Abstract
In human and many other animals, estrogens inhibit food intake and increases spontaneous activity. Previous studies hypothesized that the anorexigenic effect of estrogens is mediated by the cholecystokinin (CCK)-induced satiety effect. In the present study, we investigated whether estrogens-induced anorexigenic and hyper-active effects are present in Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rat, which is deficient in the CCK1 receptor. In OLETF rats with a regular 4-day estrous cycle, food intake decreased and spontaneous activity increased significantly more during estrus than diestrus as compared to control Long-Evans-Tokushima-Otsuka (LETO) rats. Subcutaneous injection of estradiol benzoate into ovariectomized OLETF rats significantly decreased feeding and increased spontaneous activity to the same extent as in LETO rats. These results suggest that the anorexigenic and hyper-active effects of estrogen can be mediated via pathways other than CCK-CCK1 receptor signaling pathway in CCK1 receptor-deficient rats.
Keywords: Estrogen, OLETF rat, Food intake, CCK
Introduction
Estrogens are ovarian steroid hormones known to inhibit eating and to stimulate locomotor activity, in addition to their action in the reproductive system. Daily food intake in women is lowest during the peri-ovulatory period when plasma estradiol (E2) levels are maximal during the menstrual cycle [1]. In female rats, estrus is associated with increased locomotor activity as well as decreased feeding [1–4]. On estrus, meal size is smaller and running wheel activity is more than on diestrus [5, 6]. Ovariectomy eliminates the cyclic decrease in feeding during the estrous cycle and tonically increases food intake [1, 7, 8]. Furthermore, estrogen treatment in ovariectomized rats decreases meal size [1, 3] and increases running wheel activity [9]. Thus, there are estrogens that suppress meal size and increase locomotor activity and bring cyclic change in feeding and locomotor activity during the estrous cycle.
The mechanism by which estrogens affect food intake is not well understood, but there is a hypothesis that the anorexigenic effect of estrogens is mediated by cholecystokinin (CCK) [10]. CCK is released from the duodenum and jejunum in response to intraluminal presence of nutrient-digestive products, and acts as a satiety signal to limit meal size. Intraperitoneal injection of CCK decreases food intake and produces the earlier appearance of a behavioral satiety sequence [11]. CCK’s satiating action is mediated by CCK1 (CCKA) receptors, which are present in abdominal vagal afferent fibers [12]. CCK1 receptor antagonists competitively block the feeding-inhibitory effect of exogenous CCK [13, 14]. Furthermore, an administration of CCK1 antagonists increases food intake [14, 15]. The satiating signal conveyed by the vagus nerve is processed in the nucleus tractus solitarius (NTS) in the dorsal hindbrain [16, 17]. Recently, estrogens have been reported to affect this processing in the NTS of the incoming vagal CCK satiation signal [18, 19].
Since CCK cannot explain all the anorexigenic effect of estrogen, we intended to investigate further the factor(s) mediating estrogens’ anorexigenic effect in addition to CCK. For the first step, we tried to confirm the dependency on the CCK satiety signaling of the orexigenic action of estrogen using Otsuka-Long-Evans-Tokushima-Fatty (OLETF) rats that do not express the CCK1 receptor [20]. In addition, we monitored locomotor activity as well compared the estrogens’ effect on food intake that might be CCK-dependent and on locomotor activity that might be CCK-independent.
Methods
Twenty female OLETF rats and 20 Long-Evans-Tokushima-Otsuka (LETO) rats, that are the inbred intact Long-Evans strain, of 4 weeks of age were obtained from the Tokushima Research Institute of Otsuka Pharmaceutical Company (Tokushima, Japan) and were kept in an environmentally controlled room (23 ± 3°C, light on 0600–1800 hours) with free access to tap water and pelleted food (NMF; Oriental Yeast, Tokyo, Japan). Food intake and body weight were measured at 1000 hours every morning. The estrous cycle was monitored by taking vaginal smears every morning at 1000 hours. The amount of locomotor activity was checked by the activity measurement device using far infrared rays (MDC-W01, MDC-VA; Brain Science Idea, Tokyo, Japan). The device was fixed on the assigned position, and measured the amount of the spontaneous activity. The locomotor activity was expressed in terms of cumulative values every 24 h from 1000 hours. We used the rats that showed at least two consecutive regular 4-day estrous cycles. After the experiment using cyclic rats, all rats were bilaterally ovariectomized under ether anesthesia. Three weeks after ovariectomy, they were used for the experiment of estrogen treatment. Twenty microgram of estradiol benzoate (Sigma, St. Louis, USA) or sesame oil (vehicle 0.2 ml) was subcutaneously injected at 1000–1100 hours. CCK-8 was purchased from Peptide Institute, Osaka, Japan and dissolved in physiological saline.
The experimental protocol was approved by the Animal Care Committee of University of Fukui.
The data are expressed as the mean ± SEM. The data were statistically evaluated with one-way ANOVA followed with Tukey HSD test or Student’s t test.
Results
OLETF rats with regular estrous cycle (n = 16) ate significantly (P < 0.05) less on estrus (E) than on diestrus II (D II). Cyclic LETO rats (n = 14) also took food significantly (P < 0.05) less on estrus than on D II (Fig. 1). The decrement of food intake on between E and D II was not statistically significant between OLETF and LETO rats at 25.4 and 22.8%, respectively. In cyclic OLETF rats, spontaneous activity was significantly (P < 0.05) greater on estrus than on D I or D II similar to cyclic LETO rats, whose spontaneous activity was significantly (P < 0.05) greater on estrus than D II (Fig. 2).
Fig. 1.
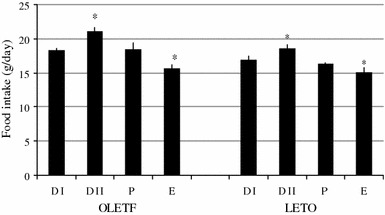
Changes of daily food intake during the estrous cycle in OLETF and LETO rats. Food intake was smallest on estrus in both groups. *Statistically significant at P < 0.05
Fig. 2.
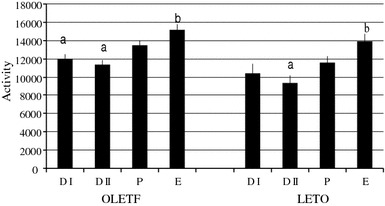
Changes of locomotor activity during the estrous cycle in OLETF and LETO rats. The activity was higher on estrus than diestrus. *Statistically significant at P < 0.05
In ovariectomized OLETF rats, estradiol benzoate (EB, 20 μg) administration (n = 10) significantly (P < 0.05) decreased food intake compared with vehicle injection (n = 9) from 1 to 5 days after injection (Fig. 3a). EB (20 μg) injected into ovariectomized LETO rats (n = 9) significantly (P < 0.05) decreased food intake compared with vehicle injection (n = 9) from 2 to 5 days after injection (Fig. 3b). The mean daily food intake 3 days immediately before EB injection was decreased by 21.5% in OLETF rats and 22.3% in LETO rats 2 days after the injection. The decrements were not statistically significant.
Fig. 3.
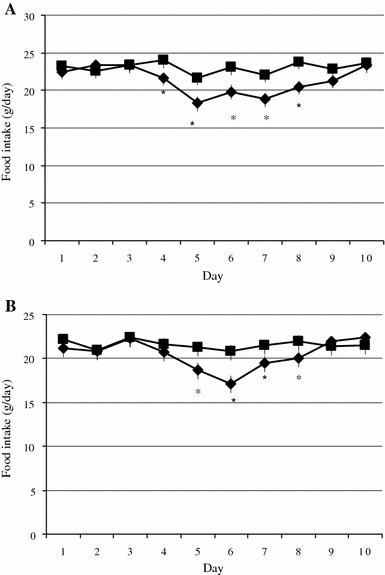
a Estrogens decreased food intake in ovariectomized OLETF rats. An injection of estradiol benzoate (20 μg) at Day 3 significantly inhibited the daily food intake in ovariectomized OLETF rats (diamonds) compared with vehicle injected OLETF rats (squares). *Statistically significant at P < 0.05. b Estrogens decreased food intake in ovariectomized LETO rats. An injection of estradiol benzoate (20 μg) at Day 3 significantly inhibited the daily food intake in ovariectomized LETO rats (diamonds) compared with vehicle injected LETO rats (squares). *Statistically significant at P < 0.05
Estrogen administration into ovariectomized OLETF rats (n = 9, mean body weight: 320.0 g) significantly (P < 0.05) increased spontaneous activity compared with vehicle injection (n = 9) from 2 to 4 days after injection (Fig. 4a). In ovariectomized LETO rats (n = 9, mean body weight: 303.8 g), estrogen administration significantly (P < 0.05) increased spontaneous activity compared with vehicle injection (n = 9) from 2 to 4 days after injection (Fig. 4b).
Fig. 4.
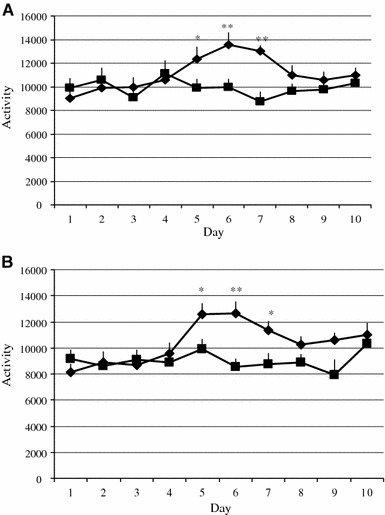
a Estrogens increased locomotor activity in ovariectomized OLETF rats. An injection of estradiol benzoate (20 μg) at Day 3 significantly increased locomotion in ovariectomized OLETF rats (diamonds) compared with vehicle injected OLETF rats (squares). Statistical significance: *P < 0.05, **P < 0.01. b Estrogens increased locomotor activity in ovariectomized LETO rats. An injection of estradiol benzoate (20 μg) at Day 3 significantly increased daily locomotion in ovariectomized LETO rats (diamonds) compared with vehicle injected OLETF rats (squares). Statistical significance: *P < 0.05, **P < 0.01
Because we got the unexpected result that both OLETF and LETO rats responded equally to estradiol benzoate in terms of not only locomotor activity but also food intake, we tried to confirm that the OLETF rats really were unresponsive to CCK. Thus, 3 weeks after the estradiol benzoate experiment, the rats were fasted for 24 h and injected with CCK-8 (1 μg/kg BW) or saline intraperitoneally, and 10 min later, food was returned. Control LETO rats injected with saline ate 4.02 ± 0.71 g (n = 8) in 60 min. LETO rats injected with CCK-8 (n = 8) ate significantly (P < 0.01) less food (0.46 ± 0.12 g) than control rats. The same treatment in the OLETF rats induced similar food intakes (5. 28 ± 0.68 and 5.41 ± 0.84 g, respectively) in both the rats treated with CCK-8 (n = 8) and with saline (n = 8), confirming that the OLETF rats we used were unresponsive to exogenous CCK to inhibit food intake.
Discussion
We demonstrated that estrogen could inhibit eating and stimulate spontaneous motor activity in the OLETF rats as well as in the LETO control rats. In both OLETF and LETO rats with a 4-day estrous cycle, food intake was significantly less on estrus than on diestrus II, and locomotion was significantly greater on estrus than on diestrus I and II. After ovariectomy, the estrogen injection to OLETF and LETO rats inhibited food intake and increased activity. These results suggest that the estrogen’s anorexigenic and increasing spontaneous active effects are expressed in OLETF rats whose CCK1 receptors are deficient [20].
The hypothesis that anorexigenic effect of estrogens is mediated by CCK’s satiating signal has been supported by several evidences. Estradiol treatment in ovariectomized rats potentiates the inhibitory effect of CCK on food intake [21–23]. In addition, a selective CCK1 receptor antagonist, devazepide, increases feeding more during estrus than during diestrus [24, 25]. Estradiol treatment increases feeding-induced [26] and CCK-induced [27] c-Fos expression in the brain. Recent reports suggest that local administration of estradiol to the surface of the brainstem just caudal to the area postrema was sufficient to decrease eating and to increase c-Fos expression in the caudal NTS of ovariectomized rats [19]. From these studies, it is proposed that estrogen inhibits meal size by increasing the central processing of the vagal CCK satiation signal, probably at the NTS.
Our present results, that feeding was inhibited by estrogen to the same extent in both OLETF and LETO rats, are not in accordance with these previous results. The CCK effect mentioned above must be mediated by CCK1 receptors. However, since OLETF rats are deficient in CCK1 receptors, the inhibitory effect of estrogen on feeding cannot be mediated by CCK’s satiation signal. It is difficult to explain the reason for the contradiction.
One possible explanation for the discrepancy is the compensation for CCK’s satiation effect by another feeding regulation system. Feeding is regulated by multiple factors. Regarding only those influenced by estrogens that affect meal size, these include orosensory signals, satiation signals from the alimentary tract, and adiposity signals [1]. In addition, estrogens have a profound influence on neural activity and neurotransmitter release. In terms of estrogen’s feeding inhibitory action on feeding, in addition to the NTS, many brain areas are reported to be involved. These are the ventromedial hypothalamus [28], the paraventricular nucleus [29–31], and the medial preoptic area [32]. Recent studies suggest that estrogens stimulate proopiomelanocortin (an anorectic peptide) expression [33, 34] and the decrease of neuropeptide Y (an orexigenic neuropeptide) expression [34] in the arcuate nucleus, resulting in the decrease of food intake. Furthermore, the study of the role of CCK in OLETF rats, demonstrated that neuropeptide Y mRNA expression was inhibited both in the arcuate nucleus and the dorsomedial hypothalamus in the LETO rats but only in the arcuate nucleus in the OLETF rats in response to high fat diet [35]. Genetic deficiency of CCK 1 receptor in the OLETF rats may induce compensatory developmental changes in these and other regulatory functions controlling food intake and metabolism. In addition, OLETF rats have the mutation of G protein-coupled receptor gene GPR10 as well as being CCK1 receptor-deficient [36]. Since the ligand for GPR10 receptor protein, prolactin-releasing peptide, has an anorexigenic effect, the difference in food regulation observed between OLETO and LETO rats cannot all be attributed to CCK-1 receptor deficiency.
In conclusion, we have demonstrated that anorectic effects of estrogen occurred in the OLETF rats whose CCK1 receptor is deficient to the same extent as control LETO rats.
Acknowledgments
The authors thank Dr. Kazuya Kawano of Tokushima Research Institute of Otsuka Pharmaceutical Company (Tokushima, Japan) for providing us the OLETF and LETO rats.
References
- 1.Asarian L, Geary N. Modulation of appetite by gondola steroid hormones. Philos Trans R Soc Lond B. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tarttelin MF, Gorski RA. Variations in food and water intake in the normal and acyclic female rat. Physiol Behav. 1971;7:847–852. doi: 10.1016/0031-9384(71)90050-3. [DOI] [PubMed] [Google Scholar]
- 3.Kenney NJ, Mook DG. Effects of ovariectomy on meal pattern in the albino rat. J Comp Physiol Psychol. 1974;87:302–309. doi: 10.1037/h0036863. [DOI] [PubMed] [Google Scholar]
- 4.Blaustein JD, Wade GN. Ovarian influences on the meal patterns of female rats. Physiol Behav. 1976;17:201–208. doi: 10.1016/0031-9384(76)90064-0. [DOI] [PubMed] [Google Scholar]
- 5.Wade GN. Gonadal hormones and behavioral regulation of body weight. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-X. [DOI] [PubMed] [Google Scholar]
- 6.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/S0031-9384(00)00278-X. [DOI] [PubMed] [Google Scholar]
- 7.Drewet RF. Oestrous and dioestrous components of the ovarian inhibition on hunger in the rat. Anim Behav. 1973;21:772–780. doi: 10.1016/S0003-3472(73)80103-4. [DOI] [PubMed] [Google Scholar]
- 8.Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J Comp Physiol Psychol. 1976;90:747–754. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- 9.Kawashima S, Shinoda A. Spontaneous activity of neonatally estrogenized female rats. Endocrinol Jpn. 1968;15:305–312. doi: 10.1507/endocrj1954.15.305. [DOI] [PubMed] [Google Scholar]
- 10.Geary N. Estradiol, CCK and satiation. Peptides. 2001;22:1251–1263. doi: 10.1016/S0196-9781(01)00449-1. [DOI] [PubMed] [Google Scholar]
- 11.Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol. 1975;89:784–790. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- 12.Cox JE. Cholecystokinin satiety involves CCKA receptors perfused by the superior pancreaticoduodenal artery. Am J Physiol. 1998;274:R1390–R1396. doi: 10.1152/ajpregu.1998.274.5.R1390. [DOI] [PubMed] [Google Scholar]
- 13.Dourish CT, Ruckert AC, Tattersall FD, Iversen SD. Evidence that decreased feeding induced by systemic injection of cholecystokinin is mediated by CCK-A receptors. Eur J Pharmacol. 1989;173:233–234. doi: 10.1016/0014-2999(89)90528-1. [DOI] [PubMed] [Google Scholar]
- 14.Moran TH, Ameglio PJ, Schwartz GJ, McHugh PR. Blockade of type A, not type B, CCK receptors attenuates satiety actions of exogenous and endogenous CCK. Am J Physiol. 1992;262:R46–R50. doi: 10.1152/ajpregu.1992.262.1.R46. [DOI] [PubMed] [Google Scholar]
- 15.Reidelberger RD, O’Rourke MF. Potent cholecystokinin antagonist L364718 stimulates food intake in rats. Am J Physiol. 1989;257:R1512–R1518. doi: 10.1152/ajpregu.1989.257.6.R1512. [DOI] [PubMed] [Google Scholar]
- 16.Ritter RC. Gastrointestinal mechanisms of satiation for food. Physiol Behav. 2004;81:249–273. doi: 10.1016/j.physbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Investig. 2007;117:13–23. doi: 10.1172/JCI30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asarian L, Geary N. Estradiol enhances cholecystokinin-dependent lipid-induced satiation and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2007;148:5656–5666. doi: 10.1210/en.2007-0341. [DOI] [PubMed] [Google Scholar]
- 19.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates ER-α-expressing cells in the NTS of ovariectomized rats. Endocrinology. 2007;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takiguchi S, Takata Y, Funakoshi A, Miyasaka K, Kataoka K, Fujimura Y, Goto T, Kono A. Disrupted cholecystokinin type-A receptor (CCKAR) gene in OLETF rats. Gene. 1997;197:169–175. doi: 10.1016/S0378-1119(97)00259-X. [DOI] [PubMed] [Google Scholar]
- 21.Linden A, Uvnas-Moberg K, Forsberg G, Bednar I, Sodersten P. Involvement of cholecystokinin in food intake: III. Oestoradiol potentiates the inhibitory effect of cholecystokinin octapeptide on food intake in ovariectomized rats. J Neuroendocrinol. 1990;2:797–801. doi: 10.1111/j.1365-2826.1990.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 22.Butera PC, Bradway DM, Cataldo NJ. Modulation of the satiety effect of cholecystokinin by estradiol. Physiol Behav. 1993;53:1235–1238. doi: 10.1016/0031-9384(93)90387-U. [DOI] [PubMed] [Google Scholar]
- 23.Geary N, Trace D, McEwen B, Smith GP. Cyclic estradiol replacement increases the satiety effect of CCK-8 in ovariectomized rats. Physiol Behav. 1994;56:281–289. doi: 10.1016/0031-9384(94)90196-1. [DOI] [PubMed] [Google Scholar]
- 24.Asarian L, Geary N. Cyclic estradiol treatment phasically potentiates endogenous cholecystokinin’s satiating action in ovariectomized rats. Peptides. 1999;20:445–450. doi: 10.1016/S0196-9781(99)00024-8. [DOI] [PubMed] [Google Scholar]
- 25.Eckel LA, Geary N. Endogenous cholecystokinin’s satiating action increases during estrus in female rats. Peptides. 1999;20:451–456. doi: 10.1016/S0196-9781(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 26.Eckel LA, Geary N. Estradiol treatment increases feeding-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2001;281:R738–R746. doi: 10.1152/ajpregu.2001.281.3.R738. [DOI] [PubMed] [Google Scholar]
- 27.Eckel LA, Houpt TA, Geary N. Estradiol treatment increases CCK-induced c-Fos expression in the brains of ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:1378–1385. doi: 10.1152/ajpregu.00300.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wade GN, Zucker I. Modulation of food intake and locomotor activity in female rats by diencephalic hormone implants. J Comp Physiol Psychol. 1970;72:328–336. doi: 10.1037/h0029461. [DOI] [PubMed] [Google Scholar]
- 29.Butera PC, Willard DM, Raymond SA. Effects of PVN lesions on the responsiveness of female rats to estradiol. Brain Res. 1992;576:304–310. doi: 10.1016/0006-8993(92)90694-5. [DOI] [PubMed] [Google Scholar]
- 30.Butera PC, Beikirch RJ. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- 31.Butera PC, Xiong M, Davis RJ, Platania SP. Central implants of dilute estradiol enhance the satiety effect of CCK-8. Behav Neurosci. 1996;110:823–830. doi: 10.1037/0735-7044.110.4.823. [DOI] [PubMed] [Google Scholar]
- 32.Dagnault A, Richard D. Involvement of the medial preoptic area in the anorectic action of estrogens. Am J Physiol. 1997;272:R311–R317. doi: 10.1152/ajpregu.1997.272.1.R311. [DOI] [PubMed] [Google Scholar]
- 33.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao XB, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 34.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol. 2007;19:426–431. doi: 10.1111/j.1365-2826.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 35.Bi S, Chen J, Behles RR, Hyun J, Kopin AS, Moran TH. Differential body weight and feeding responses to high-fat diets in rats and mice lacking cholecystokinin 1 receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R55–R63. doi: 10.1152/ajpregu.00002.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe TK, Suzuki M, Yamasaki Y, Okuno S, Hishigaki H, Ono T, Oga K, Mizoguchi-Miyakita A, Tsuji A, Kanemoto N, Wakitani S, Takagi T, Nakamura Y, Tanigami A. Mutated G-protein-coupled receptor GPR10 is responsible for the hyperphagia/dyslipidaemia/obesity locus of Dmo1 in the OLETF rat. Clin Exp Pharmacol Physiol. 2005;32:355–366. doi: 10.1111/j.1440-1681.2005.04196.x. [DOI] [PubMed] [Google Scholar]


