Abstract
Increased oxidative stress resulting from enhanced production of reactive oxygen species and/or inadequate mechanisms of antioxidant defenses has been recognized as an important factor contributing to the initiation and progression of cardiac dysfunction under a wide variety of pathophysiological conditions. The main objective of this study was to examine the effect of electrically induced tachycardia on oxidative stress and the capacity of antioxidant defenses in the normal and hypertrophied left ventricle (LV) in the rat. Left ventricular hypertrophy (LVH) was produced by banding the descending abdominal aorta. The activities of antioxidant enzymes, concentrations of non-enzymatic antioxidants, and biomarkers of oxidative stress were measured in the LV of aortic-banded animals (LVH), untreated or banded rats subjected to short-term (45 min) atrial pacing [(CTR + S) and (LVH + S), respectively], and untreated (CTR) or sham-operated (SHAM) controls. The results indicate that the increase in heart rate in vivo as a result of atrial pacing to a maximum level, independent of sympathetic nerve activity, leads to a substantial increase in oxidative stress and a marked decline in the activities of antioxidant enzymes in both the normal and hypertrophied left ventricle of the rat. The accompanying increase in tissue content of α- and γ-tocopherols seem to contribute to attenuation of the oxidant stress-related loss of thiol stores in the LV. Stable left ventricular hypertrophy induced by aortic banding for six weeks has a minor impact on the capacity of the endogenous antioxidant defense system in the LV, but significantly and negatively affects the ability of the heart LV to tolerate the stress of tachycardia.
Keywords: Left ventricular hypertrophy, Tachycardia, Rat
Introduction
There is a large body of evidence that an increase in oxidative stress because of enhanced formation of reactive oxygen species (ROS) or a relative deficit in endogenous antioxidant defense capacity plays a crucial role in cardiac dysfunction in a wide variety of pathophysiological conditions [1]. Although the ROS are also generated during normal metabolism as an integral part of normal cellular signaling functions [2], their overproduction is harmful to the cells. The most important potential sources of ROS generation in cardiac tissue include the mitochondrial electron-transport chain [3, 4], xanthine oxidase [5], dysfunctional nitric oxide synthases (NOSs), and endothelial and myocardial NADPH oxidase [6]. Intracellular defense mechanisms that attenuate their damaging effects include ROS scavenging enzymes and a wide range of non-enzymatic antioxidants [7, 8].
An increase in oxidative stress and a decline in the capacity of antioxidant defense have been reported in animal models of heart failure subsequent to pressure-overload-induced left ventricular hypertrophy (LVH) [1, 9–11]. Moreover, several observations suggest that the hypertrophied left ventricle is at a higher risk for ischemia-induced damage during enhanced cardiac work [12, 13]. Indeed, it was demonstrated that perfusion abnormalities occurring in the chronically hypertrophied heart during rapid cardiac pacing resulted in a substantial increase in myocardial ischemia [14]. Worthy of note is that rapid cardiac pacing is known to be associated with increased oxygen demand to compensate for greater energy expenditure of the heart [15], which cannot be fully satisfied, despite increased oxygen consumption, and may thus lead to the development of ischemia [8, 13, 16]. Despite the well documented finding that ROS are produced primarily with the reintroduction of oxygen following ischemia, several investigators observed increased ROS generation also during ischemia prior to reperfusion [17–19], mainly because of direct transfer of electrons from the redox-reduced respiratory cytochromes to oxygen [8]. There is strong experimental evidence that the increase in heart rate per se caused by rapid atrial electrical stimulation, independent of sympathetic nerve activation, enhances the cardiac oxidative stress that results from increased generation of superoxide radicals, because of activation of myocardial NADPH oxidase [20], and leads to the activation of mitogen-activated protein kinases (MAPK) potentially involved in tachycardia-induced cardiac injury [13]. On the other hand, treatment with antioxidant vitamins [21] or antioxidant drugs such as probucol [22] resulted in attenuation of cardiac dysfunction in tachycardia-induced heart failure through prevention of the increase in myocardial oxidative stress. Similarly, the antioxidants edaravone and N-2-mercaptopropionyl glycine seemed to be effective in attenuation of pressure overload-induced LVH [23, 24].
This study was carried out to examine the capacity of the antioxidant defense system in the normal and moderately hypertrophied rat left ventricle. We used an experimental model of LVH induced by pressure overload imposed by banding of the infrarenal descending abdominal aorta in adult male rats [25–27]. We have chosen the right atrial pacing model to increase heart rate in vivo as a tool to investigate processes that may occur in response to alteration of the local environment, in the absence of systemic metabolic changes such as sympathetic activation in response to physical workload exposure [13]. By this method, we obtained almost a twofold increase in the heart rate, from about 330–350 beats/min under resting conditions to about 600 beats/min, which was comparable with that (580 ± 9 beats/min) observed in rats subjected to maximum exercise [28]. Measurements of activities of antioxidant enzymes and concentrations of non-enzymatic antioxidants or biomarkers of oxidative stress were obtained both under basal conditions and after short-term rapid cardiac pacing to determine whether hypertrophy compromises the ability of the heart to tolerate the stress of tachycardia.
Methods
Experimental protocol
Adult male Wistar rats weighing 250–300 g obtained from a breeding facility of the Medical University of Białystok were fed standard rat chow (Murigran; Agropol, Motycz, Poland) and water ad libitum and maintained on a 12-h light/12-h dark cycle. Body weights were measured weekly over the six-week experimental period. The animals were randomly assigned to three groups designated control (CTR, N = 24), sham-operated (SHAM, N = 16), and rats with left ventricular hypertrophy (LVH, N = 24). Pressure-overload LVH was induced by constriction of the abdominal aorta under anesthesia (Vetbutal, 80 mg/kg i.p.). Briefly, to produce aortic constriction access to the aorta was gained through a midline abdominal incision. The descending abdominal aorta was isolated, cleared from adhering tissues and constricted, 1.2–1.5 cm below kidney artery, with silk suture tied against a 0.9 mm blunt steel needle. The needle was then removed, leaving the aorta constricted to about 75% of its original diameter. Finally, the incision was closed and the animals were allowed to recover. Sham-operated animals were subjected to the same surgical treatment, except that no band was placed around the aorta. After six weeks, randomly selected animals from both the control (CTR) and experimental (LVH) groups, designated, respectively, CTR + S (N = 12) and LVH + S (N = 12), were additionally subjected to electrically induced tachycardia. Briefly, the animals were anesthetized by i.p. injection of Vetbutal (80 mg/kg) and laid ventrally on a heating pad kept at 38°C. The polyethylene catheter containing bipolar electrodes was then inserted through a cervical midline incision into the right jugular vein and further advanced to the right atrium. The heart was stimulated through electrodes to perform 600 beats/min (10-ms trains of 10 Hz, impulse duration 100 ms, 4 V) for 45 min using a stimulator (model SC-04; COTM, Poland), while the heart rate was monitored using an ECG monitor (Simplicard E10; X-Ray Apparatus and Medical Equipment Works Farum, Poland) connected to needle electrodes implanted into four limbs of the animal tested. Finally, after opening the chest to expose the heart, the descending aorta was cannulated and the jugular veins cut open in order to allow coronary perfusion in situ with normothermic 1 mM EDTA–saline to wash out the residual blood. When the perfusate was free from blood, the heart was immediately excised and weighed, the LV was separated and weighed, then cut into four or five pieces (4–6 mm in size) which were snap-frozen in liquid nitrogen and then kept at −80°C until biochemical analyses. All experimental procedures were approved by the Ethical Committee for Animal Experiments at the Medical University of Białystok.
Analytical procedures
For measurements of activities of antioxidant enzymes and concentrations of non-enzymatic antioxidants, the LV samples were homogenized in ice-cold buffers, according to the instructions provided by the manufacturers of diagnostic test kits, using an Ultra-Turrax T8 homogenizer (IKA Labortechnik, Staufen, Germany). Homogenization was performed in an ice–water bath with six or seven bursts of 15 s each at the maximum speed (setting 6), with 15-s intervals between bursts. The supernatants obtained by centrifugation at 4000g for 15 min were then assayed for activities of superoxide dismutase (SOD; EC 1.15.1.1), glutathione peroxidase (GPX; EC. 1.11.1.9), glutathione reductase (GR; EC.1.6.4.2), and concentration of reduced glutathione (GSH) with Bioxytech (Oxis International, Portland, OR, USA) diagnostic kits (SOD-525, GPx-340, GR-340, GSH-400, respectively) according to the manufacturers’ instructions. The activity of catalase (CAT; EC 1.11.1.6) was assessed according to Aebi [29] and expressed as k (first-order rate of reaction) per g of protein. The thiol (–SH) group content of proteins was quantified according to Ellman [30]. Lipid peroxide formation was determined by the thiobarbituric acid (TBA) reaction, with extraction of the produced chromogen with n-butanol–pyridine (15:1, v/v) mixture as described by Okhawa et al. [31]. In order to minimize peroxidation during the assay procedure, 1% butylated hydroxytoluene (BHT) was added to the TBA reagent immediately prior to incubation with LV tissue homogenate. The level of TBA-reactive substances formed in tissue samples was expressed as μmol malondialdehyde (MDA) per gwet wt. The antioxidants α and γ-tocopherols were extracted from tissue homogenates (1:5 w/v) in 0.1 M phosphate-buffered saline (PBS, pH 7.0) and then assayed by reversed-phase high-performance liquid chromatography (LaChrom HPLC; Merck Hitachi with L-7100 pump and D-7000 interface) with fluorimetric detection (L-7485 fluorescence detector) according to Sobczak et al. [32]. Protein concentration in supernatant samples was determined by use of the 2,2′-bicinchoninic acid method with a Sigma protein assay kit BCA1.
Statistics
The results are expressed as means ± SD. All data were tested for homogeneity of variances by using the Levene test. In order to evaluate the effect of the treatment (six weeks long pressure overload and short-term electrically induced tachycardia) on each variable of the antioxidant defense system in the rat LV, two-way ANOVA was performed followed by Tukey’s multiple comparison test where appropriate. Differences were considered significant at P < 0.05. All statistical analyses were performed using StatSoft Statistica 6.0 software.
Results
Body weights (BW), whole heart (HW) and left ventricle (LV) weights were assessed in control, sham-operated, and aortic-banded animals six weeks after the surgery. The results are presented in Table 1. Because the pressure-overload induced by aortic banding is known to affect the left ventricle only [33], the LV/BW ratio was used as a measure of hypertrophy. A significant (P < 0.05) increase in the LV/BW ratio, compared with sham-operated animals, provided evidence of the development of LVH in the banded group of animals (group LVH). There was no difference in LV/BW ratios between the control and sham-operated rats. The LV/BW ratio in aortic-banded animals additionally subjected to short-term (45 min) rapid atrial pacing was apparently less; the difference compared with sham-operated rats was close to the significance level, however. Importantly, a significant effect of aortic banding on development of LVH was revealed by two-way ANOVA (F = 24.57; P < 0.005).
Table 1.
Body and heart weights in control and experimental groups of animals
| Variable | CTR N = 12 | CTR + S N = 12 | SHAM N = 16 | LVH N = 12 | LVH + S N = 12 |
|---|---|---|---|---|---|
| Body weight (g) | 405.4 (27.1) | 499.2** (41.4) | 411.1 (29.1) | 396.1 (45.0) | 459.4* (29.1) |
| Heart weight (g) | 1.04 (0.09) | 1.18* (0.11) | 1.07 (0.11) | 1.09 (0.10) | 1.12 (0.08) |
| LV weight (g) | 0.48 (0.06) | 0.60* (0.08) | 0.48 (0.05) | 0.57* (0.07) | 0.59* (0.06) |
| LV/BW (mg/g) | 1.19 (0.14) | 1.20 (0.11) | 1.16 (0.11) | 1.44** (0.08) | 1.28$ (0.09) |
Data are means (SD)
LV left ventricle, BW body weight
CTR, controls; SHAM, sham-operated controls; LVH, animals with pressure-overload-induced left ventricular hypertrophy
CTR + S, control animals subjected to electrically induced tachycardia; LVH + S, animals with pressure overload-induced LV hypertrophy subjected to electrically stimulated tachycardia
* P < 0.05 and ** P < 0.001 versus the appropriate controls (CTR or SHAM); $ P = 0.08 versus SHAM
All groups of hypertrophied and control animals were examined for activities of antioxidant enzymes (SOD, GPX, CAT, and GR), concentrations of non-enzymatic antioxidants (reduced glutathione-GSH, protein thiols, α and γ-tocopherols), and MDA content as a biomarker of oxidative stress in the LV tissue samples. The results are shown in Figs. 1 and 2. As expected, there were no differences between the control and sham-operated groups of animals in any of the values investigated. As revealed by two-way ANOVA, rapid cardiac pacing to 600 beats/min for 45 min was a stimulus sufficient to significantly and negatively affect the activities of SOD (F = 45.1, P < 0.001), GPX (F = 108.3, P < 0.0001), and GR (F = 62.7, P < 0.001). The differences in the activities of these enzymes, with regard to appropriate control groups (CTR or SHAM), were significant (Fig. 1). The between-group differences in CAT activities did not reach significance, although the main effect of rapid cardiac pacing seemed to negatively affect the activity of the enzyme (F = 6.5, P < 0.05). The highest decline in activities of antioxidant enzymes in response to tachycardia was observed in the hypertrophied LV tissues. Interestingly, this effect was associated with significant increases in tissue concentrations of α and γ-tocopherols in both normal and hypertrophied LV (Fig. 2). The effect of rapid cardiac pacing on tissue concentrations of α and γ-tocopherols in the LV myocardium seemed to be significant in both cases (F = 37.4, P < 0.001 and F = 56.3, P < 0.0001, respectively). Tissue contents of protein thiols and GSH tended toward slightly lower values, but without reaching significance (Fig. 2). Rapid atrial pacing led to a significant increase in lipid peroxidation, as assessed by MDA content, in normal and, especially, hypertrophied LV tissue, whereas aortic banding seemed to be practically without effect (Fig. 2). Pressure overload-induced LVH did not significantly affect the activities of antioxidant enzymes and amounts of thiol antioxidants, and only a slight tendency toward lower values, compared with sham-operated animals, was recorded in the LV of aortic-banded animals (Fig. 1).
Fig. 1 .
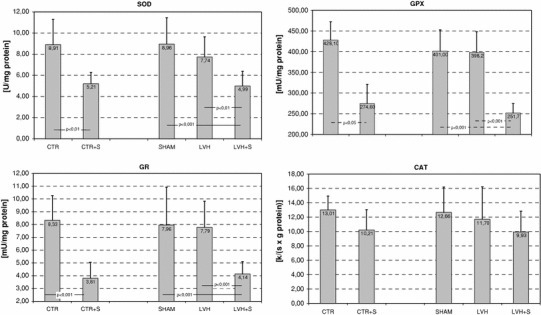
Effects of pressure overload-induced left ventricular hypertrophy (LVH) and/or electrically stimulated tachycardia on activities of antioxidants enzymes in the rat left ventricle. Group abbreviations are the same as in Table 1. SOD, activity of superoxide dismutase (EC.1.15.1.1); GPX, activity of glutathione peroxidase (EC.1.11.1.9); CAT, activity of catalase (EC.1.11.1.6); GR, activity of glutathione reductase (EC.1.6.4.2)
Fig. 2 .
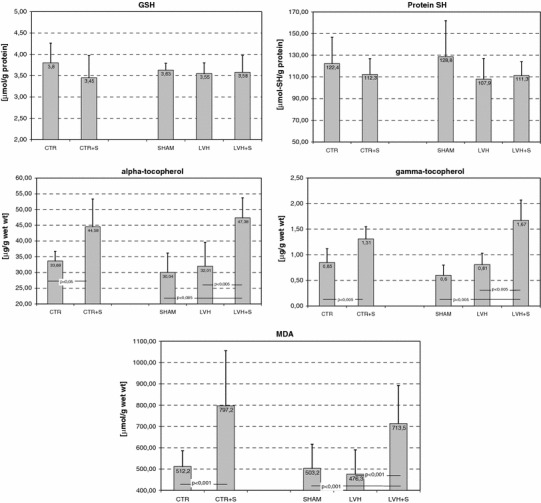
Effects of pressure overload-induced left ventricular hypertrophy (LVH) and/or electrically stimulated tachycardia on non-enzymatic antioxidants and biomarkers of oxidative stress in the rat left ventricle. Group abbreviations are the same as in Table 1. GSH glutathione reduced form, MDA malondialdehyde
Discussion
The most important finding of this study was that short-term (45 min) atrial pacing to increase heart rate to 600 beats/min resulted in significant enhancement of oxidative stress and negatively affected the capacity of the antioxidant defense system in the rat left ventricle. An increase in the oxidative stress was suggested by several observations made in this study, including a highly significant reduction in the activities of antioxidant enzymes (SOD, GPX, GR) and an increase in ROS-induced lipid peroxidation as assessed by the MDA level in the LV of animals subjected to tachycardia. Interestingly, the activities of CAT were only slightly affected by the treatment, which may be related to the small amount of this enzyme in the heart [34] or the lesser sensitivity of this enzyme to tachycardia-induced oxidative stress. Presumably, a significant decline in activity of SOD may result from a proteolytic inactivation of the enzyme [35] following its oxidative modification by hydroxyl radicals (·OH) derived from electron exchange between superoxide (·O−2) and hydrogen peroxide (H2O2). Direct evidence of increased production of ·OH radicals in the failing myocardium was provided by Ide et al. [36]. Of further importance is our finding that a decreased capacity of enzymatic antioxidant defense was, at least partially, compensated by highly significant increases in LV tissue concentrations of α and γ-tocopherols. A similar, although much weaker, tendency toward higher tissue content of both tocopherols was observed in the LV of the aortic-banded animals. A similar phenomenon, i.e. a tendency toward higher α-tocopherol content in the hypertrophied hearts of banded animals not receiving vitamin E was also reported by Dhalla et al. [37]. These findings conform to the observations reported by Elsayed [38], who suggested that the mobilization of antioxidants, for example tocopherols, from their whole body pool to the target organs of the oxidant attack helps them to cope efficiently with enhanced oxidative stress. Worthy of note is that γ-tocopherol is considered superior to α-tocopherol in protection against both general oxidation reactions and nitrosative stress damage [39], particularly in the heart [40]. In this regard, our results support previous reports providing evidence of the enhanced mobilization of vitamin E from its body pool to the organs exposed to the oxidant attack [38, 41, 42]. It may be presumed that the mobilization of tocopherols could also contribute to attenuation of oxidative damage to proteins, as assessed by the relatively low decrease in GSH and protein thiol concentrations observed in our study. Such a presumption is based on a well known concerted action of thiols and vitamin E in the cellular antioxidant network (called “the thiol redox cycle”) where thiols support the continuous recycling of vitamins E and C [43]. There are also reports documenting the protective effect of α-tocopherol, which helps to maintain protein thiols in the reduced state in rat hepatocytes treated with tert-butyl hydroperoxide [44].
Another finding of this study aimed at evaluating changes in the capacity of antioxidant defenses to counteract oxidative stress induced by chronic pressure-overload is that the activities of antioxidant enzymes in the hypertrophied LV tissue samples remained practically unchanged compared with the values recorded in sham-operated controls. Moreover, despite a small tendency toward slightly lower activities of the antioxidant enzymes examined in this study, the effect of pressure overload-induced LVH, as assessed by a two-way ANOVA, was insignificant in each case. In this regard, our finding cannot fully support previous observations made by other authors [34, 37, 45], who found that myocardial adaptation to increased pressure load is accompanied by an increase in the activities of SOD and GPX and greater endogenous antioxidant reserve. However, these authors reported that the transition of compensated heart hypertrophy to the decompensated stage, such as heart failure, was associated with increased oxidative stress, decline in activities of radical scavenging enzymes, increased lipid peroxidation, and a marked deficit in cardiac antioxidant reserve [1, 34, 37, 46, 47]. In contrast with these observations, in this study no increase in either activities of antioxidant enzymes or lipid peroxidation, compared with sham controls, were seen. Worthy of note is that other reports [48, 49] provided evidence of enhanced production of hydroxyl radicals, the existence of a deficit of the antioxidant defense, and reduced tolerance of ischemia/reperfusion in the rat hypertrophied myocardium. The reason for these discrepancies is not clear, but it may be related to different experimental procedures, e.g. the site of constriction of the artery.
In conclusion, this study has provided evidence that the increase in heart rate in vivo as a result of atrial pacing to a level corresponding to the maximum exercise heart rate, independent of sympathetic nerve activity, leads to a substantial increase in oxidative stress and a marked decline in activities of antioxidant enzymes in both the normal and hypertrophied left ventricle of the rat. The accompanying increase in the tissue content of α and γ-tocopherols seems to contribute to the attenuation of oxidant stress-related loss of thiol stores in the LV. Stable left ventricular hypertrophy induced by banding of the abdominal aorta for six weeks has a minor impact on the capacity of the endogenous antioxidant defense system in the LV, but significantly and negatively affects the ability of the heart LV to tolerate the stress of tachycardia.
Acknowledgment
This work was supported by grant 6P05A-086-21 from the State Committee for Scientific Research (Warsaw, Poland).
Footnotes
An erratum to this article can be found online at 10.1007/s12576-012-0242-7.
References
- 1.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/S0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 3.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ide T, Tsutsui H, Kinugawa S, Utsumi H, Kang D, Hattori N, Uchida K, Arimura K, Egashira K, Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.RES.85.4.357. [DOI] [PubMed] [Google Scholar]
- 5.Berry CE, Hare JM. Xanthine oxidoreductase and cardiovascular disease: molecular mechanisms and pathophysiological implications. J Physiol. 2004;555:589–606. doi: 10.1113/jphysiol.2003.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.HYP.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 7.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 8.Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer DB, Siwik DA, Xiao L, Pimentel DR, Singh K, Colucci WS. Role of oxidative stress in myocardial hypertrophy and failure. J Mol Coll Cardiol. 2002;34:379–388. doi: 10.1006/jmcc.2002.1526. [DOI] [PubMed] [Google Scholar]
- 10.Singal PK, Khaper N, Palace V, Kumar D. The role of oxidative stress in the genesis of heart disease. Cardiovasc Res. 1998;40:426–432. doi: 10.1016/S0008-6363(98)00244-2. [DOI] [PubMed] [Google Scholar]
- 11.Gupta S, Das B, Sen S. Cardiac hypertrophy: mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2007;9:623–652. doi: 10.1089/ars.2007.1474. [DOI] [PubMed] [Google Scholar]
- 12.Gillman MW, Kannel WB, Belanger A, D’Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham Study. Am Heart J. 1993;125:1148–1154. doi: 10.1016/0002-8703(93)90128-V. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto E, Lai ZF, Yamashita T, Tanaka T, Kataoka K, Tokutomi Y, Ito T, Ogawa H, Kim-Mitsuyama S. Enhancement of cardiac oxidative stress by tachycardia and its critical role in cardiac hypertrophy and fibrosis. J Hypertens. 2006;24:2057–2069. doi: 10.1097/01.hjh.0000244956.47114.c1. [DOI] [PubMed] [Google Scholar]
- 14.Bache RJ, Arentzen CE, Simon AB, Vrobel TR. Abnormalities in myocardial perfusion during tachycardia in dogs with left ventricular hypertrophy: metabolic evidence for myocardial ischemia. Circulation. 1984;69:409–417. doi: 10.1161/01.CIR.69.2.409. [DOI] [PubMed] [Google Scholar]
- 15.Badeer HS, Feisal KA. Effect of atrial and ventricular tachycardia on cardiac oxygen consumption. Circ Res. 1965;17:330–335. doi: 10.1161/01.RES.17.4.330. [DOI] [PubMed] [Google Scholar]
- 16.Furman E, Sonn J, Acad BA, Dvir S, Kedem J. Relation between myocardial substrate utilization, oxygen consumption and regional oxygen balance in the dog heart in vivo. Arch Int Physiol Biochim. 1986;94:285–293. doi: 10.3109/13813458609071428. [DOI] [PubMed] [Google Scholar]
- 17.Vanden Hoek TL, Li C, Shao Z, Schumacker PT, Becker LB. Significant levels of oxidants are generated by isolated cardiomyocytes during ischemia prior to reperfusion. J Mol Cell Cardiol. 1997;29:2571–2583. doi: 10.1006/jmcc.1997.0497. [DOI] [PubMed] [Google Scholar]
- 18.Kevin LG, Camara AKS, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radical in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003;284:H566–H574. doi: 10.1152/ajpheart.00711.2002. [DOI] [PubMed] [Google Scholar]
- 19.Vegh A, Szekeres L, Parratt JR. Transient ischaemia induced by rapid cardiac pacing results in myocardial preconditioning. Cardiovasc Res. 1991;25:1051–1053. doi: 10.1093/cvr/25.12.1051. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez G, Pedrozo Z, Domenech RJ, Hidalgo C, Donoso P. Tachycardia increases NADPH oxidase activity and RyR2 S-glutathionylation in ventricular muscle. J Mol Cell Cardiol. 2005;29:982–991. doi: 10.1016/j.yjmcc.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Shite J, Qin F, Mao W, Kawai H, Stevens SY, Liang C. Antioxidant vitamins attenuate oxidative stress and cardiac dysfunction in tachycardia-induced cardiomyopathy. J Am Coll Cardiol. 2001;38:1734–1740. doi: 10.1016/S0735-1097(01)01596-0. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura R, Egashira K, Machida Y, Hayashidani S, Takeya M, Utsumi H, Tsutsui H, Takeshita A. Probucol attenuates left ventricular dysfunction and remodeling in tachycardia-induced heart failure. Roles of oxidative stress and inflammation. Circulation. 2002;106:362–367. doi: 10.1161/01.CIR.0000021430.04195.51. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto I, Hikoso S, Yamaguchi O, Kashiwase K, Nakai A, Takeda T, Watanabe T, Taniike M, Matsumura Y, Nishida K, Hori M, Kogo M, Otsu K. The antioxidant edaravone attenuates pressure overload-induced left ventricular hypertrophy. Hypertension. 2005;45:921–926. doi: 10.1161/01.HYP.0000163461.71943.e9. [DOI] [PubMed] [Google Scholar]
- 24.Higuchi Y, Otsu K, Nishida K, Yamaguchi O, Higuchi Y, Hirotani S, Matsumura Y, Hori M, Tada M, Otsu K. The antioxidant N-2-mercaptopropionyl glycine attenuates left ventricular hypertrophy in in vivo murine pressure-overload model. J Am Coll Cardiol. 2002;39:907–912. doi: 10.1016/S0735-1097(01)01826-5. [DOI] [PubMed] [Google Scholar]
- 25.Czarnowski D, Wójcik B, Langfort J, Górski J. 5′-Nucleotidase and adenosine deaminase activities in hypertrophied rat heart, the effect of tachycardia. Rocz Akad Med Bialymst. 1996;41:334–340. [PubMed] [Google Scholar]
- 26.Dogrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res. 1998;39:90–105. doi: 10.1016/S0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 27.Goldblatt H, Kahn JR, Hanzal RF. Studies on experimental hypertension. IX. The effect on blood pressure of constriction of the abdominal aorta above and below the site of origin of both main renal arteries. J Exp Med. 1939;69:649–674. doi: 10.1084/jem.69.5.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corre KA, Cho H, Barnard RJ. Maximum exercise heart rate reduction with maturation in the rat. J Appl Physiol. 1976;40:741–744. doi: 10.1152/jappl.1976.40.5.741. [DOI] [PubMed] [Google Scholar]
- 29.Aebi H. Catalase in vitro, Microsomal lipid peroxidation. Meth Enzymol. 1984;105:121–125. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 30.Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 31.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 32.Sobczak A, Skop B, Kula B. Simultaneous determination of serum retinol and alpha and gamma-tocopherol levels in type II diabetic patients using HPLC with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1999;730:265–271. doi: 10.1016/S0378-4347(99)00141-3. [DOI] [PubMed] [Google Scholar]
- 33.Cooper G. Basic determinants of myocardial hypertrophy. A review of molecular mechanisms. Annu Rev Med. 1997;48:13–23. doi: 10.1146/annurev.med.48.1.13. [DOI] [PubMed] [Google Scholar]
- 34.Gupta M, Singal PK. Higher antioxidative capacity during a chronic stable heart hypertrophy. Circ Res. 1989;64:398–406. doi: 10.1161/01.RES.64.2.398. [DOI] [PubMed] [Google Scholar]
- 35.Salo DC, Pacifici RE, Lin SW, Giulivi C, Davies KJ. Superoxide dismutase undergoes proteolysis and fragmentation following oxidative modification and inactivation. J Biol Chem. 1990;265:11919–11927. [PubMed] [Google Scholar]
- 36.Ide T, Tsutsui H, Kinugawa S, Suematsu N, Hayashidani S, Ichikawa K, Utsumi H, Machida Y, Egashira K, Takeshita A. Direct evidence for increased hydroxyl radicals originating from superoxide in the failing myocardium. Circ Res. 2000;86:152–157. doi: 10.1161/01.RES.86.2.152. [DOI] [PubMed] [Google Scholar]
- 37.Dhalla AK, Hill MF. Singal PK Role of oxidative stress in transition of hypertrophy to heart failure. J Am Coll Cardiol. 1996;28:506–514. doi: 10.1016/0735-1097(96)00140-4. [DOI] [PubMed] [Google Scholar]
- 38.Elsayed NM. Antioxidant mobilization in response to oxidative stress: A dynamic environmental-nutritional interaction. Nutrition. 2001;17:828–834. doi: 10.1016/S0899-9007(01)00646-3. [DOI] [PubMed] [Google Scholar]
- 39.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custer LJ, Mordan LJ. γ-Tocopherol detoxification of nitrogen dioxide: Superiority to α-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohrvall M, Sundlof G, Vessby B. Gamma, but not alpha, tocopherol levels in serum are reduced in coronary heart disease patients. J Intern Med. 1996;239:111–117. doi: 10.1046/j.1365-2796.1996.410753000.x. [DOI] [PubMed] [Google Scholar]
- 41.Antosiewicz J, Matuszkiewicz A, Olek RA, Kaczor JJ, Ziółkowski W, Wakabayashi T, Popinigis J. Content and redistribution of vitamin E in tissues of Wistar rats under oxidative stress induced by hydrazine. Arch Environ Contam Toxicol. 2002;42:363–368. doi: 10.1007/s00244-001-0033-2. [DOI] [PubMed] [Google Scholar]
- 42.Kłapcińska B, Jagsz S, Sadowska-Krępa E, Górski J, Kempa K, Langfort J. Effects of castration and testosterone replacement on the antioxidant defense system in the rat left ventricle. J Physiol Sci. 2008;58:173–177. doi: 10.2170/physiolsci.RP002208. [DOI] [PubMed] [Google Scholar]
- 43.Sen CK, Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72:653S–669S. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- 44.Wang ST, Kuo JH, Chou RG, Lii CK. Vitamin E protection of cell morphology and protein thiols in rat hepatocytes treated with tert-butyl hydroperoxide. Toxicol Lett. 1996;89:91–98. doi: 10.1016/S0378-4274(96)03793-9. [DOI] [PubMed] [Google Scholar]
- 45.Dhalla AK, Singal PK. Antioxidant changes in hypertrophied and failing guinea pig hearts. Am J Physiol. 1994;266:H1280–H1285. doi: 10.1152/ajpheart.1994.266.4.H1280. [DOI] [PubMed] [Google Scholar]
- 46.Singh N, Dhalla AK, Seneviratne C, Singal PK. Oxidative stress and heart failure. Mol Cell Biochem. 1995;147:77–81. doi: 10.1007/BF00944786. [DOI] [PubMed] [Google Scholar]
- 47.Kirshenbaum LA, Singal PK. Increase in endogenous antioxidant enzymes protects hearts against reperfusion injury. Am J Physiol. 1993;265:H484–H493. doi: 10.1152/ajpheart.1993.265.2.H484. [DOI] [PubMed] [Google Scholar]
- 48.Kalenikova EI, Gorodetskaya EA, Murashev AN, Ruuge EK. Medvedev OS Role of reactive oxygen species in the sensitivity of rat hypertrophied myocardium to ischemia. Biochemistry (Mosc) 2004;69:311–316. doi: 10.1023/B:BIRY.0000022063.32185.7c. [DOI] [PubMed] [Google Scholar]
- 49.Luo JD, Zhang WW, Zhang GP, Zhong BH, Ou HJ. Effects of simvastatin on activities of endogenous antioxidant enzymes and angiotensin-converting enzyme in rat myocardium with pressure-overload cardiac hypertrophy. Acta Pharmacol Sin. 2002;23:124–128. [PubMed] [Google Scholar]


