Abstract
Cellular reactive oxygen species (ROS) production is increased by both temperature and anticancer drugs. Antioxidants are known to suppress ROS production while cancer patients may take them as dietary supplement during chemotherapy and hyperthermic therapy. We examined changes in ROS production in prostate cancer cells in the presence of various anticancer drugs and antioxidants at different temperatures. ROS production was increased with temperature in cancer cells, but not in normal cells; this increase was potently inhibited by ascorbic acid. ROS production was also increased in the presence of some anticancer drugs, such as vinblastine, but not by others. Dietary antioxidant supplements, such as β-carotene, showed variable effects. Ascorbic acid potently inhibited ROS production, even in the presence of anticancer drugs, while β-carotene showed no inhibition. Accordingly, our results suggest that cancer patients should carefully choose antioxidants during their cancer chemotherapy and/or hyperthermic therapy.
Keywords: Reactive oxygen species, Prostate cancer cells, Hyperthermia, Ascorbic acid, Anti-oxidants, Anti-cancer drugs
Introduction
Physiology of cancer cells has been extensively studied, and the understanding of mechanisms for their rapid growth and proliferation has been advanced in the past decade [1–3]. Accordingly, various therapeutic strategies in cancer treatment have been developed [1, 4]. Although surgical removal of the cancer tissue is still the golden standard for complete cure, it is not always feasible in cases with advanced or metastatic cancer. Surgical stress may be too large for geriatric and/or exhausted patients. In such cases, combination of various therapeutic strategies has been recommended. Among such strategies, hyperthermic therapy may be applied on the top of the conventional cancer chemotherapy or radiation therapy [5, 6]. Although it may not achieve complete remission of cancer by itself, clinical studies have demonstrated that the survival and quality of life may be significantly improved [3, 7].
Molecular mechanism of hyperthermic therapy includes the overstimulation metabolism of rapidly proliferating cancer cells, leading to the induction of apoptosis [8]. Increased production of reactive oxygen species (ROS) from mitochondria may also be involved [9]. Because ROS production may be increased in the presence of anticancer drugs on their own, the combination of chemotherapy and hyperthermic therapy will synergistically increase ROS production, leading to effective cancer cell death [6]. However, ROS production is inhibited in the presence of various antioxidants [10]. In this regard, various antioxidants, which are also used as dietary supplements, may interfere with the efficacy of such chemotherapy and/or hyperthermic therapy. Unfortunately, however, evaluation of the effect of such antioxidants in the combination of cancer chemotherapy has not been well performed [11, 12]. Ascorbic acid, for example, is often used as a dietary supplement. Because ascorbic acid may improve immunity or peripheral circulation [13], people, including cancer patients, take this antioxidant. However, the use of ascorbic acid in cancer patients remains controversial; ascorbic acid may enhance [10] or suppress [13] the efficacy of chemotherapy.
In this study, we examined the effect of temperature, anticancer drugs, and antioxidants on ROS production. We used MAT-Lu prostate cancer cells since hyperthermia therapy has often been applied to prostatic cancer patients [14, 15], and thus it is necessary to evaluate the effect of hyperthermia on this cancer cell type. We demonstrated their effect on ROS production, and make potential suggestions for future use of antioxidants in cancer patients.
Materials and methods
Materials
We used the following anticancer drugs; vinblastine (VBL) (Nihon Kayaku, Japan), cisplatin (CIS), (Pfizer, Japan), adriamycin (ADR), (Wako, Japan), docetaxel (DTX), (Sanofi Aventis, Japan). Similarly, as antioxidants, we used N-acetyl-cysteine (NAC), (Sigma, Japan), retinoic acid (Sigma), quercetin (Sigma), catechin (Wako), lutein (Sigma), β-carotene (Sigma), and ascorbic acid (Wako).
Cell culture
Rat prostatic adenocarcinoma cells (R3327-MAT-Lu) were cultured in RPMI-1640 medium supplemented with 10% FBS and 250 nM dexamethasone, which were kindly provided by Dr. J. T. Isaacs (Johns Hopkins University, MD, USA). Cells were incubated at 37°C in 5% CO2. In some experiments, cells were incubated at 42°C as hyperthermic treatment (see below). Rat cardiac fibroblasts were isolated from adult rats (250–300 g, male) by using a modification of published methods [16]. Fibroblasts were separated from cardiac myocytes by gravity separation and grown to confluence on 10-cm cell culture dishes at 37°C with 90% air with 10% CO2 in growth media (DMEM with 10% FBS, 1% penicillin, and 1% streptomycin).
Hyperthermic stress and measurement of reactive oxygen species
Cells were plated in 24-well culture plates (5.0 × 104 cells/well) overnight. Cells were then treated with various agents, including anticancer drugs, at 37°C for 3 h. For hyperthermic treatment, cells were further incubated in the presence or absence of various reagents at 42°C for 1 h. The intracellular ROS level was then measured using a fluorescent dye 2′,7′-dichlorofluorescein diacetate (DCFH-DA) (Life technologies, Japan) as previously described [17]. In the presence of oxidant, DCFH is converted into the highly fluorescent 2′,7′-dichlorofluorescein. Cells were first washed with PBS, and serum-free DMEM containing 10 μM DCFH-DA was added to each well. Cells were then incubated at 37°C for 45 min. ROS production was measured using a microplate reader equipped with a spectrofluorometer (PerkinElmer ARVO MX, Japan) at an emission wavelength of 538 nm and extinction wavelength of 485 nm.
Statistical analysis
Data are expressed as means ± SEM. Data was analyzed by one-way ANOVA followed by Tukey post hoc using Graph-pad Prism software. Statistical significance was set at p < 0.05.
Results
Effect of temperature on ROS generation
It is known that cancer cells exhibit higher metabolism than normal cells. High metabolic rate may be reflected by increased ROS generation, in particular, upon hyperthermia. Accordingly, we compared the effect of temperature on ROS production between MAT-Lu prostate cancer cells and normal fibroblasts obtained from the cardiac tissue. It is known that fibroblasts grow rapidly and thus possess high metabolic rate in comparison to other normal cell types.
As shown in Fig. 1a, ROS production was lower at 32°C than at 37°C while it was higher at 42°C. Thus, ROS production was increased in a temperature-dependent manner, at least in prostate cancer cells. In contrast, ROS production in cardiac fibroblasts was not increased at 42°C in comparison to that at 37°C (Fig. 1b). Thus, ROS production by hyperthermia was increased only in cancer cells.
Fig. 1.
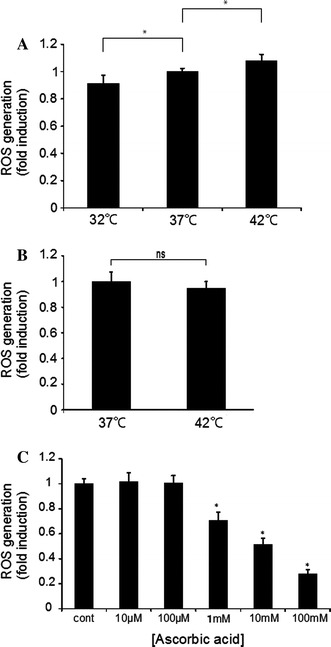
ROS production in cancer cells and normal cells at different temperatures. a ROS production in cancer cells at 32, 37, and 42°C. Prostate cancer cells were incubated at different temperatures, followed by determination of ROS production (mean ± SEM; n = 4, *p < 0.05). b ROS production in cardiac fibroblasts at 37 and 42°C. Cardiac fibroblasts were incubated at different temperatures similarly, followed by determination of ROS production (mean ± SEM; n = 4, *p < 0.05). c ROS production was determined with cancer cells in the presence of an increasing concentration of ascorbic acid (10 μM–100 mM). Prostate cancer cells were incubated at 37°C, followed by determination of ROS production (mean ± SEM; n = 4, *p < 0.05)
Effect of ascorbic acid on ROS production
We then examined the effect of ascorbic acid, which has been used in cancer treatment as part of chemotherapy, but is also known as a major antioxidant. In the presence of an increasing concentration of ascorbic acid (10 μM–100 mM), ROS production was decreased in a concentration-dependent manner at 37°C (Fig. 1c). Similar inhibition was observed at 42°C. Thus, ascorbic acid potently inhibited the production of ROS.
Effect of anticancer drugs on ROS production
Anticancer drugs may induce cytotoxicity through various mechanisms. We examined the effect of these anticancer drugs, which have been widely used in many cancer cell types, including prostate cancer, on ROS production. We first determined the EC50 values of these drugs in prostate cancer cells, which were 200 nM for VBL, 15 μM for CIS, 7.5 μM for ADR, and 1 mM for DTX. When prostate cancer cells were incubated with these drugs at the EC50 value concentration, ROS production was slightly, but significantly, increased with VBL and CIS, but not with DTX and ADR at 37°C (Fig. 2a). When hyperthermic treatment at 42°C was added, ROS production by VBL and CIS became even greater (Fig. 2a). Thus, hyperthermia by itself can increase ROS production, which is further enhanced in the presence of certain anticancer drugs.
Fig. 2.
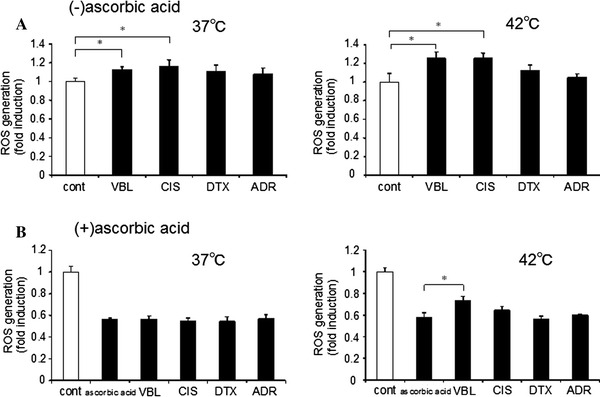
Effect of anticancer drugs and ascorbic acid on ROS production. a ROS production was determined at 37 or 42°C in the presence of 200 nM VBL, 15 μM CIS, 7.5 μM DTX or 1 μM ADR (mean ± SEM; n = 4, *p < 0.05). b ROS production was similarly determined in the presence of 1 mM ascorbic acid at 37 or 42°C (mean ± SEM; n = 4, *p < 0.05)
We then examined the effect of ascorbic acid in the presence of anticancer drugs. ROS production was potently inhibited by 1 mM ascorbic acid in the presence of any anticancer drugs (Fig. 2b). ROS production at 37°C was similar among these anticancer drugs. However, when hyperthermic treatment at 42°C was added, ROS production was significantly greater with VBL (Fig. 2b). Thus, ascorbic acid may negate ROS production induced by certain anticancer drugs at 37°C; however, it cannot negate ROS production of VBL at 42°C. Accordingly, anticancer drug-induced ROS enhancement may be retained in hyperthermia for VBL, but not others.
Effect of ascorbic acid on ROS production by Resovist
Resovist is super-paramagnetic iron oxide nanoparticle that has been used as MRI contrast agent. Because of its magnetic property, similar compounds have been used as source of heat production in hyperthermic therapy. We found that the ROS production was increased in the presence of 10 μM Resovist at 37°C, suggesting that Resovist can produce ROS with cancer cells. When ascorbic acid was added, ROS production was negated or instead decreased (Fig. 3). Thus, ascorbic acid could potently inhibit ROS production induced by Resovist.
Fig. 3.
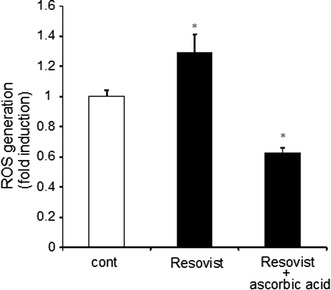
Effect of Resovist on ROS production. ROS production was determined in the presence of 10 μM Resovist and/or 1 mM ascorbic acid at 37°C. Prostate cancer cells were incubated for 45 min, followed by ROS production assays (mean ± SEM; n = 4, *p < 0.05)
Effect of various antioxidants on ROS production
Patients may take various dietary supplements during cancer chemotherapy. In some cases, patients may take supplementary antioxidants on the top of anticancer drugs. We thus examined the effect of these antioxidants and related drugs, namely, N-acetyl cysteine (NAC), retinoic acid, quercetin, catechin, lutein, and β-carotene, on ROS production. We used these antioxidants at concentrations as previously demonstrated to be effective in various assays [11, 18, 19]. We examined their effect on VBL and CIS, which increased ROS production in the above assays.
As shown in Fig. 4a–f, these antioxidative compounds exhibited various degrees of antioxidative effects. NAC showed the most potent inhibition on ROS production; ROS production was decreased by a quarter in prostate cancer cells. VBL or CIS did not further increase ROS production in the presence of NAC at either 37 or 42°C, suggesting the ROS production by these anticancer drugs was completely suppressed by NAC. Thus, NAC showed similar, but perhaps greater, antioxidative effect compared to ascorbic acid. Retinoic acid, quercetin, and lutein showed comparable results to each other. They inhibited ROS production at both 37 and 42°C. However, both VBL and CIS could increase ROS production in the presence of these antioxidants, suggesting that these antioxidants could not inhibit anticancer drug-mediated ROS production. Catechin and β-carotene are best known as general antioxidants. However, they did not inhibit ROS production, at either 37 or 42°C, in the absence or presence of anticancer drugs. Thus, the effects of many antioxidants are not always the same.
Fig. 4.
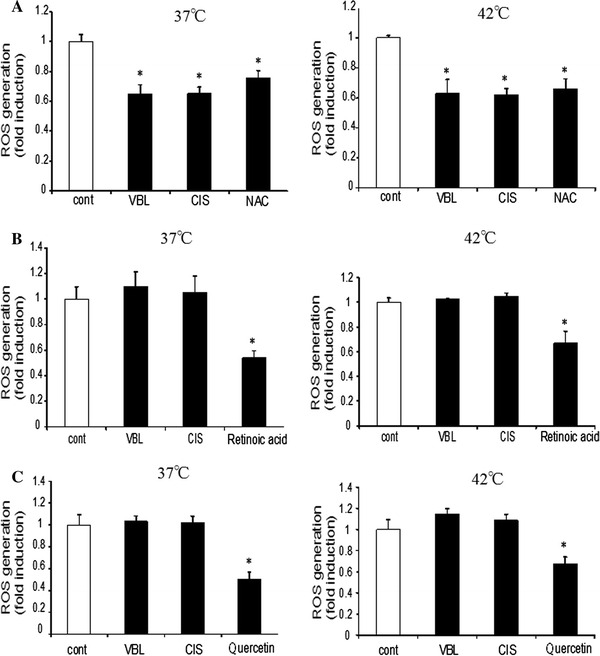
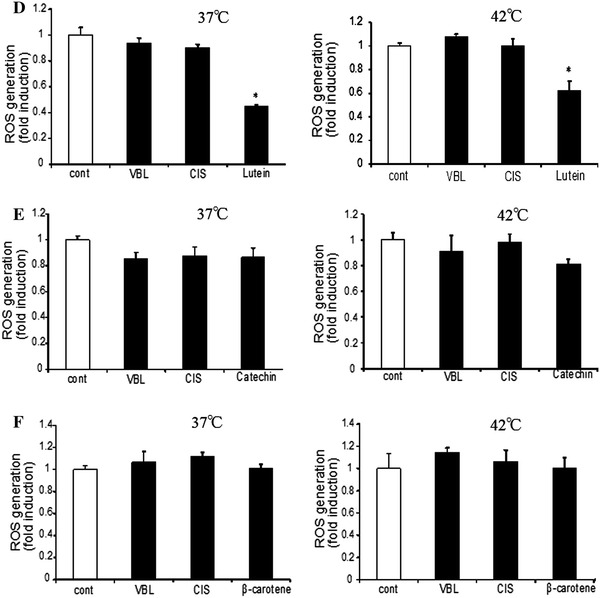
Effect of various antioxidants on ROS production. ROS production was determined in the presence of 200 nM VBL or 15 μM CIS at 37 or 42°C. Various antioxidants, i.e., 10 mM NAC (N-acetyl-cysteine), 50 nM retinoic acid, 100 nM quercetin, 50 μM catechin, 100 nM lutein, and 20 μM, β-carotene, were added. Cells were incubated for 45 min, followed by determination of ROS production (mean ± SEM; n = 4, *p < 0.05)
Discussion
The current study has demonstrated that ROS production was higher in cancer cells than in normal cells, and was further increased with temperature. Ascorbic acid exhibited the potent inhibition of ROS production regardless of temperature. ROS production was also increased in the presence of anticancer drugs, such as VBL and CIS, but not by DTX or ADR. Importantly, ROS production of these anticancer drugs was inhibited in the presence of ascorbic acid regardless of temperature. In contrast, antioxidants, some of which have been used as dietary supplements among the general population, showed variable effects. NAC inhibited ROS production regardless of the presence of anticancer drugs, while catechin or β-carotene did not inhibit ROS production. Lutein, quercetin, and retinoic acid inhibited ROS production in the absence of anticancer drugs, while they did not inhibit the ROS production as induced by anticancer drug. Thus, these antioxidants should be taken carefully by patients since they may variably affect the effect of anticancer drugs, at least in their ROS production.
ROS as a cause of cytotoxicity of anticancer drugs has been extensively studied in the past [20, 21]. CIS may interfere with mitochondrial membrane function and thus increases ROS production. Paclitaxel, which is comparable to DTX, may regulate membrane NOX release, and increases ROS production [22–25]. We found that both CIS and VBL increased ROS production in prostate cancer cells. Hyperthermic therapy potentiates ROS production, leading to enhanced cytotoxicity [26]. We also found that increased temperature enhanced ROS production by CIS and VBL. Thus, both cancer chemotherapy and hyperthermic treatment enhanced ROS production, at least in prostate cancer cells.
With increasing public interest in antioxidant therapy, many nutritional supplements have been taken by the general public including cancer patients. There have been multiple studies that have examined the interaction between anticancer drugs and antioxidants. However, the results of these studies are not in agreement with each other. Anticancer drugs may produce ROS, which may damage cancer cells [27, 28]. Thereby, some studies demonstrated that antioxidants reduced the effect of these anticancer drugs [29]. In contrast, others demonstrated that ROS production was enhanced by antioxidants [30]. More specifically, ascorbic acid can quench ROS within the cell, and thus stabilize mitochondrial membrane, leading to protection of the cell [13, 26]. Although previous studies have demonstrated that ascorbic acid increased the effect of anticancer drugs, more recently attenuation of anticancer drug effect has also been reported [26].
We found that antioxidants indeed exhibited various effects on ROS production. NAC, which by itself scavenges ROS [18], potently decreased ROS production, and ROS production by anticancer drugs was also negated. Thus, the use of NAC may hamper the effect of anticancer drugs. In contrast, lutein, quercetin, and retinoic acid, which are also known as ROS scavengers, decreased ROS production. However, they were not potent enough to inhibit the ROS-producing effect of anticancer compounds. Thus, these antioxidants may be taken safely by cancer patients during chemotherapy and hyperthermic therapy. Catechin and β-carotene are known as antioxidants and are contained in various kinds of foods, such as green tea or carrot [11, 12]. However, they did not exhibit inhibitory effect on ROS production regardless of the presence of anticancer drugs, suggesting that they do not interfere with such drug effects. Thus, cancer patients may take these antioxidants as well as foods containing these antioxidants.
Putting it together, administration of NAC and ascorbic acid may need caution while other antioxidants may not require major attention, at least in terms of ROS production in cancer patients. In particular, ascorbic acid is widely used for multiple purposes, including for viral infection. Accordingly, the current study has suggested that the use of ascorbic acid may be considered carefully by both cancer patients and oncologists. Further, with our findings, the effects of ascorbic acid and its related antioxidants need to be clinically examined in future in cancer patients who are to be treated with chemotherapy and/or hyperthermic therapy.
References
- 1.Johnson KA, Brown PH. Drug development for cancer chemoprevention: focus on molecular targets. Semin Oncol. 2010;37(4):345–358. doi: 10.1053/j.seminoncol.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 2.Zhang H, Wang G, Yang H. Drug delivery systems for differential release in combination therapy. Expert Opin Drug Deliv. 2011;8:171–190. doi: 10.1517/17425247.2011.547470. [DOI] [PubMed] [Google Scholar]
- 3.Suit HD, Shwayder M. Hyperthermia: potential as an anti-tumor agent. Cancer. 1974;34:122–129. doi: 10.1002/1097-0142(197407)34:1<122::AID-CNCR2820340118>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Li LF, Wang HQ, Liu XM, Zhang HL, Qiu LH, Qian ZZ, Li W. Nimotuzumab in combination with chemotherapy in patients with advanced non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi. 2011;33:626–628. [PubMed] [Google Scholar]
- 5.Rodriguez-Luccioni HL, Latorre-Esteves M, Mendez-Vega J, Soto O, Rodriguez AR, Rinaldi C, Torres-Lugo M. Enhanced reduction in cell viability by hyperthermia induced by magnetic nanoparticles. Int J Nanomed. 2011;6:373–380. doi: 10.2147/IJN.S14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen F, Wang CC, Kim E, Harrison LE. Hyperthermia in combination with oxidative stress induces autophagic cell death in HT-29 colon cancer cells. Cell Biol Int. 2008;32:715–723. doi: 10.1016/j.cellbi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz MD, Hansen JL, Prokopios-Davos S, Manola J, Wang Q, Bornstein BA, Hynynen K, Kaplan ID. Hyperthermia combined with radiation for the treatment of locally advanced prostate cancer: long-term results from Dana–Farber Cancer Institute study 94–153. Cancer. 2011;117:510–516. doi: 10.1002/cncr.25619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arai Y, Kondo T, Tanabe K, Zhao QL, Li FJ, Ogawa R, Li M, Kasuya M. Enhancement of hyperthermia-induced apoptosis by local anesthetics on human histiocytic lymphoma U937 cells. J Biol Chem. 2002;277:18986–18993. doi: 10.1074/jbc.M108084200. [DOI] [PubMed] [Google Scholar]
- 9.Chan SW, Nguyen PN, Ayele D, Chevalier S, Aprikian A, Chen JZ. Mitochondrial DNA damage is sensitive to exogenous H(2)O(2) but independent of cellular ROS production in prostate cancer cells. Mutat Res. 2011;716:40–50. doi: 10.1016/j.mrfmmm.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 10.Kurbacher CM, Wagner U, Kolster B, Andreotti PE, Krebs D, Bruckner HW. Ascorbic acid (vitamin C) improves the antineoplastic activity of doxorubicin, cisplatin, and paclitaxel in human breast carcinoma cells in vitro. Cancer Lett. 1996;103:183–189. doi: 10.1016/0304-3835(96)04212-7. [DOI] [PubMed] [Google Scholar]
- 11.Kim IS, Jin JY, Lee IH, Park SJ. Auranofin induces apoptosis and when combined with retinoic acid enhances differentiation of acute promyelocytic leukaemia cells in vitro. Br J Pharmacol. 2004;142:749–755. doi: 10.1038/sj.bjp.0705708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh SL, Wang WY, Huang CS, Hu ML. Flavonoids suppresses the enhancing effect of beta-carotene on DNA damage induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A549 cells. Chem Biol Interact. 2006;160:175–182. doi: 10.1016/j.cbi.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Heaney ML, Gardner JR, Karasavvas N, Golde DW, Scheinberg DA, Smith EA, O’Connor OA. Vitamin C antagonizes the cytotoxic effects of antineoplastic drugs. Cancer Res. 2008;68:8031–8038. doi: 10.1158/0008-5472.CAN-08-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maluta S, Dall’oglio S, Nadalini L. Treatment for intermediate and high-risk prostate cancer: controversial issues and the role of hyperthermia. Int J Hyperthermia. 2010;26:765–774. doi: 10.3109/02656736.2010.509749. [DOI] [PubMed] [Google Scholar]
- 15.Venkataraman S, Wagner BA, Jiang X, Wang HP, Schafer FQ, Ritchie JM, Patrick BC, Oberley LW, Buettner GR. Overexpression of manganese superoxide dismutase promotes the survival of prostate cancer cells exposed to hyperthermia. Free Radic Res. 2004;38:1119–1132. doi: 10.1080/10715760400010470. [DOI] [PubMed] [Google Scholar]
- 16.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuznetsov AV, Kehrer I, Kozlov AV, Haller M, Redl H, Hermann M, Grimm M, Troppmair J. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal Bioanal Chem. 2011;400:2383–2390. doi: 10.1007/s00216-011-4764-2. [DOI] [PubMed] [Google Scholar]
- 18.Supabphol A, Muangman V, Chavasiri W, Supabphol R, Gritsanapan W. N-acetylcysteine inhibits proliferation, adhesion, migration and invasion of human bladder cancer cells. J Med Assoc Thai. 2009;92:1171–1177. [PubMed] [Google Scholar]
- 19.Jimenez-Aliaga K, Bermejo-Bescos P, Benedi J, Martin-Aragon S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89:939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Sinha BK, Mimnaugh EG. Free radicals and anticancer drug resistance: oxygen free radicals in the mechanisms of drug cytotoxicity and resistance by certain tumors. Free Radic Biol Med. 1990;8:567–581. doi: 10.1016/0891-5849(90)90155-C. [DOI] [PubMed] [Google Scholar]
- 21.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/A:1009616228304. [DOI] [PubMed] [Google Scholar]
- 22.Alexandre J, Hu Y, Lu W, Pelicano H, Huang P. Novel action of paclitaxel against cancer cells: bystander effect mediated by reactive oxygen species. Cancer Res. 2007;67:3512–3517. doi: 10.1158/0008-5472.CAN-06-3914. [DOI] [PubMed] [Google Scholar]
- 23.Fukui M, Yamabe N, Zhu BT. Resveratrol attenuates the anticancer efficacy of paclitaxel in human breast cancer cells in vitro and in vivo. Eur J Cancer. 2010;46:1882–1891. doi: 10.1016/j.ejca.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayana K. Cisplatin induces duplex 3′ overhangs and 5′ blunt ends in epididymal epithelium in a Bax-dependent manner without any protection from l-ascorbic acid. Eur J Pharmacol. 2010;641:238–245. doi: 10.1016/j.ejphar.2010.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Kim HJ, Lee JH, Kim SJ, Oh GS, Moon HD, Kwon KB, Park C, Park BH, Lee HK, Chung SY, et al. Roles of NADPH oxidases in cisplatin-induced reactive oxygen species generation and ototoxicity. J Neurosci. 2010;30:3933–3946. doi: 10.1523/JNEUROSCI.6054-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verrax J, Calderon PB. The controversial place of vitamin C in cancer treatment. Biochem Pharmacol. 2008;76:1644–1652. doi: 10.1016/j.bcp.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 27.Jackson IL, Batinic-Haberle I, Sonveaux P, Dewhirst MW, Vujaskovic Z. ROS production and angiogenic regulation by macrophages in response to heat therapy. Int J Hyperthermia. 2006;22:263–273. doi: 10.1080/02656730600594027. [DOI] [PubMed] [Google Scholar]
- 28.Manda G, Nechifor MT, Neagu TM. Reactive oxygen species, cancer and anti-cancer therapies. Curr Chem Biol. 2009;3:342–366. doi: 10.2174/187231309787158271. [DOI] [Google Scholar]
- 29.Labriola D, Livingston R. Possible interactions between dietary antioxidants and chemotherapy. Oncology (Williston Park) 1999;13:1003–1008. [PubMed] [Google Scholar]
- 30.Block KI, Koch AC, Mead MN, Tothy PK, Newman RA, Gyllenhaal C. Impact of antioxidant supplementation on chemotherapeutic efficacy: a systematic review of the evidence from randomized controlled trials. Cancer Treat Rev. 2007;33:407–418. doi: 10.1016/j.ctrv.2007.01.005. [DOI] [PubMed] [Google Scholar]


