Abstract
Appropriate cardiovascular adjustment is necessary to meet the metabolic demands of working skeletal muscle during exercise. The sympathetic nervous system plays a crucial role in the regulation of arterial blood pressure and blood flow during exercise, and several important neural mechanisms are responsible for changes in sympathetic vasomotor outflow. Changes in sympathetic vasomotor outflow (i.e., muscle sympathetic nerve activity: MSNA) in inactive muscles during exercise differ depending on the exercise mode (static or dynamic), intensity, duration, and various environmental conditions (e.g., hot and cold environments or hypoxic). In 1991, Seals and Victor [6] reviewed MSNA responses to static and dynamic exercise with small muscle mass. This review provides an updated comprehensive overview on the MSNA response to exercise including large-muscle, dynamic leg exercise, e.g., two-legged cycling, and its regulatory mechanisms in healthy humans.
Keywords: MSNA, Leg cycling, Sympathetic nerve activity, Blood pressure
Introduction
Precise cardiovascular and hemodynamic adjustments are necessary to meet the metabolic demand of active skeletal muscle. An appropriate regulation of sympathetic vasomotor outflow is key for maintaining arterial blood pressure and to facilitate the delivery of blood flow to active skeletal muscle. Central command (a feedforward mechanism originating from the cerebral cortex and/or subcortical nuclei), the exercise pressor reflex (a feedback mechanism originating from skeletal muscle, i.e., metaboreflex and mechanoreflex), the arterial baroreflex (a negative feedback mechanism originating from the carotid sinus and aortic arch), and cardiopulmonary baroreflex (a negative feedback mechanism originating from low-pressure mechanically sensitive stretch receptors located in the heart, vena cava and blood vessels of the lungs) work in concert creating complex interactions that regulate sympathetic vasomotor outflow during exercise [1–4]. Alternations in sympathetic nerve activity during exercise have been inferred from changes in plasma norepinephrine concentrations. The main interpretive limitation of this measurement is that plasma levels are influenced by norepinephrine release and reuptake of norepinephrine [5, 6]. In addition, changes in plasma norepinephrine are progressing slowly, resulting in low time resolution. Therefore, direct measurement of sympathetic nervous activity is needed to provide more definitive insight into the effect of exercise. In 1972, Delius et al. [7] reported, for the first time, an increase in muscle sympathetic nerve activity (MSNA) during sustained muscle contractions (handgrip and leg adduction). Thereafter, numerous investigators have reported the MSNA response to exercise. These studies have revealed that the change in MSNA differs considerably depending on the exercise mode (static or dynamic), exercise intensity, duration of exercise, and environment (normoxia or hypoxia). In 1991, Seals and Victor [6] reviewed articles concerning MSNA responses to exercise in humans. At that time, no data on the MSNA responses to dynamic leg exercise with large muscle mass were available. This review aimed to provide an updated comprehensive overview on the MSNA response to exercise including large-muscle, dynamic leg exercise, e.g., two-legged cycling, and its regulatory mechanisms in healthy humans.
Microneurography
Microneurography is a technique that can be used to measure and record the electrical activity of the postganglionic sympathetic nerve [8–10]. In humans, MSNA can be measured from the radial, median, and ulnar nerves in the upper limb and from the peroneal and tibial nerves in the lower limb [10, 11]. Although microneurography has several limitations [9, 12], it is a gold standard for the assessment of sympathetic vasomotor outflow. The limb of the MSNA recording site must remain relaxed to avoid electromyographic contamination; thus, measurements of MSNA to contracting muscle are not possible, and MSNA has been recorded from inactive limb. The most recorded peripheral nerve is the peroneal nerve at the fibular head, and the subjects perform handgrip [13–21], arm cycling [13, 18, 22–24] or one-legged dynamic exercise [25–27]. Additionally, several researchers have measured MSNA from the radial [28, 29] or median nerve [30–35] during two-legged cycling. Real-time ultrasound guidance with microneurography has recently been utilized [11]. The assessment of MSNA is often an integrated signal of multiple nerve fibers presented as burst frequency (bursts/min), burst incidence (bursts/100 heart beats), and total activity (mean burst amplitude or burst area × number of bursts) [9]. In addition to the difficulty of recording MSNA during dynamic two-legged cycling, in some cases it is difficult to analyze burst amplitude and, thereby, total activity because electromyographic, efferent, and afferent nerve activities alter the baseline of the integrated neurogram during dynamic leg cycling [30, 31, 35]. For a more in-depth discussion of MSNA measurement methods and analyses, we refer the reader to previous, more detailed reviews [9, 36].
Regulation of MSNA
In this review, we focus on representative studies on MSNA regulation during exercise in humans. The reader is referred to a number of excellent reviews [1–4] that provide a much higher level of detail on individual topics.
Central command
The concept that signals from the higher brain contribute to the cardiovascular and hemodynamic adjustments to exercise has been well presented for over a century [1, 3, 4]. Originally termed “cortical irradiation”, and now known as “central command”, this refers to descending neural signals that involve the parallel activation of the somatomotor, respiratory, and cardiovascular systems [37]. The traditional supposition has been that central command sets the initial pattern of autonomic activation to the heart and blood vessels at the onset of exercise [37–39]. To determine the influence of central command on sympathetic vasomotor outflow, Mark et al. [14] compared MSNA responses during voluntary and involuntary (percutaneous electrical stimulation) biceps contraction at 20% maximal voluntary contraction (MVC). MSNA increased during involuntary contraction but decreased during voluntary contraction. This result suggests that during static exercise with small muscle mass, central command does not increase but, rather, inhibits MSNA. Victor et al. [40, 41] investigated whether central command affects MSNA during static and intense intermittent isometric handgrip exercise with and without partial neuromuscular blockade. During static handgrip exercise at 15% and 30% MVC and intermittent isometric handgrip exercise at 25% and 50% MVC, central command had very little effect on MSNA. In contrast, intermittent isometric handgrip exercise at 75% MVC induced an increase in MSNA [41]. These results suggest that central command increases MSNA only during higher intensities of exercise [1, 3, 4]. To date, no data on the influence of central command on MSNA responses to dynamic exercise with large muscle mass are available.
Exercise pressor reflex
A lot of researchers have devoted themselves to understanding the feedback mechanisms emanating from active skeletal muscle, and the importance of the exercise pressor reflex to the arterial blood pressure during dynamic exercise has been demonstrated [1, 3, 4, 42]. Skeletal muscle afferents are comprised of mechanically and metabolically sensitive sensory fibers (myelinated group III and unmyelinated group IV afferent fibers) that provide feedback to cardiovascular control areas in the brain stem [43–45]. Importantly, these temporal profiles for the activation of chemically sensitive receptors and channels are not absolute, since both group III and IV afferent fibers exhibit polymodal qualities [3, 43]. It has been suggested that the activation of both mechanically and metabolically sensitive afferent fibers contributes to exercise pressor reflex-mediated sympathetic vasomotor outflow [1, 3, 4]. It is very challenging to isolate the stimulation of mechanoreceptors in humans; primary adopted strategies include passive muscle stretch or passive limb movement. Indeed, Middlekauff et al. [46] recorded MSNA during passive arm extension/flexion movement, and MSNA remained unchanged from baseline during passive movement in healthy subjects. Cui et al. [47] investigated whether isolated stimulation of the mechanoreceptors can induce responses in MSNA. They found that passive leg calf muscle stretching induced transient increases in MSNA, supporting the idea that mechanoreceptors in muscles play a role in evoking the sympathetic response. During fatiguing isometric muscle contraction, the activity of muscle mechanoreceptors typically increases [48]. Thus, the accumulation of metabolites within active skeletal muscle can sensitize mechanically sensitive skeletal muscle afferents [4]. In contrast to the mechanoreflex, numerous human studies have shown a rather robust ability of the muscle metaboreflex to induce increases in MSNA. The majority of studies have used post-exercise ischemia to trap exercise-induced metabolites within the previously active muscle [4, 14, 49], e.g., an occlusion cuff placed over the upper arm is inflated to suprasystolic pressure (> 240 mmHg) after handgrip exercise. Thus, their stimulatory effect on metabolically sensitive skeletal muscle afferents is preserved in the absence of the muscle mechanoreflex and central command [1]. This maneuver has been consistently shown to maintain a major portion (~ 85%) of the exercise-induced increase in MSNA [1, 14] in an exercise intensity-dependent manner [50].
Arterial baroreflex
Arterial baroreceptors are located in the carotid artery and aorta and play a key role in the rapid sympathetic vasomotor adjustments to acute cardiovascular stressors [2]. These baroreceptors are mechanically sensitive and function as sensors in a negative feedback control loop that responds to beat-to-beat changes in arterial blood pressure [1, 3, 4]. When arterial blood pressure is elevated, baroreceptors are stretched, leading to further increases in afferent firing and resulting in a reflex-mediated decrease in MSNA [2]. In contrast, when arterial blood pressure decreases, tonic afferent firing is decreased, resulting in an increase in MSNA [2]. These neural adjustments affect blood vessels (altering total vascular conductance), to return arterial blood pressure to its original set point pressure [2, 3]. The ability of the arterial baroreflex to regulate arterial blood pressure is critically dependent on alterations in vascular tone both at rest and during exercise. Indeed, some studies have reported a progressive resetting of the baroreflex control of MSNA, to operate around the exercise-induced elevations in arterial blood pressure with maintained or increased sensitivity [23, 24, 51, 52]. Therefore, the arterial baroreflex control of MSNA is well maintained throughout a bout of exercise, and the arterial baroreflex plays an important role in the regulation of MSNA during exercise [1–4].
Cardiopulmonary baroreflex
Cardiopulmonary baroreceptors are mechanically sensitive stretch receptors located in the heart, vena cava, and blood vessels of the lungs that sense changes in central blood volume and pressure [53–55] and reflexively modulate MSNA. These phenomena have been revealed by multiple studies that recorded MSNA during lower body negative pressure and lower body positive pressure at resting conditions [15–17, 50, 56–58]. The cardiopulmonary baroreflex during dynamic exercise has been but seldom studied, and most representative work was performed by Ray et al. [25] who showed that MSNA was affected by changes in central blood volume mediated by the cardiopulmonary baroreflex during dynamic one-legged knee extension exercise in the sitting and supine positions. They found a reduction in MSNA below resting levels when exercise was performed in the sitting position, while no changes in MSNA were observed when exercise was performed in the supine position. Based on these data, it is believed that a decrease in MSNA in dynamic leg exercise is linked to the loading of cardiopulmonary baroreceptors, which is attributable to muscle pump-induced increases in venous return or central blood volume [2, 25, 55, 59, 60]. We [61] recently demonstrated that the enhanced muscle pump-induced increase in central blood volume led to decreased MSNA during two-legged cycling. Ogoh et al. [62] revealed that increasing the central blood volume, which loads the cardiopulmonary baroreceptors, reduces the magnitude of the exercise-induced increase in arterial blood pressure with the arterial baroreflex resetting. Importantly, the effect of the cardiopulmonary baroreflex on MSNA would be overcome by the skeletal muscle metaboreflex during high-intensity exercise [2, 50]. Overall, the cardiopulmonary baroreflex contributes to the regulation of MSNA during dynamic exercise at light or mild intensity.
Respiratory modulation
The respiratory modulation of MSNA is very clear in humans [63], and MSNA declines during inspiration, reaching its nadir at peak inspiration (when the lung volume is the highest and central respiratory motor output is at its peak) and then rises, reaching its peak at end expiration (when respiratory motor output and lung volume are the lowest) [64–67]. Potential mediators of the fluctuations in MSNA during respiration include coincident small changes in systemic blood pressure and lung volume. It seems, most likely, that the greatest influence of afferent input from pulmonary stretch receptors is in modulating sympathetic responsiveness to baroreceptor influences, although the respiratory modulation of MSNA remains complex and incompletely understood [63]. In addition, it has been assumed that carotid chemoreceptors are related to exercise-induced increases in MSNA [68]. To test this assumption, Stickland et al. [69] applied hyperoxic gas (inspired oxygen fraction [FIO2] = 1.00) to inhibit carotid chemoreceptor activity at rest and during rhythmic handgrip exercise with MSNA measurement. Transient hyperoxia had no significant effect on MSNA at rest, while MSNA decreased with hyperoxia during exercise. These results support the assumption that carotid chemoreceptors contribute to sympathetic vasoconstrictor outflow during exercise in humans. It is well known that high-intensity whole-body exercise leads to respiratory muscle fatigue [70, 71]. Fatiguing respiratory muscle work and the accumulation of metabolites are associated with neural and cardiovascular consequences, resulting in a redistribution of blood flow during exercise [68, 72]. Indeed, Dempsey and colleagues [35, 73–75] have reported that imposing a high work of breathing modulates MSNA at rest and during mild leg cycling with corresponding increases in arterial blood pressure and leg vascular resistance. This sympathoexcitation occurs through an inspiratory muscle-induced metaboreflex [76–78]. Dominelli et al. [79] recently confirmed a decrease in MSNA during two-legged cycling at moderate and high intensity when the work of breathing was reduced by a proportional assist ventilator. Based on these results, the respiratory muscle-induced metaboreflex contributes to the regulation of MSNA during dynamic leg exercise above moderate intensity.
MSNA responses to exercise
Static exercise
Upper limb exercise
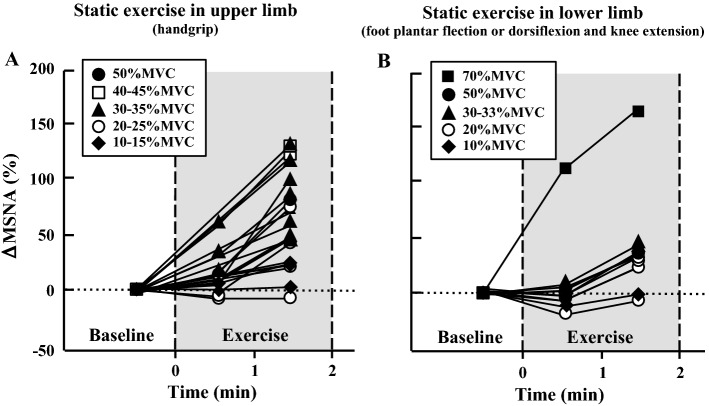
Numerous studies have confirmed increased MSNA during sustained handgrip exercise [14, 19, 21, 40, 50, 80–83]. The change in MSNA during sustained handgrip exercise increases in proportion to exercise intensity [6, 15, 17, 21, 40, 80] (Fig. 1a). The main mechanism of the increased MSNA during sustained exercise is associated with the accumulation of metabolites in active muscle, i.e., the muscle metaboreflex.
Fig. 1.
Changes in MSNA during static exercise in the upper (a) and lower limb (b) (isometric handgrip exercise and isometric foot planter flexion or dorsiflexion and isometric knee extension, respectively). Percent changes in MSNA from baseline were calculated by MSNA data (bursts/min) in published articles. a [14, 17, 19, 21, 40, 50, 80, 81, 83, 86], b [81, 84, 86]
Lower limb exercise
Some researchers have reported increased MSNA during sustained foot plantar flexion or dorsiflexion [81, 84] and isometric knee extension [85, 86]. Interestingly, when the static exercise performed in lower limb (knee extension) and exercise intensity is below 30% of maximal voluntary contraction (MVC), a temporal decrease in MSNA appears relative to that at rest (Fig. 1b). Then a time-dependent increase in MSNA appeared during static leg exercise [85].
Generally, MSNA does not increase during sustained exercise in the upper or lower limb if the exercise intensity is below 15% MVC and if the exercise duration lasts several minutes [17, 21, 40]. At the same relative exercise intensity and same duration, the magnitude (percentage) of the increased MSNA from baseline appears larger during upper limb than lower limb exercise. There are some cases in which MSNA decreased slightly over the first minutes during static light leg exercise when exercise was performed in a sitting, but not supine, position [85, 86]. Therefore, stimulating cardiopulmonary baroreceptors by increasing venous return may be related to decreased MSNA during static light leg exercise, although the mechanisms are unclear.
Dynamic exercise
Upper limb exercise
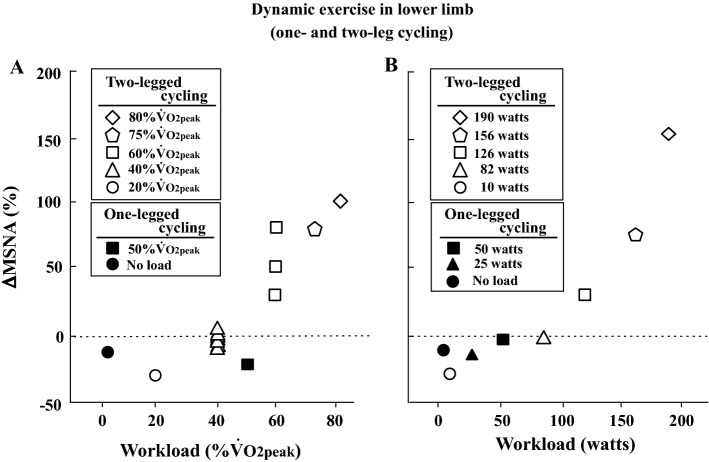
MSNA does not increase during dynamic (rhythmic) exercise with small muscle mass (handgrip) for a brief duration (~ 2 min) when the exercise intensity is less than 50% MVC (Fig. 2) [13, 18, 41, 69, 87–89]. When rhythmic handgrip exercise at 25% MVC is prolonged (~ 30 min), a progressive increase in MSNA appears [90] (Fig. 2). Regarding arm cycling, MSNA does not increase up to 30% maximal workload or ~ 30 watts over the first several minutes [13, 18], while MSNA does increase above 40 watts and 50% peak oxygen uptake (VO2peak) [13, 23, 24] (Fig. 3).
Fig. 2.
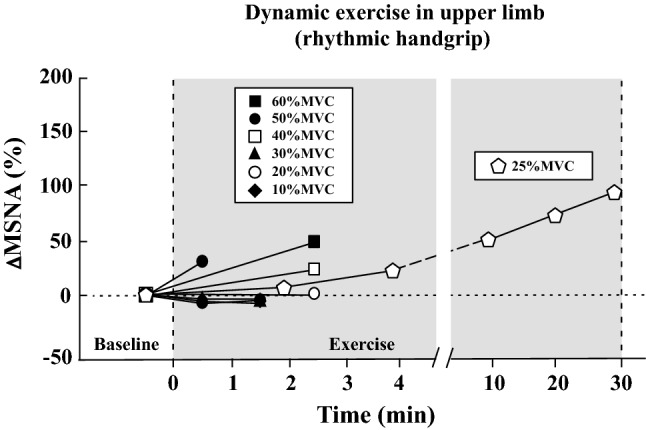
Changes in MSNA during dynamic exercise in the upper limb (rhythmic handgrip exercise). Percent changes in MSNA from baseline were calculated by MSNA data (bursts/min or total activity/min) in published articles [13, 18, 69, 89, 90]
Fig. 3.
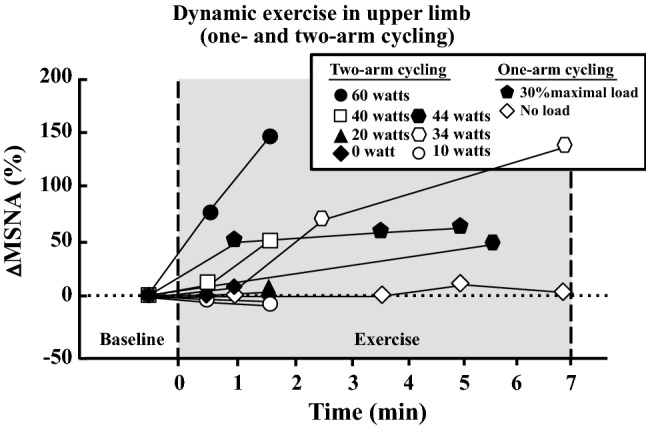
Changes in MSNA during dynamic exercise in the upper limb (one- and two-arm cycling). Percent changes in MSNA from baseline were calculated by MSNA data (bursts/min) in published articles [13, 18, 23, 24]
Lower limb exercise
Several research groups have attempted to record MSNA during dynamic leg exercise (Fig. 4). In 1993, Saito et al. [30] recorded MSNA from the median nerve during two-legged cycling at different intensities; MSNA decreased at a light intensity (20% VO2peak) relative to that at rest and returned to the resting level at mild intensity (40% VO2peak). These results were consistent with those obtained during one-legged cycling in their previous study [91]. This result indicates an inhibition of sympathetic vasomotor outflow during leg cycling at light and mild intensities. Above moderate intensity, MSNA during two-legged exercise gradually rose in proportion to the increase in workload [30]. This interesting phenomenon confirmed later studies that utilized one-legged [27, 28] and two-legged cycling [29, 35, 61, 74, 75, 92, 93]. The mechanism of the inhibition of MSNA during dynamic leg exercise at light and mild intensity is due to loading of the cardiopulmonary baroreceptors, which is induced by a muscle pump-induced increase in venous return and central blood volume [2, 25, 55, 59, 60]. The above-mentioned studies recorded MSNA less than 7 min at each intensity, and the changes in MSNA were strongly influenced by exercise duration. Ray et al. [26] reported changes in MSNA during prolonged dynamic exercise. The subjects performed dynamic one-legged knee extension exercise at 30 watts in an upright posture for 40 min. MSNA decreased during exercise during the first 10 min and returned to resting levels by 20 min and remained by 40 min (heart rate [HR] = 110 beats/min at 40 min) (Fig. 5). Moreover, Saito et al. [31] revealed changes in MSNA during two-legged cycling at 40% VO2peak for 30 min in a semirecumbent position; MSNA decreased during the first 10 min of cycling and then gradually increased above baseline by 30 min (HR = 113 beats/min at 30 min) (Fig. 5). The gradual increase in MSNA during prolonged leg cycling at mild intensity could be related to cardiovascular drift. Cardiovascular drift is defined as a continuous time-dependent change in some cardiovascular variables after ~ 10 min of prolonged moderate-intensity exercise [94–96]. General responses include reductions in stroke volume and arterial blood pressure and a parallel increase in HR, which are due to progressive increases in cutaneous blood flow and cutaneous vasodilation, as the body temperature rises. Thus, it is likely that a reduction of arterial blood pressure elicits an increase in MSNA during prolonged two-legged cycling [94]. In addition, central command and/or exercise pressor reflex may also contribute to a gradual increase in MSNA during prolonged exercise.
Fig. 4.
Changes in MSNA during dynamic exercise in the lower limb (two-legged or one-legged cycling). Exercise duration is within 6 min. Percent changes in MSNA from baseline were calculated by MSNA data (bursts/min) in published articles. a [27, 30, 31, 35, 61, 74, 75, 79, 93], b [51, 91]
Fig. 5.
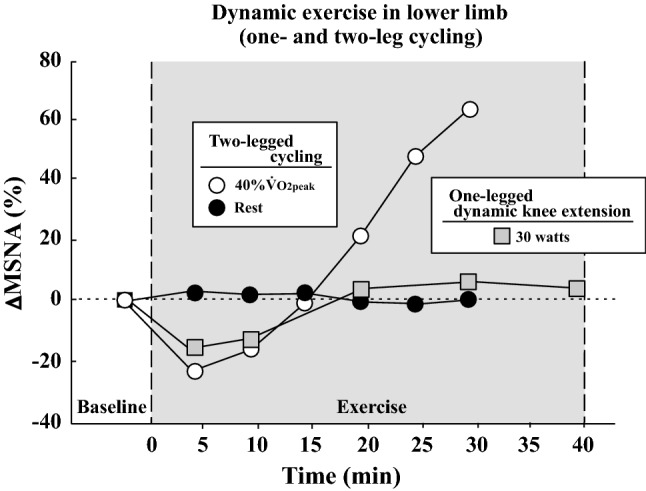
Changes in MSNA during dynamic prolonged exercise in the lower limb (two-legged cycling and one-legged dynamic knee extension). Percent changes in MSNA from baseline were calculated by MSNA data (bursts/min) in published articles [26, 31]
Environment
Heat stress induces increases in MSNA with raises in body temperature. Niimi et al. [97] measured resting MSNA with ambient temperatures of 29, 34, and 40 °C in an artificial climate chamber. They found progressive increases in MSNA during heat stress with rises in tympanic temperature. The increases in MSNA in response to acute heat stress could cause the redistribution of the circulatory blood flow from the muscles to the skin. Recently, Cui et al. [98] examined whether neural and cardiovascular response to stimulation of muscle metaboreceptors and mechanoreceptors are modified if body temperature is elevated before exercise. The magnitude increase in MSNA during handgrip exercise and post-exercise ischemia did not alter under whole body heat stress. However, MSNA response to exercise depends on the duration and intensity of the activity. Even if under normothermic conditions (21–25 °C), body temperature rises when dynamic leg cycling is prolonged, eliciting progressive increases in MSNA [31]. Thus, an increase in MSNA during whole-body exercise may potentiate in hot environment.
Sympathetic vasomotor outflow regulating arterial blood pressure changes with exposure to a cold environment. A representative maneuver is the cold pressure test whereby one hand is immersed in cold water for several minutes [99], and immersion evokes an increase in MSNA [100]. Fagius et al. [101] examined the effect of whole-body exposure to a cold environment (22.7–10.5 °C) on MSNA at rest, and a significant increase in MSNA appeared at a low environmental temperature, with simultaneous increase in arterial blood pressure. The increase in MSNA may play a role in body temperature regulation by preserving heat within the central core [101]. To our knowledge, little is known about the possible modulatory effects of cold environment on MSNA during exercise in humans. Further investigations are needed to clarify the MSNA responses during whole-body exercise in hot and cold environments.
Hypoxia produces a significant increase in sympathetic tone, to redistribute the blood to supply oxygen to vital organs. It is well known that acute hypoxia at rest induces an increase in MSNA in humans [102–104], and the magnitude of the MSNA response to brief hypoxia depends on the level and duration of hypoxic exposure. In healthy humans, a significant increase in MSNA appears when arterial oxygen saturation (SaO2) levels are below 80% [105] (FIO2 is below 0.125 or the altitude is above 4000 m), although there are wide individual differences. There are a lot of situations in which people perform exercise under hypoxic conditions, e.g., at high altitude or with pathophysiological conditions. Seals et al. [106] found a significant increase in MSNA during rhythmic handgrip exercise in hypoxia (FIO2 = 0.10). The magnitude of the increase in MSNA during hypoxic exercise was greater than the sum of the separate MSNA responses to the same exercise in normoxia and at resting level in hypoxia, suggesting an interaction between hypoxia and exercise effects. However, when rhythmic exercise was performed with blood flow occlusion, the extent of the MSNA response in normoxia did not differ from that in hypoxia [6, 106]. The latter results are consistent with those by Saito et al. [107] who showed that the magnitude of the MSNA response to static exercise was not altered under hypoxic conditions relative to normoxic conditions. These data indicate that the mechanism for the potentiation of the response to dynamic exercise with small muscle mass under hypoxic conditions originates in the contracting muscle [6, 106]. It would be interesting to evaluate the MSNA during dynamic leg exercise under hypoxic conditions. We [33] attempted to record MSNA during mild and moderate two-legged cycling under normoxic (FIO2 = 0.209) and hypoxic conditions (FIO2 = 0.127). Consequently, MSNA increased at 40% VO2peak exercise in hypoxia, but not in normoxia. These results suggest that acute hypoxia potentiates the MSNA response during dynamic exercise with large muscle mass and that hypoxia-induced heightened sympathetic nerve activity during dynamic leg exercise attenuates the cardiopulmonary baroreflex control of sympathetic vasomotor outflow.
Relationship between MSNA and plasma norepinephrine concentrations
Changes in sympathetic nerve activity during exercise have been estimated from alterations in plasma norepinephrine concentrations [108–111]. A significant relationship between plasma norepinephrine concentration and MSNA during arm cycling and static handgrip exercise were reported under normoxic conditions [22, 112], although the relative (%) increase in above baseline levels was larger in MSNA than in plasma norepinephrine concentrations. However, the changes in plasma norepinephrine concentrations during dynamic leg cycling at light and mild exercise differed from that in MSNA [33], suggesting that plasma norepinephrine concentrations during hypoxic exercise could be an imprecise and even misleading index of sympathetic nerve activity [103, 113, 114]. This reason may be attributed to increased norepinephrine concentration reuptake [115] and the inhibited neuronal release of norepinephrine during acute hypoxemia [103].
Conclusions
In this review, we provide an updated comprehensive overview on the MSNA response to exercise. Several groups have revealed changes in MSNA during large-muscle, dynamic leg exercise, e.g., two-legged cycling. MSNA decreases or is unchanged during two-legged cycling at light or mild intensity, suggesting the inhibition of sympathetic vasomotor outflow. This phenomenon is due to a muscle pump-induced increase in venous return and central blood volume, which loads cardiopulmonary baroreceptors. It is clear that the cardiopulmonary baroreflex plays a significant role in the regulation of sympathetic vasomotor outflow during dynamic exercise at light or mild intensity. During higher intensity dynamic exercise, it is plausible that the exercise pressor reflex from the active limb muscle in conjunction with the respiratory muscle-induced metaboreflex dominates to regulate sympathetic vasomotor outflow. Additionally, during high-intensity two-leg cycling, the respiratory muscle metaboreflex contributes to regulate sympathetic vasomotor outflow. Future studies are necessary to clarify 1) the interactions between central command, the exercise pressor reflex, the arterial baroreflex, the cardiopulmonary baroreflex, and the respiratory muscle metaboreflex, and 2) the MSNA responses during whole-body exercise in hot and cold environments or hypoxia.
Acknowledgements
This paper was supported, in part, by JSPS KAKENHI Grant No., JP16KK0201.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fadel PJ. Reflex control of the circulation during exercise. Scand J Med Sci Sports. 2015;25(Suppl 4):74–82. doi: 10.1111/sms.12600. [DOI] [PubMed] [Google Scholar]
- 2.Fadel PJ, Raven PB. Human investigations into the arterial and cardiopulmonary baroreflexes during exercise. Exp Physiol. 2012;97:39–50. doi: 10.1113/expphysiol.2011.057554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mueller PJ, Clifford PS, Crandall CG, Smith SA, Fadel PJ. Integration of central and peripheral regulation of the circulation during exercise: acute and chronic adaptations. Compr Physiol. 2017;8:103–151. doi: 10.1002/cphy.c160040. [DOI] [PubMed] [Google Scholar]
- 4.Fisher JP, Young CN, Fadel PJ. Autonomic adjustments to exercise in humans. Compr Physiol. 2015;5:475–512. doi: 10.1002/cphy.c140022. [DOI] [PubMed] [Google Scholar]
- 5.Esler M, Jennings G, Korner P, Willett I, Dudley F, Hasking G, Anderson W, Lambert G. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.HYP.11.1.3. [DOI] [PubMed] [Google Scholar]
- 6.Seals DR, Victor RG. Regulation of muscle sympathetic nerve activity during exercise in humans. Exerc Sport Sci Rev. 1991;19:313–349. doi: 10.1249/00003677-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand. 1972;84:82–94. doi: 10.1111/j.1748-1716.1972.tb05158.x. [DOI] [PubMed] [Google Scholar]
- 8.Joyner MJ, Wallin BG, Charkoudian N. Sex differences and blood pressure regulation in humans. Exp Physiol. 2016;101:349–355. doi: 10.1113/EP085146. [DOI] [PubMed] [Google Scholar]
- 9.White DW, Shoemaker JK, Raven PB. Methods and considerations for the analysis and standardization of assessing muscle sympathetic nerve activity in humans. Auton Neurosci. 2015;193:12–21. doi: 10.1016/j.autneu.2015.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol. 2004;96:1262–1269. doi: 10.1152/japplphysiol.00470.2003. [DOI] [PubMed] [Google Scholar]
- 11.Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Auton Neurosci. 2011;162:89–93. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter JR, Ray CA. Sympathetic neural adaptations to exercise training in humans. Auton Neurosci. 2015;188:36–43. doi: 10.1016/j.autneu.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest. 1987;79:508–516. doi: 10.1172/JCI112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res. 1985;57:461–469. doi: 10.1161/01.RES.57.3.461. [DOI] [PubMed] [Google Scholar]
- 15.Scherrer U, Vissing SF, Victor RG. Effects of lower-body negative pressure on sympathetic nerve responses to static exercise in humans. Microneurographic evidence against cardiac baroreflex modulation of the exercise pressor reflex. Circulation. 1988;78:49–59. doi: 10.1161/01.CIR.78.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Sanders JS, Ferguson DW. Cardiopulmonary baroreflexes fail to modulate sympathetic responses during isometric exercise in humans: direct evidence from microneurographic studies. J Am Coll Cardiol. 1988;12:1241–1251. doi: 10.1016/0735-1097(88)92607-1. [DOI] [PubMed] [Google Scholar]
- 17.Seals DR. Cardiopulmonary baroreflexes do not modulate exercise-induced sympathoexcitation. J Appl Physiol. 1988;64:2197–2203. doi: 10.1152/jappl.1988.64.5.2197. [DOI] [PubMed] [Google Scholar]
- 18.Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol. 1989;257:H2017–H2024. doi: 10.1152/ajpheart.1989.257.6.H2017. [DOI] [PubMed] [Google Scholar]
- 19.Seals DR, Enoka RM. Sympathetic activation is associated with increases in EMG during fatiguing exercise. J Appl Physiol. 1989;66:88–95. doi: 10.1152/jappl.1989.66.1.88. [DOI] [PubMed] [Google Scholar]
- 20.Seals DR. Sympathetic neural discharge and vascular resistance during exercise in humans. J Appl Physiol. 1989;66:2472–2478. doi: 10.1152/jappl.1989.66.5.2472. [DOI] [PubMed] [Google Scholar]
- 21.Seals DR, Chase PB, Taylor JA. Autonomic mediation of the pressor responses to isometric exercise in humans. J Appl Physiol. 1988;64:2190–2196. doi: 10.1152/jappl.1988.64.5.2190. [DOI] [PubMed] [Google Scholar]
- 22.Seals DR, Victor RG, Mark AL. Plasma norepinephrine and muscle sympathetic discharge during rhythmic exercise in humans. J Appl Physiol. 1988;65:940–944. doi: 10.1152/jappl.1988.65.2.940. [DOI] [PubMed] [Google Scholar]
- 23.Fadel PJ, Ogoh S, Watenpaugh DE, Wasmund W, Olivencia-Yurvati A, Smith ML, Raven PB. Carotid baroreflex regulation of sympathetic nerve activity during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2001;280:H1383–H1390. doi: 10.1152/ajpheart.2001.280.3.H1383. [DOI] [PubMed] [Google Scholar]
- 24.Ogoh S, Fisher JP, Raven PB, Fadel PJ. Arterial baroreflex control of muscle sympathetic nerve activity in the transition from rest to steady-state dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 2007;293:H2202–H2209. doi: 10.1152/ajpheart.00708.2007. [DOI] [PubMed] [Google Scholar]
- 25.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to dynamic one-legged exercise: effect of body posture. Am J Physiol. 1993;264:H1–H7. doi: 10.1152/ajpcell.1993.264.1.C1. [DOI] [PubMed] [Google Scholar]
- 26.Ray CA. Muscle sympathetic nerve responses to prolonged one-legged exercise. J Appl Physiol. 1993;74:1719–1722. doi: 10.1152/jappl.1993.74.4.1719. [DOI] [PubMed] [Google Scholar]
- 27.Notarius CF, Millar PJ, Murai H, Morris BL, Marzolini S, Oh P, Floras JS. Divergent muscle sympathetic responses to dynamic leg exercise in heart failure and age-matched healthy subjects. J Physiol. 2015;593:715–722. doi: 10.1113/jphysiol.2014.281873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Callister R, Ng AV, Seals DR. Arm muscle sympathetic nerve activity during preparation for and initiation of leg-cycling exercise in humans. J Appl Physiol. 1994;77:1403–1410. doi: 10.1152/jappl.1994.77.3.1403. [DOI] [PubMed] [Google Scholar]
- 29.Moralez G, Jouett NP, Tian J, Zimmerman MC, Bhella P, Raven PB. Effect of centrally acting angiotensin converting enzyme inhibitor on the exercise-induced increases in muscle sympathetic nerve activity. J Physiol. 2018;596:2315–2332. doi: 10.1113/JP274697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito M, Tsukanaka A, Yanagihara D, Mano T. Muscle sympathetic nerve responses to graded leg cycling. J Appl Physiol. 1993;75:663–667. doi: 10.1152/jappl.1993.75.2.663. [DOI] [PubMed] [Google Scholar]
- 31.Saito M, Sone R, Ikeda M, Mano T. Sympathetic outflow to the skeletal muscle in humans increases during prolonged light exercise. J Appl Physiol. 1997;82:1237–1243. doi: 10.1152/jappl.1997.82.4.1237. [DOI] [PubMed] [Google Scholar]
- 32.Ichinose M, Saito M, Kitano A, Hayashi K, Kondo N, Nishiyasu T. Modulation of arterial baroreflex dynamic response during mild orthostatic stress in humans. J Physiol. 2004;557:321–330. doi: 10.1113/jphysiol.2003.057133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katayama K, Ishida K, Iwamoto E, Iemitsu M, Koike T, Saito M. Hypoxia augments muscle sympathetic neural response to leg cycling. Am J Physiol Regul Integr Comp Physiol. 2011;301:R456–R464. doi: 10.1152/ajpregu.00119.2011. [DOI] [PubMed] [Google Scholar]
- 34.Katayama K, Ishida K, Saito M, Koike T, Ogoh S. Hypoxia attenuates cardiopulmonary reflex control of sympathetic nerve activity during mild dynamic leg exercise. Exp Physiol. 2016;101:377–386. doi: 10.1113/EP085632. [DOI] [PubMed] [Google Scholar]
- 35.Katayama K, Iwamoto E, Ishida K, Koike T, Saito M. Inspiratory muscle fatigue increases sympathetic vasomotor outflow and blood pressure during submaximal exercise. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1167–R1175. doi: 10.1152/ajpregu.00006.2012. [DOI] [PubMed] [Google Scholar]
- 36.Shoemaker JK, Klassen SA, Badrov MB, Fadel PJ. Fifty years of microneurography: learning the language of the peripheral sympathetic nervous system in humans. J Neurophysiol. 2018;119:1731–1744. doi: 10.1152/jn.00841.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodwin GM, McCloskey DI, Mitchell JH. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krogh A, Lindhard J. The regulation of respiration and circulation during the initial stages of muscular work. J Physiol. 1913;47:112–136. doi: 10.1113/jphysiol.1913.sp001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell JH. Cardiovascular control during exercise: central and reflex neural mechanisms. Am J Cardiol. 1985;55:34D–41D. doi: 10.1016/0002-9149(85)91053-7. [DOI] [PubMed] [Google Scholar]
- 40.Victor RG, Pryor SL, Secher NH, Mitchell JH. Effects of partial neuromuscular blockade on sympathetic nerve responses to static exercise in humans. Circ Res. 1989;65:468–476. doi: 10.1161/01.RES.65.2.468. [DOI] [PubMed] [Google Scholar]
- 41.Victor RG, Secher NH, Lyson T, Mitchell JH. Central command increases muscle sympathetic nerve activity during intense intermittent isometric exercise in humans. Circ Res. 1995;76:127–131. doi: 10.1161/01.RES.76.1.127. [DOI] [PubMed] [Google Scholar]
- 42.Strange S, Secher NH, Pawelczyk JA, Karpakka J, Christensen NJ, Mitchell JH, Saltin B. Neural control of cardiovascular responses and of ventilation during dynamic exercise in man. J Physiol. 1993;470:693–704. doi: 10.1113/jphysiol.1993.sp019883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mitchell JH. J.B. Wolffe memorial lecture. Neural control of the circulation during exercise. Med Sci Sports Exerc. 1990;22:141–154. doi: 10.1249/00005768-199004000-00535. [DOI] [PubMed] [Google Scholar]
- 44.Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol. 2006;91:89–102. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman MP, Foster HV. Reflexes controlling circulatory, ventilatory, and airway responses to exercise. In: Rowell LB, Shepherd JT, editors. Exercise: regulation and integration of multiple systems. Bethsda: American Physiological Society; 1996. [Google Scholar]
- 46.Middlekauff HR, Chiu J, Hamilton MA, Fonarow GC, Maclellan WR, Hage A, Moriguchi J, Patel J. Muscle mechanoreceptor sensitivity in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1937–H1943. doi: 10.1152/ajpheart.00330.2004. [DOI] [PubMed] [Google Scholar]
- 47.Cui J, Blaha C, Moradkhan R, Gray KS, Sinoway LI. Muscle sympathetic nerve activity responses to dynamic passive muscle stretch in humans. J Physiol. 2006;576:625–634. doi: 10.1113/jphysiol.2006.116640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- 49.Holwerda SW, Restaino RM, Manrique C, Lastra G, Fisher JP, Fadel PJ. Augmented pressor and sympathetic responses to skeletal muscle metaboreflex activation in type 2 diabetes patients. Am J Physiol Heart Circ Physiol. 2016;310:H300–H309. doi: 10.1152/ajpheart.00636.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katayama K, Kaur J, Young BE, Barbosa TC, Ogoh S, Fadel PJ. High-intensity muscle metaboreflex activation attenuates cardiopulmonary baroreflex-mediated inhibition of muscle sympathetic nerve activity. J Appl Physiol. 2018;125:812–819. doi: 10.1152/japplphysiol.00161.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ichinose M, Saito M, Fujii N, Ogawa T, Hayashi K, Kondo N, Nishiyasu T. Modulation of the control of muscle sympathetic nerve activity during incremental leg cycling. J Physiol. 2008;586:2753–2766. doi: 10.1113/jphysiol.2007.150060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Keller DM, Fadel PJ, Ogoh S, Brothers RM, Hawkins M, Olivencia-Yurvati A, Raven PB. Carotid baroreflex control of leg vasculature in exercising and non-exercising skeletal muscle in humans. J Physiol. 2004;561:283–293. doi: 10.1113/jphysiol.2004.071944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mark AL, Mancia G. Cardiopulmonary baroreflexes in humans. In: Berne RM, editor. Handbook of physiology, the cardiovascular system, peripheral circulation and organ blood flow. Bethesda: American Physiological Society; 1983. pp. 795–813. [Google Scholar]
- 54.Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- 55.Ray CA, Saito M. The cardiopulmonary baroreflex. In: Saltin B, Boushel R, Secher NH, Mitchell JH, editors. Exercise and circulation in health and disease. Champaign: Human Kinetics; 1999. pp. 43–51. [Google Scholar]
- 56.Rea RF, Wallin BG. Sympathetic nerve activity in arm and leg muscles during lower body negative pressure in humans. J Appl Physiol. 1989;66:2778–2781. doi: 10.1152/jappl.1989.66.6.2778. [DOI] [PubMed] [Google Scholar]
- 57.Fu Q, Sugiyama Y, Kamiya A, Shamsuzzaman AS, Mano T. Responses of muscle sympathetic nerve activity to lower body positive pressure. Am J Physiol. 1998;275:H1254–H1259. doi: 10.1152/ajpheart.1998.275.4.H1254. [DOI] [PubMed] [Google Scholar]
- 58.Fu Q, Sugiyama Y, Kamiya A, Mano T. A comparison of autonomic responses in humans induced by two simulation models of weightlessness: lower body positive pressure and 6 degrees head-down tilt. J Auton Nerv Syst. 2000;80:101–107. doi: 10.1016/S0165-1838(00)00081-3. [DOI] [PubMed] [Google Scholar]
- 59.Mack G, Nose H, Nadel ER. Role of cardiopulmonary baroreflexes during dynamic exercise. J Appl Physiol. 1988;65:1827–1832. doi: 10.1152/jappl.1988.65.4.1827. [DOI] [PubMed] [Google Scholar]
- 60.Raven PB, Chapleau MW. Blood pressure regulation XI: overview and future research directions. Eur J Appl Physiol. 2014;114:579–586. doi: 10.1007/s00421-014-2823-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katayama K, Ishida K, Saito M, Koike T, Hirasawa A, Ogoh S. Enhanced muscle pump during mild dynamic leg exercise inhibits sympathetic vasomotor outflow. Physiol Rep. 2014;2:e12070. doi: 10.14814/phy2.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ogoh S, Fisher JP, Fadel PJ, Raven PB. Increases in central blood volume modulate carotid baroreflex resetting during dynamic exercise in humans. J Physiol. 2007;581:405–418. doi: 10.1113/jphysiol.2006.125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dempsey JA, Sheel AW, St Croix CM, Morgan BJ. Respiratory influences on sympathetic vasomotor outflow in humans. Respir Physiol Neurobiol. 2002;130:3–20. doi: 10.1016/S0034-5687(01)00327-9. [DOI] [PubMed] [Google Scholar]
- 64.Eckberg DL, Nerhed C, Wallin BG. Respiratory modulation of muscle sympathetic and vagal cardiac outflow in man. J Physiol. 1985;365:181–196. doi: 10.1113/jphysiol.1985.sp015766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Seals DR, Suwarno NO, Dempsey JA. Influence of lung volume on sympathetic nerve discharge in normal humans. Circ Res. 1990;67:130–141. doi: 10.1161/01.RES.67.1.130. [DOI] [PubMed] [Google Scholar]
- 66.Macefield VG, Wallin BG. Modulation of muscle sympathetic activity during spontaneous and artificial ventilation and apnoea in humans. J Auton Nerv Syst. 1995;53:137–147. doi: 10.1016/0165-1838(94)00173-H. [DOI] [PubMed] [Google Scholar]
- 67.St Croix CM, Satoh M, Morgan BJ, Skatrud JB, Dempsey JA. Role of respiratory motor output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ Res. 1999;85:457–469. doi: 10.1161/01.RES.85.5.457. [DOI] [PubMed] [Google Scholar]
- 68.Dempsey JA. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J Physiol. 2012;590:4129–4144. doi: 10.1113/jphysiol.2012.233908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stickland MK, Morgan BJ, Dempsey JA. Carotid chemoreceptor modulation of sympathetic vasoconstrictor outflow during exercise in healthy humans. J Physiol. 2008;586:1743–1754. doi: 10.1113/jphysiol.2007.147421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol. 2008;104:879–888. doi: 10.1152/japplphysiol.01157.2007. [DOI] [PubMed] [Google Scholar]
- 72.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson NP, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 73.St Croix CM, Morgan BJ, Wetter TJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katayama K, Itoh Y, Saito M, Koike T, Ishida K. Sympathetic vasomotor outflow and blood pressure increase during exercise with expiratory resistance. Physiol Rep. 2015;3:e12421. doi: 10.14814/phy2.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katayama K, Smith JR, Goto K, Shimizu K, Saito M, Ishida K, Koike T, Iwase S, Harms CA. Elevated sympathetic vasomotor outflow in response to increased inspiratory muscle activity during exercise is less in young women compared with men. Exp Physiol. 2018;103:570–580. doi: 10.1113/EP086817. [DOI] [PubMed] [Google Scholar]
- 76.Dempsey JA, Amann M, Romer LM, Miller JD. Respiratory system determinants of peripheral fatigue and endurance performance. Med Sci Sports Exerc. 2008;40:457–461. doi: 10.1249/MSS.0b013e31815f8957. [DOI] [PubMed] [Google Scholar]
- 77.Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856:240–244. doi: 10.1016/S0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- 78.Sheel AW, Boushel RC, Dempsey JA. Competition for blood flow distribution between respiratory and locomotor muscles: implications for muscle fatigue. J Appl Physiol. 2018;125:820–831. doi: 10.1152/japplphysiol.00189.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dominelli PB, Katayama K, Vermeulen TD, Stuckless TJR, Brown CV, Foster GE, William Sheel A. Work of breathing influences muscle sympathetic nerve activity during semi-recumbent cycle exercise. Acta Physiol. 2018;225:e13212. doi: 10.1111/apha.13212. [DOI] [PubMed] [Google Scholar]
- 80.Saito M, Mano T, Abe H, Iwase S. Responses in muscle sympathetic nerve activity to sustained hand-grips of different tensions in humans. Eur J Appl Physiol Occup Physiol. 1986;55:493–498. doi: 10.1007/BF00421643. [DOI] [PubMed] [Google Scholar]
- 81.Saito M. Differences in muscle sympathetic nerve response to isometric exercise in different muscle groups. Eur J Appl Physiol Occup Physiol. 1995;70:26–35. doi: 10.1007/BF00601805. [DOI] [PubMed] [Google Scholar]
- 82.Wallin BG. Muscle sympathetic activity and plasma concentrations of noradrenaline. Acta Physiol Scand Suppl. 1984;527:21–24. [PubMed] [Google Scholar]
- 83.Seals DR. Influence of muscle mass on sympathetic neural activation during isometric exercise. J Appl Physiol. 1989;67:1801–1806. doi: 10.1152/jappl.1989.67.5.1801. [DOI] [PubMed] [Google Scholar]
- 84.Kamiya A, Michikami D, Fu Q, Niimi Y, Iwase S. Muscle sympathetic nerve activity (MSNA) during high-intensity, isometric leg exercise in humans. Environ Med. 2000;44:49–52. [PubMed] [Google Scholar]
- 85.Ray CA, Mark AL. Augmentation of muscle sympathetic nerve activity during fatiguing isometric leg exercise. J Appl Physiol. 1993;75:228–232. doi: 10.1152/jappl.1993.75.1.228. [DOI] [PubMed] [Google Scholar]
- 86.Ray CA, Rea RF, Clary MP, Mark AL. Muscle sympathetic nerve responses to static leg exercise. J Appl Physiol. 1992;73:1523–1529. doi: 10.1152/jappl.1992.73.4.1523. [DOI] [PubMed] [Google Scholar]
- 87.Saito M, Kagaya A, Ogita F, Shinohara M. Changes in muscle sympathetic nerve activity and calf blood flow during combined leg and forearm exercise. Acta Physiol Scand. 1992;146:449–456. doi: 10.1111/j.1748-1716.1992.tb09446.x. [DOI] [PubMed] [Google Scholar]
- 88.Pott F, Ray CA, Olesen HL, Ide K, Secher NH. Middle cerebral artery blood velocity, arterial diameter and muscle sympathetic nerve activity during post-exercise muscle ischaemia. Acta Physiol Scand. 1997;160:43–47. doi: 10.1046/j.1365-201X.1997.00126.x. [DOI] [PubMed] [Google Scholar]
- 89.Incognito AV, Doherty CJ, Nardone M, Lee JB, Notay K, Seed JD, Millar PJ. Evidence for differential control of muscle sympathetic single units during mild sympathoexcitation in young healthy humans. Am J Physiol Heart Circ Physiol. 2019;316:H13–H23. doi: 10.1152/ajpheart.00675.2018. [DOI] [PubMed] [Google Scholar]
- 90.Batman BA, Hardy JC, Leuenberger UA, Smith MB, Yang QX, Sinoway LI. Sympathetic nerve activity during prolonged rhythmic forearm exercise. J Appl Physiol. 1994;76:1077–1081. doi: 10.1152/jappl.1994.76.3.1077. [DOI] [PubMed] [Google Scholar]
- 91.Saito M, Mano T. Exercise mode affects muscle sympathetic nerve responsiveness. Jpn J Physiol. 1991;41:143–151. doi: 10.2170/jjphysiol.41.143. [DOI] [PubMed] [Google Scholar]
- 92.Dominelli PB, Katayama K, Vermuelen TD, Stuckless TJ, Brown CV, Foster GE, Sheel AW. Work of breathing influences muscle sympathetic nerve activity during whole-body execise. Med Sci Sports Exerc. 2018;50:122. doi: 10.1249/01.mss.0000535489.43958.a5. [DOI] [PubMed] [Google Scholar]
- 93.Katayama K, Yamashita S, Ishida K, Iwamoto E, Koike T, Saito M. Hypoxic effects on sympathetic vasomotor outflow and blood pressure during exercise with inspiratory resistance. Am J Physiol Regul Integr Comp Physiol. 2013;304:R374–R382. doi: 10.1152/ajpregu.00489.2012. [DOI] [PubMed] [Google Scholar]
- 94.Coyle EF, Gonzalez-Alonso J. Cardiovascular drift during prolonged exercise: new perspectives. Exerc Sport Sci Rev. 2001;29:88–92. doi: 10.1097/00003677-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 95.Rowell LB. Control of regional blood flow during dynamic exercise. In: Rowell LB, editor. Human cardiovascular control. New York: Oxford University Press; 1993. pp. 204–254. [Google Scholar]
- 96.Ekelund LG. Circulatory and respiratory adaptation during prolonged exercise. Acta Physiol Scand Suppl. 1967;292:1–38. [PubMed] [Google Scholar]
- 97.Niimi Y, Matsukawa T, Sugiyama Y, Shamsuzzaman AS, Ito H, Sobue G, Mano T. Effect of heat stress on muscle sympathetic nerve activity in humans. J Auton Nerv Syst. 1997;63:61–67. doi: 10.1016/S0165-1838(96)00134-8. [DOI] [PubMed] [Google Scholar]
- 98.Cui J, Blaha C, Sinoway LI. Whole body heat stress attenuates the pressure response to muscle metaboreceptor stimulation in humans. J Appl Physiol. 2016;121:1178–1186. doi: 10.1152/japplphysiol.00212.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hines EA, Brown GE. The cold pressor test for measuring the reactibility of the blood pressure: data concerning 571 normal and hypertensive subjects. Am Heart J. 1936;11:1–9. doi: 10.1016/S0002-8703(36)90370-8. [DOI] [Google Scholar]
- 100.Fagius J, Karhuvaara S, Sundlof G. The cold pressor test: effects on sympathetic nerve activity in human muscle and skin nerve fascicles. Acta Physiol Scand. 1989;137:325–334. doi: 10.1111/j.1748-1716.1989.tb08760.x. [DOI] [PubMed] [Google Scholar]
- 101.Fagius J, Kay R. Low ambient temperature increases baroreflex-governed sympathetic outflow to muscle vessels in humans. Acta Physiol Scand. 1991;142:201–209. doi: 10.1111/j.1748-1716.1991.tb09148.x. [DOI] [PubMed] [Google Scholar]
- 102.Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol. 1988;65:1548–1552. doi: 10.1152/jappl.1988.65.4.1548. [DOI] [PubMed] [Google Scholar]
- 103.Rowell LB, Johnson DG, Chase PB, Comess KA, Seals DR. Hypoxia raises muscle sympathetic activity but not norepinephrine in resting humans. J Appl Physiol. 1989;66:1736–1743. doi: 10.1152/jappl.1989.66.4.1736. [DOI] [PubMed] [Google Scholar]
- 104.Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol. 1989;67:2095–2100. doi: 10.1152/jappl.1989.67.5.2095. [DOI] [PubMed] [Google Scholar]
- 105.Smith ML, Muenter NK. Effects of hypoxia on sympathetic neural control in humans. Respir Physiol. 2000;121:163–171. doi: 10.1016/S0034-5687(00)00141-9. [DOI] [PubMed] [Google Scholar]
- 106.Seals DR, Johnson DG, Fregosi RF. Hypoxia potentiates exercise-induced sympathetic neural activation in humans. J Appl Physiol. 1991;71:1032–1040. doi: 10.1152/jappl.1991.71.3.1032. [DOI] [PubMed] [Google Scholar]
- 107.Saito M, Abe H, Iwase S, Koga K, Mano T. Muscle sympathetic nerve responsiveness to static contraction is not altered under hypoxia. Jpn J Physiol. 1991;41:775–783. doi: 10.2170/jjphysiol.41.775. [DOI] [PubMed] [Google Scholar]
- 108.Bubb WJ, Howley ET, Cox RH. Effects of various levels of hypoxia on plasma catecholamines at rest and during exercise. Aviat Space Environ Med. 1983;54:637–640. [PubMed] [Google Scholar]
- 109.Clancy LJ, Critchley JA, Leitch AG, Kirby BJ, Ungar A, Flenley DC. Arterial catecholamines in hypoxic exercise in man. Clin Sci Mol Med. 1975;49:503–506. doi: 10.1042/cs0490503. [DOI] [PubMed] [Google Scholar]
- 110.Escourrou P, Johnson DG, Rowell LB. Hypoxemia increases plasma catecholamine concentrations in exercising humans. J Appl Physiol. 1984;57:1507–1511. doi: 10.1152/jappl.1984.57.5.1507. [DOI] [PubMed] [Google Scholar]
- 111.Rowell LB, Blackmon JR, Kenny MA, Escourrou P. Splanchnic vasomotor and metabolic adjustments to hypoxia and exercise in humans. Am J Physiol. 1984;247:H251–H258. doi: 10.1152/ajpheart.1984.247.2.H251. [DOI] [PubMed] [Google Scholar]
- 112.Wallin BG, Morlin C, Hjemdahl P. Muscle sympathetic activity and venous plasma noradrenaline concentrations during static exercise in normotensive and hypertensive subjects. Acta Physiol Scand. 1987;129:489–497. doi: 10.1111/j.1748-1716.1987.tb08088.x. [DOI] [PubMed] [Google Scholar]
- 113.Seals DR, Jones PP, Davy KP. Autonomic nervous system. In: Hornbein TF, Schoene RB, editors. High altitude. NewYork: Mercel Dekker; 2001. pp. 425–442. [Google Scholar]
- 114.Folkow B, Di Bona GF, Hjemdahl P, Toren PH, Wallin BG. Measurements of plasma norepinephrine concentrations in human primary hypertension. A word of caution on their applicability for assessing neurogenic contributions. Hypertension. 1983;5:399–403. doi: 10.1161/01.HYP.5.4.399. [DOI] [PubMed] [Google Scholar]
- 115.Leuenberger U, Gleeson K, Wroblewsski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol. 1991;261:H1659–H1664. doi: 10.1152/ajpheart.1991.261.5.H1659. [DOI] [PubMed] [Google Scholar]