Abstract
The neuronal K+–Cl− cotransporter KCC2 maintains a low intracellular Cl− concentration and facilitates hyperpolarizing GABAA receptor responses. KCC2 also plays a separate role in stabilizing and enhancing dendritic spines in the developing nervous system. Using a conditional transgenic mouse strategy, we examined whether overexpression of KCC2 enhances dendritic spines in the adult nervous system and characterized the effects on spine dynamics in the motor cortex in vivo during rotarod training. Mice overexpressing KCC2 showed significantly increased spine density in the apical dendrites of layer V pyramidal neurons, measured in vivo using two-photon imaging. During modest accelerated rotarod training, mice overexpressing KCC2 displayed enhanced spine formation rates, greater balancing skill at higher rotarod speeds and a faster rate of learning in this ability. Our results demonstrate that KCC2 enhances spine density and dynamics in the adult nervous system and suggest that KCC2 may play a role in experience-dependent synaptic plasticity.
Keywords: Two-photon microscopy, In vivo, Motor learning, KCC2, Synaptic plasticity
Introduction
The neuron-selective isoform of the K+–Cl− cotransporter KCC2 plays an important role in regulating the intracellular chloride concentration. In most adult neurons, KCC2 simultaneously exports K+ and Cl− and thereby maintains a low intracellular [Cl−] that facilitates subsequent gamma-amino butyric acid receptor (GABAARs)-mediated Cl− influx, hyperpolarization and neuronal inhibition [1–3]. Indeed, GABAergic inhibition can be diminished when KCC2 is absent or has reduced function, such as occurs during neuronal development and/or after injury. Given this role in influencing GABA function, there has been extensive investigation into the contributions that KCC2 may make to neuronal circuit development, the pathogenesis of different diseases and the mechanisms that regulate KCC2 expression and transport function (reviewed in Kaila et al. [3], Watanabe et al. [4] and Kahle et al. [5]).
However, KCC2 has also been reported to have additional morphogenic effects on neuronal circuits independent of its Cl− transport function. In neuronal cultures from KCC2 knockout mice, dendritic spines exhibited a more immature filopodia-like phenotype, and the number of immunohistochemically and functionally identified excitatory synapses was markedly decreased [6]. Remarkably, this immature dendritic spine phenotype was rescued by overexpression of Cl− transport-deficient KCC2 mutant constructs [6]. Furthermore, in utero overexpression of KCC2, or a Cl− transport-defective mutant KCC2, resulted in an increase in the density of mature dendritic spines in the somatosensory cortex (layer II/III) that was sustained at least until 90 days after birth [7]. Pertinently, KCC2 appears to form a complex with the actin-associated proteins 4.1 N and βPIX and can interact with the kinase and cofilin signaling pathways associated with dendritic spine development and stabilization [6, 8, 9]. Thus, it would appear that KCC2 supports the development of excitatory synapses by enhancing spinogenesis and/or maturation. Such a role is consistent with the upregulation of KCC2 expression in development coinciding with the start of spinogenesis and may (at least partly) explain the presence of KCC2 on dendritic spines at glutamatergic synapses [4, 10–12].
Maintenance of dendritic spines in the adult nervous system is also highly dynamic, underpinned by numerous molecular mechanisms, and facilitates the functional and structural plasticity required for memory and behavioral adaptations in response to training [13–16]. Specifically, in vivo experiments have demonstrated a strong correlation between the rate of newly formed dendritic spines in the motor cortex and the extent and rate at which mice can improve specific motor behaviors during learning [17, 18]. In addition, long-term potentiation (LTP) of synaptic transmission is associated with the appearance of new dendritic spines and an increase in the volume of dendritic spine heads, while long-term depression (LTD) is correlated with dendritic spine shrinkage and removal (reviewed in Kasai et al. [16] and Bosch and Hayashi [19]). Together this suggests an activity-dependent modulation of dendritic spine appearance, growth and stability during learning. Recent evidence suggests that KCC2 can directly contribute to aspects of dendritic spine plasticity. Suppression of KCC2 expression in juvenile rats and in cultured neurons prevented the induction of LTP and the associated increases in dendritic spine volume and AMPA receptor insertion [9], pointing to a possible link between KCC2 and learning-induced synaptic plasticity in adult neuronal circuits.
Therefore, to address whether altered KCC2 modulates dendritic spine and synaptic plasticity in adult animals, we have utilized a transgenic mouse wherein KCC2 can be conditionally overexpressed by altering dietary doxycycline. We overexpressed KCC2 and quantified the effects of this on dendritic spine density. Furthermore, we examined how overexpressed KCC2 affects dendritic spine plasticity during motor learning and the functional implications of this. We hypothesized that increasing KCC2 levels in the adult brain would increase the density of dendritic spines, as seen in developing brains. We also proposed that increasing KCC2 would enhance the dendritic spine plasticity associated with motor learning. Using in vivo two-photon imaging of dendrites and spines in apical dendrites of layer V motor cortex pyramidal neurons and Golgi-Cox staining of hippocampal and layer II/III somatosensory cortex pyramidal neurons in fixed tissues, we show that overexpression of KCC2 does indeed increase dendritic spine density. Using time-lapse imaging, we then demonstrate that KCC2 overexpression increases dendritic spine formation in the motor cortex during rotarod motor learning and increases the extent and rate at which rotarod performance is enhanced during training.
Materials and methods
All animal experiments were carried out according to the guidelines defined by the National Institutes of Natural Sciences (NIPS) and approved by the Okazaki Institutional Animal Care and Use Committee or by the UNSW Sydney Animal Care and Ethics Committee.
Animals
We used a conditional transgenic mouse in which overexpression of KCC2 can be induced by cessation of dietary supplementation with doxycycline [20]. KCC2 expression was driven by the tetracycline transactivator (tTA) binding to the tetracycline operator construct (tet-O), and this interaction is prevented by doxycycline [21]. The tet-O construct was introduced upstream of the KCC2 gene, and KCC2 overexpression was restricted to excitatory neurons of the forebrain by using the CaMKIIα promoter to drive tTA expression [22]. Mice were housed in standard cages with a reversed light/dark cycle (light on 6:00 a.m., light off 6:00 p.m.) and fed with doxycycline (Dox, 100 mg/kg) laced chow. Adult (8–12 weeks old) male mice were used for experiments, and feed was exchanged to Dox-free standard chow prior to experiments as indicated (typically 2–3 weeks).
Accelerating rotarod experiments
One week prior to behavioral experiments, mice were identified, weighed and health-checked, before being acclimatized to the behavioral rooms for at least 30 min prior to testing. The accelerating rotarod test used a rotarod with five lanes, as previously described in Kakegawa et al. [23] and Rothwell et al. [24]. Mice were placed into the rotarod, and the rotation speed was increased from 4 to 40 rotations per minute (r.p.m) over 5 min. The time at which a mouse fell from the rotarod was recorded. Six trials were performed over a period of 90 min, and this was repeated daily over 5 successive days.
Surgery and virus injection for in vivo imaging
To facilitate in vivo imaging, we made cranial windows over the M1 motor cortex using the open skull approach as described in Kim et al. [25] and Wake et al. [26]. Surgery was performed over 2 days, 2–3 weeks before imaging and rotarod experiments. On the 1st surgery day, a metal head-plate, used to secure the mouse head under the microscope objective lens, was mounted with dental cement onto exposed skull under anesthesia (ketamine:xyalizine mix, 0.13: 0.01 mg/g, i.p.). On the 2nd surgery day, the skull over the M1 motor cortex was removed under anesthesia (isoflurane, 1.5% vol/vol in air), leaving a 2.0–3.0-mm-diameter window centered at 1.5 mm lateral to the bregma and corresponding to the mouse’s forepaw. Subsequently, adeno-associated virus (AAV) containing an enhanced green fluorescent protein (eGFP) coupled to a CaMKIIα promoter (AAV2-CaMK2-eGFP; University of Pennsylvania vector core) was injected into layer V of the motor cortex (250 nl over 5 min). After injection, the cortical surface was washed with artificial cerebrospinal fluid for > 20 min before the open window was sealed using double-cover glass and 2–5% agarose. The cover glass was then secured to the adjacent skull using dental cement and cyanoacrylate glue.
Two-photon imaging of dendrites and spines in vivo
Mice were anesthetized with isoflurane (~ 1.5% v/vol in air) and secured via their head-plate under the microscope objective lens. The motor cortex was initially imaged at low resolution at a depth of 500–600 μm to identify eGFP-expressing layer V pyramidal neurons. The apical dendrites of these neurons were traced toward the brain surface and higher resolution imaging of dendrites and spines performed within 100 μm from the cortical surface. On average, 2–5 apical dendrites were imaged in each mouse.
Dendritic spine analysis in vivo
Dendrite and spine structures were analyzed as described in Kim et al. [25] and Kim and Nabekura [27] using ImageJ (http://rsbweb.nih.gov/ij/). Briefly, we manually identified any dendritic protrusion and classified all these protrusions as spines regardless of length or shape. Thin filopodia without clear spine heads were excluded. Spine formation and elimination rates were determined as the percentages of spines in a single dendrite that appeared or disappeared, respectively, between two successive imaging sessions and were expressed relative to the total spine number in the former session. Spine turnover was defined as the average number of formed and eliminated spines.
Golgi-Cox staining and in vitro spine analysis
Golgi-Cox staining was performed using a method modified from Levine et al. [28]. Mice were deeply anesthetized (sodium pentobarbital, 33 mg/kg, i.p.) and cardiac perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were then dissected out and sectioned at 100 µm thickness in PBS using a vibratome. These sections were then sandwiched between two microscope slides and immersed in Golgi-Cox fixative (1:1:1% w/vol HgCl2:K2CrO4:K2Cr2O7) for 14 days and 1% K2Cr2O7 for a subsequent 24 h in a light-free environment. Following this, the sections were removed from the microscope slide sandwich and successively exposed to 28% vol/vol NH3 for 20 min and 15% Kodak Polymax T Fixer for 8 min. After air drying for 1 week, the sections were dehydrated through an increasing alcohol series, incubated for 5 min in limonene and mounted on glass microscope slides with EntellanNew (Merck). Spine density was manually counted for 30–100 μm sections of the first or second dendritic branch off the apical dendrites.
Immunostaining
Mice were deeply anesthetized (ketamine:xyalizine mix, 0.13: 0.01 mg/g, i.p.) and cardiac perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde. Brains were dissected out and stored in 4% paraformaldehyde at 4 °C overnight, which was replaced with 30% w/vol sucrose the next day. Brains were then snap frozen with liquid nitrogen and sections cut at 35 μm thickness using a microtome and stored at 4 °C in PBS. Immunostaining was conducted using primary antibodies anti-NeuN (Mouse, Monoclonal, Millipore, 1:500 dilution) and anti-VP16, amino acids 413–490 (Rabbit, Polyclonal, Abcam, 1:100 dilution) and secondary antibodies Alexa Fluor 564 (Anti-Mouse, Life Technologies, 1:2000 dilution) and Alexa Fluor 488 (Anti-Rabbit, Life Technologies, 1:2000 dilution).
Western blotting
Mice were deeply anesthetized with ketamine (0.13 mg/g, i.p.) and xylazine (0.01 mg/g, i.p.) and cardiac perfused with PBS. After most of the blood was cleared, brains were dissected out and sectioned at 300 μm thickness in carbogenated artificial cerebrospinal fluid (aCSF) using a vibratome. Motor cortex was isolated from these sections and homogenized in cold lysis buffer (0.5 M Tris-HCl, 150 mM NaCl, 0.1% Triton x-100, 10% sodium dexycholate, pH 6.8) with protease inhibitor (11697498001, Roche). Cell lysates were centrifuged (for 12,000 rpm for 10 min at 4 °C), with the supernatants collected as samples and protein concentration determined using the Pierce BCA assay kit (Pierce, Rockford, IL). The samples were then diluted in SDS sample buffer before being heated to 100 °C for 5 min. Sample proteins were then separated by gel electrophoresis (7% SDS-PAGE gel, 0.04 A, 100 min) and transferred to a nitrocellulose membrane. This was then incubated with anti-KCC2 primary antibody (rabbit, 1:1000, 07-432, Millipore) and anti-βactin (mouse, 1:1000, 030M4788, Sigma–Aldrich) and secondary antibody (anti-rabbit immunoglobulin; 1:5000, HAF008, R&D systems, and anti-mouse immunoglobulins; 1:1000, HAF007, R&D systems). Protein bands were analyzed for protein concentration using ImageJ.
Data analysis and statistics
Data are presented as mean ± SEM. Comparisons between control and KCC2 upregulated mice used an unpaired t test or a one- or two-way ANOVA, followed by a post hoc Tukey’s or Bonferroni’s test as indicated. Significant differences were defined by p < 0.05.
Results
Overexpression of KCC2 in cortex
To overexpress KCC2, we utilized a conditional transgenic mouse that incorporates the tetracycline operator construct (tet-O) upstream of the KCC2 gene. Overexpression of KCC2 was driven by the expression of the tetracycline trans-activator (tTA) protein and restricted to excitatory neurons of the forebrain (cortex, hippocampus, amygdala) by coupling to the CaMKIIα promoter [22, 29]. In mice that expressed both tet-O and tTA, overexpression of KCC2 was prevented by dietary supplementation of doxycycline (Dox) and triggered by cessation of Dox supplementation. Details of this transgenic mouse have been recently reported as a preprint in Goulton et al. [20], which demonstrates that Dox cessation increases KCC2 mRNA and protein expression throughout the cortex, amygdala and hippocampus. Overexpression of KCC2 does not induce obvious changes in any fundamental physiologic parameters. For example, in 7–11-week-old male mice without KCC2 overexpression, body weight was 25.1 ± 1.4 g (n = 13), while in age-matched mice with KCC2 overexpression, body weight was 27.0 ± 1.0 g (n = 11), with both values similar to the average weight of 11-week-old male C57Bl6 mice supplied by the Jackson Laboratory (26.7 ± 1.7 g). Furthermore, increasing KCC2 by withdrawing Dox for 1 week has no significant effect on basal locomotor activity or anxiety as measured in open field and elevated plus maze tests, respectively (see Goulton et al. [20]). In the current study, we first confirmed the overexpression of KCC2 protein in the motor cortex 7 days after cessation of Dox supplementation, using Western blot experiments (Fig. 1a). The expression of KCC2 (relative to β-actin intensity) was significantly increased compared with control mice, from 40.4 ± 9.3% (n = 3 mice) to 87.8 ± 9.8% (n = 3), representing an approximate doubling of protein expression (unpaired t test, p = 0.025; Fig. 1b). The control or wild-type (WT) mice used in this study were from the same colony and with the same diet, which lacked both of the two gene constructs required to induce KCC2 overexpression (tet-O, tTA). To confirm that KCC2 overexpression was reliable across the majority of excitatory pyramidal neurons in the motor cortex, we immunostained neurons using a label against the virion protein 16 (VP16), a component of the tTA construct [21], with neurons identified by NeuN expression. As shown in Fig. 1c, the majority of excitatory pyramidal neurons in layer V of the M1 motor cortex co-expressed VP16. (e.g., Fig. 1c). In four KCC2-overexpressing mice, 62 ± 3.4% of layer II/III and 69 ± 3.3% of layer V neurons that expressed NeuN also expressed VP16. Given that about 15% of these NeuN-positive cells should be inhibitory neurons [30], we estimate that withdrawal of doxycycline would overexpress KCC2 in about 75% of all excitatory neurons. Such mosaicism for tTA/tet-O-driven constructs in the absence of Dox has been previously characterized, with between 34 and 72% of CA1 pyramidal neurons expressing tet-O-driven reporter proteins in different transgenic lines [31].
Fig. 1.
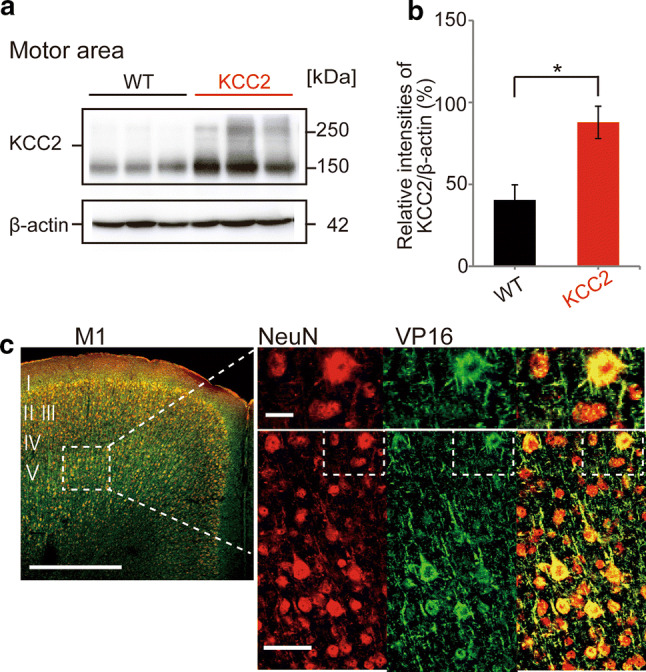
Conditional overexpression of KCC2 in motor cortex in transgenic mice. a Representative Western blots for KCC2 immunoreactivity in tissue from the motor cortex obtained from three WT (left) and KCC2-overexpressing (right) mice. Samples were obtained from mice 7 days after withdrawal of doxycycline (Dox) from the diet to induce overexpression in KCC2 mice. b Quantification of the intensity of KCC2 immunoreactivity, expressed relative to β-actin. The relative expression of KCC2 was significantly greater in KCC2-overexpressing mice compared with WT mice, with the absolute KCC2/β-actin ratios being 40.4 ± 9.3% for WT (n = 3) and 87.8 ± 9.8% for KCC2 mice (n = 3; unpaired t test,*p < 0.05). This corresponded to a 117% increase in KCC2 protein expression levels. c Representative immunohistochemical images of a brain slice from a KCC2-overexpressing transgenic mouse showing the primary motor cortex (left) stained positive for NeuN (red, neuronal marker) and VP16 (green, marker for the tetO-KCC2 construct). Most larger layer V pyramidal neurons were double-labeled (yellow). Bar; 500 μm (left), 10 μm (upper right) and 50 μm (bottom right)
Overexpression of KCC2 increases spine density and the formation of new spines during motor learning
We initially conducted preliminary experiments in Golgi-Cox-stained brain slices from adult (≈ 6 months) mice to examine whether overexpression of KCC2 affected spine density. For these experiments, mice (and mothers) were raised in the absence of Dox, and thus KCC2 overexpression would have occurred from 1 to 2 weeks after birth, paralleling CaMKIIα expression [32]. Dendritic spines were manually counted from the primary branch of apical dendrites. As shown in Fig. 2a, b, the density of dendritic spines in hippocampal neurons was significantly increased (unpaired t test, p = 0.003) by ≈ 50%. Dendritic spine counts in the somatosensory cortex were more variable, and no significant differences were detected (WT: 0.70 ± 0.11 spines/µm, 9 dendrites, 4 mice; KCC2: 0. 82 ± 0.08 spines/µm, 14 dendrites, 5 mice; not shown).
Fig. 2.
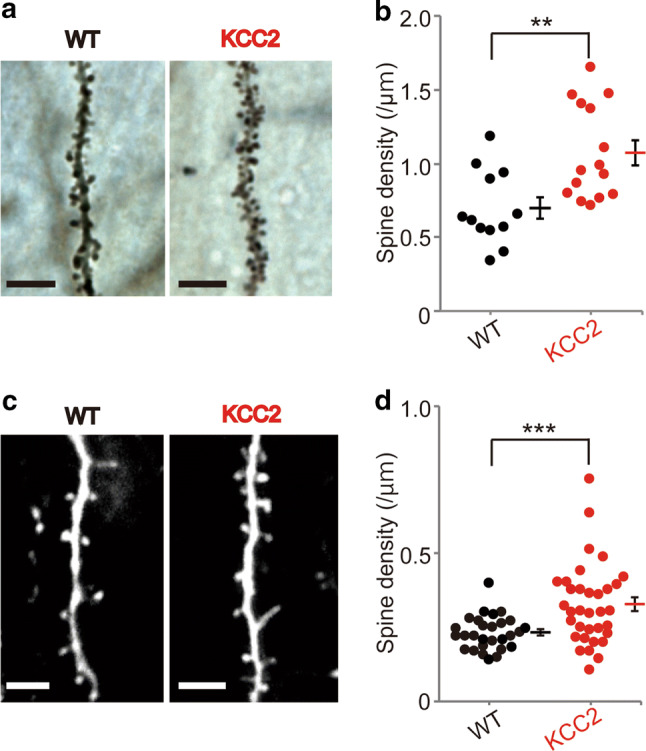
Overexpression of KCC2 increases spine density. a Representative CA1 pyramidal neuron dendrites in Golgi-stained hippocampal slices from WT and KCC2-overexpressing mice. Scale bars, 5 μm. b The density of spines in CA1 hippocampal pyramidal neurons was significantly increased in KCC2-overexpressing mice. Spine density in the KCC2 mice was 1.06 ± 0.10 spines/μm (15 dendrites from 5 mice), while the spine density in the control group was 0.69 ± 0.07 (12 dendrites from 4 mice), corresponding to a 54% increase (**p < 0.01, unpaired t test). c Representative images of single dendrites in WT and KCC2-overexpressing mice using in vivo two-photon imaging of dendrites and spines in apical dendrites of layer V pyramidal neurons of the motor cortex. Scale bars, 5 μm. d In KCC2-overexpressing mice (34 dendrites, 16 animals), there was a significant increase in spine density compared with WT mice (18 dendrites, 11 animals ***p < 0.001, unpaired t test). Graphs show individual data with mean ± SEM)
To evaluate whether increasing KCC2 in the adult brain could also increase dendritic spine density, we used in vivo two-photon imaging. Using this approach, we could also examine whether spine density changed over time and during motor learning (see below). As shown in Fig. 2c, d, basal spine density (pre-training levels) in KCC2-overexpressing mice was significantly increased (≈ 40%) compared with WT mice. Under pre-training control conditions, WT mice had 0.23 ± 0.01 spines/µm (28 dendrites, 11 animals), while KCC2-overexpressing mice had 0.32 ± 0.02 spines/µm (34 dendrites, 16 animals; unpaired t test, p = 0.0007). There was more variability in spine counts for KCC2-overexpressing mice (Fig. 2c), perhaps reflecting that KCC2 was not overexpressed in every pyramidal neuron (see above). Nevertheless, our data confirm that KCC2 overexpression can increase dendritic spine density and notably confirm that this capability is still retained in the adult brain. We did not examine whether the increase in spine density was reversible, although returning Dox to the diet for 3 weeks returns KCC2 levels back to control values [20].
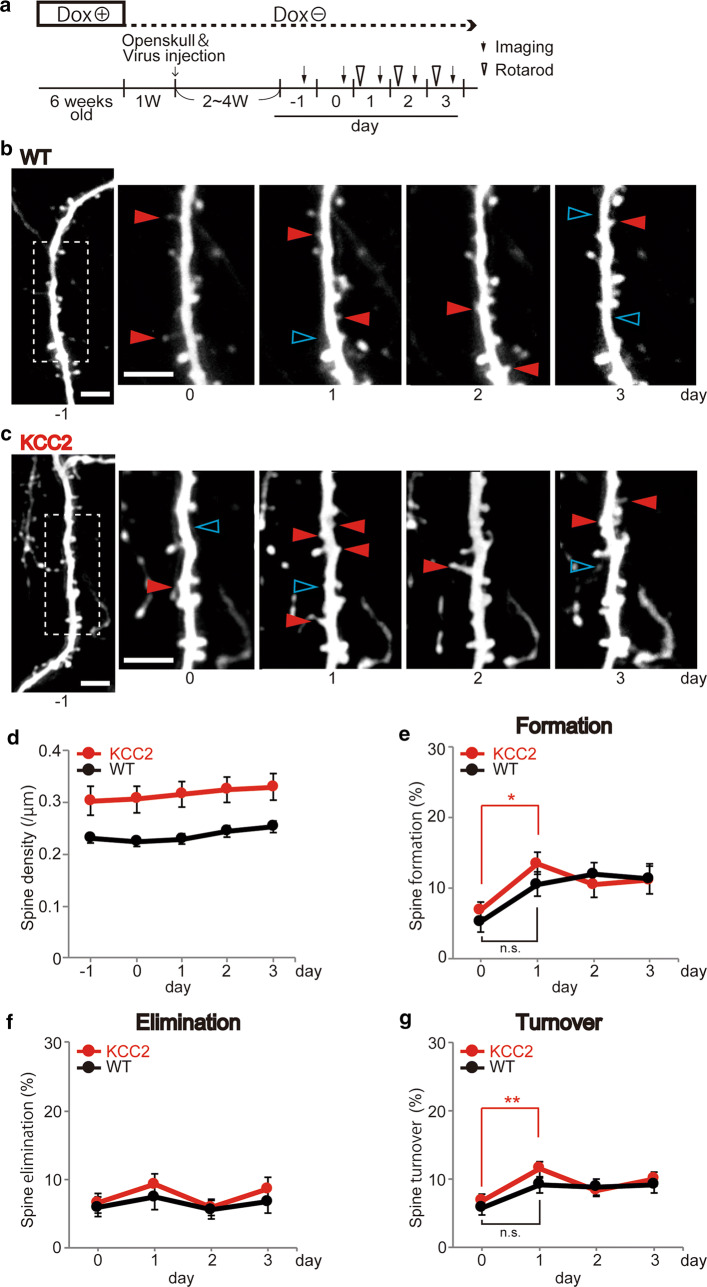
Subjecting mice to repeated trials on an accelerating rotarod enables them to increase the time/speed before they fall off, and this motor learning has been associated with an increased formation rate of new dendritic spines specifically in layer V pyramidal neurons within the forelimb-associated region of the M1 motor cortex [17]. Previous studies have shown that motor learning and other forms of plasticity may be associated with modest increases in dendritic spine density (approximately 5–10% in Yang et al. [18] and Xu et al. [17]) or may be associated with no overall change depending on the balance of formation and elimination [15]. Under our conditions, total dendritic spine density was relatively stable throughout 5 successive days of repeated imaging of the same dendrite, with mice undergoing rotarod training during the latter 3 days (see below, Fig. 3a). Over the 5 imaging days, dendritic spine density was consistently higher in KCC2-overexpressing mice (by 30–40%) across each of these days (Fig. 3b–d). When images of dendrites and their spines were compared between 2 consecutive days, a significant increase in the proportion of newly appeared dendritic spines (dendritic spine formation rate) was seen in mice overexpressing KCC2 following a single day of rotarod training (Fig. 3b, c, e). Between the 2 pre-training control days (day − 1 to day 0), the dendritic spine formation rate in KCC2 mice was 6.94 ± 1.11%, whereas after 1 day of training the dendritic spine formation rate increased to 13.49 ± 1.58% (day 0 to day 1, 23 dendrites, 7 animals; one-way ANOVA, Tukey’s test, p = 0.036, Fig. 3e). The corresponding rates of new dendritic spine formation pre- and post-1 day training in WT mice was 5.28 ± 1.52% and 12.00 ± 1.57% (18 dendrites, 7 animals; one-way ANOVA, Tukey’s test, not significantly different, Fig. 3e). In both WT and KCC2 mice, the proportion of dendritic spines that disappeared across consecutive imaging days (dendritic spine elimination rate) was relatively constant across the 5 days of imaging (between 5 and 10%) and not significantly different between WT and KCC2 mice (Fig. 3f). Corresponding with the increased dendritic spine formation in KCC2 mice, the dendritic spine turnover rate (an average of elimination and formation) seen after 1 day of training was also significantly increased in KCC2 mice (11.45 ± 1.13; one-way ANOVA, Tukey’s test, p = 0.0097; Fig. 3g), but not in WT mice (6.76 ± 0.98%).
Fig. 3.
Increasing KCC2 induces a sustained increase in spine density and a more rapid rate of spine formation during rotarod learning. a Schematic diagram of experimental protocol. Imaging and rotarod training began 3 weeks after withdrawing Dox from the diet. The same dendrites were imaged over 5 consecutive days. From days 1 to 3, rotarod training was performed in the morning prior to imaging later that day. b, c Representative single dendrites of pyramid neurons from WT (b) and KCC2-overexpressing (c) mice during repeated in vivo imaging before and during rotarod training. Red arrowheads indicate spines that newly appeared compared with the previous day’s image (newly formed spines), while blue arrowheads indicate those that disappeared (eliminated spines). Scale bars in each image in b and c, 5 μm. d Spine density measured at each imaging day was consistently higher in KCC2-overexpressing mice compared with that in WT mice. e The relative number of new spines that formed during the 1st day of rotarod learning was significantly greater in KCC2-overexpressing mice, but not in WT mice. However, subsequent new spine formation rates were not different. The graph plots the percentage of spines in a dendrite that was not present at the previous day’s imaging (7 KCC2 over-expressing mice, 23 dendrites; 7 WT mice, 18 dendrites; one-way ANOVA followed by Tukey’s test, 0 day vs. 1 day KCC2 mice, *p < 0.05). f The proportion of spines that disappeared (spine elimination) across the rotarod training period was not significantly different between WT and KCC2-overexpressing mice. g The spine turnover rate (numerical addition of formation and elimination) was significantly increased following 1 day of rotarod training for KCC2-overexpressing mice (one-way ANOVA, Tukey’s test, **p < 0.01), but not for WT mice—nor was it significantly different for WT or KCC2 mice at 2 and 3 days
KCC2-overexpressing mice show a more rapid acquisition of improved motor performance
Given the correlations between motor learning and dendritic spine formation rates previously reported, we examined and quantified the performance of mice during the rotarod task. We used the same mouse cohort as subjected to 5 days of imaging, but also included mice in which reliable imaging across the entire protocol was unsuccessful. A series of six rotarod trials was performed over 5 consecutive days, which included imaging on days 1–3 in Fig. 3a and 2 additional training days. Performance was measured by quantifying the time it took for mice to be no longer able to hold on to the rotarod as its speed was gradually increased in a step-wise fashion. As shown in Fig. 4a, both WT and KCC2-overexpressing mice showed similar motor performance levels in the first rotarod session, and both cohorts improved in their motor performance during the 5 days of training. Nevertheless, the motor performance curves across the 5 days of training were significantly different between KCC2-overexpressing mice and WT mice (Fig. 4a; n = 15 WT mice with 6 trials per day, n = 15 KCC2 mice with 6 trials per day, p = 0.00095, two-way ANOVA with Bonferroni’s test). Furthermore, the pooled motor performance from days 4 and 5 of training was significantly better in KCC2-overexpressing mice compared with WT mice (Fig. 4a; n = 15 WT mice, 15 KCC2 mice; p = 0.0267, unpaired t test). Motor performance across trials for each day was also averaged and compared with the initial motor performance level at day 1 (Fig. 4b). In WT mice, a significant improvement in performance (i.e., motor learning) was first seen at day 4 of training, whereas for KCC2-overexpressing mice, a significant improvement in performance was first seen at day 2 of training. Thus, a significant improvement in motor performance occurred earlier in KCC2-overexpressing mice compared with WT mice, which suggests an enhanced learning capacity.
Fig. 4.
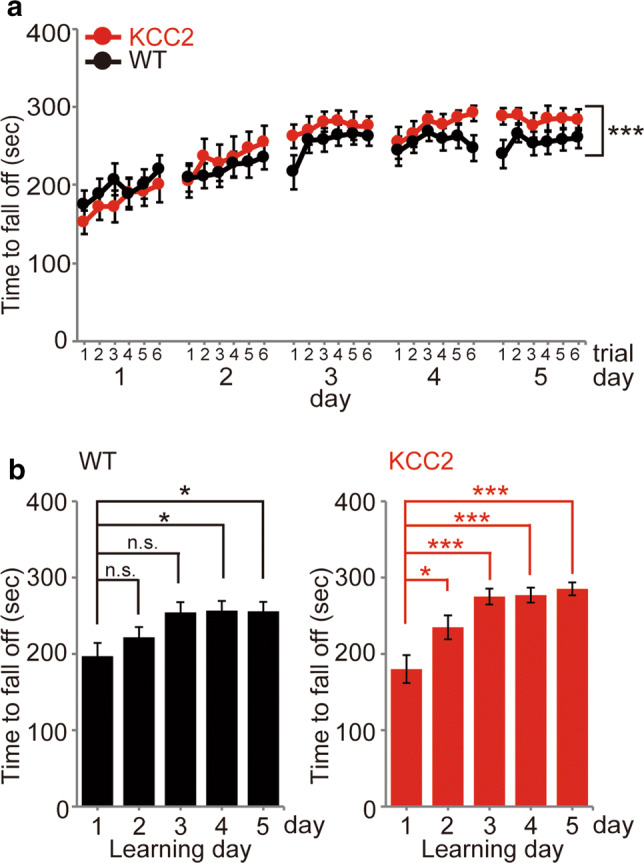
Performance in rotarod training trials over 5 days of training for KCC2 mice and WT mice. Following withdrawal of Dox for 2–3 weeks, mice underwent 6 trials of accelerating rotarod training per day over 5 successive days (WT mice: black; n = 15; KCC2-overexpressing mice: red; n = 15). a Rotarod performance was measured as the time before falling off as the rotarod was accelerated from 4 to 40 rpm over 300 s in each trial. The improvement of performance during the successive 5-day training sessions was significantly greater in KCC2 mice compared with WT mice (***p < 0.001, 2-way ANOVA). b Comparison of the increase in rotarod performance across each day of training in WT (left, black) and KCC2-overexpressing mice (right, red). The performance in each of the six trials was averaged for each day and the averaged performance compared with that on day 1 to evaluate the time course of the motor learning. For WT mice a significant improvement was seen by day 4 (n = 15, *p < 0.05, one-way ANOVA, Bonferroni’s test), while for KCC2-overexpressing mice, a significant increase in performance was seen earlier, on the 2nd day of training (n = 15, *p < 0.05, ***p < 0.001, one-way ANOVA, Bonferroni’s test). Data are shown as mean ± SEM
Stability of newly formed and existing dendritic spines
Most newly formed dendritic spines are labile and are eliminated over the subsequent weeks. However, a proportion of dendritic spines that is newly formed during learning of a specific motor task, such as accelerating rotarod training or forelimb reaching, persists for a longer time, suggesting they help encode the storage of that learning information [17, 18]. In addition, training can enhance the elimination of existing dendritic spines [18]. Hence, we next examined the stability of existing and newly formed dendritic spines. Although the absolute and relative numbers of new dendritic spines seen in KCC2-overexpressing mice were greater than in WT mice (Fig. 2), these new dendritic spines were similarly labile, and both WT and KCC2 mice had similar rates of elimination of dendritic spines that existed prior to the rotarod training (Fig. 5a). We then examined the stability of dendritic spines that newly formed over the first 2 days of training (Fig. 5b). In both WT and KCC2-overexpressing mice, approximately 20% of the new dendritic spines seen after 1 or 2 days of rotarod training had disappeared by about 24 h later (Fig. 5c). Hence, under our conditions, we could not detect a population of more stable dendritic spines associated with the enhanced learning in KCC2-overexpressing mice.
Fig. 5.
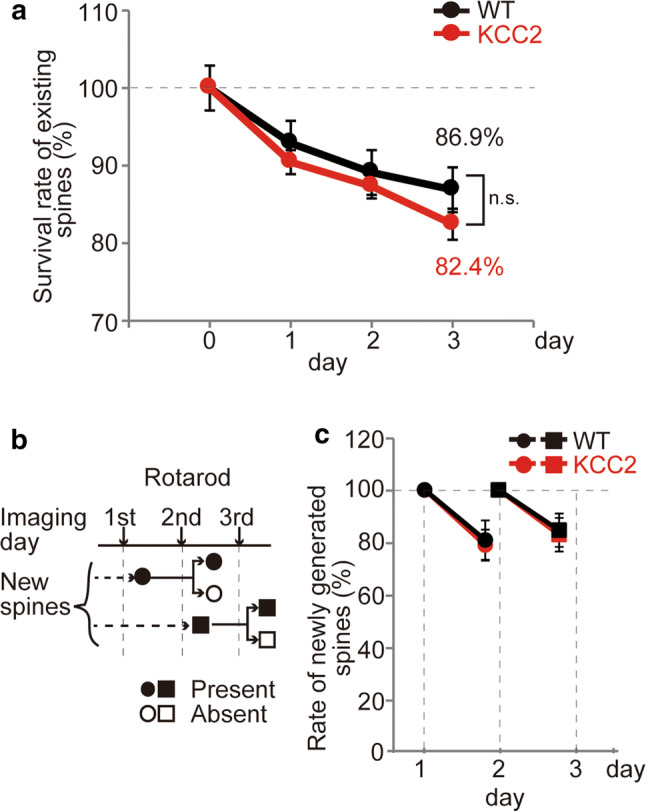
Stability of newly formed and pre-existing spines. a Survival rates of spines that existed prior to the rotarod training in both WT and KCC2 mice indicate a similar rate of spine elimination. b Schematic of the protocol used to define the stability of newly formed spines. Spines that appeared on the 1st day of training (closed circles, relative to 1 day pre-training) were either present (closed circle) or eliminated by the next day (open circle). Similarly, new spines observed after day 2 of rotarod training (closed squares, relative to day 1) were either present (closed square) or eliminated (open square) by the next day. c The relative elimination rates of newly formed spines indicate that about 80% of the new spines survive the next day with no difference between WT and KCC2-overexpressing mice
Discussion
Using a conditional transgenic mouse where KCC2 is overexpressed in excitatory pyramidal neurons by ceasing dietary doxycycline supplementation, we have shown that: (1) increasing KCC2 in the brain after the major developmental period of synaptogenesis can increase dendritic spine density, (2) increasing KCC2 enhances the capacity of motor learning to form new dendritic spines, and (3) increasing KCC2 increases the extent and rate of performance increase during training. Hence, our data strongly support the growing appreciation of the role of KCC2 in dendritic spine development, maturation and/or maintenance and extend this to a putative role in enhancing synaptic plasticity and performance during learning in vivo.
In utero transfection of KCC2 into the cortex results in a permanent increase in dendritic spine density of between 50 and 100% when measured at different postnatal times and in different regions of layer II/III neurons. For example, in apical and basal dendrites from mice at 3 months of age, spine density was approximately 90% higher [7]. This increase in dendritic spines was supported by an approximately 70% increase in the frequency of miniature excitatory postsynaptic currents (mEPSCs; at postnatal day 30–34; [7]). Conversely, neurons cultured from KCC2 knockout mice showed an approximate halving of synapses identified by both glutamate transporter (VGLUT) staining and mEPSC frequency [6]. It has been debated whether these striking effects of KCC2 on synapse numbers may be at least partly due to an impact on developmental spinogenesis. Knocking down KCC2 in more mature hippocampal cultures has not been associated with significant changes in dendritic spine density or mEPSC frequency, although knocking out KCC2 in developing neuronal cultures did alter dendritic spine volume, AMPA receptor aggregation and quantal size [12]. Our present results show that overexpressing KCC2 in the postnatal brain in vivo leads to a significant increase in dendritic spine density in the hippocampus and motor cortex (but not somatosensory cortex). However, the extent of this increase in the motor cortex was 40–50% less than that achieved when KCC2 was manipulated prior to spinogenesis in development [7, 33]. While two reports have shown that shRNA-induced knockdown of KCC2 decreases hippocampal dendritic spine density [8], a more recent report demonstrated that overexpression of KCC2 decreases dendritic spine density in CA1 neurons, both in vivo and in slice cultures. In the same study, dendritic spine density was increased in pyramidal neurons of the cortex after KCC2 overexpression, indicating regional differences [34]. The effects of KCC2 on dendritic spine density may depend on the extent of effects of KCC2 on spinogenesis and neuronal excitability, which may further relate to regional and age-dependent differences in relative membrane levels of KCC2 and on other neuroactive molecules such as brain-derived neurotrophic factor [34]. It will be important to also determine if adult KCC2 expression is associated with an increased dendritic spine head diameter and the number of functional excitatory synapses, as has been observed with in utero electroporation of KCC2 [7]. In the present study, while we did not specifically compare the morphology of dendritic spines or the number of functional synapses, the elimination rates of the dendritic spines in KCC2-overexpressing and WT mice over the 3-day imaging period were similar, which suggests that the additional dendritic spines in KCC2 mice could be similar to mature dendritic spines in WT mice.
Experience-dependent changes in dendritic spine number, size and spatial arrangements are important components of the structural synaptic plasticity that accompanies learning and memory formation [35, 36]. In the adult brain, most dendritic spines are generally very stable with a small proportion (≈ 5%) being continuously formed and eliminated. Learning can increase this new dendritic spine formation rate and promote elimination of pre-existing dendritic spines. Motor training on an accelerated rotarod, for example, causes an approximate 5% increase in dendritic spine formation in the forelimb area of the motor cortex in adult mice [18]. Similar increases in dendritic spine formation rates are seen in other motor tasks, such as learning to reach for a pellet [17, 37]. The extent of the increase in formation rate can depend on many factors including age, specific cortical layer and dendritic branch as well as sleep and glucocorticoid status [37–39]. Some of the newly formed dendritic spines persist, with the degree of dendritic spine stability correlated with the degree of learning [17]. In our current study, we used a more modest rotarod training regime and did not detect significant increases in dendritic spine formation rates in WT mice, nor did we detect correlations between the extent of performance increments and new dendritic spine formation or stability, as has been previously reported in Yang et al. [18] and Liston et al. [38]. Our rotarod accelerated to a maximum of 40 rpm, and we trained with six trials per session (for 5 days), whereas Yang et al. [18], for example, accelerated to 100 rpm and trained with 20 trials per session (for 2 days). Despite this modest training protocol, we still observed a significant increase in dendritic spine formation rate in mice overexpressing KCC2. Hence, as well as increasing basal spine levels, KCC2 overexpression may facilitate the capacity for motor training to form new dendritic spines. Related to this, KCC2 overexpression was also associated with subtle yet significant increases in the rate and extent of performance increase resulting from the rotarod training. We speculate that the significant increase in performance seen at day 2 in the KCC2-overexpressing mice may be related to the increase in dendritic spine formation seen following training on day 1, enabling at least part of the structural component of synapses to be more readily available to encode the learning associated with subsequent training. Indeed, daily significant increments in motor performance improvement and dendritic spine formation were only seen on all days of the training protocol in KCC2-overexpressing mice. This assumes that the locus of the rotarod learning resides in the motor cortex, although other regions related to motor activity, e.g., thalamus and cerebellum, may be affected by KCC2 and also be involved in the motor performance changes. Clearly KCC2 overexpression will also affect Cl− homeostasis, GABAergic inhibition and neuronal excitability, and we are as yet unable to distinguish the aspects of enhanced behavioral learning that are solely attributable to the dendritic spine effects. Transport-deficient KCC2 mutations may be able to probe this, as described in Li et al. [6] and Fiumelli et al. [7], but see Awad et al. [34]. Nevertheless, our tantalizing results raise the possibility that KCC2 may be involved in synaptic plasticity in the adult nervous system and should encourage further studies to more closely examine this hypothesis.
Acknowledgements
We thank Ms. Tatsuko Oba for the support of animal maintenance and preparation and Dr. Miho Watanabe at Hamamatsu Medical University for a useful suggestion for the analysis of KCC2 expression and staining.
Author contributions
KN, DLC, PWR, KE and IT conducted experiments; KN, AJM and JN wrote the paper; AJM and JN conceived the study. All authors approved the final version of the manuscript.
Funding
This study was supported by JSPS KAKENHI grant no. JP17H01530 and JP25253017 to JN. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants. All animal experiments were approved by the Okazaki Institutional Animal Care and Use Committee or by the UNSW Sydney Animal Care and Ethics Committee.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 2.Blaesse P, Airaksinen MS, Rivera C, Kaila K. Cation-chloride cotransporters and neuronal function. Neuron. 2009;61:820–838. doi: 10.1016/j.neuron.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Kaila K, Price TJ, Payne JA, Puskarjov M, Voipio J. Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat Rev Neurosci. 2014;15:637–654. doi: 10.1038/nrn3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe M, Fukuda A. Development and regulation of chloride homeostasis in the central nervous system. Cell Neurosci Front. 2015 doi: 10.3389/fncel.2015.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kahle KT, Delpire E. Kinase-KCC2 coupling: Cl− rheostasis, disease susceptibility, therapeutic target. J Neurophysiol. 2016;115:8–18. doi: 10.1152/jn.00865.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li H, Khirug S, Cai C, Ludwig A, Blaesse P, Kolikova J, Afzalov R, Coleman SK, Lauri S, Airaksinen MS, Keinänen K, Khiroug L, Saarma M, Kaila K, Rivera C. KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron. 2007;56:1019–1033. doi: 10.1016/j.neuron.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 7.Fiumelli H, Briner A, Puskarjov M, Blaesse P, Belem BJT, Dayer AG, Kaila K, Martin J-L, Vutskits L. An ion transport-independent role for the cation-chloride cotransporter KCC2 in dendritic spinogenesis in vivo. Cereb Cortex. 2013;23:378–388. doi: 10.1093/cercor/bhs027. [DOI] [PubMed] [Google Scholar]
- 8.Llano O, Smirnov S, Soni S, Golubtsov A, Guillemin I, Hotulainen P, Medina I, Nothwang HG, Rivera C, Ludwig A. KCC2 regulates actin dynamics in dendritic spines via interaction with β-PIX. J Cell Biol. 2015;209:671–686. doi: 10.1083/jcb.201411008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chevy Q, Heubl M, Goutierre M, Backer S, Moutkine I, Eugène E, Bloch-Gallego E, Lévi S, Poncer JC. KCC2 gates activity-driven AMPA receptor traffic through cofilin phosphorylation. J Neurosci. 2015;35:15772–15786. doi: 10.1523/JNEUROSCI.1735-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamma I, Heubl M, Chevy Q, Renner M, Moutkine I, Eugene E, Poncer JC, Levi S. Activity-dependent regulation of the K/Cl transporter KCC2 membrane diffusion, clustering, and function in hippocampal neurons. J Neurosci. 2013;33:15488–15503. doi: 10.1523/JNEUROSCI.5889-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulyas AI, Sik A, Payne JA, Kaila K, Freund TF. The KCl cotransporter, KCC2, is highly expressed in the vicinity of excitatory synapses in the rat hippocampus. Eur J Neurosci. 2001;13:2205–2217. doi: 10.1046/j.0953-816x.2001.01600.x. [DOI] [PubMed] [Google Scholar]
- 12.Gauvain G, Chamma I, Chevy Q, Cabezas C, Irinopoulou T, Bodrug N, Carnaud M, Lévi S, Poncer JC. The neuronal K-Cl cotransporter KCC2 influences postsynaptic AMPA receptor content and lateral diffusion in dendritic spines. Proc Natl Acad Sci USA. 2011;108:15474–15479. doi: 10.1073/pnas.1107893108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Zuo Y. Spine plasticity in the motor cortex. Curr Opin Neurobiol. 2011;21:169–174. doi: 10.1016/j.conb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 16.Kasai H, Fukuda M, Watanabe S, Hayashi-Takagi A, Noguchi J. Structural dynamics of dendritic spines in memory and cognition. Trends Neurosci. 2010;33:121–129. doi: 10.1016/j.tins.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goulton CS, Watanabe M, Cheung DL, Wang KW, Oba T, Khoshaba A, Lai D, Inada H, Eto K, Nakamura K, Power JM, Lewis TM, Housley GD, Wake H, Nabekura J, Moorhouse AJ. Conditional upregulation of KCC2 selectively enhances neuronal inhibition during seizures. bioRxiv. 2018 doi: 10.1101/253831. [DOI] [Google Scholar]
- 21.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 23.Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K, Emi K, Motohashi J, Konno R, Zaitsu K, Yuzaki M. d-serine regulates cerebellar LTD and motor coordination through the delta2 glutamate receptor. Nat Neurosci. 2011;14:603–611. doi: 10.1038/nn.2791. [DOI] [PubMed] [Google Scholar]
- 24.Rothwell PE, Fuccillo MV, Maxeiner S, Hayton SJ, Gokce O, Lim BK, Fowler SC, Malenka RC, Sudhof TC. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell. 2014;158:198–212. doi: 10.1016/j.cell.2014.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim SK, Hayashi H, Ishikawa T, Shibata K, Shigetomi E, Shinozaki Y, Inada H, Roh SE, Kim SJ, Lee G, Bae H, Moorhouse AJ, Mikoshiba K, Fukazawa Y, Koizumi S, Nabekura J. Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain. J Clin Invest. 2016;126:1983–1997. doi: 10.1172/JCI82859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim SK, Nabekura J. Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J Neurosci. 2011;31:5477–5482. doi: 10.1523/JNEUROSCI.0328-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine ND, Rademacher DJ, Collier TJ, O’Malley JA, Kells AP, Sebastian WS, Bankiewicz KS, Steece-Collier K. Advances in thin tissue Golgi-Cox impregnation: fast, reliable methods for multi-assay analyses in rodent and non-human primate brain. J Neurosci Methods. 2013;213:214–227. doi: 10.1016/j.jneumeth.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutoo D, Akiyama K, Yabe K. Comparison analysis of distributions of tyrosine hydroxylase, calmodulin and calcium/calmodulin-dependent protein kinase II in a triple stained slice of rat brain. Brain Res. 2002;933:1–11. doi: 10.1016/S0006-8993(02)02271-0. [DOI] [PubMed] [Google Scholar]
- 30.Meyer HS, Schwarz D, Wimmer VC, Schmitt AC, Kerr JND, Sakmann B, Helmstaedter M. Inhibitory interneurons in a cortical column form hot zones of inhibition in layers 2 and 5A. Proc Natl Acad Sci. 2011;108:16807–16812. doi: 10.1073/pnas.1113648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krestel HE, Mayford M, Seeburg PH, Sprengel R. A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res. 2001;29:E39–E39. doi: 10.1093/nar/29.7.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xue J, Li G, Bharucha E, Cooper NGF. Developmentally regulated expression of CaMKII and iGluRs in the rat retina. Dev Brain Res. 2002;138:61–70. doi: 10.1016/S0165-3806(02)00460-1. [DOI] [PubMed] [Google Scholar]
- 33.Awad PN, Sanon NT, Chattopadhyaya B, Carriço JN, Ouardouz M, Gagné J, Duss S, Wolf D, Desgent S, Cancedda L, Carmant L, Di Cristo G. Reducing premature KCC2 expression rescues seizure susceptibility and spine morphology in atypical febrile seizures. Neurobiol Dis. 2016;91:10–20. doi: 10.1016/j.nbd.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 34.Awad PN, Amegandjin CA, Szczurkowska J, Carriço JN, do Nascimento ASF, Baho E, Chattopadhyaya B, Cancedda L, Carmant L, Di Cristo G. KCC2 regulates dendritic spine formation in a brain-region specific and BDNF dependent manner. Cereb Cortex. 2018;28:4049–4062. doi: 10.1093/cercor/bhy198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fu M, Zuo Y. Experience-dependent structural plasticity in the cortex. Trends Neurosci. 2011;34:177–187. doi: 10.1016/j.tins.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu J, Zuo Y. A local rebalancing act leads to global benefit. Neuron. 2017;96:712–713. doi: 10.1016/j.neuron.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Tjia M, Yu X, Jammu LS, Lu J, Zuo Y. Pyramidal neurons in different cortical layers exhibit distinct dynamics and plasticity of apical dendritic spines. Neural Circuits Front. 2017 doi: 10.3389/fncir.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan W-B. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nat Neurosci. 2013;16:698. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan W-B. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344:1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]