Abstract
In adult rats (4–9 months), chronic nicotine infusion increases the basal level of acetylcholine (ACh) release in the cerebral cortex and enhances responses of cortical ACh release and cortical vasodilation elicited by nucleus basalis of Meynert (NBM) stimulation. In the present study, we examined whether these effects of nicotine are detected in aged rats. Aged rats (27–30 months) received sustained subcutaneous nicotine (100 μg/kg/h) or saline for 14 days. Under urethane anesthesia, ACh release and regional blood flow in the parietal cortex were measured. The basal level of ACh release in the cerebral cortex was not changed by chronic nicotine. In addition, the magnitudes of ACh release and vasodilation by NBM stimulation were similar between the saline-treated and nicotine-treated groups. The lack of an effect of chronic nicotine in aged rats may be due to a decrease in nicotinic receptors in the cerebral cortex during aging (Nordberg et al., J Neurosci Res 31:103–111, 1992).
Keywords: Chronic nicotine, Nucleus basalis of Meynert, Cortical cerebral blood flow, Acetylcholine release, Aging, Rat
Introduction
Long-term stimulation of nicotinic receptors by chronic nicotine administration for about 1–2 weeks in adult rats has been reported to up-regulate (increase in number) of nicotinic receptors (measured by the binding of [3H] nicotine [2, 3]), but not muscarinic receptors (measured by the binding of [3H] QNB [3]) in the cerebral cortex, and to increase the basal level of acetylcholine release in the cerebral cortex [2, 4]. The majority of cholinergic fibers in the cerebral cortex originate in the nucleus basalis of Meynert (NBM) [5, 6]. Chronic nicotine infusion enhances the response of cortical acetylcholine release elicited by stimulation of the NBM [4]. These data indicate that long-term stimulation of nicotinic receptors activates the cholinergic system originating in the NBM and projecting to the cerebral cortex.
A physiological function of acetylcholine released from NBM cholinergic nerve endings in the cerebral cortex is cerebral vasodilator action, which produces an increase in regional cerebral cortical blood flow [7, 8]. The vasodilation response in the cortex elicited by stimulation of the NBM is independent of systemic blood pressure and metabolic vasodilation, and mediates muscarinic and nicotinic receptors in the parenchyma of the cortex. This cerebral cortical vasodilation response by NBM stimulation was found to be enhanced by long-term stimulation of nicotinic receptors [4].
Cholinergic neurons in the basal forebrain show degeneration in patients with Alzheimer’s disease [9] and in healthy aged people [10]. Previously, we reported that the vasodilation response of the cortex by stimulation of the NBM declined in aged rats [11].
Therefore, long-term stimulation of nicotinic receptors in aged individuals may be beneficial in maintaining the cholinergic system originating in the NBM and projecting to the cerebral cortex. It has been reported that long-term stimulation of nicotinic receptors in aged rats up-regulates nicotinic receptors in the cerebral cortex [12]. The present study aimed to clarify whether long-term stimulation of nicotinic receptors increases basal acetylcholine release in the cerebral cortex and enhances both responses of cortical acetylcholine release and cortical vasodilation elicited by NBM stimulation in aged rats (27–30 months old), as observed in adult rats (4–9 months old).
Materials and methods
The experiments were performed on 12 male and female, aged Wistar rats (27–30 months old). All animals were obtained from the animal farm of Tokyo Metropolitan Institute of Gerontology. This study was approved by the Animal Committee of our Institution.
Sustained subcutaneous infusion of nicotine
The rats were divided into two groups: (1) a saline-treated control group (n = 6), and (2) a nicotine-treated group (n = 6). The rats were anesthetized with halothane, and a mini-pump (Alzet, model #2002, 0.5 μl/h, 14 days duration) containing either (−) nicotine (Tokyo Kasei Kogyo, Japan; 13–18 mg/200 μl, calculated as the free base) or saline was inserted into a subcutaneous pocket via a small incision over the shoulders (Fig. 1). The dose (100 μg/kg/h) and duration (14 days) of nicotine infusion were the same as those utilized in our previous study using adult rats [4]. The wound was sutured with cotton thread and the rats were returned to their cage. After awakening, the animals were housed at an ambient temperature of 22 ± 2 °C and fed laboratory food with water ad libitum.
Fig. 1.

The time course of the present experiments
General surgery and anesthesia
Fourteen days after minipump implantation, the responses of acetylcholine release in the cerebral cortex and cerebral cortical blood flow to focal electrical stimulation of the NBM were examined under general anesthesia (Fig. 1), as described previously [4]. Briefly, the rats were anesthetized with urethane (0.7–1.1 g/kg, i.p.). Respiration was maintained by means of an artificial respirator (model 683; Harvard, USA) through a tracheal cannula. End-tidal CO2 concentration was maintained at 3.0–4.0 % by monitoring with a respiratory gas monitor (Microcap; Oridion Medical, Jerusalem, Israel). Arterial blood pressure was measured through a catheter with a pressure transducer (TP-400T; Nihon Kohden, Tokyo, Japan) inserted into a femoral artery. Body temperature was measured rectally and continuously using a thermistor, and maintained at approximately 37.5 °C by means of an infrared lamp and a heater system (ATB-1100; Nihon Kohden). The depth of anesthesia was adjusted by additional urethane doses (100 mg/kg, i.v. via a catheter inserted into a femoral vein) when necessary and by monitoring body movement, stability of blood pressure; and respiratory movement.
Measurement of acetylcholine release in the cerebral cortex
The animals were mounted on a stereotaxic instrument (SR-5; Narisige) in a prone position, and the parietal cortex was exposed by removing a portion of the skull and dura. Extracellular acetylcholine in the parietal cortex was collected by a microdialysis technique. A microdialysis probe (outer diameter, 0.5 mm; length of perfusion, 3 mm; CMA/12; CMA/Microdialysis, Sweden) was inserted in the right parietal cortex at an angle of 30° to the vertical line to a depth of 3.5 mm from the cortical surface at AP = + 0.2, L = +4, as originally described by Kurosawa et al. [13]. The microdialysis probe was perfused at a speed of 2 μl/min with artificial cerebral spinal fluid (aCSF) containing (in mM) NaCl (122.7), KCl (2.4), CaCl2 (1.5), MgCl2 (1.1), NaHCO3 (27.5), KH2PO4 (0.6), Na2SO4 (0.5), glucose (6), and the acetylcholinesterase inhibitor physostigmine (5 μM). The aCSF was bubbled with 95 % O2/5 % CO2 to adjust the pH to 7.4. The recovery rate of acetylcholine by the microdialysis probe in vitro at room temperature is usually 23–25 %. The recovery rates of acetylcholine of four microdialysis probes were 14–16 %. In these four probes, the recovery rates were adjusted to 24 % by calculation. The perfused fluid was collected every 3 min in a sample cup kept on ice. The perfusate in each sample (6 μl) was mixed with 6 μl (120 fmol) of isopropylhomocholine, an internal standard, dissolved in aCSF. Acetylcholine was measured by high-performance liquid chromatography (HPLC) using an electrochemical detector (HTEC-500; Eicom, Kyoto). The mobile phase, consisting of 50 mM KHCO3, 400 mg/l sodium 1-decanesulfonate (Tokyo Kasei Kogyo, Japan), and 50 mg/l EDTA·2Na, was pumped at a rate of 150 μl/min through a microbore separation column (AC-GEL, 2 × 150 mm). The acetylcholine was converted to hydrogen peroxide and betaine by immobilized acetylcholinesterase and choline oxidase packed into a column (AC-ENZYMEPAKII, 1.0 × 4 mm). Both the separation column and enzyme column were maintained at 33 °C. Hydrogen peroxide was measured using an electrochemical detector, and acetylcholine was calculated by hydrogen peroxide measurement. The platinum working electrode was held at 0.45 V versus Ag/AgCl.
Measurement of cortical cerebral blood flow
The probe (diameter 0.8 mm) of a laser Doppler flowmeter (ALF21D; Advance, Tokyo) was placed on the surfaces of the right or left parietal lobe (AP = + 1 to −2 mm, L = +4 to +6 mm), and was fixed with a balancing holder (ALF-B; Advance). The output of the laser Doppler flowmeter was expressed in mV and recorded on a polygraph. In all 12 rats, cortical blood flow and acetylcholine release in the parietal cortex were measured simultaneously.
Stimulation of the NBM
A coaxial metal electrode of 0.3 mm outer diameter was stereotactically inserted into the NBM ipsilateral to the parietal cortex where acetylcholine and blood flow were measured. Focal electrical stimulation of the NBM was performed by means of a stimulator (SEN-7203; Nihon Kohden) and stimulus isolation unit (SS-202J; Nihon Kohden). The parameters of electric stimulation were 0.5 ms duration and 50 Hz frequency for 3 min. Stimulus intensity was varied from 50 to 200 μA. Histological verification of the tip position of the stimulating electrode was performed after the end of experiments on frozen transverse brain sections. The stimulated area was located 1.6–2.4 mm posterior to the bregma, 3.4–4.0 mm lateral from the midline, and 6.4–7.4 mm vertical under the bregma height in both the control and nicotine-treated rats. All the tip positions in 12 rats were confirmed within the regions of the NBM according to Paxinos and Watson’s atlas [14].
Statistical analysis
All values are presented as mean ± SEM. Statistical comparisons were carried out by means of one-way repeated-measures ANOVA followed by a Dunnett’s multiple comparison test, two-way factorial ANOVA and unpaired t test. A P value of <0.05 was considered to be statistically significant.
Results
Effect of sustained subcutaneous infusion of nicotine on the NBM stimulation-induced increase of acetylcholine release in the cerebral cortex
Basal acetylcholine release at the beginning of the experiments in the parietal cortex of both the saline-treated control rats and nicotine-treated rats was 78 ± 14 fmol/3 min and 68 ± 14 fmol/3 min, respectively (Fig. 2). There were no significant differences in the basal acetylcholine release between the two groups. The basal level of acetylcholine release was stable throughout the experiments.
Fig. 2.

Basal level of extracellular acetylcholine (ACh) release in the parietal cortex in aged rats of the saline-treated control group and nicotine-treated group. Values of two samples at the beginning of experiments were averaged in each rat. Each column and vertical bar represents the mean ± SEM. n.s. non-significant difference between the values by unpaired t test
Figure 3 demonstrates the time course of acetylcholine release in the parietal cortex in response to the NBM stimulation at 200 μA in saline-treated control rats and nicotine-treated rats. In both groups, and in all rats tested (n = 6 rats in each group), acetylcholine release was increased during NBM stimulation, and returned to the pre-stimulus level after the stimulation ended (Fig. 3a, b). However, the increase of acetylcholine release during NBM stimulation varied between rats in both groups, and was 92–468 fmol/3 min in the control group and 207–758 fmol/3 min in the nicotine-treated group. In the 6 rats in each group during NBM stimulation at 200 μA, acetylcholine release was significantly increased to 337 ± 63 fmol/3 min in the control group and 365 ± 82 fmol/3 min in the nicotine-treated group (Fig. 3c, d). There were no significant differences in the magnitudes and time course of the response of acetylcholine release to NBM stimulation between the two groups.
Fig. 3.

Responses of acetylcholine (ACh) release in the parietal cortex to focal electrical stimulation (at 200 μA) of the unilateral NBM ipsilateral to the parietal cortex in aged rats of the control group and nicotine-treated group. The level of extracellular acetylcholine measured every 3 min are plotted as the absolute values in the saline-treated control group (a, c, closed circles, n = 6) and the nicotine-treated group (b, d, open circles, n = 6). a, b ACh release measured in 6 rats from each group. c, d Summarized graph. Each point and vertical bar represents the mean ± SEM. **P < 0.01; significantly different from pre-stimulus control values (−3 to 0 min) using one-way repeated-measures ANOVA followed by Dunnett’s multiple comparison test
The changes in acetylcholine release during the NBM stimulation at different intensities of stimulation (50–200 μA) are summarized in Fig. 4. Cortical acetylcholine release was increased by NBM stimulation in a stimulus intensity-dependent manner in both the saline-treated control rats and nicotine-treated rats. The stimulus intensity-dependent acetylcholine release by NBM stimulation was not significantly different between in the control group and in the nicotine-treated group.
Fig. 4.
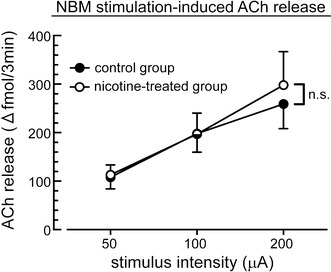
Changes in acetylcholine (ACh) release in the parietal cortex in response to electrical stimulation of the unilateral NBM at different intensities in aged rats of the saline-treated control group and nicotine-treated group. Relationships between stimulus intensity (abscissa) and magnitude of increase in ACh release from the basal level of each rat (ordinate) are summarized as the mean ± SEM. n.s. non-significant difference between the responses by two-way factorial ANOVA
Effect of sustained subcutaneous infusion of nicotine on the NBM stimulation-induced increase in cortical blood flow
The basal cortical blood flow in the 6 saline-treated control rats at the beginning of the experiments was 357 ± 45 mV. This value was similar to that in the 6 nicotine-treated rats (287 ± 33 mV).
Figure 5 demonstrates the time course of cortical cerebral blood flow in the parietal cortex in response to NBM stimulation at 200 μA in the saline-treated control rats and nicotine-treated rats. In both groups, and in all rats tested (n = 6 rats in each group), cortical cerebral blood flow was increased during NBM stimulation, and returned to the pre-stimulus level after the stimulation ended (Fig. 5a, b). However, the magnitude of increase in cortical cerebral blood flow during the NBM stimulation varied between rats in both groups, and was 136–229 % of the pre-stimulus basal value in the control group and 125–238 % of the pre-stimulus basal value in the nicotine-treated group. In the 6 rats in each group, cortical cerebral blood flow was increased significantly during NBM stimulation at 200 μA, reaching 186 ± 13 % of the pre-stimulus basal value in the control rats and 174 ± 19 % of the pre-stimulus basal value in the nicotine-treated rats (Fig. 5c, d). There were no significant differences in the magnitude and time course of the response of cortical cerebral blood flow to NBM stimulation between the two groups.
Fig. 5.
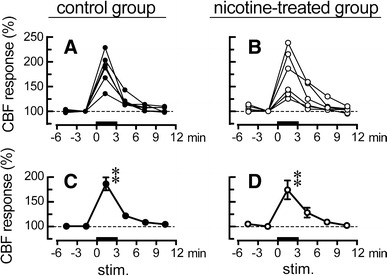
Responses of cerebral blood flow (CBF) in the parietal cortex to focal electrical stimulation (at 200 μA) of the unilateral NBM ipsilateral to the parietal cortex in aged rats of the control group and nicotine-treated group. Reponses of CBF during a 3-min period were plotted every 3 min as a percentage of the pre-stimulus values in the saline-treated control group (a, c, closed circles, n = 6) and the nicotine-treated group (b, d, open circles, n = 6). a, b CBF response measured in 6 rats from each group, c, d Summarized graph. Each point and vertical bar represents the mean ± SEM. **P < 0.01; significantly different from pre-stimulus control values (−3 to 0 min) using one-way repeated-measures ANOVA followed by Dunnett’s multiple comparison test
The cortical cerebral blood flow responses during NBM stimulation at different intensities of stimulation (50–200 μA) are summarized in Fig. 6. Cortical cerebral blood flow was increased by NBM stimulation in a stimulus intensity-dependent manner in both the control rats and nicotine-treated rats. The stimulus intensity-dependent increase in cortical cerebral blood flow by NBM stimulation was not significantly different between the control group and the nicotine-treated group.
Fig. 6.
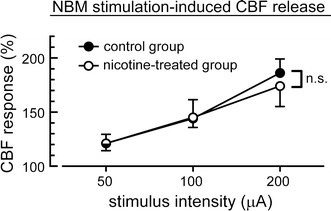
Relationships between stimulus intensity (x-axis) and magnitude of cerebral blood flow (CBF) responses (y-axis) in aged rats of the saline-treated control group and nicotine-treated group. The magnitude of the response during the stimulus period is expressed as a percentage of the pre-stimulus control value for 3 min just before the stimulation, and are summarized as the mean ± SEM. n.s. non-significant difference between the responses by two-way factorial ANOVA
Discussion
Our previous study, using adult rats of 4–9 months old, demonstrated that long-term stimulation of nicotinic receptors by sustained subcutaneous infusion of nicotine for 2 weeks increases basal acetylcholine release in the cerebral cortex and enhances both the response of cortical acetylcholine release and cortical vasodilation evoked by NBM stimulation [4] (Fig. 7). The present study employed the same experimental procedures as those utilized in our previous study on adult rats, and showed that long-term stimulation of nicotinic receptors did not significantly affect the NBM cholinergic function of acetylcholine release and vasodilation in the cerebral cortex in aged rats of 27–30 months old (Fig. 7). Below, we discuss the effect of long-term stimulation of nicotinic receptors on NBM cholinergic system function in the cerebral cortex of aged rats, by comparing the present results with our previous data in adult rats [4].
Fig. 7.

Summary of the effect of long-term stimulation of nicotinic receptors by chronic nicotine infusion on basal acetylcholine release in the cerebral cortex and both the response of cortical acetylcholine release and the cortical vasodilation evoked by NBM stimulation in adult and aged rats. The present results of aged rats are summarized together with our previous results of adult rats [4]
Our present results of acetylcholine release in the parietal cortex examining both the basal level and the stimulus intensity-dependent increase in response to NBM stimulation in aged rats of the control group were not significantly different from those in control adult rats reported previously [4]. Well-maintained cortical acetylcholine release at both the basal level and in response to NBM stimulation in aged rats of 32–42 months old has been reported [11], and the present results confirm those findings. In contrast, our present results of the cortical vasodilation response to NBM stimulation in a stimulus intensity-dependent manner in aged rats of control group was about 70 % of those in adult rats of the control group reported previously [4]. The cortical vasodilation response to NBM stimulation has been reported to be well maintained in aged rats of 24–28 months old [11, 15], but declined significantly in aged rats of 32–42 months old (about 36 % of adult rat response) [11]. Rats at 27–30 months old used in present study seem to be the age at which a decline the cortical vasodilation response to NBM stimulation is detected.
Our previous study in adult rats of 4–9 months old demonstrated that sustained subcutaneous infusion of nicotine enhances cholinergic vasodilation in the cerebral cortex originating in the NBM [4] (Fig. 7). Enhancement of the NBM cholinergic system in the cerebral cortex by sustained nicotine treatment in adult rats may be due to enhancement of trophic support to basal forebrain cholinergic neurons by nerve growth factor (NGF) [16–18], which is essential for the survival and function of cholinergic basal forebrain neurons [19, 20] and up-regulation of nicotinic receptors in the cerebral cortex [2, 3] by sustained nicotine treatments. Unlike in the case of adult rats, our present study showed that sustained subcutaneous infusion of nicotine did not significantly affect cholinergic vasodilation system in the cerebral cortex originating in the NBM in aged rats 27–30 months old (Fig. 7). The basal level of acetylcholine release in the parietal cortex and the responsiveness of both cortical acetylcholine release and cerebral cortical vasodilation to NBM stimulation were not significantly affected by sustained nicotine treatment in aged rats. The lack of a chronic nicotine effect on NBM cholinergic vasodilation system in the cerebral cortex in aged rats found in the present study may be due to (1) a diminished response of cortical NGF secretion by nicotinic receptor activation in aged rats [21], and (2) the decrease in the number of nicotinic receptors in the cerebral cortex during aging [1, 22]. Sustained long-term nicotine delivery may enhance NBM cholinergic vasodilation system in aged rats, if initiated before age-related nicotinic receptor decline. Chronic treatment with nicotine has been reported to up-regulate nicotinic receptors in the cerebral cortex of aged rats of 24–25 months [12]. In our present experiments, chronic nicotine treatment of aged rats of 27–30 months seems insufficient to compensate for the age-related decline in the number and/or function of nicotinic receptors, because the chronic nicotine had no effect on the NBM cholinergic system in aged rats of 27–30 months.
In the present study, using aged rats of 27–30 months, 2.4 mg/kg/day of nicotine (calculated as the free base) was delivered subcutaneously for 2 weeks. Similar chronic nicotine infusion is known to affect behavioral performance such as working memory of adult rats and aged rats, depending on the dose and period of nicotine [23, 24]. Levin and Terry [23] reported that a sustained subcutaneous infusion of nicotine (5 mg/kg/day, calculated as nicotine base) for 4 weeks improves working memory in adult rats, but not in aged rats of 24–28 months. In contrast, French et al. [24] showed that a lower dose of nicotine (0.3 mg/kg/day, calculated as nicotine base) for 4 weeks improves working memory in aged rats of 24 months. Since the metabolism of nicotine declines in aged rats [25], the effective dose and period of nicotine may differ between adult and aged rats.
In conclusion, the present study provides important information that long-term stimulation of nicotinic receptors by sustained subcutaneous infusion of nicotine does not affect basal acetylcholine release in the cerebral cortex, the response of cortical acetylcholine release, and cortical vasodilation evoked by NBM stimulation in aged rats of 27–30 months, when the dose and period of nicotine that are known to enhance the cholinergic system in adult rats (4–9 months old) is used.
Acknowledgments
This work was supported by funds from the Smoking Research Foundation of Japan.
References
- 1.Nordberg A, Alafuzoff I, Winblad B. Nicotinic and muscarinic subtypes in the human brain: changes with aging and dementia. J Neurosci Res. 1992;31:103–111. doi: 10.1002/jnr.490310115. [DOI] [PubMed] [Google Scholar]
- 2.Nordberg A, Romanelli L, Sundwall A, Bianchi C, Beani L. Effect of acute and subchronic nicotine treatment on cortical acetylcholine release and on nicotinic receptors in rats and guinea-pigs. Br J Pharmacol. 1989;98:71–78. doi: 10.1111/j.1476-5381.1989.tb16864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanderson EM, Drasdo AL, McCrea K, Wonnacott S. Upregulation of nicotinic receptors following continuous infusion of nicotine is brain-region-specific. Brain Res. 1993;617:349–352. doi: 10.1016/0006-8993(93)91104-Z. [DOI] [PubMed] [Google Scholar]
- 4.Uchida S, Hotta H, Misawa H, Kawashima K. Sustained subcutaneous infusion of nicotine enhances cholinergic vasodilation in the cerebral cortex induced by stimulation of the nucleus basalis of Meynert in rats. Eur J Pharmacol. 2011;654:235–240. doi: 10.1016/j.ejphar.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 5.Herrera-Marschitz M, Goiny M, Utsumi H, Ferre S, Håkansson L, Nordberg A, Ungerstedt U. Effect of unilateral nucleus basalis lesion on cortical and striatal acetylcholine and dopamine release monitored in vivo with microdialysis. Neurosci Lett. 1990;110:172–179. doi: 10.1016/0304-3940(90)90807-L. [DOI] [PubMed] [Google Scholar]
- 6.Johnston MV, McKinney M, Coyle JT. Neocortical cholinergic innervation: a description of extrinsic and intrinsic components in the rat. Exp Brain Res. 1981;43:159–172. doi: 10.1007/BF00237760. [DOI] [PubMed] [Google Scholar]
- 7.Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- 8.Sato A, Sato Y. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neurosci Res. 1992;14:242–274. doi: 10.1016/0168-0102(92)90071-J. [DOI] [PubMed] [Google Scholar]
- 9.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, DeLong MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 10.McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer’s disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–745. doi: 10.1212/WNL.34.6.741. [DOI] [PubMed] [Google Scholar]
- 11.Uchida S, Suzuki A, Kagitani F, Hotta H. Effects of age on cholinergic vasodilation of cortical cerebral blood vessels in rats. Neurosci Lett. 2000;294:109–112. doi: 10.1016/S0304-3940(00)01556-1. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Tian JY, Svensson AL, Gong ZH, Meyerson B, Nordberg A. Chronic treatments with tacrine and (−)-nicotine induce different changes of nicotinic and muscarinic acetylcholine receptors in the brain of aged rat. J Neural Transm. 2002;109:377–392. doi: 10.1007/s007020200030. [DOI] [PubMed] [Google Scholar]
- 13.Kurosawa M, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases acetylcholine release in the cerebral cortex in rats. Neurosci Lett. 1989;98:45–50. doi: 10.1016/0304-3940(89)90371-6. [DOI] [PubMed] [Google Scholar]
- 14.Paxinos G, Watson C (2009) The rat brain in stereotaxic coordinates, 6th edn. Academic, Amsterdam
- 15.Kurosawa M, Sato A, Sato Y. Well-maintained responses of acetylcholine release and blood flow in the cerebral cortex to focal electrical stimulation of the nucleus basalis of Meynert in aged rats. Neurosci Lett. 1989;100:198–202. doi: 10.1016/0304-3940(89)90684-8. [DOI] [PubMed] [Google Scholar]
- 16.Brown RW, Perna MK, Schaefer TL, Williams MT. The effects of adulthood nicotine treatment on D2-mediated behavior and neurotrophins of rats neonatally treated with quinpirole. Synapse. 2006;59:253–259. doi: 10.1002/syn.20237. [DOI] [PubMed] [Google Scholar]
- 17.Formaggio E, Fazzini F, Dalfini AC, Di Chio M, Cantù C, Decimo I, Fiorini Z, Fumagalli G, Chiamulera C. Nicotine increases the expression of neurotrophin receptor tyrosine kinase receptor A in basal forebrain cholinergic neurons. Neuroscience. 2010;166:580–589. doi: 10.1016/j.neuroscience.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 18.Martínez-Rodríguez R, Toledano A, Álvarez MI, Turégano L, Colman O, Rosés P, Gómez de Segura I, De Miguel E. Chronic nicotine administration increases NGF-like immunoreactivity in frontoparietal cerebral cortex. J Neurosci Res. 2003;73:708–716. doi: 10.1002/jnr.10688. [DOI] [PubMed] [Google Scholar]
- 19.Cuello AC, Bruno MA, Bell KFS. NGF-cholinergic dependency in brain aging, MCI and Alzheimer’s disease. Curr Alzheimer Res. 2007;4:351–358. doi: 10.2174/156720507781788774. [DOI] [PubMed] [Google Scholar]
- 20.Rylett RJ, Goddard S, Schmidt BM, Williams LR. Acetylcholine synthesis and release following continuous intracerebral administration of NGF in adult and aged Fischer-344 rats. J Neurosci. 1993;13:3956–3963. doi: 10.1523/JNEUROSCI.13-09-03956.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hotta H, Kagitani F, Kondo M, Uchida S. Basal forebrain stimulation induces NGF secretion in ipsilateral parietal cortex via nicotinic receptor activation in adult, but not aged rats. Neurosci Res. 2009;63:122–128. doi: 10.1016/j.neures.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Araujo DM, Lapchak PA, Meaney MJ, Collier B, Quirion R. Effects of aging on nicotinic and muscarinic autoreceptor function in the rat brain: relationship to presynaptic cholinergic markers and binding sites. J Neurosci. 1990;10:3069–3078. doi: 10.1523/JNEUROSCI.10-09-03069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levin ED, Torry D. Acute and chronic nicotine effects on working memory in aged rats. Psychopharmacology. 1996;123:88–97. doi: 10.1007/BF02246285. [DOI] [PubMed] [Google Scholar]
- 24.French KL, Granholm AC, Moore AB, Nelson ME, Bimonte-Nelson HA. Chronic nicotine improves working and reference memory performance and reduces hippocampal NGF in aged female rats. Behav Brain Res. 2006;169:256–262. doi: 10.1016/j.bbr.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto M, Kita T, Okuda H, Tanaka T, Nakashima T. Effects of aging on acute toxicity of nicotine in rats. Pharmacol Toxicol. 1994;75:1–6. doi: 10.1111/j.1600-0773.1994.tb00316.x. [DOI] [PubMed] [Google Scholar]


