Abstract
To investigate whether high-intensity interval training (HIIT) and continuous moderate-intensity training (CMT) have different impacts on exercise performance and cardiac function and to determine the influence of these exercise protocols on modulating basal autophagy in the cardiac muscle of rats. Rats were assigned to three groups: sedentary control (SC), CMT, and HIIT. Total exercise volume and mean intensity were matched between the two protocols. After a 10-week training program, rats were evaluated for exercise performance, including exercise tolerance and grip strength. Blood lactate levels were measured after an incremental exercise test. Cardiac function and morphology were assessed by echocardiography. Western blotting was used to evaluate the expression of autophagy and mitochondrial markers. Transmission electron microscopy was used to evaluate mitochondrial content. The results showed that time to exhaustion and grip strength increased significantly in the HIIT group compared with the SC and CMT groups. Both training interventions significantly increased time to exhaustion, reduced blood lactate level (after an incremental exercise test) and induced adaptive changes in cardiac morphology, but without altering cardiac systolic function. The greater improvements in exercise performance with the HIIT than with the CMT protocol were related to improvement in basal autophagic adaptation and mitochondria function in cardiac muscle. Mitochondria markers were positively correlated with autophagy makers. This study shows that HIIT is more effective for improving exercise performance than CMT and this improvement is related to mitochondrial function and basal autophagic adaptation in cardiac muscle.
Keywords: High-intensity interval training, Continuous moderate-intensity training, Exercise performance, Autophagy
Introduction
High-intensity interval training (HIIT) involves aerobic exercise performed at a high intensity interspersed with active or passive recovery periods [1]. HIIT is superior to continuous moderate-intensity training (CMT) for improving cardiovascular function and aerobic capacity in healthy men and rodents [2]. These findings also highlight cardiovascular remodeling as a potentially modifiable adaptive response to HIIT [2, 3]. Human and animal studies have identified cardiovascular adaptations due to HIIT as being correlated with improved circulatory function [3], myocardial antioxidant capacity, and mitochondrial respiratory capacity in cardiac muscle [2]. However, the underlying cellular and molecular metabolic adaptations in the heart remain unclear.
Autophagy is an evolutionary intracellular catalytic process of targeted cellular degradation, including the elimination of redundant or damaged cellular structures, such as mitochondria, by selectively targeting them into a double-membrane structure called the phagophore, which subsequently matures into an autophagosome and eventually fuses with a lysosome to form an autolysosome [4, 5]. Increased autophagy, which is particularly involved in cellular adaptation to endurance exercise training, is a crucial precursor to mitochondrial biogenesis and remodeling, revealing an essential role of autophagy in the maintenance of mitochondrial morphology and function [4]. Several proteins are involved in autophagosome formation, including mammalian homologues of autophagy-related gene (ATG) proteins 3/5/6/7/12/16L1, Beclin 1, and microtubule-associated protein A/BE-light chain 3 (LC3). The knockout of various proteins associated with autophagy leads to dysregulation of various tissue functions [6]. Moreover, during the dynamic process of autophagosome formation, the conversion of LC3B from the free form (LC3-I) to the phosphatidylethanolamine-conjugated form (LC3-II) represents a key step in autophagosome formation. Thus, LC3-II immunoblotting is the most commonly used biochemical method to evaluate autophagy in tissues. The ratio of LC3-II to LC3-I (LC3-II/LC3-I ratio) is used as a direct marker of autophagic flux in tissues [7].
Recently, it has been shown that exercise promotes cellular adaptation through the activation of signaling pathways, particularly those associated with mitochondrial biogenesis due to the peroxisome proliferator-activated receptor gamma coactivator 1-mediated synthesis of proteins and the autophagy-mediated clearance of damaged proteins in the mitochondria [8], both of which are extremely important in maintaining mitochondrial homeostasis. Exercise training induces autophagy in different organs, including skeletal muscle, heart, liver, and brain [9]. Several animal studies have reported that a single bout of exercise activates autophagy and increases autophagy flux in cardiac muscles [9–11], while autophagy inhibition results in dysfunctional mitochondria and defective oxidative metabolism, compromising ATP generation and reducing exercise capacity during a single bout of exercise to exhaustion [11]. However, little is known about the relationship between chronic exercise-induced cardiac muscle adaptation and the alteration of basal autophagy [5], which is defined as a housekeeping mechanism that cleans cells of aberrant and dysfunctional organelles, thereby maintaining cell homeostasis [12]. Endurance training has also been reported to increase autophagosome content in cardiac muscle in some [5, 13–15] but not all animal studies [16]. Sun et al. [16] reported that increasing the volume of endurance training by augmenting the duration while maintaining intensity at a stable level increases cardiac muscle efficiency and basal autophagic activities, including increased autophagy marker content (e.g., LC3-II, ATG-7, Beclin 1, and LC3-II/LC3-I ratio).
The enhancement of cardiac mechanoenergetic and maximal mitochondrial respiratory capacity during cardiac adaptation in rat models following HIIT has received considerable attention [2], with evidence that HIIT increases basal autophagic activity in CD4 lymphocytes, which potentially contributes to greater improvement in physical performance compared to an intensity- and volume-matched CMT protocol in sedentary men [17]. Hence, it is plausible that the increase in basal autophagic activity caused by HIIT may be associated with the enhancement of cardiac mitochondrial respiratory capacity, thereby improving exercise performance. With the above in mind, the objective of this study was to compare the basal autophagic activity and mitochondrial respiratory capacity changes in the heart between 10-week intensity- and volume-matched HIIT or CMT protocols in a rat model.
Methods
Animal care
Six-week-old, male, Sprague–Dawley rats and standard laboratory chow were purchased from Guangdong Medical Laboratory Animal Center. The rats were kept on an artificial 12-h light–dark cycle (6:00 am–6:00 pm) at constant room temperature (23 ± 1 °C) in the Laboratory Animal Center, School of Sports Science and Physical Education, South China Normal University. Water and food were available ad libitum. The animals were housed in their respective groups in a collective cage and received water and standard laboratory chow. All animal care and experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and approved by the Ethics Committee on Animal Experimentation of the Guangdong Medical Laboratory Animal Center.
After 1 week of preconditioning feeding and 2 weeks of a preconditioning running regimen, all rats were randomly assigned to three groups: sedentary control (SC, n = 10), CMT (n = 12), and HIIT (n = 12). The training protocol was performed over a 10-week period. The mean initial body weight (BW) was comparable between the SC, CMT, and HIIT groups: 225 ± 4.0, 224 ± 6.0, and 222 ± 3.0 g, respectively. Energy intake (EI) was calculated according to the following formula [18]: EI = WFI × RCV N−1, where WFI is the weekly food intake (g), RCV is the ration caloric value (g × kcal), and N is the number of animals in the cage. For the RCV calculation, 1 g of chow is equivalent to 3.5 kcal.
Exercise training protocols
The rats performed exercises based on a protocol described previously, with some modifications [19]. Briefly, before the exercise program, all animals underwent a preconditioning running regimen which consisted, in the first week, of 10–30 min of daily running on a treadmill with a 10% grade at a speed of 10 m min−1 and, in the second week, of 30–40 min of daily running at 10 m min−1, which was progressively increased by 2 m min−1, ending at 20 m min−1. CMT included 3 min of warm-up at a constant running speed of 18 m min−1, which corresponded to 35–40% of maximal oxygen uptake (VO2max), followed by 34 min of constant running speed of 28 m min−1 (corresponding to 60–70% of VO2max) [20, 21] and cool-down at a constant running speed of 18 m min−1 for 3 min. HIIT included 4 min of high-speed running at 42 m min−1 at a 10% grade (four repetitions), which corresponded to 95–99% of VO2max [19, 20], followed by 5 min of low-speed running at 18 m min−1 (40–45% of VO2max; five repetitions) and cool-down at a constant running speed of 7 m min−1 for 2 min. Thus, the total exercise volume and mean intensity for HIIT were 1060 m day−1 and 70% of VO2max, which matched the total volume and intensity for the CMT protocol. Both exercise protocols included five sessions per week for 10 weeks. An electrified grid (0.6-mA intensity) was placed behind the belt of the treadmill to induce running. The rats that failed to run regularly were excluded from the training session. SC group rats remained in their cages during the training sessions.
Echocardiography
At the end of the exercise program, the rats rested for 48 h and were anesthetized using 12 μl g−1 BW of 2.5% avertin (Sigma-Aldrich). Left ventricular (LV) morphometry and cardiac function were determined using echocardiography (VisualSonics VeVo 2100) as previously described [18, 22]. Using the M-mode image, LV end systolic diameter (LVSD), diastolic diameter (LVDD), LV anterior wall thickness in diastole (LVAWd) and systole (LVAWs), LV internal diameter in diastole (LVIDd) and systole (LVIDs), LV posterior wall thickness in diastole (LVPWd) and systole (LVPWd), LV end diastolic (LVD. vol) and systolic (LVS. vol) volume, LV mass, LV ejection fraction (EF), cardiac output (CO), and fractional shortening (FS) were measured as per the American Society of Echocardiography guidelines. The relative wall thickness (RWT) was calculated as (2 × PWT–LVDD), where PWT is the posterior wall thickness and LVDD is the left ventricular end-diastolic diameter, with the cardiac index calculated as the cardiac output normalized to BW [22]. LVSD, LVDD, LVAWd, LVAWs, LVIDd, LVIDs, LVPWd, LVPWs, and LV mass were normalized to BW [18, 22].
Sample collection and preparation
BW and body length were measured and Lee’s index was calculated as BW (g) 3−1×1000 length−1 (cm). After echocardiography, the rats were made to fast for 12 h and sacrificed under carbon dioxide anesthesia. The entire cardiac muscle was rapidly dissected and weighed to determine the relative heart weight (relative heart weight [%] = heart weight [g] BW−1 [g] × 100). Each cardiac muscle was divided into two parts: one part was fixed in 2.5% glutaraldehyde in phosphate-buffered saline for transmission electron microscopy while the other part was used for molecular detection.
Physical performance measures
Graded exercise test
After 10 weeks of training, all rats underwent physical performance testing, including exercise tolerance and blood lactate level measurements [23]. The exercise tolerance test consisted of walking at 12 m min−1 for 3 min followed by 1.2 m min−1 increases in speed every 2 min until the rat reached exhaustion. Time to exhaustion (s) was identified as the time till the rat sat at the lower end of the treadmill, near a shock bar, for 5 s. Blood lactate level was measured prior to exercise (baseline), immediately after exercise (I-EX), 10 min post-exercise (10minPOST), and 3 h post-exercise (3hPOST) [18]. A lactate Scout analyzer (EKF Diagnostics, Magdeburg, Germany) was employed to analyze 0.2 μl of blood obtained using a tail nick.
Grip strength test
Briefly, all rats were allowed to grasp the steel wire grid attached to the force gauge and were subsequently pulled back from the gauge. The force was recorded once they released the grid. Three trials were conducted, with the greatest force value recorded using a grip strength meter (Bioseb, Shanghai, China) as the maximum grip strength and the force relative to BW (N g−1) [23].
Transmission electron microscopy
Cardiac muscle samples were fixed at 4 °C in 2.5% glutaraldehyde in phosphate-buffered saline and processed using conventional techniques. Ultrathin sections (0.08 μm) were stained with lead citrate and uranyl acetate and examined with transmission electron microscopy (model EM10A, Zeiss, Jena, Germany). The morphometric analysis of the mitochondria of the myocardium was performed according to published methods [9]. The volumetric density of mitochondria was computed based on micrographs at × 6800 using Image J software (NIH, Bethesda, MD, USA).
Western blotting analysis
Whole cardiac muscle was homogenized in tissue-lysing buffer. The primary antibodies used in this study were rabbit anti-LC3A/B (1:1000, CST 12741), rabbit anti-ATG-5 (1:1000, CST 12994), rabbit anti-ATG-3 (1:1000, CST 3415), rabbit anti-Beclin 1 (1:1000, CST 3495), rabbit anti-ATG-16L1 (1:1000, CST 8089), rabbit anti-ATG-7 (1:1000, CST 8558), rabbit anti-ATG-12 (1:1000, CST 4180), rabbit anti-acetaldehyde dehydrogenase 2 (ALDH2) (1:1000, Abcam, ab108306), rabbit anti-sirtuin 3 (SIRT3) (1:1000, CST 2627), rabbit anti-GAPDH (1:1000, CST 2118), rabbit anti–cytochrome C oxidase subunit IV (COX IV: EC number 1.9.3.1; 1:1000, CST 4844), and rabbit anti-succinate dehydrogenase (SDH: EC number 1.3.5.1; 1:1000, CST 11998). Horseradish peroxidase-conjugated donkey anti-rabbit IgG (H + L) (711-035-152, Jackson ImmunoResearch Europe, Newmarket, UK) was used as the secondary antibody. Immunoreactivity was detected using an Enhanced Chemiluminescence western blot detection kit (Amersham Pharmacia Biotech) according to the manufacturer’s instructions. Protein expression levels were quantitatively analyzed with Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Results are presented as mean ± SD, with P values < 0.05 accepted as statistically significant. All data were analyzed using Graph Pad Prism (version 6.07; Graph Pad Prism Software, La Jolla, CA, United States). Prior to statistical analysis, the normality of the distribution of the data was evaluated using a one-sample Kolmogorov–Smirnov test and homogeneity of variances was tested using Levene’s test for equality of variances. Blood lactate levels were evaluated using two-way repeated measure analysis of variance (ANOVA). Pearson’s correlation coefficient was employed to analyze the correlations between parametrical data. One-way ANOVA followed by a Tukey’s post hoc test was used to analyze other measures.
Results
Morphological characteristics
The morphological characteristics of rats in the three groups after the 10-week program are shown in Table 1. The final BW (P < 0.01), EI (P < 0.01), Lee’s index (P < 0.05), liver weight (P < 0.01), and perirenal adipose tissue weight (P < 0.01) were lower for the CMT and HIIT groups than those for the SC group. The relative liver weight (P < 0.01), relative perirenal adipose tissue weight (P < 0.01), relative quadriceps weight (P < 0.05), relative gastrocnemius weight (P < 0.05), and relative epididymis weight (P < 0.01) were higher for the CMT and HIIT groups than those for the SC group. The weight of the heart, quadriceps, and gastrocnemius decreased significantly by 18.2, 15.6, and 19.2%, respectively, in the HIIT group relative to those in the SC group (P < 0.05). However, the relative heart weight was comparable among the three groups (P > 0.05).
Table 1.
Morphological characteristics of the experimental groups
| SC (n = 10) | CMT (n = 12) | HIIT (n = 12) | |
|---|---|---|---|
| BW (g) | 674 ± 45 | 525 ± 24b | 520 ± 38b |
| Lee’s index | 0.31 ± 0.01 | 0.29 ± 0.01a | 0.29 ± 0.01a |
| EI (kcal day−1) | 113 ± 2.9 | 88.5 ± 2.5b | 89.3 ± 2.8b |
| Liver weight (g) | 17.4 ± 1.30 | 12.2 ± 2.10b | 10.6 ± 1.20b |
| Heart weight (g) | 1.59 ± 0.12 | 1.40 ± 0.12 | 1.30 ± 0.13a |
| Perirenal adipose tissue weight (g) | 17.0 ± 3.20 | 5.73 ± 2.80b | 5.04 ± 1.85b |
| Quadriceps weight (g) | 10.4 ± 1.10 | 9.25 ± 0.28 | 8.78 ± 0.68a |
| Gastrocnemius weight (g) | 7.76 ± 0.84 | 6.95 ± 0.45 | 6.27 ± 0.63a |
| Soleus muscles weight (g) | 0.65 ± 0.22 | 0.57 ± 0.08 | 0.61 ± 0.14 |
| Extensor digitorum longus weight (g) | 0.68 ± 0.16 | 0.58 ± 0.08 | 0.60 ± 0.05 |
| Epididymis weight (g) | 4.10 ± 0.18 | 3.76 ± 0.17 | 3.75 ± 0.38 |
| Relative liver weight (%) | 2.63 ± 0.27 | 2.42 ± 0.42b | 2.16 ± 0.12b |
| Relative heart weight (%) | 0.24 ± 0.02 | 0.28 ± 0.03 | 0.26 ± 0.01 |
| Relative perirenal adipose tissue weight (%) | 2.60 ± 0.50 | 1.10 ± 0.50b | 1.00 ± 0.40b |
| Relative quadriceps weight (%) | 1.61 ± 0.14 | 1.81 ± 0.13a | 1.78 ± 0.09a |
| Relative gastrocnemius weight (%) | 1.16 ± 0.09 | 1.41 ± 0.09a | 1.32 ± 0.08a |
| Relative soleus muscles weight (%) | 0.10 ± 0.03 | 0.11 ± 0.02 | 0.13 ± 0.03 |
| Relative extensor digitorum longus weight (%) | 0.10 ± 0.03 | 0.12 ± 0.01 | 0.12 ± 0.01 |
| Relative epididymis weight (%) | 0.62 ± 0.02 | 0.75 ± 0.03b | 0.76 ± 0.06b |
Values are reported as the mean ± standard deviation
Relative tissue weight (%) = tissue weight (g)/final body weight (g) × 100%. ap < 0.05, b p < 0.01. Means with different superscripts in each row are significantly different from SC (one–way ANOVA followed by a Tukey’s post-hoc test)
SC sedentary control, CMT continuous moderate-intensity training, HIIT high intensity interval training, BW body weight, EI the energy intake
Exercise performance and blood lactate levels after the graded exercise test
The mean endurance times, grip strength, and blood lactate levels from the rats in the three groups after 10 weeks of exercise training are shown in Fig. 1. Two-way ANOVA revealed that the two exercise modalities decreased blood lactate levels after running to exhaustion. A significant two-way interaction (exercise-time interaction; P = 0.0002), main effect for time (P < 0.0001), and main effect for exercise (p = 0.02) for blood lactate levels were observed (Fig. 1a). Significant differences in blood lactate levels at I-EX (HIIT 2.81 ± 0.6 mol l−1, SC 6.48 ± 3.8 mol l−1; P < 0.001) and 10 minPOST (HIIT 1.65 ± 0.75 mol l−1, SC 3.28 ± 2.4 mol l−1; P < 0.05) between the HIIT and SC groups were identified. Significant differences in blood lactate levels at I-EX (CMT 3.28 ± 0.70 mol l−1, SC 6.48 ± 3.8 mol l−1; P < 0.001) between the CMT and SC groups were also noted.
Fig. 1.
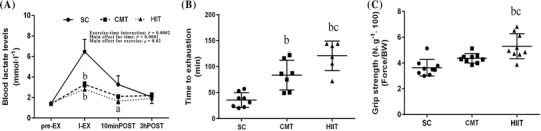
Effect of the CMT and HIIT protocols on physical performance. Changes in blood lactate levels (mmol l−1) after one-time exhaustive exercise (a), time to exhaustion (min) (b), and grip strength (N g−1) (c). Groups: SC sedentary control; CMT continuous moderate-intensity training, HIIT high-intensity interval training. Data are presented as mean ± SD (n = 8). aP < 0.05 versus SC; bP < 0.01 versus SC; cP < 0.05 versus CMT
One-way ANOVA also indicated higher time to exhaustion (HIIT 120.8 ± 28 s, SC 35.6 ± 14 s, P < 0.01; CMT 83.6 ± 28 s, SC 35.6 ± 14 s, P < 0.01; Fig. 1b) and grip strength (HIIT 5.31 ± 0.9 N g−1, SC 3.64 ± 0.6 N g−1, P < 0.01; CMT 4.37 ± 0.3 N g−1, SC 3.64 ± 0.6 N g−1, P > 0.05; Fig. 1c) in both the HIIT and CMT groups compared to the SC group. Similarly, time to exhaustion and grip strength were significantly higher in the HIIT group than in the CMT group (P < 0.05).
Echocardiographic morphological and functional characteristics
The echocardiographic data of the heart structural and function parameters of the three groups after 10 weeks of training are shown in Table 2. CO and SV in both exercise modality groups were significantly lower than those in the SC group (P < 0.05). The decrease in heart rate after 10 weeks was significantly greater in the HIIT than in the SC group (P < 0.01), with no difference between the CMT and SC groups (P > 0.05). The LVDD/BW, LVPWd/BW, LVPWs/BW, LVAWs/BW, LVAWd/BW, and LVIDd/BW ratios were significantly higher for the HIIT and CMT groups than for the SC group (P < 0.01). The LV mass to BW ratio was also significantly higher for the HIIT than for the SC group (P < 0.05).
Table 2.
Cardiac function and morphology parameters normalized to body weight
| SC (n = 6) | CMT (n = 6) | HIIT (n = 6) | |
|---|---|---|---|
| LV function parameters | |||
| CO (ml min−1) | 107 ± 18.0 | 79.1 ± 14.0a | 72.2 ± 11.0a |
| Cardiac index (ml min−1 kg−1) | 0.167 ± 0.03 | 0.166 ± 0.04 | 0.152 ± 0.02 |
| LVEF (%) | 74.8 ± 9.0 | 71.7 ± 3.8 | 71.7 ± 6.4 |
| HR (bpm) | 332 ± 24 | 310 ± 31 | 286 ± 9.7b |
| LVFS (%) | 46.2 ± 8.4 | 42.6 ± 3.3 | 42.8 ± 5.6 |
| LVSV (μl) | 323 ± 41 | 255 ± 32a | 252 ± 35a |
| LVSV index (ml beat kg−1) | 0.50 ± 0.06 | 0.53 ± 0.09 | 0.53 ± 0.05 |
| LV structural parameters | |||
| LV mass/final BW (mg g−1) | 1.51 ± 0.21 | 1.82 ± 0.18 | 1.88 ± 0.25a |
| RWT (mm kg−1) | 0.40 ± 0.05 | 0.42 ± 0.10 | 0.45 ± 0.07 |
| LVDD/final BW (mm kg−1) | 13.8 ± 1.4 | 17.0 ± 1.9b | 17.1 ± 1.1b |
| LVSD/final BW (mm kg−1) | 7.51 ± 1.8 | 9.73 ± 1.4 | 9.80 ± 1.5 |
| LVPWs/final BW (mm kg−1) | 4.63 ± 0.51 | 6.24 ± 0.45b | 6.20 ± 0.70b |
| LVPWd/final BW (mm kg−1) | 2.74 ± 0.15 | 3.53 ± 0.66b | 3.78 ± 0.41b |
| LVIDs/final BW (mm kg−1) | 7.45 ± 1.4 | 9.66 ± 1.3 | 9.68 ± 1.5 |
| LVIDd/final BW (mm kg−1) | 13.9 ± 1.4 | 16.9 ± 1.8b | 17.0 ± 1.1b |
| LVAWd/final BW (mm kg−1) | 2.73 ± 0.34 | 3.53 ± 0.17b | 3.53 ± 0.27b |
| LVAWs/final BW (mm kg−1) | 4.85 ± 0.43 | 6.11 ± 0.21b | 0.61 ± 0.56b |
| LVD vol (μl) | 443 ± 98 | 356 ± 48 | 351 ± 61 |
| LVS vol (μl) | 115 ± 65 | 99.4 ± 21 | 99.3 ± 40 |
Values are reported as the mean ± standard deviation
SC sedentary control, CMT continuous moderate–intensity training, HIIT high–intensity interval training, BW body weight, CO cardiac output, LVEF left ventricular ejection fraction, LVFS left ventricular fractional shortening, LVSV left ventricular stroke volume, cardiac index cardiac output/BW, LVSV index LV stroke volume/BW, HR heart rate, LV left ventricular, LVSD left ventricular end systolic diameters, LVDD left ventricular end diastolic diameters, LVAWd LV anterior wall thickness in diastole, LVAWs LV anterior wall thickness in systole, RWT relative wall thickness, LVIDd LV internal diameter in diastole, LVIDs LV internal diameter in systole, LVPWs LV posterior wall thickness in systole, LVD. vol LV end diastolic volume, LVS. vol LV end systolic volume
ap < 0.05, b p < 0.01. Means with different superscripts in each row are significantly different from SC (one–way ANOVA followed by a Tukey’s post-hoc test)
Protein expression of autophagic factors in the cardiac muscle
The autophagic factors in the cardiac muscle of the three groups after 10 weeks of training are shown in Fig. 2. Ratio of LC3-II to LC3-I (Fig. 2a) and the protein content of LC3-II (Fig. 2b), LC3-I (Fig. 2c), Beclin 1 (Fig. 2f), ATG-3 (Fig. 2d), ATG-16L1 (Fig. 2h), and ATG-12 (Fig. 2i) significantly increased by 61.6% (P < 0.05) and 116% (P < 0.01), 154% (P < 0.01), 48.5% (P < 0.05), 39.9% (P < 0.05), 139% (P < 0.01), and 99.3% (P < 0.01), respectively, in the cardiac muscle of the HIIT group relative to that of the SC group. No significant difference was found in the protein content of ATG-5 (Fig. 2g) and ATG-7 (Fig. 2e) in the cardiac muscle between the SC and exercise groups (P > 0.05). The protein content of LC3-II, LC3-I, ATG-16L1, and ATG-12 also significantly increased by 62.9, 89.4, 57.4, and 23.9%, respectively, in the cardiac muscle of the HIIT group relative to that of the CMT group (P < 0.05).
Fig. 2.
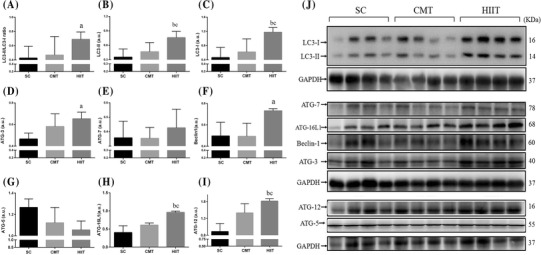
Effect of CMT and HIIT protocols on the content of autophagic factors in cardiac muscle in the three experimental groups assayed by western blotting. Levels of detected proteins were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Densitometry analysis was performed to quantify the expression levels of detected proteins (a.u.). Groups: SC sedentary control, CMT continuous moderate-intensity training, HIIT high-intensity interval training. Data are presented as mean ± SD (n = 4). aP < 0.05 versus SC; bP < 0.01 versus SC; cP < 0.05 versus CMT
Protein expression of mitochondrial biogenesis markers in the cardiac muscle
No significant differences were found in the protein contents of ALDH2 (Fig. 3a) and SIRT3 (Fig. 3c) in the cardiac muscle between the SC and exercise training groups. The protein content of SDH (Fig. 3b) and COX IV (Fig. 3d) significantly increased by 51% (P < 0.05) and 4.26-fold (P < 0.05) in the cardiac muscle of the HIIT group relative to that of the SC group, while the protein content of SDH and COX IV significantly increased by 37.6 and 61.5%, respectively, in the cardiac muscle of the HIIT group relative to that of the CMT group (P < 0.05). The ultrastructural morphology of rat myocardial mitochondria in myocardiocytes was observed using transmission electron microscopy (Fig. 3j, k). Transmission electron microscopy images showed that HIIT, but not CMT, induced signs of mitochondrial biogenesis with an elevation in mitochondrial volume density (Fig. 3j). However, myocardial mitochondrial damage in rats, such as mitochondrial swelling, disruptions of the crest and membrane, mitochondrial deficiency, were not observed in the HIIT and CMT groups.
Fig. 3.
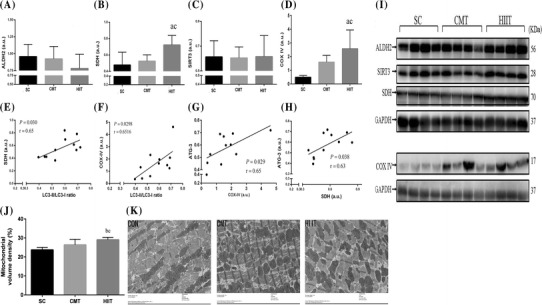
Effect of the CMT and HIIT protocols on protein levels of ALDH2 (a), SDH (b), SIRT3 (c), and COX IV (d) in cardiac muscle in the three experimental groups assayed by western blotting (i). Levels of detected proteins were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Densitometry analysis was performed to quantify the expression levels of detected proteins (a.u.). Positive correlations of SDH content with LC3-II/LC3-I ratio (e) and ATG-3 (h) in cardiac muscle. Positive correlations of COX IV content with ATG-3 (g) and LC3-II/LC3-I ratio (f) in cardiac muscle. Mitochondrial volume density (j, k) determined using a transmission electron micrograph (at ×6800) of an ultra-thin section of the left ventricle of rat hearts (n = 5). Groups: SC sedentary control, CMT continuous moderate-intensity training, HIIT high-intensity interval training. Data are presented as mean ± SD (n = 4). aP < 0.05 versus SC; bP < 0.01 versus SC; cP < 0.05 versus CMT
Moreover, the protein content of SDH was positively correlated with that of ATG-3 (r = 0.63, P = 0.038; Fig. 3h) and the LC3-II/LC3-I ratio (r = 0.65, P = 0.03; Fig. 3e); similar associations were found between COX IV content and that of ATG-3 (r = 0.65, P = 0.029; Fig. 3g) and the LC3-II/LC3-I ratio (r = 0.6516, P = 0.0298; Fig. 3f) in the cardiac muscle.
Correlation between autophagy and mitochondria markers and exercise performance
Time to exhaustion was positively correlated with LC3-II/LC3-I ratio (r = 0.48, P = 0.0174; Fig. 4a), ATG-3 (r = 0.70, P = 0.017; Fig. 4b), ATG-12 (r = 0.75, P = 0.0074; Fig. 4c), and ATG-16L (r = 0.83, P = 0.016; Fig. 4d). SDH content was positively correlated with time to exhaustion (r = 0.70, P = 0.016; Fig. 4e) and negatively correlated with blood lactate levels at I-EX (r = − 0.66, P = 0.028; Fig. 4g); similar associations were found between COX IV content and time to exhaustion (r = 0.76, P = 0.006; Fig. 4f) and blood lactate levels at I-EX (r = − 0.60, P = 0.05; Fig. 4h).
Fig. 4.
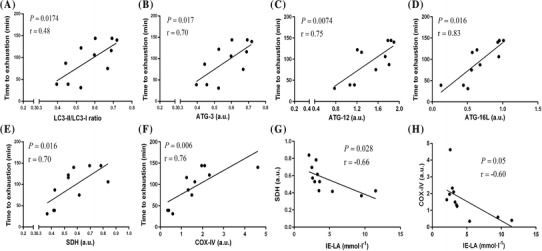
Correlations between autophagy or mitochondria marker proteins in cardiac muscles and exercise performance after 10 weeks of exercise training. Positive correlations of time to exhaustion with LC3-II/LC3-I ratio (a), ATG-3 (b), ATG-12 (c), and ATG-16L (d). Positive correlations of time to exhaustion with SDH (e) and COX IV content (f) in cardiac muscles. Negative correlation of IE-LA with SDH (g) and COX IV (h) in cardiac muscles. Pearson correlations were conducted for the above analyses (n = 12, pooled samples of SC, CMT, and HIIT groups). IE-LA, blood lactate levels immediately post-exercise
Discussion
This study revealed differential effects of HIIT and CMT on physical fitness, myocardial remodeling, mitochondrial gene expression, and basal autophagic activity. The major findings were as follows: when compared to the SC group, the HIIT protocol resulted in a larger improvement in physical performance and cardiac hypertrophy as assessed by the ratio of LV mass to BW; autophagy and mitochondria makers were significantly increased in the cardiac muscle in the HIIT group compared with those of the SC group; both basal autophagic activity and mitochondria biogenetic makers were positively related to time to exhaustion, indicative of the superiority of HIIT training over CMT (matched for intensity and volume of training) in improving physical performance via an elevation in mitochondria oxidation capacity and basal autophagic activity in cardiac muscle.
Physical performance
The large increase in physical fitness of the rats after the 10-week training protocol with both HIIT and CMT protocols, as evidenced by an increase in time to exhaustion (Fig. 1b) and relative skeletal muscle weight (relative quadriceps and gastrocnemius weight), reduction of body fat, attenuation of body weight gain, and Lee’s index (Table 1), is consistent with the results of other animal studies [24, 25]. This study indicated that HIIT can also lead to significantly greater increases in exercise performance (e.g., grip strength as well as time to exhaustion) than a volume- and intensity-matched CMT protocol (Fig. 2b–d). Our results are compatible with the studies by Helgerud et al. [26] and Weng et al. [17], who concluded that a HIIT protocol led to greater improvements in VO2max than a total work- and training intensity-matched CMT protocol. However, Pereira et al. [25] reported no significant change in time to exhaustion between volume- and intensity-matched CMT and HIIT protocols. Of note, their training protocols were performed at the same training intensity (70% of VO2max) as those in the present study but at a lower volume, suggesting that their low-volume HIIT protocol was insufficient to elicit an increase in endurance performance when compared with the CMT protocol. This is supported by the study of Laursen et al. [24], which reported no improvement in endurance performance after low-volume HIIT compared to an intensity-matched CMT protocol in well-trained rats [2, 27]. However, both of these studies demonstrated that a higher intensity of training could compensate for lower-volume exercise [2, 27]. Previously, it has also been observed that an 8- or 6-week CMT program consisting of training on a mechanically braked cycle ergometer improved maximal aerobic power but not anaerobic capacity and that adequate high-intensity intermittent training can significantly improve both anaerobic and aerobic capacity [28, 29]. Our findings are in agreement with those of a previous study [30] showing positive effects of HIIT on repeated-sprint ability and anaerobic power as well as a positive training effect on maximal peak power output [28], with the HIIT group showing a greater increase in forelimb grip strength than the CMT group.
Muscle lactate production during exercise and its transport into the bloodstream, which exponentially increases its concentration, is a limiting factor of aerobic metabolism. Indeed, a faster rate of disposal and clearance of lactate from the blood during submaximal exercise with endurance training can prolong exercise duration before exercised-induced lactate accumulation is sustained [31]. The measurement of blood lactate during the incremental exercise test to exhaustion in our study provides a more accurate and convincing display of the positive adaptations to exercise and shows that the exercise protocols used in this study were appropriate for the physical conditions of the rats in the HIIT and CMT groups. Both the HIIT and CMT protocols lowered the blood lactate level immediately after the incremental exercise test to exhaustion, with only the HIIT protocol producing a significant decrease at 10-min after exhaustion. Therefore, the HIIT protocol was superior to the volume- and intensity-matched CMT protocol in accelerating blood lactate disposal and removal after exercise to exhaustion (Fig. 1a). This effect may have contributed to greater improvements in exercise performance after the HIIT protocol compared to the CMT protocol. Thus, the improvements in exercise tolerance for both protocols likely occurred, at least in part, due to an improvement in the oxidative capacity of the heart (see below) and an increase in mitochondrial oxidative metabolism, with the faster blood lactate transport, disposal, and removal occurring 10 min after the incremental exercise test only being associated with the HIIT protocol.
Cardiac morphological and functional parameters
Notably, the superior effect of HIIT on the enhancement of exercise capacity is also influenced by the efficiency of oxygen transport to the skeletal muscle, which can be mediated by enhanced cardiovascular adaptation [2, 32]. Increases in VO2max during the HIIT protocol were mediated, in part, through an increase in cardiac output provided by an increase in heart rate and stroke volume, resulting from positive training effects on the sympathetic control of the heart during exercise in humans [26, 33]. Human studies have provided strong evidence that improvements in VO2max with HIIT correlates positively with cardiac output and stroke volume [34, 35]. In contrast to these studies, however, both our HIIT and CMT protocols led to similar significant decreases in absolute cardiac output, heart rate, and stroke volume (P < 0.01) compared to those in the SC group but with no effect on the stroke volume index and cardiac index (P > 0.05) at rest (Table 2). These observations are in line with previous findings in animal models that showed a reduction in stroke volume after a 10-week CMT protocol, with a tendency for a decrease in cardiac output and heart rate at rest [36]. A recent study also failed to report a significant improvement in cardiac output, stroke volume, and cardiac index in rats after a 10-week swimming program of moderate-intensity aerobic training [22]. Despite these findings, our HIIT and CMT exercise protocols did lower cardiac output, possibly by reducing resting heart rate and stroke volume, although changes in the stroke volume index and cardiac index were not significantly different from those in the SC group. This could be the result of our normalization to BW, with both exercise protocols inducing a rapid decrease in BW. Our finding of a decreased resting heart rate only in the HIIT group is consistent with the finding that HIIT, but not CMT, significantly decreases resting heart rate [32, 36] in healthy rats. However, this finding is not in agreement with the findings of Neves et al., who reported no change in the resting heart rate of rats after HIIT [18]. This discrepancy is explained by differences in training duration, intensity, and volume for the HIIT protocol.
LV systolic function is generally assessed by measuring the LV shortening fraction and ejection fraction, both of which have been reported to significantly increase at rest and after HIIT and CMT in healthy males [37]. However, a meta-analysis indicated that there were no significant differences in the LV ejection fraction and fractional shortening between sedentary controls and athletes [38]. This evidence supports our finding of unchanged resting LV shortening fraction and ejection fraction among rats in both the HIIT and CMT groups. This is also consistent with recent studies [18, 32, 36, 39] that did not identify an improvement in LV fractional shortening and ejection fraction in rats with various training protocols: HIIT (8 min at a speed corresponding to 80% of the maximum speed and 2 min at a speed corresponding to 20% of the maximum speed, 5 day week−1, and 60 min day−1 for 60 days); 6-month period of incremental endurance exercise training; 10 weeks of CMT; and 12 weeks of high-volume and low-intensity CMT. Thus, conventional systolic indices, such as LV shortening fraction and ejection fraction, are limited in their assessment of LV systolic function.
Cardiac hypertrophy increases the heart’s pumping capacity during physiological adaptation to exercise, thereby meeting the increased needs of the whole body. The ratio of heart weight to BW (relative heart weight) is widely used to assess exercise-induced heart hypertrophy. However, this measure also has limitations that should not be ignored. As an example, the decrease in the amount of fatty tissue located around the heart after long-term exercise, independent of the size of the heart, would render relative heart weight inaccurate as an index of heart hypertrophy [21]. While both our HIIT and CMT exercise protocols failed to induce a significant difference in relative heart weight compared to the value in the SC group, both HIIT and CMT decreased heart weight by 18.2% (P < 0.05) and 12.0% (P > 0.05), respectively, (Table 1), with these decreases being primarily attributed to a decrease in fatty tissue. Several studies have reported the relative heart weight, in addition to other indices of heart hypertrophy in rats, such as the ratio of LV mass to BW, as being significantly different between sedentary controls and athletes [38]. Our data agree with these findings, with HIIT leading to a significant increase in the ratio of LV mass to BW (P < 0.05) compared to that in the SC group [18], which can be regarded as an adverse precursor to LV hypertrophy. Thus, it is reasonable to speculate that the ratio of LV mass to BW provides a more accurate index of heart hypertrophy in rats than the relative heart weight, supporting an increase in LV function after exercise. Previous studies have also reported a larger ratio of LV mass to BW, LVDD to BW, LVPWd to BW, and LVPWs to BW in endurance-trained individuals compared to untrained controls [38, 40]. Our findings on the assessment of heart morphology are consistent with those of previous reports of a similar increase in the ratio of LVDD to BW, LVAWs to BW, LVAWd to BW, ratio of LVIDd to BW, ratio of LVPWd to BW, and ratio of LVPWs to BW in the HIIT and CMT groups relative to those in the SC group (P < 0.01) (Table 2). This is consistent with observations in rats of an increase in the LVPWd to BW ratio of 12.7% for HIIT and 9% for CMT, with an increase in LVPWs to BW ratio of 20% for both exercise modalities [32]. However, our results disagree with previous human and rat observations, with no improvement in the ratio of LVDD to BW, LVIDd to BW, LVPWs to BW, and LVPWd to BW identified with either the HIIT or CMT protocol [18, 35, 37, 41]. Overall, these contradictory results are probably due to differences in training duration, type of training, methods, and animals/subjects.
With respect to morphological and functional parameters, apart from the LV mass to BW ratio, we observed that both exercise protocols induced similar adaptive changes of cardiac morphology but LV systolic function was unaltered according to measurements of LV shortening fraction and ejection fraction. This finding is in agreement with those of previous studies, which have proposed that the magnitude of LV structure remodeling during exercise is not sufficient to enhance LV systolic function [32]. Kemi et al. [32] also indicated that HIIT yielded greater effects on cardiomyocyte contractility and Ca2+ handling than CMT, with no changes observed in LV fractional shortening, including in the ratio of the peak mitral flow velocity (E-wave) or the peak velocity during the late filling wave of atrial contraction (A-wave). Thus, these earlier findings and our results strongly support the idea that the increased exercise performance observed in HIIT and CMT rats was not associated with a significant improvement in LV ejection fraction and fractional shortening and cardiac output at rest, with myocardial mechanisms likely to be more important to increases in aerobic fitness [32]. Indeed, recent evidence has suggested that the superior hypertrophic response to HIIT than to CMT is associated with activation of myocardial antioxidant capacity and maximal mitochondrial respiration in healthy rats [2], which has been shown to ameliorate myocardial glucolipid metabolism and enhances myocardial ATP production in a rat model of myocardium infarction [42].
Basal autophagic activity
Exercise has been implicated in the regulation of basal autophagic activity in a tissue-specific manner. For instance, in rats, endurance training induced a significant increase in autophagy makers, such as Beclin 1, LC3-II, and p62 content, in cerebral tissues but not in the liver or gastrocnemius and cardiac muscles [13]. It has been suggested that alteration of autophagy depends on the metabolic characteristics of the muscle during exercise training and that increased basal autophagic activity is a crucial precursor to mitochondrial biogenesis and mitochondrial remodeling, which has been shown to increase with endurance training in the plantaris muscle (composed of mixed fibers) to improve oxidative function [15]. Previous studies have also confirmed that long-term endurance training resulting in fiber-type shifting from type IIX to IIA was associated with an increase in LC3-II in the plantaris muscle [5, 43], but not in the soleus muscle, which is an oxidative muscle [5]. However, our results showed no significant change in the LC3-II, LC3-II/LC3-I ratio, ATG-3/5/7/12/16L, and Beclin 1 expression in the cardiac muscle of rats in the CMT group compared to those in the SC group, suggesting that the basal autophagy and mitochondrial metabolic characteristics in the cardiac muscle remain stable after a 10-week CMT intervention. This has also been confirmed by other studies. For instance, Smuder et al. [15] reported that the ATG-7, ATG-12, Beclin 1, and LC3-II content and the LC3-II/LC3-I ratio in the cardiac muscle did not increase using a CMT protocol, which was of comparable intensity to our protocol (5 consecutive days of treadmill exercise for 60 min day−1 at 30 m min−1, 70% of VO2max) but with a higher volume (60 min day−1). In addition, as reported in other studies [14, 27], protein expression of Beclin 1, ATG-5, ATG-7, and p62 and the LC3-II/LC3-I ratio in the cardiac muscle were unaltered after habitual wheel-running exercise [5], long-term low-intensity exercise (12 m min−1, 4–5 days week−1 for 36 weeks) [27], and CMT (intensity and duration were progressively increased until week 3, when the animals were running at 21 m min−1 for 45 min day−1 at a 4.5% slope; this training intensity was then maintained for the final 3 weeks) [14].
Of particular note, the paper published by McMillan et al. [14] was compatible with the study of Sun et al. [16], who reported that increasing the volume and duration of exercise and matched intensity (intensity and duration were progressively increased until week 4, when the animals were running at 20 m min−1, 5% slope, for 60 min day−1; this training intensity was then maintained for the final 4 weeks) compared to that of this study, increased autophagy flux, including elevated LC3-II, ATG-7, and Beclin 1 protein expression and LC3-II/LC3-I ratio, inferring that alteration of basal autophagy seems to rely on both training duration and volume. Additionally, an aforementioned study also reported that high-intensity endurance training (cycling for 2 h at 70% of VO2max) is a more potent stimulus than CMT for increasing autophagic flux (55% of VO2max) in fast twitch-type human skeletal muscle during cycling exercise [15]. However, there is relatively little information regarding the influence of exercise intensity, duration, and volume on the adaptive response of autophagy to exercise training in cardiac muscles. Further research is required to understand the importance of exercise intensity, duration, and volume in mediating basal autophagy in cardiac muscles.
This study is the first to show that a HIIT protocol can increase LC3-II and LC3-I protein expression, as well as the LC3-II/LC3-I ratio, in cardiac muscle compared to the levels in the SC group, which is the most commonly used marker in monitoring autophagic flux (Fig. 4). We also measured autophagy using other marker proteins promoting the initial assembly of the autophagosomal membrane, including ATG-3/5/7/12/16L and Beclin 1, which are involved in the up-regulation of autophagosome synthesis and autophagosome formation of the first ubiquitin-like conjugation system [6], indicating that ATG-3/12/16L and Beclin 1 protein expression increases in cardiac muscle after a 10-week HIIT protocol compared to the value in the SC group.
Importantly, to our knowledge, this is the first study to provide evidence that the HIIT protocol was superior to a volume- and intensity-matched CMT protocol for activating basal autophagy in cardiac muscle. Our findings are in agreement with those of previous studies that have demonstrated a greater effectiveness of HIIT than CMT in reducing the extent of exercised-induced decline in autophagy via a down-regulation of Beclin 1, LC3-II, ATG-1, and ATG-12 in CD4 lymphocytes caused by hypoxic exercise, alleviating CD4 lymphocyte death and improving endurance performance [17]. We also found that the basal autophagic activity in the myocardium is closely associated with improvements in endurance performance (Fig. 4a–d). These results, together with those presented here, demonstrate in part that the superior elevation of basal autophagic activity in both lymphocytes and myocardium induced by a HIIT protocol may significantly contribute to improved exercise performance compared to the use of a matched CMT protocol. Further research is warranted to investigate these results in a tissue-specific manner and in ATG knockout model animals.
Mitochondria function markers
Endurance training results in adaptations in both exercise performance and skeletal muscle metabolism associated with changes in fiber-type distribution and fiber-type specific oxidative and glycolytic capacity [44]. However, compared with other types of skeletal muscle fibers, the cardiac muscle, which is a continually contracting muscle, has the highest aerobic capacity and highest metabolic demand of all muscles and is subsequently rich in mitochondria [45]. Several recent reports have suggested that improvements in the mitochondrial biogenesis and oxidative phosphorylation capacity in cardiac muscles were associated with improvements in aerobic capacity [2]. Indeed, in the current study, CMT did not alter the number of myocardial mitochondria nor the expression of mitochondrial biogenetic marker proteins, SDH and COX IV, in cardiac muscle. These findings are similar to the those of Kainulainen et al. [46] in hearts from exercise-trained rats, which showed similarly unaltered SDH and COX IV activity after a speed-increased (running 1 h at an increased speed to 20 m min−1) and speed-constant (running 1 h at a constant speed of 25 m min−1) 5-week endurance exercise regime. Several studies have also implied that increasing the volume and intensity of endurance exercise (speed of 25 m min−1 at 10% or 16% for 30 min day−1 in the first week before being progressively increased by 15 min week−1 until week 4, at which point the animals were running at 120 m min−1 which was continued for 4 weeks) was insufficient in altering the maximal mitochondrial respiratory capacity, citrate synthase activity, mitochondrial biogenesis, and antioxidant-related gene expression in the myocardium of rats [2, 34, 39]. Therefore, metabolic adaptation of the heart may not easily respond to the CMT protocol. The response in mitochondrial biogenesis and respiratory capacity of the myocardium to the CMT protocol is not a prerequisite for exercise capacity improvement but may be related to changes in fiber-type specific oxidative and glycolytic capacity in skeletal muscles [15].
Our data also show that the HIIT protocol was more effective in increasing the expression of SDH and COX IV protein and mitochondrial content than the intensity- and volume-matched CMT protocol. Previous studies have indicated that HIIT improves cardiac efficiency by increasing myocardial glucose oxidation with a concomitant decrease in fatty acid oxidation, and increases the maximal mitochondrial respiratory capacity and citrate synthase activity of the myocardium, effects which are not induced by the volume-matched CMT [2]. These results suggest that the myocardium exhibits less metabolic plasticity in response to exercise training than skeletal muscles but the intensity- and volume-matched HIIT protocol facilitates cardiac adaptations that increase mitochondrial oxidative capacity and cardiac efficiency due to decreased unloaded myocardial oxygen consumption, a switch toward a faster cardiac myosin isoform, and the ability to catabolize carbohydrates over fats. Our data showed that autophagy markers (LC3-II/LC3-I ratio and ATG-3) have significant positive relationships with the SDH and COX IV content in cardiac muscles (Fig. 3e–h), which suggests that elevated basal autophagic adaptation is involved in increased mitochondrial biogenesis and respiratory capacity in cardiac muscle during exercise training [9, 11].
Mechanically, autophagic activities and mitochondrial biogenesis in cardiomyocytes can be regulated by mitochondrial sirtuin 3 (SIRT3) through the regulation of the forkhead box O1 pathway [47]. Recent studies have indicated that 12 weeks of CMT (60 min day−1 with velocity increased gradually from 18 to 30 m min−1) increased SIRT3 contents in the cerebellum and brain cortex of young male rats, which was related to mitochondrial biogenesis and alteration in proteins involved in mitochondrial dynamics and autophagy signaling [48]. However, we did not identify any change in the expression of SIRT3 of cardiac muscle after either exercise modality. Rather, we hypothesize that the CMT protocol increased SIRT3 protein expression in a tissue-specific manner. Several lines of evidence suggest that ALDH2 in the cardiac muscle could act on the detoxification of acetaldehyde and may be a key mitochondrial enzyme involved in the regulation of oxidative stress and autophagy activities, the maintenance of mitochondrial homeostasis, and the occurrence of apoptosis, which are involved in protection against various cardiac injuries [49]. However, ALDH2 levels were not modulated by either of the exercise models in the present study. These findings are in accordance with a study that reported no changes in ALDH2 protein expression in hypertensive rats following endurance training [50]. However, a recent study implied that ALDH2 protein expression decreased in the skeletal muscle of rats during exhaustive exercise but that overexpression of ALDH2 restored exhaustive exercise-induced mitochondrial dysfunction in skeletal muscle [51]. It can thus be hypothesized that these changes in basal autophagy activity and mitochondrial oxidative function indicate that the observed exercise-induced improvements in cardiac muscle were not dependent on SIRT3 and ALDH2 after the HIIT protocol. Whether these proteins take part in mechanistic events involved in autophagic adaptation in other tissues with exercise needs to be addressed in future studies.
Conclusions
HIIT was more effective for improving physical performance than an intensity- and volume-matched CMT protocol and these improvements were related to basal autophagic adaptation and mitochondrial oxidative function in cardiac muscle.
Acknowledgements
The authors greatly appreciate the technical expertise and support of Hong-ying Pan, Huan-chun Li, Xue Wang, Ding-guo Ruan, and the extraordinary research participants for their enthusiasm to participate in this project.
Abbreviations
- ALDH2
Acetaldehyde dehydrogenase 2
- ANOVA
Analysis of variance
- ATG
Autophagy-related gene
- CMT
Continuous moderate-intensity training
- COX IV
Cytochrome C oxidase subunit IV
- HIIT
High-intensity interval training
- LC3
Microtubule-associated protein A/BE-light chain 3
- LV
Left ventricular
- LVAWd
LV anterior wall thickness in diastole
- LVAWs
LV anterior wall thickness in systole,
- LVDD
LV end diastolic diameters
- LVPWd
LV posterior wall thickness in diastolic
- LVPWs
LV posterior wall thickness in systolic
- LVSD
LV end systolic diameters
- SDH
Succinate dehydrogenase
- SIRT3
Sirtuin 3
Author contributions
Fang-Hui Li, Tao Li, and Ying-min Su performed the experiments; Fang-Hui Li and Ying-min Su analyzed the data; Tao Li interpreted the results of experiments; Fang-Hui Li and Jing-yi Ai prepared the figures; Fang-Hui Li, Rui Duan, and Timon Cheng-Yi Liu drafted, edited and revised the manuscript; Fang-Hui Li and Tao Li conceived and designed the research.
Funding
This study was funded by the National Natural Science Foundation of China (grant numbers 31500961, 61575065, 31771256) and Guangdong Scientific Project (grant numbers 2014A020220015, 2015A020219015).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
References
- 1.Fox EL, Bartels RL, Billings CE, Mathews DK, Bason R, Webb WM. Intensity and distance of interval training programs and changes in aerobic power. Med Sci Sports. 1973;5(1):18–22. [PubMed] [Google Scholar]
- 2.Hafstad AD, Boardman NT, Lund J, Hagve M, Khalid AM, Wisløff U, Larsen TS, Aasum E. High intensity interval training alters substrate utilization and reduces oxygen consumption in the heart. J Appl Physiol. 2011;111(5):1235–1241. doi: 10.1152/japplphysiol.00594.2011. [DOI] [PubMed] [Google Scholar]
- 3.Trilk JL, Singhal A, Bigelman KA, Cureton KJ. Effect of sprint interval training on circulatory function during exercise in sedentary, overweight/obese women. Eur J Appl Physiol. 2011;111(8):1591–1597. doi: 10.1007/s00421-010-1777-z. [DOI] [PubMed] [Google Scholar]
- 4.Ju JS, Jeon SI, Park JY, Lee JY, Lee SC, Cho KJ, Jeong JM. Autophagy plays a role in skeletal muscle mitochondrial biogenesis in an endurance exercise-trained condition. J Physiol Sci. 2016;66(5):417–430. doi: 10.1007/s12576-016-0440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee Youngil, Kwon Insu, Jang Yongchul, Song Wankeun, Cosio-Lima Ludmila M., Roltsch Mark H. Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. The Journal of Physiological Sciences. 2017;67(6):639–654. doi: 10.1007/s12576-017-0555-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaizuka T, Mizushima N. Atg13 is essential for autophagy and cardiac development in mice. Mol Cell Biol. 2015;36(4):585–595. doi: 10.1128/mcb.01005-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Yang, Pan Shan-Shan, Shen Yu-Jun. Cardioprotection of exercise preconditioning involving heat shock protein 70 and concurrent autophagy: a potential chaperone-assisted selective macroautophagy effect. The Journal of Physiological Sciences. 2016;68(1):55–67. doi: 10.1007/s12576-016-0507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loverso F, Carnio S, Vainshtein A, Sandri M. Autophagy is not required to sustain exercise and PRKAA1/AMPK activity but is important to prevent mitochondrial damage during physical activity. Autophagy. 2014;10(11):1883–1894. doi: 10.4161/auto.32154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He C, Rhea Sumpter J, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8(10):1548–1551. doi: 10.4161/auto.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogura Y, Iemitsu M, Naito H, Kakigi R, Kakehashi C, Maeda S, Akema T. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem Biophys Res Commun. 2011;414(4):756–760. doi: 10.1016/j.bbrc.2011.09.152. [DOI] [PubMed] [Google Scholar]
- 11.Qiao S, Dennis M, Song X, Vadysirisack DD, Salunke D, Nash Z, Yang Z, Liesa M, Yoshioka J, Matsuzawa S, Shirihai OS, Lee RT, Reed JC, Ellisen LW. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat Commun. 2011;6:7014. doi: 10.1038/ncomms8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat Med. 2007;13(5):619–624. doi: 10.1038/nm1574. [DOI] [PubMed] [Google Scholar]
- 13.Bayod S, Del Valle J, Pelegri C, Vilaplana J, Canudas AM, Camins A, Jimenez A, Sanchez-Roige S, Lalanza JF, Escorihuela RM, Pallas M. Macroautophagic process was differentially modulated by long-term moderate exercise in rat brain and peripheral tissues. J Physiol Pharmacol. 2014;65(2):229–239. [PubMed] [Google Scholar]
- 14.Mcmillan EM, Paré MF, Baechler BL, Graham DA, Rush JW, Quadrilatero J. Autophagic signaling and proteolytic enzyme activity in cardiac and skeletal muscle of spontaneously hypertensive rats following chronic aerobic exercise. PLoS One. 2015;10(3):e0119382. doi: 10.1371/journal.pone.0119382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smuder AJ, Kavazis AN, Min K, Powers SK. Doxorubicin-induced markers of myocardial autophagic signaling in sedentary and exercise trained animals. J Appl Physiol. 2013;115(2):176–185. doi: 10.1152/japplphysiol.00924.2012. [DOI] [PubMed] [Google Scholar]
- 16.Sun M, Huang C, Wang C, Zheng J, Zhang P, Xu Y, Chen H, Shen W. Ginsenoside Rg3 improves cardiac mitochondrial population quality: mimetic exercise training. Biochem Biophys Res Commun. 2013;441(1):169–174. doi: 10.1016/j.bbrc.2013.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Weng TP, Huang SC, Chuang YF, Wang JS. Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One. 2013;8(11):e80248. doi: 10.1371/journal.pone.0080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neves CH, Tibana RA, Prestes J, Voltarelli FA, Aguiar AF, Ferreira Mota GA, de Sousa SL, Leopoldo AS, Leopoldo AP, Mueller A, Aguiar DH, Navalta JW, Sugizaki MM. Digoxin induces cardiac hypertrophy without negative effects on cardiac function and physical performance in trained normotensive rats. Int J Sports Med. 2017;38(4):263–269. doi: 10.1055/s-0042-119727. [DOI] [PubMed] [Google Scholar]
- 19.Criswell D, Powers S, Dodd S, Lawler J, Edwards W, Renshler K, Grinton S. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Med Sci Sports Exerc. 1993;25(10):1135–1140. doi: 10.1249/00005768-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Bedford TG, Tipton CM, Wilson NC, Oppliger RA, Gisolfi CV. Maximum oxygen consumption of rats and its changes with various experimental procedures. J Appl Physiol Respir Environ Exerc Physiol. 1979;47(6):1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- 21.Liao J, Li Y, Zeng F, Wu Y. Regulation of mTOR pathway in exercise-induced cardiac hypertrophy. Int J Sports Med. 2015;36(5):343–350. doi: 10.1055/s-0034-1395585. [DOI] [PubMed] [Google Scholar]
- 22.Felix AC, Dutra SG, Tezini GC, Simões MV, de Souza HC. Aerobic physical training increases contractile response and reduces cardiac fibrosis in rats subjected to early ovarian hormone deprivation. J Appl Physiol. 2015;118(10):1276–1285. doi: 10.1152/japplphysiol.00483.2014. [DOI] [PubMed] [Google Scholar]
- 23.Marzetti E, Groban L, Wohlgemuth SE, Lees HA, Lin M, Jobe H, Giovannini S, Leeuwenburgh C, Carter CS. Effects of short-term GH supplementation and treadmill exercise training on physical performance and skeletal muscle apoptosis in old rats. Am J Physiol Regul Integr Comp Physiol. 2008;294(2):558–567. doi: 10.1152/ajpregu.00620.2007. [DOI] [PubMed] [Google Scholar]
- 24.Laursen PB, Marsh SA, Jenkins DG, Coombes JS. Manipulating training intensity and volume in already well-trained rats: effect on skeletal muscle oxidative and glycolytic enzymes and buffering capacity. Appl Physiol Nutr Metab. 2007;32(3):434–442. doi: 10.1139/H07-006. [DOI] [PubMed] [Google Scholar]
- 25.Pereira F, de Moraes R, Tibiriçá E. Interval and continuous exercise training produce similar increases in skeletal muscle and left ventricle microvascular density in rats. Biomed Res Int. 2013;2013:752817. doi: 10.1155/2013/752817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- 27.Haram PM, Kemi OJ, Lee SJ, Bendheim MØ, Al-Share QY, Waldum HL, Gilligan LJ, Koch LG, Britton SL, Najjar SM, Wisløff U. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovasc Res. 2009;81(4):723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazurek K, Zmijewski P, Krawczyk K, Czajkowska A, Kęska A, Kapuściński P, Mazurek T. High intensity interval and moderate continuous cycle training in a physical education programme improves health-related fitness in young females. Biol Sport. 2016;33(2):139–144. doi: 10.5604/20831862.1198626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tabata I, Nishimura K, Kouzaki M, Hirai Y, Ogita F, Miyachi M, Yamamoto K. Effects of moderate-intensity endurance and high-intensity intermittent training on anaerobic capacity and VO2max. Med Sci Sports Exerc. 1996;28(10):1327–1330. doi: 10.1097/00005768-199610000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Naimo MA, de Souza EO, Wilson JM, Carpenter AL, Gilchrist P, Lowery RP, Averbuch B, White TM, Joy J. High-intensity interval training has positive effects on performance in ice hockey players. Int J Sports Med. 2015;36(1):61–66. doi: 10.1055/s-0034-1382054. [DOI] [PubMed] [Google Scholar]
- 31.Donovan CM, Brooks GA. Endurance training affects lactate clearance, not lactate production. Am J Physiol. 1983;244(1):83–92. doi: 10.1152/ajpendo.1983.244.1.E83. [DOI] [PubMed] [Google Scholar]
- 32.Kemi OJ, Haram PM, Loennechen JP, Osnes JB, Skomedal T, Wisløff U, Ellingsen Ø. Moderate vs. high exercise intensity: differential effects on aerobic fitness, cardiomyocyte contractility, and endothelial function. Cardiovasc Res. 2005;67(1):161–172. doi: 10.1016/j.cardiores.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 33.Astorino TA, Edmunds RM, Clark A, King L, Gallant RA, Namm S, Fischer A, Wood KM. High-intensity interval training increases cardiac output and VO2max. Med Sci Sports Exerc. 2017;49(2):265–273. doi: 10.1249/MSS.0000000000001099. [DOI] [PubMed] [Google Scholar]
- 34.Ascensão A, Magalhães J, Soares JM, Ferreira R, Neuparth MJ, Marques F, Oliveira PJ, Duarte JA. Moderate endurance training prevents doxorubicin-induced in vivo mitochondriopathy and reduces the development of cardiac apoptosis. Am J Physiol Heart Circ Physiol. 2005;289(2):722–731. doi: 10.1152/ajpheart.01249.2004. [DOI] [PubMed] [Google Scholar]
- 35.Esfandiari S, Sasson Z, Goodman JM. Short-term high-intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. Eur J Appl Physiol. 2014;114(2):331–343. doi: 10.1007/s00421-013-2773-x. [DOI] [PubMed] [Google Scholar]
- 36.Dolinsky VW, Jones KE, Sidhu RS, Haykowsky M, Czubryt MP, Gordon T, Dyck JR. Improvements in skeletal muscle strength and cardiac function induced by resveratrol during exercise training contribute to enhanced exercise performance in rats. J Physiol. 2012;590(11):2783–2799. doi: 10.1113/jphysiol.2012.230490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mahdiabadi J, Gaeini AA, Kazemi T, Mahdiabadi MA. The effect of aerobic continuous and interval training on left ventricular structure and function in male non-athletes. Biol Sport. 2013;30(3):207–211. doi: 10.5604/20831862.1059302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101(3):336–344. doi: 10.1161/01.CIR.101.3.336. [DOI] [PubMed] [Google Scholar]
- 39.Schoepe M, Schrepper A, Schwarzer M, Osterholt M, Doenst T. Exercise can induce temporary mitochondrial and contractile dysfunction linked to impaired respiratory chain complex activity. Metabolism. 2012;61(1):117–126. doi: 10.1016/j.metabol.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 40.Laaksonen MS, Heinonen I, Luotolahti M, Knuuti J, Kalliokoski KK. VO(2peak), myocardial hypertrophy, and myocardial blood flow in endurance-trained men. Med Sci Sports Exerc. 2014;46(8):1498–1505. doi: 10.1249/MSS.0000000000000264. [DOI] [PubMed] [Google Scholar]
- 41.Eskelinen JJ, Heinonen I, Löyttyniemi E, Hakala J, Heiskanen MA, Motiani KK, Virtanen K, Pärkkä JP, Knuuti J, Hannukainen JC, Kalliokoski KK. Left ventricular vascular and metabolic adaptations to high-intensity interval and moderate intensity continuous training: a randomized trial in healthy middle-aged men. J Physiol. 2016;594(23):7127–7140. doi: 10.1113/jp273089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu K, Wang L, Wang C, Yang Y, Hu D, Ding R. Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis, oxidative stress and metabolism of the infarcted myocardium in a rat model. Mol Med Rep. 2015;12(2):2374–2382. doi: 10.3892/mmr.2015.3669. [DOI] [PubMed] [Google Scholar]
- 43.Schwalm C, Jamart C, Benoit N, Naslain D, Prémont C, Prévet J, Van Thienen R, Deldicque L, Francaux M. Activation of autophagy in human skeletal muscle is dependent on exercise intensity and AMPK activation. FASEB J. 2015;29(8):3515–3526. doi: 10.1096/fj.14-267187. [DOI] [PubMed] [Google Scholar]
- 44.Scribbans TD, Edgett BA, Vorobej K, Mitchell AS, Joanisse SD, Matusiak JB, Parise G, Quadrilatero J, Gurd BJ. Fibre-specific responses to endurance and low volume high intensity interval training: striking similarities in acute and chronic adaptation. PLoS One. 2014;9(6):e98119. doi: 10.1371/journal.pone.0098119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SY, Gifford JR, Andtbacka RH, Trinity JD, Hyngstrom JR, Garten RS, Diakos NA, Ives SJ, Dela F, Larsen S, Drakos S, Richardson RS. Cardiac, skeletal, and smooth muscle mitochondrial respiration: are all mitochondria created equal? Am J Physiol Heart Circ Physiol. 2014;307(3):346–352. doi: 10.1152/ajpheart.00227.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kainulainen H, Komulainen J, Leinonen A, Rusko H, Vihko V. Regional differences of substrate oxidation capacity in rat hearts: effects of extra load and endurance training. Basic Res Cardiol. 1990;85(6):630–639. doi: 10.1007/BF01907897. [DOI] [PubMed] [Google Scholar]
- 47.Li J, Chen T, Xiao M, Li N, Wang S, Su H, Guo X, Liu H, Yan F, Yang Y, Zhang Y, Bu P. Mouse Sirt3 promotes autophagy in AngII-induced myocardial hypertrophy through the deacetylation of FoxO1. Oncotarget. 2016;7(52):86648–86659. doi: 10.18632/oncotarget.13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marques-Aleixo I, Santos-Alves E, Balça MM, Rizo-Roca D, Moreira PI, Oliveira PJ, Magalhães J, Ascensão A. Physical exercise improves brain cortex and cerebellum mitochondrial bioenergetics and alters apoptotic, dynamic and auto (mito) phagy markers. Neuroscience. 2015;301:480–495. doi: 10.1016/j.neuroscience.2015.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, Qin Y, Zhang P, Chen Y, Harada M, Isse T, Kawamoto T, Fan H, Yang P, Akazawa H, Nagai T, Takano H, Ping P, Komuro I, Ge J. Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc. 2014;3(5):e000779. doi: 10.1161/jaha.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campos JC, Fernandes T, Bechara LR, da Paixão NA, Brum PC, de Oliveira EM, Ferreira JC. Increased clearance of reactive aldehydes and damaged proteins in hypertension-induced compensated cardiac hypertrophy: impact of exercise training. Oxid Med Cell Longev. 2015;2015:464195. doi: 10.1155/2015/464195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q, Zheng J, Qiu J, Wu X, Xu Y, Shen W, Sun M. ALDH2 restores exhaustive exercise-induced mitochondrial dysfunction in skeletal muscle. Biochem Biophys Res Commun. 2017;485(4):753–760. doi: 10.1016/j.bbrc.2017.02.124. [DOI] [PubMed] [Google Scholar]


