrome oxidase [19, 80], measured in whole homogenates of unloaded and atrophied soleus muscle are generally lower than in normal controls. However, the specific activity of SDH measured in single muscle fibers is often maintained or even elevated [4–6, 9, 45, 46, 59, 82]. However, the total activity of SDH per whole fiber CSA was decreased [6, 9]. These results suggest that an unloading-related decrease of mitochondrial enzyme levels advances when muscle or muscle fibers atrophy. The data also indicate that the rates of their decreases are parallel to or less than those of the cytoplasmic volume of fibers since the relative volume of connective tissues is increased [83].
Further, the SDH activity measured in the cross section of muscle fibers is significantly higher in the subsarcolemmal than intermyofibrillar region [28]. Spaceflight caused a significant decrease in SDH activity in the subsarcolemmal region. It was also reported that the distribution of mitochondria in the subsarcolemmal area of the adductor longus muscle of flight group was 31 % less than that in ground controls [12]. These results suggest that an unloading-related decrease of mitochondrial enzymes may be of functional importance and where those enzymes are distributed within a fiber should be considered.
Glycolytic enzymes
Responses of the specific activities of glycolytic enzymes, such as lactate dehydrogenase (LDH) [16, 19, 43] and α-glycerophosphate dehydrogenase [9, 82] in rat soleus, and phosphofructokinase in human vastus lateralis [80], to gravitational unloading were also reported. The specific activities (per unit weight or protein) of LDH [16, 19, 43] and phosphofructokinase [80], measured in whole homogenates, remained stable in response to unloading. No changes [9] or slightly elevated α-glycerophosphate dehydrogenase activities [82] in single muscle fibers were reported. However, the total LDH activity in whole muscle [16, 19] was decreased. These results clearly suggest that the activities of glycolytic as well as oxidative enzymes in skeletal muscles are generally decreased in response to mechanical unloading. Further, such phenomena are parallel to or slower than the fiber atrophy.
Gene and protein expression related to metabolism
Significant downregulation of 15 mitochondria-related proteins was induced in the neck muscle of mouse following 3-month spaceflight, although four proteins were upregulated (Fig. 3) [35]. As for the glycolysis-related proteins, five proteins—fructose-bisphosphate aldolase A isoform 1, glycerol-3-phosphate dehydrogenase (NAD+) (cytoplasmic), L-LDH A chain isoform, 2, 6-phosphofructokinase (muscle type), and phosphorylase—were upregulated significantly. However, downregulation was not seen in any glycolytic proteins. These results, including the downregulation of genes related to mitochondrial metabolism, including β-adrenoceptor, as described below [34], imply that the metabolic properties were shifted from oxidative to glycolytic metabolism even in fast-twitch muscle because of unloading-related changes of the metabolic rate.
Fig. 3.
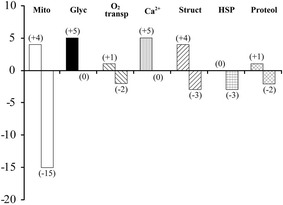
Numbers of up- and downregulated proteins in mouse neck muscle following 3-month spaceflight vs. the age-matched ground-based vivarium laboratory control. Mito, mitochondria; Glyc, glycolysis; O2 transp, oxygen transport; Ca2+, calcium metabolism; Struct, myofibrillar structure; HSP, heat shock proteins; Proteol, proteolysis. Cited from [35]
Density of β2-adrenoceptor
β2-Adrenoceptors play a regulatory role in respiratory and metabolic function, although its significance is not yet fully understood [84]. However, β2-adrenoceptors in skeletal muscles play an important role in glucose metabolism and/or homeostasis by activation of the sympathetic nervous system [85, 86]. Further, the density of β2-adrenoceptors is considerably greater in slow- than fast-twitch muscle [87] or muscle fibers [18], suggesting that they are closely related to oxidative metabolism in skeletal muscle.
β2-Adrenoceptor density in skeletal muscle is increased by exercise training [87, 88] and electrical stimulation at 10 Hz [89]. Application of these mechanical and/or neural stimuli also causes an increase of mitochondrial enzyme activities or shift of fibers toward the slow-twitch type [87–89]. However, the density of β2-adrenoceptor in soleus [23] and plantaris muscles [24] of rats was decreased in response to hindlimb suspension and spaceflight, respectively. Spaceflight-related significant downregulation of genes related to cellular metabolism (vs. ground-based laboratory control) in neck muscle (rhomboideus capitis) of wild-type mouse (male C57BL/10J, 8 weeks old at the launch of space shuttle), exposed to microgravity for 3 months on the International Space Station (ISS), was observed [34]. Downregulation of the β-adrenoceptor gene, also seen in this muscle, agrees with the previous findings observed in response to hindlimb suspension [23] and spaceflight [24]. These results suggest an unloading-related inhibition of oxidative metabolism.
Similarly, the maximal binding capacity (B max) of β2-adrenoceptor in soleus was decreased following feeding of creatine, as well as inhibition of mechanical stress on muscles by hindlimb suspension, which caused elevation of high-energy phosphate contents in rat calf muscles (Fig. 4) [23]. The B max of β2-adrenoceptor was, on the contrary, increased in response to chronic depletion of creatine by feeding β-guanidinopropionic acid, which also caused a decrease of high-energy phosphate contents in muscles [21–23, 26]. The metabolic rate, level of resting oxygen consumption [26], and mitochondrial enzyme activities of skeletal muscles in these rats [76, 90, 91] were greater than in rats fed normal food, and contractile properties were shifted from fast- to slow-twitch type [92, 93]. These findings suggest that the mechanism responsible for the regulation of muscular properties is closely related to the mechanical and metabolic factors.
Fig. 4.
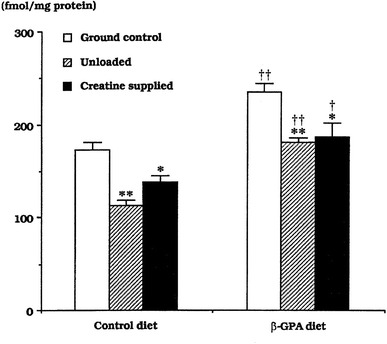
Responses of the maximum binding capacity of β2-adrenoceptors in rat soleus to hindlimb unloading and/or supplementation of creatine or its analog, β-guanidinopropionic acid (β-GPA). Mean ± SEM. * and **: p < 0.05 and 0.01 vs. ground control; † and ††: p < 0.05 and 0.01 vs. the respective group in the control diet group. Cited from [23]
Fiber phenotype
Myosin heavy chain expression
Gravitational unloading by spaceflight and/or hindlimb suspension causes a shift of fiber phenotype from slow to fast in soleus and adductor longus [1, 4, 6, 9], but not in fast-twitch muscles [39, 44, 47]. Figure 5 shows the region-specific responses of fiber types in adductor longus muscle of Wistar Hannover rats to 16 days of hindlimb unloading and reloading [6]. The percent distribution of slow fibers was generally greater in the caudal and middle regions than in the rostral region. The caudal and middle regions in the control rats were composed of approximately 97 and 92 % of fibers expressing pure type I MHC at the beginning of the experiment, respectively. Meanwhile, the mean percentage of pure type I fibers in the rostral region was 61 %, and the distribution of type I+II and II fibers was 14 and 25 %, respectively.
Fig. 5.
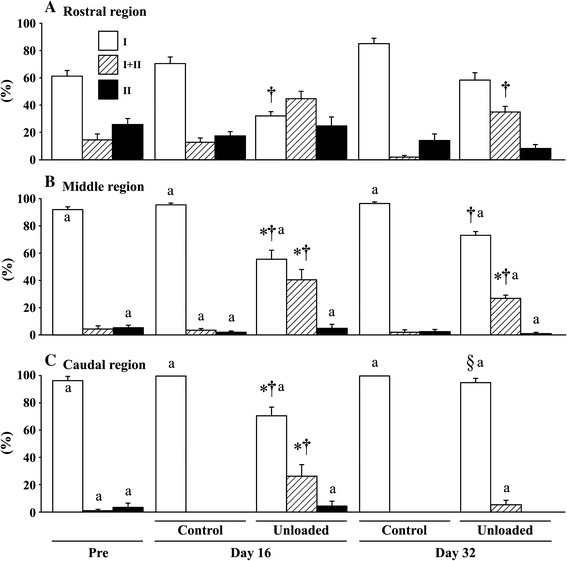
Region-specific responses of fiber phenotypes in adductor longus muscle of Wiatar Hannover rats to 16 days of hindlimb suspension and ambulation recovery. Mean ± SEM; n = 5 in each group/stage. See Fig. 2 for the symbols and abbreviations. Cited from [6]
After 16 days of growth on the floor, all fibers in the caudal region became pure type I [6]. The percentage of type I fibers in the middle and caudal region in the unloaded group decreased from the pre-experimental levels, and de novo appearance of type I+II fibers was noted. The percentage of type I fibers in all regions of the unloaded group was less than in the age-matched control. The percentages of type I+II fibers of the unloaded group in the middle and caudal, but not rostral, regions were significantly greater than those of the pre-suspension and age-matched control levels. Thus, it is suggested that unloading-related adverse effects in the caudal region are influenced by inhibition of mechanical and neural activities, as described above.
The fiber phenotypes in the caudal region were normalized after 16-day ambulation [6]. However, the unloading-related transformation toward fast type in the rostral and middle region was not completely normalized, although there was a trend to recover toward the control level. The percentages of type I and I+II fiber in the middle region and that of type I+II fiber in the rostral region were still different from those of the age-matched controls.
Figure 6 shows the responses of fiber phenotypes in soleus muscle of male Sprague-Dawley rats to 2 weeks of spaceflight (Space Transport System-58 of the Spacelab Life Sciences-2 mission) [1]. Spaceflight resulted in a significant decrease of type I and IIa + I fibers as well as the complete loss of type IIa fibers. Further, de novo expression of type IIb and neonatal MHCs and fibers coexpressing various types of MHCs was also noted. However, the distribution of these fibers disappeared after 9 days of ambulation recovery in a 1-G environment (data not shown).
Fig. 6.
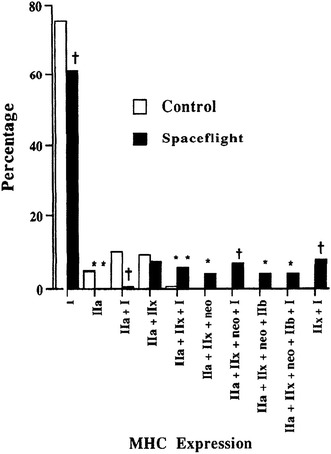
Mean percentages of rat soleus muscle fibers expressing various isoforms of myosin heavy chain (MHC) after 2 weeks of spaceflight. *, **, and †: p < 0.05, p < 0.005, and p < 0.001 vs. control. Cited from [1]
Three intracellular calcium handling proteins, i.e., calsequestrin-1 (found in fast skeletal muscle), parvalbumin alpha, and sarcoplasmic/endoplasmic reticulum calcium ATPase 1, in mouse neck muscle were significantly upregulated in response to 3-month spaceflight [35]. Ryanodine receptor 1 (skeletal muscle) was also upregulated. These responses, in general, are consistent with a shift toward a faster phenotype reported elsewhere [1, 4, 6, 9].
Relationship to myosin ATPase activity
It was reported that the activity of myosin ATPase is correlated with the speed of muscle contraction [94]. Therefore, the staining of myosin ATPase in muscle fibers were utilized to classify the properties into either slow- or fast-twitch type [9, 39]. However, the quantitative activities of myosin ATPase in muscle fibers did not change, even though the fiber phenotypes were shifted from slow to fast type in response to spaceflight [9, 35] or hindlimb suspension [6, 9, 16, 82], according to the classification using the classic myosin ATPase staining [9, 16, 82] or current method using antibodies specific for slow and fast MHC [6, 35]. These results suggest that the shift of fiber phenotypes is not directly related to the changes of myosin ATPase activity.
Contractile properties
Animal studies
Effects of unloading in mature rats
Unloading-related changes of contractile properties were observed in muscle and/or muscle fibers with atrophy and a shift of MHC phenotype, as described above. Caiozzo et al. [2] analyzed the in situ contractile properties of soleus approximately 3 h after the landing of the space shuttle in male Sprague-Dawley rats exposed to microgravity for 6 days (n = 6). The maximal isometric tension (P o) was decreased by 24 % and maximal shortening velocity (V o) was increased by 14 % following spaceflight vs. those in the ground-based controls (n = 6). At the end of the 2-min fatigue test, the flight muscles generated only 34 % of P o, whereas the control muscles generated 64 % of P o. On the contrary, it was also reported that the fatigue resistance in atrophied soleus remains remarkably high [49, 95]. Such phenomena may in part be attributable to (1) lowered absolute tension development, (2) relatively stable levels of oxidative enzyme, SDH, in fiber as described above [4, 5, 9, 45–47], and/or the shorter diffusion distance from the blood capillary to the center of the fiber due to atrophy.
The contractile speed-related properties in slow soleus muscle were shifted toward fast-type, although fast muscles were not affected [49, 96–98]. The time-to-peak tension was reduced. The V o of whole muscle [49, 96, 98] and single fibers [99–101] was increased following unloading. Interestingly, elevation of myosin ATPase activity, which could be proportional to an increase of V o, was not always observed [9, 48, 97, 102]. Further, the one-half relaxation time was decreased, perhaps due to changes in sarcoplasmic reticulum kinetics [103].
An unloading-related shift of muscle toward a faster phenotype was also suggested by changes in protein expression. As described above, three intracellular calcium handling proteins and ryanodine receptor 1 (skeletal muscle) in mouse neck muscle were significantly upregulated in response to 3-month spaceflight [35]. The shift of contractile properties is generally consistent with the shift of fiber phenotype and metabolic properties.
Effects of unloading during the growing period
Force development
Effects of hindlimb unloading during the 3-month growing period followed by 3-month ambulation recovery on the contractile properties of single fibers expressing only type I MHC were studied in rat soleus [104]. The larger fibers generally develop greater force than small fibers because of the higher number of cross bridges. Thus, the absolute tension production was ~97 % less in the unloaded muscle fibers (Fig. 7C) with smaller CSA (~−80 % vs. control) (Fig. 7B). However, the relative tension, normalized by fiber CSA, was also less in the unloaded than control fibers (Fig. 7D). All of these parameters gradually recovered toward the control levels during the 3-month ambulation.
Fig. 7.
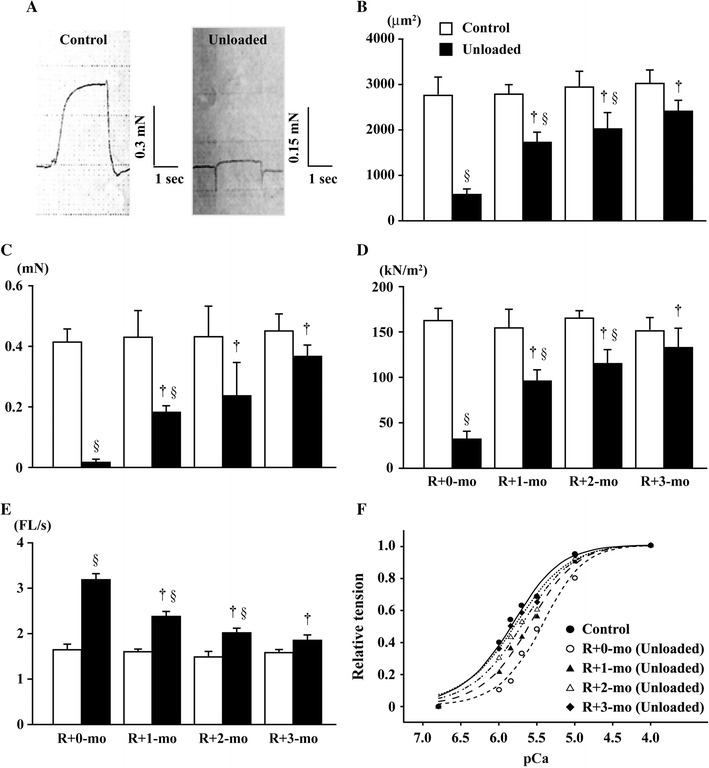
Contractile properties of single fibers of soleus muscle. A Pictures showing the typical patterns of tension development in single fibers of control and unloaded rats immediately after 3-month cage housing or unloading. B Changes in the cross-sectional area of fibers, which were used for the analysis of contractile properties. C Absolute maximally activated isometric tension (P o). D Relative maximally activated isometric tension (F max) per fiber cross-sectional area. E Unloaded shortening velocity (V o). F Sensitivity of the fibers to calcium: calcium concentration when half of the maximum tension was obtained. Mean ± SEM. † and §: p < 0.05 vs. the level immediately after 3-month unloading or cage housing (R+0-mo) and the age-matched control, respectively. FL, fiber length. R+1-mo, R+2-mo, and R+3-mo: 1, 2, and 3 months after R+0-mo, respectively. Cited from [108]
Shortening velocity
The V o was ~97 % greater at the end of 3-month unloading than the age-matched control rats (Fig. 7E). The sensitivity of fibers to Ca2+ was evaluated as pCa50, which is the Ca2+ concentration when half of the maximum tension has been obtained. The mean pCa50 in the unloaded fibers (5.44) was less than that in the controls (5.84) at the 3rd month after birth. These properties gradually recovered toward the control levels. Similar responses of the size, as well as contractile properties, of single fibers to long-term bedrest were also seen in human soleus, as described below [60].
Number of neuromuscular junctions
Interestingly, muscle fibers with multiple distributions of neuromuscular junctions, detected by staining for acetylcholine receptor (AchR) using α-bungarotoxin, were noted at the end of 3-month unloading (Fig. 8) [104]. At postnatal day 4, a single junction was noted at the middle portion in all of the fibers analyzed, suggesting that the aggregation of AchR had already formed normally (Fig. 9A). Approximately 97.2 % of muscle fibers of control rats had a single junction area at the age of 3 months, and ~2.8 % of fibers had two junctions. However, the fibers containing one junction were only ~60 % in the unloaded fibers, and ~32, 7, and 1 % of the fibers had 2, 3, and even 5 endplates. The single junction in the fiber was located at the middle region of the fiber length. Double junctions were located at the proximal and distal regions of the fibers.
Fig. 8.
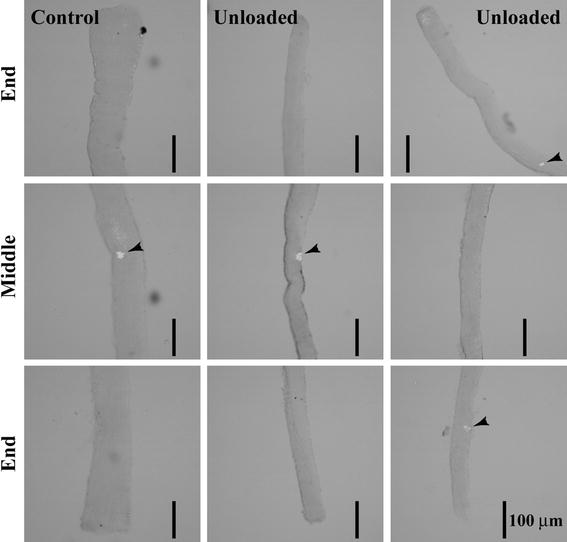
Pictures showing the location of nerve endplates, indicated by arrowheads, in single soleus muscle fibers sampled from the cage control and hindlimb-unloaded 3-month old rats. Cited from [108]
Fig. 9.
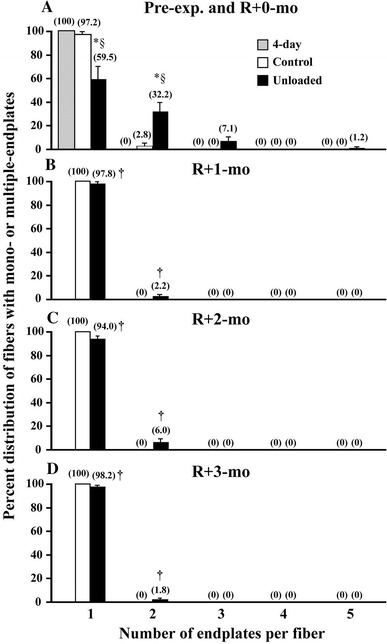
Percent distribution of muscle fibers with various numbers of nerve endplates 4 days after birth (pre-exp.) and 3 months after unloading or cage housing (R+0-mo, A), 1 (B), 2 (C), and 3 (D) months after ambulation recovery, respectively. Mean ± SEM. *, †, and §: p < 0.05 vs. the 4-day old control, R+0-mo, and the age-matched control, respectively. See Fig. 7 for other abbreviations. Cited from [108]
The total fiber number of the soleus muscle at the 4th day after birth was ~800 and was increased to ~2500 following the normal 3-month growth [61]. The growth-related fiber formation was not influenced, even if the rats were hindlimb-unloaded between postnatal day 4 and month 3. It is suggested that the unloading may cause an abnormal innervation in some newly formed fibers during the postnatal development, since all fibers in 4-day-old rats had single innervations. However, the number of junctions was normalized within 1-month reloading (Fig. 9B). It is indicated that the formation and/or location of the junction area in soleus muscle fibers may be regulated, in part, by the gravity-dependent motor activity after birth.
The fibers coexpressing the multiple isoforms of MHCs were noted in the soleus muscle fibers at the end of 3-month unloading [104]. However, these fibers disappeared within 1 month of reloading. It is speculated that the appearance of fibers coexpressing various types of MHC may be closely related to the unloading-associated abnormal activity due to the plural inputs from the multiple junctions. The relationship between the location of junctions and the phenotype or function of fiber is unclear since the matured muscle fibers are generally innervated by a single motoneuron, regardless of their phenotype [105]. However, the multiple innervations may affect the motor performance. Abnormal patterns of EMG in hindlimb muscles, such as coactivation of dorsiflexor and plantarflexor muscles, were observed during walking on the floor after chronic unloading [17]. Walton et al. [106] also reported that new-born rats that were hindlimb-unloaded from the postnatal day 8–13 could not swim. These findings suggest that the gravitational unloading during the critical period for development of the motor system causes a failure of morphological and functional growth of skeletal muscle fibers.
Human studies
Spaceflight
It was reported that the absolute maximal voluntary contraction (MVC) of the human plantarflexor muscles declined by 20–48 % following 6-month spaceflight [107]. The absolute isometric torque, measured during MVC of the same muscle group, also decreased (~17 %) following 90–180 days in microgravity [108]. Further, ~21 (12–40) % decline in the absolute peak force of the slow type I fiber was observed following a 17-day spaceflight [109, 110]. In addition, the absolute peak force of type IIa fibers in soleus was 25 % lower than the pre-flight value [111]. The effects of 180 days of spaceflight on the MVC of calf muscle [112] and the structure and function of slow and fast fibers in the gastrocnemius and soleus muscles [109] of nine ISS crew members were also reported. Prolonged weightlessness produced a decline in the MVC of calf muscle (~13 %) and in the absolute peak force, as soleus type I (35 %) > soleus type II (27 %) > gastrocnemius type I (25 %) > gastrocnemius type II fibers (7 %). Although these results indicate the fiber type-dependent decline in peak force, flight-duration-dependent effects were not observed.
Bedrest
The responses of contractile properties in human soleus to bedrest were also studied [60, 113–116]. Effects of 2 and 4 months of bedrest on the contractile properties of slow fibers in soleus muscles of male subjects, sampled by using needle biopsy, were reported [60]. The mean fiber diameters were 8 and 36 % smaller after 2 and 4 months of bedrest, respectively, than the pre-experimental level (Fig. 10). The P o and maximum activated force (F max) per CSA decreased after bedrest. Such phenomena may be related to a greater reduction of contractile proteins than other structural or metabolic proteins following unloading. However, the precise mechanism is still unknown. The common logarithm value of the free Ca2+ concentration required for half-maximal activation (pCa50) was also decreased. In contrast, the V o was increased after bedrest.
Fig. 10.
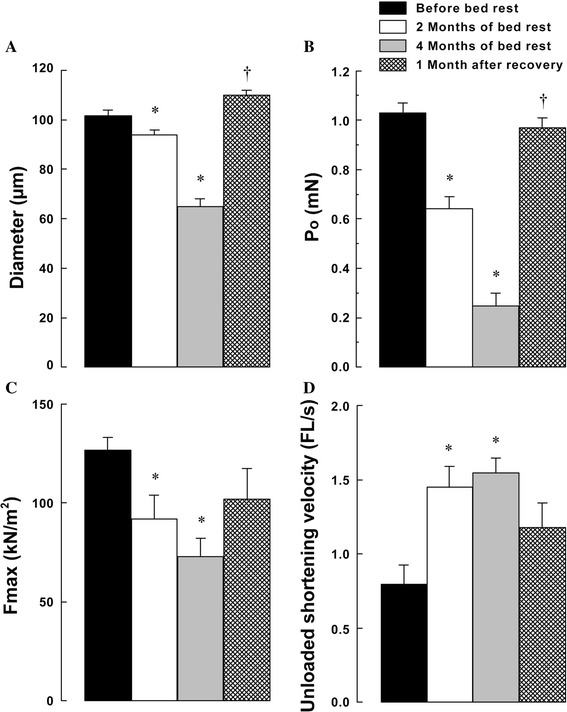
Responses of contractile profiles of slow soleus muscle fibers to 2 and 4 months of bedrest and 1 month of ambulation recovery in male subjects. A Fiber diameter. B Absolute Ca2+-activated maximal force (P o). C Relative Ca2+-activated maximal force (F max) per cross-sectional area. D Unloaded shortening velocity (V o). Mean ± SEM. FL, fiber length. * and †: p < 0.05 vs. pre-bedrest and 4 months of bedrest, respectively. Number of fibers: 25 per group. Cited from [60]
After 1 month of recovery, fiber diameters, P o, F max per CSA (p > 0.05), and pCa50 increased, and the V o decreased toward the pre-bedrest levels. Effects of 60 days of bedrest were also compared between male and female subjects [113, 114]. The diameter of soleus muscle fibers expressing type I MHC (−14 % in both males and females), P o (−38 % in males and −35 % in females), and power (−39 % in males and −42 % in females), but not of contractile velocity, decreased following bedrest. There were no sex differences in the responses of contractile properties of single muscle fibers. These contractile responses to inactivity are similar to the results obtained from animal studies describes above.
Responses to countermeasures
Effects of exercise and/or nutritional treatment as the countermeasures for prevention of bedrest-related adverse effects were also studied [60, 113, 114]. Effects of knee extension/flexion exercise by wearing an anti-G Penguin suit for 10 h daily, and the effects of loading or unloading of the plantarflexors with (Penguin-1 group) or without (Penguin-2 group) placing elastic loading elements of the suit, respectively, were investigated during 2 months of bedrest [60]. In the Penguin-1 group, mean fiber diameter, P o, F max per CSA, V o, and pCa50 were similar before and after bedrest. However, the responses of fiber size and contractile properties to bedrest were not prevented in the Penguin-2 group, although the degree of the changes was less than those induced by bedrest without any countermeasures [60]. These results indicate that long-term bedrest results in reductions of fiber size, force-generation capacity, and Ca2+ sensitivity, and enhancement of shortening velocity in slow fibers of soleus muscle, as described above. It was suggested that knee extension/flexion exercise was not useful as the countermeasure for the soleus. However, the data also indicate that continuous mechanical loading on muscle, such as stretching of muscle, is an effective countermeasure for the prevention of muscular adaptations to gravitational unloading.
Trappe et al. [113, 114] investigated the effects of aerobic and resistance training on the properties of soleus muscle fibers during bedrest. The lower body negative pressure (LBNP) treadmill device was used for aerobic training. The treadmill was positioned vertically so that all treadmill exercise activity was performed with the subject in a horizontal (zero degree) position. The subjects performed 40-min exercise ranging from 40 to 80 % of pre-bedrest peak oxygen uptake, followed by 10-min resting LBNP 2–4 days per week. During the course of 60-day bedrest, 29 exercise sessions were prescribed. Resistance training was performed by using supine squat (SS) exercises on an inertial ergometer. Resistance exercise was scheduled for each subject approximately every third day (2–3 days/week) for a total of 19 sessions. The inertial ergometer was in the 6° head-down tilt position, and all resistance exercises were performed in the supine position. Ten minutes of light supine cycling and submaximal SS repetitions were completed as warm-up. The SS exercise consisted of four sets of seven maximal concentric and eccentric repetitions. There were 2-min intervals between sets.
A nutritional countermeasure was also performed to provide an additional amount of protein and free leucine during the bedrest [113, 114]. An additional protein level was maintained +0.45 g/kg body weight/day. In addition, subjects received 3.6 g/day of free leucine, 1.8 g/day of free valine, and 1.8 g/day of free isoleucine, which were equally divided out over the three meals of the day. Thus, the total protein intake for the bedrest with nutrition countermeasure group was 1.6 g/kg body weight/day. To compensate for the additional increase in energy intake from protein, the carbohydrate content was reduced during the bedrest period.
The results showed the combination of aerobic and resistance exercise was effective for protection against adverse effects induced by bedrest in the upper (vastus lateralis) and lower leg (soleus) muscles in both males [113] and females [114], although beneficial effects of nutritional treatment alone were not obtained. However, it was reported that the exercises were not effective for prevention of the reduction of calf muscle mass and performance along with a slow-to-fast transition of fiber type in gastrocnemius and soleus muscles, although nine crew members had access to a running treadmill, cycle ergometer, and resistance exercise device on the ISS [112]. They performed aerobic exercise (~5 h/week) at moderate intensity and resistance exercise (3–6 days/wk) incorporating multiple lower leg exercises. It is speculated that mobilization of muscles during exercise may not be identical between in-flight and on-ground conditions, even if the same exercise device is utilized. Therefore, it was suggested that exercise prescription and/or hardware on the ISS should be modified.
Further investigations to be performed
Although it is generally accepted that the specific tension per unit CSA or weight is constant, some studies [49, 96] also reported that the magnitude of the decrease in the P o was greater than that in muscle mass. Therefore, the specific tension per unit weight or CSA was lowered (Figs. 7D, 10C). Such phenomena may be due to the greater decrease in myofibrillar protein [48, 117], especially in thin filament density [118], lattice spacing [119, 120], and/or the relative increase of the non-contractile tissue [81, 83] and interstitial volume [121].
The Ca2+ sensitivity in the muscle fibers is dependent on the isoform of troponin C [122, 123] and/or the cooperativity between thin and thick filaments [124]. As described above (Fig. 7F), the Ca2+ sensitivity was modified by unloading. It has been reported that the intracellular free Ca2+ concentration in soleus muscle with unloading-induced atrophy is elevated in a resting state [125]. Unloading might cause an alteration of intracellular Ca2+ movement in muscle fibers, resulting in modification of the Ca2+ sensitivity. Reloading promoted the normalization of the fiber characteristics. However, the molecular responses of Ca2+ sensitivity-modification are still unclear.
The activity of myosin ATPase is correlated with the speed of muscle contraction [94]. However, the quantitative activities of myosin ATPase in muscle fibers did not change, even though the fiber phenotypes were shifted from slow to fast type in response to spaceflight [9, 35] or hindlimb suspension [6, 9, 16, 82]. These results suggest that the shift of fiber phenotypes is not directly related to the changes of myosin ATPase activity.
The precise mechanisms responsible for these phenomena seen following gravitational unloading and/or reloading are still unclear. Therefore, further investigations should be carried out to elucidate these mechanisms.
Conclusion
Adaptation of morphological, metabolic, and contractile properties of skeletal muscles to inhibition of antigravity activities was reviewed. Generally, studies have suggested that atrophy of muscles and/or muscle fibers is closely related to the decrease of tension development due to passive shortening of muscle fibers and sarcomeres. Such responses are also associated with inhibition of the activities of EMG and motoneurons, mainly afferent input. Further, these physiological adaptations were associated with inhibited mitochondrial energy metabolism. The studies also suggested that the shift of fiber phenotypes and contractile properties toward the fast-twitch type was influenced by neural (shift from tonic to phasic activity) and metabolic factors.
References
- 1.Allen L, Yasui W, Tanaka T, Ohira Y, Nagaoka S, Sekiguchi C, Hinds WE, Roy RR, Edgerton VR. Myonuclear number and myosin heavy chain expression in rat soleus single muscle fibers after spaceflight. J Appl Physiol. 1996;81:145–151. doi: 10.1152/jappl.1996.81.1.145. [DOI] [PubMed] [Google Scholar]
- 2.Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. Effects of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of slow muscle. J Appl Physiol. 1994;76:1764–1773. doi: 10.1152/jappl.1994.76.4.1764. [DOI] [PubMed] [Google Scholar]
- 3.Edgerton VR, Roy RR (1996) Neuromuscular adaptations to actual and simulated spaceflight. In: Fregly MJ, Blatteis CM (eds) Handbook of physiology, Sect 4, Environmental physiology, III. The gravitational environment, Oxford University Press, New York, pp 721–763
- 4.Martin TP, Edgerton VR, Grindland RE. Influence of spaceflight on rat skeletal muscle. J Appl Physiol. 1988;65:2318–2325. doi: 10.1152/jappl.1988.65.5.2318. [DOI] [PubMed] [Google Scholar]
- 5.Miu B, Martin TP, Roy RR, Oganov V, Ilyina-Kakueva E, Marini JF, Leger JJ, Bodine-Fowler SC, Edgerton VR. Metabolic and morphologic properties of single muscle fibers in the rat after spaceflight, Cosmos 1887. FASEB J. 1990;4:64–72. doi: 10.1096/fasebj.4.1.2136839. [DOI] [PubMed] [Google Scholar]
- 6.Ohira T, Wang XD, Terada M, Kawano F, Nakai N, Ogura A, Ohira Y. Region-specific responses of adductor longus muscle to gravitational load-dependent activity in Wistar Hannover rats. PLoS One. 2011;6:e21044. doi: 10.1371/journal.pone.0021044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohira Y. Neuromuscular adaptation to microgravity environment. Jpn J Physiol. 2000;50:303–314. doi: 10.2170/jjphysiol.50.303. [DOI] [PubMed] [Google Scholar]
- 8.Ohira Y, Edgerton VR. Neuromuscular adaptation to gravitational unloading or decreased contractile activity. Adv Exerc Sports Physiol. 1994;1:1–12. [Google Scholar]
- 9.Ohira Y, Jiang B, Roy RR, Oganov V, Ilyina-Kakueva E, Martin JF, Edgerton VR. Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J Appl Physiol. 1992;73:51S–57S. doi: 10.1152/jappl.1992.73.2.S51. [DOI] [PubMed] [Google Scholar]
- 10.Riley DA, Ellis S, Giometti CS, Hoh JF, Ilyina-Kakueva EI, Oganov VS, Slocum GR, Bain JL, Sedlak FR. Muscle sarcomere lesions and thrombosis after spaceflight and suspension unloading. J Appl Physiol. 1992;73:33S–43S. doi: 10.1152/jappl.1992.73.2.S33. [DOI] [PubMed] [Google Scholar]
- 11.Riley DA, Ellis S, Slocum GR, Sedlak FR, Bain JLW, Krippendorf BB, Lehman CT, Macias MY, Thompson JL, Vijayan K, De Bruin JA. In-flight and postflight changes in skeletal muscles of SLS-1 and SLS-2 spaceflown rats. J Appl Physiol. 1996;81:133–144. doi: 10.1152/jappl.1996.81.1.133. [DOI] [PubMed] [Google Scholar]
- 12.Riley DA, Ilyina-Kakueva EI, Ellis S, Bain JL, Slocum GR, Sedlak FR. Skeletal muscle fiber, nerve, and blood vessel breakdown in space-flown rats. FASEB J. 1990;4:84–91. doi: 10.1096/fasebj.4.1.2153085. [DOI] [PubMed] [Google Scholar]
- 13.Alford EK, Roy RR, Hodgson JA, Edgerton VR. Electromyography of rat soleus, medial gastrocnemius, and tibialis anterior during hindlimb suspension. Exp Neurol. 1987;96:635–649. doi: 10.1016/0014-4886(87)90225-1. [DOI] [PubMed] [Google Scholar]
- 14.De-Doncker L, Kasri M, Picquet F, Falempin M. Physiologically adaptive changes of the L5 afferent neurogram and of the rat soleus EMG activity during 14 days of hindlimb unloading and recovery. J Exp Biol. 2005;208:4585–4592. doi: 10.1242/jeb.01931. [DOI] [PubMed] [Google Scholar]
- 15.Kawano F, Ishihara A, Stevens JL, Wang XD, Ohshima S, Horisaka M, Maeda Y, Nonaka I, Ohira Y. Tension- and afferent-input-associated responses of neuromuscular system of rats to hindlimb unloading and/or tenotomy. Am J Physiol Regul Integr Comp Physiol. 2004;287:R76–R86. doi: 10.1152/ajpregu.00694.2003. [DOI] [PubMed] [Google Scholar]
- 16.Ohira M, Hanada H, Kawano F, Ishihara A, Nonaka I, Ohira Y. Regulation of the properties of rat hind limb muscles following gravitational unloading. Jpn J Physiol. 2002;52:235–245. doi: 10.2170/jjphysiol.52.235. [DOI] [PubMed] [Google Scholar]
- 17.Ohira Y, Nomura T, Kawano F, Sato Y, Ishihara A, Nonaka I. Effects of nine weeks of unloading on neuromuscular activities in adult rats. J Gravit Physiol. 2002;9:49–60. [PubMed] [Google Scholar]
- 18.Martin WH, Coggan AR, Spina RJ, Saffitz JE. Effects of fiber type and training on β-adrenoceptor density in human skeletal muscle. Am J Physiol. 1989;257:E736–E742. doi: 10.1152/ajpendo.1989.257.5.E736. [DOI] [PubMed] [Google Scholar]
- 19.Ohira Y, Yasui W, Kariya F, Wakatsuki T, Nakamura K, Asakura T, Edgerton VR. Metabolic adaptation of skeletal muscles to gravitational unloading. Acta Astronaut. 1994;33:113–117. doi: 10.1016/0094-5765(94)90115-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang XD, Kawano F, Matsuoka Y, Fukunaga K, Terada M, Sudoh M, Ishihara A, Ohira Y. Mechanical load-dependent regulation of satellite cell and fiber size in rat soleus muscle. Am J Physiol Cell Physiol. 2006;290:C981–C989. doi: 10.1152/ajpcell.00298.2005. [DOI] [PubMed] [Google Scholar]
- 21.Fitch CD, Jellinek M, Fitts RH, Baldwin KM, Holloszy JO. Phosphorylated β-guanidinopropionate as a substitute for phosphocreatine in rat muscle. Am J Physiol. 1975;228:1123–1125. doi: 10.1152/ajplegacy.1975.228.4.1123. [DOI] [PubMed] [Google Scholar]
- 22.Fitch CD, Jellinek M, Mueller EJ. Experimental depletion of creatine and phosphocreatine from skeletal muscle. J Biol Chem. 1974;249:1060–1063. [PubMed] [Google Scholar]
- 23.Ohira Y, Saito K, Wakatsuki T, Yasui W, Suetsugu T, Nakamura K, Tanaka H, Asakura T. Responses of β-adrenoceptor in rat soleus to phosphorus compound levels and/or unloading. Am J Physiol. 1994;266:C1257–C1262. doi: 10.1152/ajpcell.1994.266.5.C1257. [DOI] [PubMed] [Google Scholar]
- 24.Ohira Y, Yasui W, Kariya F, Tanaka T, Kitajima I, Maruyama I, Nagaoka S, Sekiguchi C, Hinds WE. Spaceflight effects on β-adrenoceptor and metabolic properties in rat plantaris. J Appl Physiol. 1996;81:152–155. doi: 10.1152/jappl.1996.81.1.152. [DOI] [PubMed] [Google Scholar]
- 25.Ohira Y, Yasui W, Wakatsuki T, Nakamura K, Asakura T (1992) Effects of stretching and shortening on mass and high-energy phosphate contents in rat antigravity muscles. In: Proc. 9th Space Utiliz Symp, pp 159–161
- 26.Tanaka T, Ohira Y, Danda M, Hatta H, Nishi I. Improved fatigue resistance not associated with maximum oxygen consumption in creatine-depleted rats. J Appl Physiol. 1997;82:1911–1917. doi: 10.1152/jappl.1997.82.6.1911. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin KM, Herrick RE, Ilyina-Kakueva E, Oganov VS. Effects of zerogravity on myofibril content and isomyosin distribution in rodent skeletal muscle. FASEB J. 1990;4:79–83. doi: 10.1096/fasebj.4.1.2136840. [DOI] [PubMed] [Google Scholar]
- 28.Bell GJ, Martin TP, Ilyina-Kakueva EI, Oganov VS, Edgerton VR. Altered distribution of mitochondria in rat soleus muscle fibers after spaceflight. J Appl Physiol. 1992;73:493–497. doi: 10.1152/jappl.1992.73.2.493. [DOI] [PubMed] [Google Scholar]
- 29.Bodine-Fowler SC, Roy RR, Rudolph W, Haque N, Kozlobskaya IB, Edgerton VR. Spaceflight and growth effects on muscle fibers in the rhesus monkey. J Appl Physiol. 1992;73(Suppl):82S–89S. doi: 10.1152/jappl.1992.73.2.S82. [DOI] [PubMed] [Google Scholar]
- 30.Camerino GM, Pierno S, Liantoni A, De Bellis M, Cannone M, Sblendorio V, Conte E, Mele A, Tricarico D, Tavella S, Ruggiu A, Cancedda R, Ohira Y, Danieli-Betto D, Ciciliot S, Germinario E, Sandonà D, Betto R, Conte Camerino D, Desaphy JF. Effects of pleiotrophin overexpression on mouse skeletal muscles in normal loading and actual and simulated microgravity. PLoS One. 2013;8(8):e72028. doi: 10.1371/journal.pone.0072028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chui LA, Castleman KR. Morphometric analysis of rat muscle fibers following space flight and hypogravity. Physiologist. 1980;23:S76–S78. [PubMed] [Google Scholar]
- 32.Greenisen MC, Edgerton VR (1993) Human capability in the space environment. In: Nicogossian AE, Huntoon CL, Pool SL (eds) Space physiology and medicine, 3rd edn. Lea & Febiger, Philadelphia, pp 194–210
- 33.Leonard JI, Leach CS, Rambaut PC. Quantitation of tissue loss during prolonged space flight. Am J Clin Nutr. 1983;38:667–679. doi: 10.1093/ajcn/38.5.667. [DOI] [PubMed] [Google Scholar]
- 34.Ohira T. (2012) Effects of 3-month gravitational loading and/or unloading on the characteristics of neck muscle in mice. Undergraduate Thesis, National Institute of Fitness and Sports
- 35.Ohira T, Ohira T, Kawano F, Shibaguchi T, Okabe H, Goto K, Ogita F, Sudoh M, Roy RR, Edgerton VR, Cancedda R, Ohira Y. Effects of gravitational loading levels on protein expression related to metabolic and/or morphologic properties of mouse neck muscles. Physiol Rep. 2014;2(1):e00183. doi: 10.1002/phy2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riley DA, Ellis S. Research on the adaptation of skeletal muscle to hypogravity: past and future directions. Adv Space Res. 1983;3:191–197. doi: 10.1016/0273-1177(83)90056-x. [DOI] [PubMed] [Google Scholar]
- 37.Sandonà D, Desaphy JF, Camerino GM, Bianchini E, Ciciliot S, Danieli-Betto D, Dobrowolny G, Furlan S, Germinario E, Goto K, Gutsmann M, Kawano F, Nakai N, Ohira T, Ohno Y, Picard A, Salanova M, Schiffl G, Blottner D, Musarò A, Ohira Y, Betto R, Conte D, Schiaffino S. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS One. 2012;7(3):e33232. doi: 10.1371/journal.pone.0033232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- 39.Jiang B, Ohira Y, Roy RR, Nguen Q, Ilyina-Kakueva EI, Oganov V, Edgerton VR. Adaptation of fibers in fast-twitch muscles of rats to spaceflight and hindlimb suspension. J Appl Physiol. 1992;73(Suppl):58S–65S. doi: 10.1152/jappl.1992.73.2.S58. [DOI] [PubMed] [Google Scholar]
- 40.Brocca L, Pellegrino MA, Desaphy JF, Pierno S, Camerino DC, Bottinelli R. Is oxidative stress a cause or consequence of disuse muscle atrophy in mice? A proteomic approach in hindlimb unloaded mice. Exp Physiol. 2011;95:331–350. doi: 10.1113/expphysiol.2009.050245. [DOI] [PubMed] [Google Scholar]
- 41.Hunter B, Kandarian SC. Dispuotion of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edgerton VR, Zhou MY, Ohira Y, Klitgaard H, Jiang B, Bell G, Harris B, Saltin B, Gollnick PD, Roy RR, Day MK, Greenisen M. Human fiber size and enzymatic properties after 5 and 11 days of spaceflight. J Appl Physiol. 1995;78:1733–1739. doi: 10.1152/jappl.1995.78.5.1733. [DOI] [PubMed] [Google Scholar]
- 43.Desplanches D, Mayet MH, Sempore B, Flandrois R. Structural and functional responses to prolonged hindlimb suspention in rat muscle. J Appl Physiol. 1987;63:558–563. doi: 10.1152/jappl.1987.63.2.558. [DOI] [PubMed] [Google Scholar]
- 44.Graham SC, Roy RR, Hauschka EO, Edgerton VR. Effects of weight support on medial gastrocnemius fibers of suspended rats. J Appl Physiol. 1989;67:945–953. doi: 10.1152/jappl.1989.67.3.945. [DOI] [PubMed] [Google Scholar]
- 45.Graham SC, Roy RR, West SP, Thomason D, Baldwin KM. Exercise effects on the size and metabolic properties of soleus fibers in hindlimb-suspended rats. Aviat Space Environ Med. 1989;60:226–234. [PubMed] [Google Scholar]
- 46.Hauschka EO, Roy RR, Edgerton VR. Size and metabolic properties of single muscle fibers in rat soleus after hindlimb suspension. J Appl Physiol. 1987;62:2338–2347. doi: 10.1152/jappl.1987.62.6.2338. [DOI] [PubMed] [Google Scholar]
- 47.Roy RR, Bello MA, Boissou P, Edgerton VR. Size and metabolic properties of fibers in fast-twitch muscles after hindlimb suspension. J Appl Physiol. 1987;62:2348–2357. doi: 10.1152/jappl.1987.62.6.2348. [DOI] [PubMed] [Google Scholar]
- 48.Thomason DB, Herrick RE, Surdyka D, Baldwin KM. Time course of soleus myosin expression during hindlimb suspension and recovery. J Appl Physiol. 1987;63:130–137. doi: 10.1152/jappl.1987.63.1.130. [DOI] [PubMed] [Google Scholar]
- 49.Winiarski AM, Roy RR, Alford EK, Ching PC, Edgerton VR. Mechanical properties of rat skeletal muscle after hind limb suspension. Exp Neurol. 1987;96:650–660. doi: 10.1016/0014-4886(87)90226-3. [DOI] [PubMed] [Google Scholar]
- 50.Ohira Y. Effects of denervation and deafferentation on mass and enzyme activity in rat skeletal muscles. Jpn J Physiol. 1989;39:21–31. doi: 10.2170/jjphysiol.39.21. [DOI] [PubMed] [Google Scholar]
- 51.Kawano F, Matsuoka Y, Oke Y, Higo Y, Terada M, Wang XD, Nakai N, Fukuda H, Imajoh-Ohmi S, Ohira Y. Role(s) of nucleoli and phosphorylation of ribosomal protein S6 and/or HSP27 in the regulation of muscle mass. Am J Physiol Cell Physiol. 2007;293:C35–C44. doi: 10.1152/ajpcell.00297.2006. [DOI] [PubMed] [Google Scholar]
- 52.Roy RR, Baldwin KM, Edgerton VR (1991) The plasticity of skeletal muscle: effects of neuromuscular activity. In: Holloszy JO (ed) Exercise and sports sciences reviews, vol 19. Williams and Wilkins, Baltimore, pp 269–312 [PubMed]
- 53.Gollvik L, Kellerth J-O, Ulfhake B. The effects of tenotomy and overload on the postnatal development of muscle fibre histochemistry in the cat triceps surae. Acta Physiol Scand. 1988;132:353–362. doi: 10.1111/j.1748-1716.1988.tb08339.x. [DOI] [PubMed] [Google Scholar]
- 54.Ohira Y, Nakajima N, Takekura H, Inoue N, Saito K (1989) Effects of aging on the atrophy and contractile properties of tenotomized gastrocnemius muscle in rat. In: Proc 6th Space Utiliz Symp, pp 126–130
- 55.Booth FW. Time course of muscular atrophy during immobilization of hindlimb in rats. J Appl Physiol Respir Environ Exerc Physiol. 1977;43:656–661. doi: 10.1152/jappl.1977.43.4.656. [DOI] [PubMed] [Google Scholar]
- 56.Booth FW. Effect of limb immobilization on skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:1113–1118. doi: 10.1152/jappl.1982.52.5.1113. [DOI] [PubMed] [Google Scholar]
- 57.Herbison GJ, Jaweed MM, Ditunno JF. Muscle fiber atrophy after cast immobilization in the rat. Arch Phys Med Rehabil. 1978;59:301–305. [PubMed] [Google Scholar]
- 58.Ohira Y, Yoshinaga T, Nonaka I, Ohara M, Yoshioka T, Yamashita-Goto K, Izumi R, Yasukawa K, Sekiguchi C, Shenkman BS, Kozlovskaya IB. Histochemical responses of human soleus muscle fibers to long-term bedrest with or without countermeasures. Jpn J Physiol. 2000;50:41–47. doi: 10.2170/jjphysiol.50.41. [DOI] [PubMed] [Google Scholar]
- 59.Ohira Y, Yoshinaga T, Ohara M, Nonaka I, Yoshioka T, Yamashita-Goto K, Shenkman BS, Kozlovskaya IB, Roy RR, Edgerton VR. Myonuclear domain and myosin phenotype in human soleus after bed rest with or without loading. J Appl Physiol. 1999;87:1776–1785. doi: 10.1152/jappl.1999.87.5.1776. [DOI] [PubMed] [Google Scholar]
- 60.Yamashita-Goto K, Okuyama R, Honda M, Kawasaki K, Fujita K, Yamada T, Nonaka I, Ohira Y, Yoshioka T. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J Appl Physiol. 2001;91:417–424. doi: 10.1152/jappl.2001.91.1.417. [DOI] [PubMed] [Google Scholar]
- 61.Kawano F, Takeno Y, Nakai N, Higo Y, Terada M, Ohira T, Nonaka I, Ohira Y. Essential role of satellite cells in the growth of rat soleus muscle fibers. Am J Physiol Cell Physiol. 2008;295:C458–C467. doi: 10.1152/ajpcell.00497.2007. [DOI] [PubMed] [Google Scholar]
- 62.Ohira Y, Tanaka T, Yoshinaga T, Kawano F, Nomura T, Nonaka I, Allen DL, Roy RR, Edgerton VR. Ontogenetic, gravity-dependent development of rat soleus muscle. Am J Physiol Cell Physiol. 2001;280:C1008–C1016. doi: 10.1152/ajpcell.2001.280.4.C1008. [DOI] [PubMed] [Google Scholar]
- 63.Kawano F, Nomura T, Ishihara A, Nonaka I, Ohira Y. Afferent input-associated reduction of muscle activity in microgravity environment. Neuroscience. 2002;114:1133–1138. doi: 10.1016/s0306-4522(02)00304-4. [DOI] [PubMed] [Google Scholar]
- 64.Romanul FC, Van der Meulen JP. Slow and fast muscle cross innervation. Enzymatic and physiological changes. Arch Neurol. 1967;17:387–402. doi: 10.1001/archneur.1967.00470280053006. [DOI] [PubMed] [Google Scholar]
- 65.Naito H, Powers SK, Demirel HA, Sugiura T, Dodd SL, Aoki J. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J Appl Physiol. 2000;88:359–363. doi: 10.1152/jappl.2000.88.1.359. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Lormes W, Baur C, Opitz-Gress A, Altenburg D, Lehmann M, Steinacker JM. Human skeletal muscle HSP70 response to physical training depends on exercise intensity. Int J Sports Med. 2000;21:351–355. doi: 10.1055/s-2000-3784. [DOI] [PubMed] [Google Scholar]
- 67.Locke M, Noble EG, Atkinson BG. Exercising mammals synthesize stress proteins. Am J Physiol. 1990;258:C723–C729. doi: 10.1152/ajpcell.1990.258.4.C723. [DOI] [PubMed] [Google Scholar]
- 68.Goto K, Honda M, Kobayashi T, Uehara K, Kojima A, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T. Heat stress facilitates the recovery of atrophied soleus muscle in rat. Jpn J Physiol. 2004;54:285–293. doi: 10.2170/jjphysiol.54.285. [DOI] [PubMed] [Google Scholar]
- 69.Uehara K, Goto K, Kobayashi T, Kojima A, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T, Aoki H. Heat stress enhances proliferative potential in rat soleus muscle. Jpn J Physiol. 2004;54:263–271. doi: 10.2170/jjphysiol.54.263. [DOI] [PubMed] [Google Scholar]
- 70.Kojima A, Goto K, Morioka S, Naito T, Akema T, Fujiya H, Sugiura T, Ohira Y, Beppu M, Aoki H, Yoshioka T. Heat stress facilitates the regeneration of injured skeletal muscle in rats. J Orthop Sci. 2007;12:74–82. doi: 10.1007/s00776-006-1083-0. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi T, Goto K, Kojima A, Akema T, Uehara K, Aoki H, Sugiura T, Ohira Y, Yoshioka T. Possible role of calcineurin in heating-related increase of rat muscle mass. Biochem Biophys Res Commun. 2005;331:1301–1309. doi: 10.1016/j.bbrc.2005.04.096. [DOI] [PubMed] [Google Scholar]
- 72.Goto K, Oda H, Kondo H, Igaki M, Suzuki A, Tsuchiya S, Murase T, Hase T, Fujiya H, Matsumoto I, Naito H, Sugiura T, Ohira Y, Yoshioka T. Responses of muscle mass, strength and gene transcripts to long-term heat stress in healthy human subjects. Eur J Appl Physiol. 2011;111:17–27. doi: 10.1007/s00421-010-1617-1. [DOI] [PubMed] [Google Scholar]
- 73.Goto K, Oda H, Morioka S, Naito T, Akema T, Kato H, Fujiya H, Nakajima Y, Sugiura T, Ohira Y, Yoshioka T. Skeletal muscle hypertrophy induced by low-intensity exercise with heat-stress in healthy human subjects. Jpn J Aerosp Environ Med. 2007;44:13–18. [Google Scholar]
- 74.Goto K, Okuyama R, Sugiyama H, Honda M, Kobayashi T, Uehara K, Akema T, Sugiura T, Yamada S, Ohira Y, Yoshioka T. Effects of heat stress and mechanical stretch on protein expression in cultured skeletal muscle cells. Pflügers Arch. 2003;447:247–253. doi: 10.1007/s00424-003-1177-x. [DOI] [PubMed] [Google Scholar]
- 75.Ohira M, Ohira Y. Effects of exposure to cold on the metabolic characteristics in gastrocnemius muscle of frog (Rana pipiens) J Physiol. 1988;395:589–595. doi: 10.1113/jphysiol.1988.sp016936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ohira Y, Wakatsuki T, Inoue N, Nakamura K, Asakura T, Ikeda K, Tomiyoshi T, Nakajoh M (1992) Non-exercise-related stimulation of mitochondrial protein synthesis in creatine-depleted rats. In: Sato Y, Poortmans J, Hashimoto I, Oshida Y (eds) Integration of medical and sports sciences, vol 37. Karger, Basel, pp 318–323
- 77.Ohira T, Terada M, Kawano F, Nakai N, Ochiai T, Gyotoku J, Ogura A, Ohira Y. Neural and/or mechanical responses of adductor longus muscle to exposure to microgravity in Wistar Hannover rats. Jpn J Aerosp Environ Med. 2009;46:21–28. [Google Scholar]
- 78.Wakatsuki T, Hirata F, Ohno H, Yamamoto M, Sato Y, Ohira Y. Thermogenic responses to high-energy phosphate contents and/or hindlimb suspension in rats. Jpn J Physiol. 1996;46:171–175. doi: 10.2170/jjphysiol.46.171. [DOI] [PubMed] [Google Scholar]
- 79.Stump CS, Overton JM, Tipton CM. Influence of single hindlimb support during stimulated weightlessness in the rat. J Appl Physiol. 1990;68:627–634. doi: 10.1152/jappl.1990.68.2.627. [DOI] [PubMed] [Google Scholar]
- 80.Berg HE, Dudley GA, Hather B, Tesch PA. Work capacity and metabolic and morphological characteristics of the human quadriceps muscle in response to unloading. Clin Physiol. 1993;13:337–347. doi: 10.1111/j.1475-097x.1993.tb00334.x. [DOI] [PubMed] [Google Scholar]
- 81.Flynn DE, Max SR. Effects of suspention hypokinesia/hypodynamia on rat skeletal muscle. Aviat Space Environ Med. 1985;56:1065–1069. [PubMed] [Google Scholar]
- 82.Ohira Y, Yasui W, Roy RR, Edgerton VR. Effects of muscle length on the response to unloading. Acta Anat. 1997;159:90–98. doi: 10.1159/000147971. [DOI] [PubMed] [Google Scholar]
- 83.Martin TP. Protein and collagen content of rat skeletal muscle following space flight. Cell Tissue Res. 1988;254:251–253. doi: 10.1007/BF00220042. [DOI] [PubMed] [Google Scholar]
- 84.Lynch GS, Ryall JG. Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- 85.Fernandes GW, Ueta CB, Fonseca TL, Gouveia CH, Lancellotti CL, Brum PC, Christoffolete MA, Bianco AC, Ribeiro MO. Inactivation of the adrenergic receptor β2 disrupts glucose homeostasis in mice. J Endocrinol. 2014;22:381–390. doi: 10.1530/JOE-13-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, Suzuki A, Bachman ES, Kim YB, Sakurai T, Yanagisawa M, Shioda S, Imoto K, Minokoshi Y. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 87.Williams RS, Caron MG, Daniel K. Skeletal muscle β-adrenergic receptors: variations due to fiber type and training. Am J Physiol. 1984;246:E160–E167. doi: 10.1152/ajpendo.1984.246.2.E160. [DOI] [PubMed] [Google Scholar]
- 88.Thomas DP, Jenkins RR. Effects of β1- vs. β1-β2-blockade on training adaptations in rat skeletal muscle. J Appl Physiol. 1986;60:1722–1726. doi: 10.1152/jappl.1986.60.5.1722. [DOI] [PubMed] [Google Scholar]
- 89.Kraus WE, Bernard TS, Williams RS. Interactions between sustained contractile activity and β-adrenergic receptors in regulation of gene expression in skeletal muscles. Am J Physiol. 1989;256:C506–C514. doi: 10.1152/ajpcell.1989.256.3.C506. [DOI] [PubMed] [Google Scholar]
- 90.Ohira Y, Kawano F, Roy RR, Edgerton VR. Metabolic modulation of muscle fiber properties unrelated to mechanical stimuli. Jpn J Physiol. 2003;53:389–400. doi: 10.2170/jjphysiol.53.389. [DOI] [PubMed] [Google Scholar]
- 91.Shoubridge EA, Challiss RAJ, Hayes DJ, Radda GK. Biochemical adaptation in the skeletal muscle of rats depleted of creatine with the substrate analogue β-guanidinopropionic acid. Biochem J. 1985;232:125–131. doi: 10.1042/bj2320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mainwood GW, Alward M, Eiselt B. Contractile characteristics of creatine-depleted rat diaphragm. Can J Physiol Pharmacol. 1982;60:120–127. doi: 10.1139/y82-020. [DOI] [PubMed] [Google Scholar]
- 93.Moerland TS, Wolf NG, Kushmerick MJ. Administration of creatine analogue increases isomyosin transitions in muscle. Am J Physiol. 1989;257:C810–C816. doi: 10.1152/ajpcell.1989.257.4.C810. [DOI] [PubMed] [Google Scholar]
- 94.Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50(Suppl):197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fell RD, Gladden LB, Steffen JM, Musacchia XJ. Fatigue and contraction of slow and fast muscles in hypokinetic/hypodynamic rats. J Appl Physiol. 1985;58:65–69. doi: 10.1152/jappl.1985.58.1.65. [DOI] [PubMed] [Google Scholar]
- 96.Pierotti DJ, Roy RR, Flores V, Edgerton VR. Influence of 7 days of hindlimb suspension and intermittent weight support on rat muscle mechanical properties. Aviat Space Environ Med. 1990;61:205–210. [PubMed] [Google Scholar]
- 97.Diffee GM, Caiozzo VJ, Herrick RE, Baldwin KM. Contractile and biochemical properties of rat soleus and plantaris after hindlimb suspension. Am J Physiol. 1991;260:C528–C534. doi: 10.1152/ajpcell.1991.260.3.C528. [DOI] [PubMed] [Google Scholar]
- 98.Fitts RH, Metzger JM, Riley DA, Unsworth BR. Models of disuse: a comparison of hindlimb suspension and immobilization. J Appl Physiol. 1986;60:1946–1953. doi: 10.1152/jappl.1986.60.6.1946. [DOI] [PubMed] [Google Scholar]
- 99.Gardetto PR, Schluter JM, Fitts RH. Contractile function of single muscle fibers after hindlimb suspension. J Appl Physiol. 1989;66:2739–2749. doi: 10.1152/jappl.1989.66.6.2739. [DOI] [PubMed] [Google Scholar]
- 100.McDonald KS, Delp MD, Fitts RH. Fatigability and blood flow in the gastrocnemius-plantaris-soleus after hindlimb suspension. J Appl Physiol. 1992;73:1135–1140. doi: 10.1152/jappl.1992.73.3.1135. [DOI] [PubMed] [Google Scholar]
- 101.Reiser PJ, Kasper CE, Moss RL. Myosin subunits and contractile properties of single fibers from hypokinetic rat muscles. J Appl Physiol. 1987;63:2293–2300. doi: 10.1152/jappl.1987.63.6.2293. [DOI] [PubMed] [Google Scholar]
- 102.Thomason DB, Herrick RE, Baldwin KM. Activity influences on soleus muscle myosin during rodent hindlimb suspension. J Appl Physiol. 1987;63:138–144. doi: 10.1152/jappl.1987.63.1.138. [DOI] [PubMed] [Google Scholar]
- 103.Yoshioka T, Shirota T, Tazoe T, Yamashita-Goto K. Calcium movement of sarcoplasmic reticulum from hindlimb suspended muscle. Acta Astronaut. 1996;38:209–212. doi: 10.1016/0094-5765(96)00010-0. [DOI] [PubMed] [Google Scholar]
- 104.Kawano F, Goto K, Wang XD, Terada M, Ohira T, Nakai N, Yoshioka T, Ohira Y. Role(s) of gravitational loading during developing period on the growth of rat soleus muscle fibers. J Appl Physiol. 2010;108:676–685. doi: 10.1152/japplphysiol.00478.2009. [DOI] [PubMed] [Google Scholar]
- 105.Brown MC, Jansen JK, Van Essen D. Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol. 1976;261:387–422. doi: 10.1113/jphysiol.1976.sp011565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walton KD, Lieberman D, Llinas A, Begin M, Llinas RR. Identification of a critical period for motor development in neonatal rats. Neuroscience. 1992;51:763–767. doi: 10.1016/0306-4522(92)90517-6. [DOI] [PubMed] [Google Scholar]
- 107.Zange J, Muller K, Schuber M, Wackerhage H, Hoffmann U, Gunther RW, Adam G, Neuerburg JM, Sinitsyn VE, Bacharev AO, Belichenko OL. Changes in calf muscle performance, energy metabolism and muscle volume caused by long term stay on space station MIR. Int J Sports Med. 1997;18:S308–S309. doi: 10.1055/s-2007-972738. [DOI] [PubMed] [Google Scholar]
- 108.Lambertz D, Perot C, Kaspranski R, Goubel F. Effects of long-term spaceflight on mechanical properties of muscles in humans. J Appl Physiol. 2000;90:179–188. doi: 10.1152/jappl.2001.90.1.179. [DOI] [PubMed] [Google Scholar]
- 109.Fitts RH, Trappe SW, Costill DL, Gallagher PM, Creer AC, Colloton PA, Peters JR, Romatowski JG, Bain JL, Riley DA. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibers. J Physiol. 2010;588:3567–3592. doi: 10.1113/jphysiol.2010.188508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JLW, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915–930. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fitts RH, Riley DA, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol. 2001;204:3201–3208. doi: 10.1242/jeb.204.18.3201. [DOI] [PubMed] [Google Scholar]
- 112.Trappe S, Costill D, Gallagher P, Creer A, Peters JR, Evans H, Riley DA, Fitts RH. Exercise in space: human skeletal muscle after 6 months aboard the International Space Station. J Appl Physiol. 2009;106:1159–1168. doi: 10.1152/japplphysiol.91578.2008. [DOI] [PubMed] [Google Scholar]
- 113.Trappe S, Creer A, Minchev K, Sliva D, Louis E, Luden N, Trappe T. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Intergr Comp Physiol. 2008;294:R939–R947. doi: 10.1152/ajpregu.00761.2007. [DOI] [PubMed] [Google Scholar]
- 114.Trappe S, Creer A, Slivka D, Minchev K, Trappe T. Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol. 2007;103:1242–1250. doi: 10.1152/japplphysiol.00560.2007. [DOI] [PubMed] [Google Scholar]
- 115.Widrick JJ, Romatowski JG, Bain JL, Trappe SW, Trappe TA, Thompson JL, Costill DL, Riley DA, Fitts RH. Effect of 17 days of bed rest on peak isometric force and unloaded shortening velocity of human soleus fibers. Am J Physiol. 1997;273:C1690–C1699. doi: 10.1152/ajpcell.1997.273.5.c1690. [DOI] [PubMed] [Google Scholar]
- 116.Widrick JJ, Norenberg KM, Romatowski JG, Blaser CA, Karhanek M, Sherwood J, Trappe SW, Trappe TA, Costill DL, Fitts RH. Force-velocity-power and force-pCa relationships of human soleus fibers after 17 days of bed rest. J Appl Physiol. 1998;85:1949–1956. doi: 10.1152/jappl.1998.85.5.1949. [DOI] [PubMed] [Google Scholar]
- 117.Tsika RW, Herrick RE, Baldwin KM. Effects of anabolic steroids on skeletal muscle mass during hindlimb suspension. J Appl Physiol. 1987;63:2122–2127. doi: 10.1152/jappl.1987.63.5.2122. [DOI] [PubMed] [Google Scholar]
- 118.Riley DA, Bain JL, Thompson JL, Fitts RH, Widrick JJ, Trappe SW, Trappe TA, Costill DL. Decreased thin filament density and length in human atrophic soleus muscle fibers after spaceflight. J Appl Physiol. 2000;88:567–572. doi: 10.1152/jappl.2000.88.2.567. [DOI] [PubMed] [Google Scholar]
- 119.Widrick JJ, Bangart JJ, Karhanek M, Fitts RH. Soleus fiber force and maximal shortening velocity after non-weight bearing with intermittent activity. J Appl Physiol. 1996;80:981–987. doi: 10.1152/jappl.1996.80.3.981. [DOI] [PubMed] [Google Scholar]
- 120.Prakash YS, Miyata H, Zhan WZ, Sieck GC. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22:307–319. doi: 10.1002/(sici)1097-4598(199903)22:3<307::aid-mus3>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 121.Kandarian SC, Boushel RC, Schulte LH. Elevated interstitial volume in rat soleus muscles by hindlimb unweighing. J Appl Physiol. 1991;71:910–914. doi: 10.1152/jappl.1991.71.3.910. [DOI] [PubMed] [Google Scholar]
- 122.Palmer S, Kentish JC. The role of troponin C in modulating the Ca2+ sensitivity of mammalian skinned cardiac and skeletal muscle fibres. J Physiol. 1994;480:45–60. doi: 10.1113/jphysiol.1994.sp020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Szczesna D, Guzman G, Miller T, Zhao J, Farokhi FK, Ellemberger H, Potter JD. The role of the four Ca2+ binding sites of troponin C in the regulation of skeletal muscle contraction. J Biol Chem. 1996;271:8381–8386. doi: 10.1074/jbc.271.14.8381. [DOI] [PubMed] [Google Scholar]
- 124.Metzger JM. Myosin binding-induced cooperative activation of the thin filament in cardiac myocytes and skeletal muscle fibers. Biophys J. 1995;68:1430–1442. doi: 10.1016/S0006-3495(95)80316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ingalls CP, Warren GL, Armstrong RB. Intracellular Ca2+ transients in mouse soleus muscle after hindlimb unloading and reloading. J Appl Physiol. 1999;87:386–390. doi: 10.1152/jappl.1999.87.1.386. [DOI] [PubMed] [Google Scholar]


