Abstract
Previous studies have demonstrated that zymosan, a cell wall component of the yeast Saccharomyces cerevisiae, induces inflammation in experimental models. However, few studies have evaluated the potential of zymosan to induce sickness behavior, a central motivational state that allows an organism to cope with infection. To determine whether zymosan administration results in sickness behavior, mice were submitted to the forced swim (FST) and open field (OFT) tests 2, 6, and 24 h after treatment with zymosan (1, 10, or 100 mg/kg). Additionally, to evaluate the possible relationship between zymosan-induced sickness behavior and prostaglandin synthesis, mice were pretreated with the cyclooxygenase inhibitors indomethacin (10 mg/kg) and nimesulide (5 mg/kg) and the glucocorticoid drug dexamethasone (1 mg/kg). Zymosan induced time-dependent decreases in locomotor activity in the OFT, and an increase in immobility in the FST, and increased plasma levels of corticosterone at 2 h. Pretreatment with indomethacin, nimesulide, or dexamethasone blocked zymosan-induced behavioral changes in both the FST and OFT at 2 h post administration. These findings confirm previous observations that zymosan induces sickness behavior. Furthermore, our results provide new evidence that prostaglandin synthesis is necessary for this effect, as anti-inflammatory drugs that inhibit prostaglandin synthesis attenuated zymosan-induced behavioral changes.
Keywords: Cyclooxygenase, Fungal infection, Prostaglandin, Saccharomyces cerevisiae, Sickness behavior, Zymosan
Introduction
Physiological and behavioral changes induced by infection and inflammation can be induced in experimental animals by exposure to yeast particles such as zymosan, which causes appetite suppression (anorexia), fever, and behavioral changes including reduced motor and exploratory activity and onset of a depressive-like state featuring anhedonia, evidenced by reduced consumption of palatable and preferred foods [1–4]. These sickness behaviors represent a motivational state that promotes the organism’s recovery and survival by modifying its priorities in order to cope with infectious agents [5, 6].
Sickness behaviors arise from a series of physiological changes mediated by the immune and neuroendocrine systems [2, 6, 7]. Infectious agents display characteristic pathogen-associated molecular patterns that are recognized by pattern recognition receptors expressed in cells of the innate immune system, primarily macrophages and dendritic cells. These cells initiate and propagate an inflammatory response by stimulating the synthesis and release of a variety of proinflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6), and tumor necrosis factor (TNF-α) [7–10]. These cytokines act as autocrine, paracrine, and endocrine factors that regulate cell proliferation, hormone secretion, hypothalamic–pituitary–adrenal (HPA) axis activity, and behavioral changes [7, 11]. Circulating cytokines influence brain activity by inducing the expression of cyclooxygenase (COX)-2 and microsomal prostaglandin E synthase-1 in brain vascular cells, which transduce inflammatory signals into a prostaglandin signaling cascade [12]. These interactions between the immune and neuroendocrine systems are bi-directional and crucial for the maintenance of homeostasis [7].
The current literature indicates that cytokines and prostaglandins are important mediators of sickness behavior induced by lipopolysaccharides (LPS) [5, 13, 14]; similarly, cytokine upregulation has been shown to play a role in the febrile response evoked by zymosan administration [11]. However, neither the ability of zymosan to induce sickness behavior nor the involvement of prostaglandins in this process is well understood. In this study, mice were submitted to well-established behavioral tests to evaluate depressive-like and exploratory behaviors following treatment with zymosan. We also measured plasma corticosterone levels as an indicator of HPA axis activity after zymosan exposure. Finally, to investigate the role of prostaglandins in zymosan-evoked behavioral changes, mice were treated with a selection of widely used anti-inflammatory drugs, including the COX inhibitors indomethacin and nimesulide and the synthetic glucocorticoid dexamethasone, prior to zymosan administration and assessment of behavioral changes.
Materials and methods
Animals
Adult male Swiss mice (30–40 g) were obtained from the Central Animal Facility of the Federal University of Alfenas and housed under controlled light (12:12 h light–dark cycle; lights on at 6:00 a.m.) and temperature conditions (23 ± 1 °C) with access to water and food ad libitum. Mice were habituated to the housing facility for at least 1 week before the experiments began.
Drugs
We assayed behavioral changes following systemic administration of zymosan from Saccharomyces cerevisiae yeast. A subset of mice were pretreated with indomethacin (a non-specific COX inhibitor), nimesulide (a highly selective COX-2 inhibitor), and dexamethasone (a glucocorticoid). All drugs were purchased from Sigma-Aldrich (St Louis, MO, USA). Zymosan was diluted in 0.9 % NaCl, and the other drugs were diluted in 0.1 M Tris, pH 8.0.
Experimental procedures
Drug treatments
The animals were subjected to zymosan treatment at doses of 1, 10, or 100 mg/kg (i.p.). The control group was treated with vehicle (0.9 % NaCl 0.9 %; 10 ml/kg, i.p.; n = 8–10 per group). Behavioral assessments were performed after 2, 6, or 24 h. These experiments were filmed using a digital video camera, recorded on DVD, and analyzed as described below.
Alternatively, to evaluate the role of prostaglandins in zymosan-induced behavioral changes, mice (n = 10 animals/group) were pretreated with indomethacin (10 mg/kg, i.p.), nimesulide (5 mg/kg, i.p.), dexamethasone (1 mg/kg, i.p.), or vehicle (0.1 M Tris; pH 8.0, i.p.) 30 min before injections of zymosan (10 mg/kg, i.p.) or (0.9 % NaCl, i.p.). Behavioral analyses were performed 2 h later. This time point was chosen based on our results following zymosan administration alone, as well as those of other studies [5, 13, 15].
Behavioral analyses
Forced swim test
The forced swim test (FST) was performed according to the method developed by Porsolt and colleagues [16]. Mice were placed in a vertical glass cylinder (26 cm high, 12 cm in diameter) filled with water (25 °C) to a depth of 16 cm. This depth was chosen in order to force the animals to swim or float without their hind limbs or tail touching the bottom of the cylinder. Each mouse was placed inside the cylinder for 6 min, and the time spent floating, defined as making only the smallest movements necessary to keep the head above water, was scored [13, 17].
Open field test
Locomotor activity was quantified for 5 min in an open field consisting of a white plexiglass box (60 × 60 cm) with its floor divided into 16 squares. Four squares were defined as the center, and the 12 squares along the walls were defined as the periphery. Each mouse was gently placed in the exact center of the box, and line-crossing activity was recorded when all 4 paws had entered another square. Line crossings among the 4 central and 12 peripheral squares were counted separately. The number of rearings (vertical exploratory activity) was observed. [13, 17].
Measurement of plasma corticosterone levels
Two, 6, or 24 h after zymosan (1, 10, or 100 mg/kg) or vehicle treatments (n = 8 animals/group), trunk blood was collected from each mouse in chilled heparinized vials. Plasma corticosterone was measured by radioimmunoassay [17].
Statistical analysis
Data were analyzed using GraphPad Prism version 6.0 software (GraphPad Software, Inc., La Jolla, CA, USA) and are expressed as the mean ± standard error of the mean (SEM). The results were analyzed by factorial analysis of variance (ANOVA). Post hoc comparisons using Student–Newman–Keuls tests were only performed when the F-value was significant for effects and/or interactions. The level of significance was set at 5 %; hence, p values less than 0.05 were considered significant.
Results
Time course of zymosan-induced behavioral changes
Two hours after 10 and 100 mg/kg zymosan treatments, we observed significant decreases in the numbers of periphery line crossings (F 3,31 = 6.38, p < 0.05 and 0.01, respectively), total line crossings (F 3,31 = 3.94; p < 0.05, 100 mg/kg group), and rearing episodes (F 3,31 = 11.48; p < 0.01 and 0.001, respectively) during the open field test (OFT) in these animals relative to saline-treated controls (Fig. 1a). Six hours after treatment, however, we observed decreases in the numbers of central (F 3,35 = 3.38; p < 0.05) and periphery line crossings (F 3,35 = 3.95; p < 0.05), total line crossings (F 3,35 = 5.09; p < 0.01), and rearing episodes (F 3,35 = 8.42; p < 0.01) only in animals treated with 100 mg/kg of zymosan relative to vehicle-treated animals (Fig. 1b). Twenty-four hours after treatment, we observed no differences among the experimental groups (Fig. 1c).
Fig. 1.
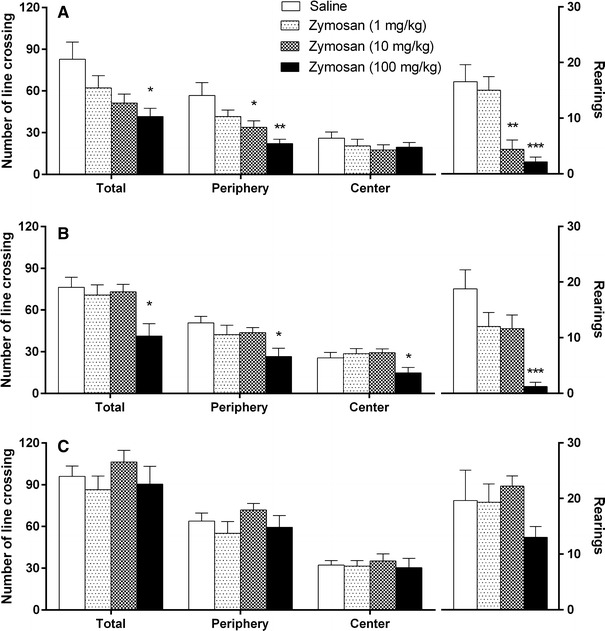
Results of the open field test obtained 2 (a), 6 (b), and 24 h (c) after the administration of zymosan (1, 10, or 100 mg/kg) or saline in mice. Columns and error bars represent mean ± standard error of the mean (SEM). *p < 0.05; **p < 0.01, and ***p < 0.001 compared with the saline-treated group
In the FST, significant increases in floating time were observed 2 h after 10 and 100 mg/kg zymosan treatments compared to animals treated with saline (F 3,34 = 7.32; p < 0.05 and 0.001, respectively; Fig. 2a). This effect remained up to 6 h after treatment; we observed increases in floating time in zymosan-treated animals at all doses tested when compared to vehicle-treated controls (F 3,34 = 7.77; p < 0.05; Fig. 2b). These results suggest that the mice exhibited both a transient decrease in locomotor activity and a sustained increase in immobility in the FST following administration of zymosan (dose of 1 and 10 mg/kg). Twenty-four hours after treatment, there were no significant differences between the animals treated with saline and those treated with zymosan (F 3,34 = 0.64; p = 0.59; Fig. 2c).
Fig. 2.
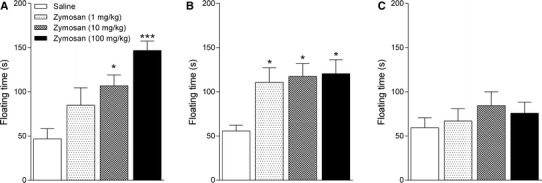
Results of the forced swim test obtained 2 (a), 6 (b), and 24 h (c) after the administration of zymosan (1, 10, or 100 mg/kg) or saline in mice. Columns and error bars represent mean ± SEM. *p < 0.05 and ***p < 0.001 compared with the saline-treated group
Zymosan-induced effects on plasma corticosterone levels
Plasma corticosterone concentrations were significantly increased 2 h after administration of 10 or 100 mg/kg doses of zymosan when compared to vehicle-treated controls (F 3,30 = 14.15; p < 0.05; Fig. 3). At 6 and 24 h after treatment, however, no significant differences among the experimental groups were observed.
Fig. 3.
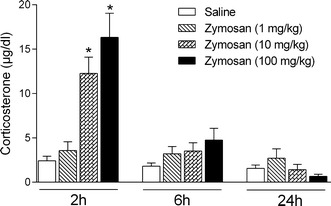
Corticosterone plasma concentrations were measured 2, 6, and 24 h after the administration of zymosan (1, 10, or 100 mg/kg) or saline in mice. Columns and error bars represent mean ± SEM. *p < 0.05 compared with the saline-treated group
Role of prostaglandins in behavioral changes induced by zymosan
Pretreatment of vehicle control-treated mice with indomethacin, nimesulide, or dexamethasone did not alter locomotor activity measured by the OFT 2 h after treatment (Fig. 4). Similar to our previous results (Fig. 1a), zymosan (10 mg/kg) significantly decreased the number of line crossings in the apparatus center and periphery, as well as the total number of line crossings and the number of rearing episodes. Nimesulide pretreatment reversed zymosan-induced decreases in the numbers of central (F 3,36 = 4.66; p < 0.05; Fig. 4a) and peripheral line crossings (F 3,36 = 8.61; p < 0.05; Fig. 4b) and total line crossings (F 3,36 = 6.37; p < 0.05; Fig. 4c). Similarly, indomethacin and dexamethasone pretreatments reversed the effects of zymosan on peripheral line crossings and total number of line crossings (Fig. 4b, c).
Fig. 4.
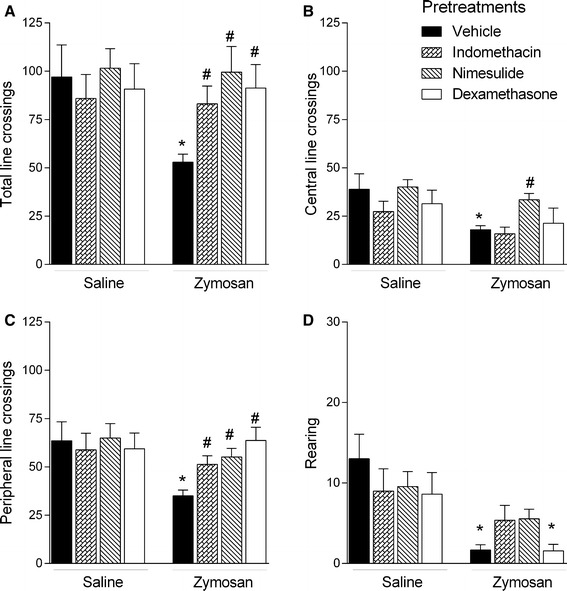
Effects of pretreatment with indomethacin (10 mg/kg), nimesulide (5 mg/kg), dexamethasone (1 mg/kg), or vehicle on locomotor behavior measured by the open field test 2 h after administration of zymosan (10 mg/kg) or saline. Columns and error bars represent mean ± SEM. *p < 0.05 compared with the control group (vehicle + saline); #p < 0.05 compared with the vehicle + zymosan-treated group
In the FST, we did not observe any differences in floating time 2 h after administration of vehicle, indomethacin, nimesulide, or dexamethasone in mice subsequently treated with saline (F 3,37 = 0.18; p = 0.91; Fig. 5). Similar to the results described above (Fig. 2a), we observed a significant increase in floating time 2 h after administration of zymosan (10 mg/kg). Pretreatment with indomethacin, nimesulide, or dexamethasone attenuated this effect (F 3,37 = 4.14; p < 0.05).
Fig. 5.
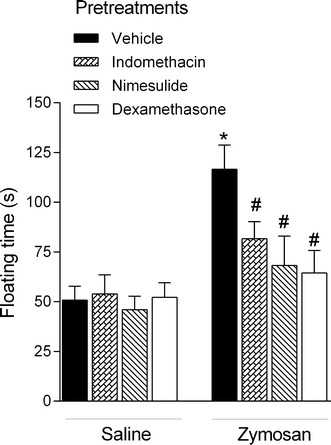
Effects of pretreatment with indomethacin (10 mg/kg), nimesulide (5 mg/kg), dexamethasone (1 mg/kg), or vehicle on floating time measured in the forced swim test 2 h after administration of zymosan (10 mg/kg) or saline. Columns and error bars represent mean ± SEM. *p < 0.05 compared with the control group (vehicle + saline); #p < 0.05 compared with the vehicle + zymosan-treated group
Discussion
The results of the present study confirm that zymosan induces sickness behavior. Our results further suggest that zymosan-induced behavioral changes may occur in a COX pathway-dependent manner, as COX inhibitors attenuated these effects.
The locomotor activity in mice decreases transiently 2 h after zymosan injection and recovers at around 6 h after injection. This short-term decrease in locomotor activity after zymosan injections is called sickness behavior, and is due to acute inflammation. Sickness is an adaptive response to infectious pathogens [2]. Although sickness and depression can share symptoms, the similarities are only partial. Sickness rather than depression is fully reversible once the pathogen has been eliminated [2, 18, 19]. The systemic administration of zymosan causes time-dependent behavioral alterations similar to behavioral response to bacteria triggered by, for example, LPS [18, 19]. Sickness behavior is evident approximately 2 h following zymosan administration, while depressive-like behavior is observed 6 h after zymosan challenge.
It is known that the constancy of the internal parameters of living organisms, such as blood volume and pressure, and extracellular fluid electrolyte composition, are necessary to the maintenance of life; the maintenance of this constant state is called homeostasis [20, 21]. Both external and internal agents may affect homeostasis by creating stressful situations. In response to the allostatic load, or the accumulating physiological responses to stress, internal control systems are activated to restore equilibrium, even in the presence of the offending agent [22]. This process is defined as allostasis [23, 24]. The internal systems involved in the maintenance of homeostasis against stressful situations, including infection, include the nervous, endocrine, and immune systems. The communication between the peripheral immune system and the nervous system is a well-described phenomenon. However, the biological mechanisms underlying the behavioral changes associated with inflammation and infection are not yet fully understood. The present study demonstrated that pretreatment with the COX inhibitors indomethacin and nimesulide reversed the zymosan-induced behavioral changes in both the FST and OFT. Pretreatment with dexamethasone, a steroidal drug that inhibits immune and inflammatory responses, achieved similar effects. Dexamethasone induces the production of lipocortin (also known as annexin-1), which inhibits phospholipase A2 and thereby prevents arachidonic acid release [25, 26] and reduces prostaglandin formation. Dexamethasone also inhibits the expression of cytokines [27–29]. Thus, our results suggest that prostaglandin synthesis plays a critical role in the development of sickness behavior following zymosan exposure.
In contrast to the behavioral response to bacteria triggered by, for example, LPS, the sickness response to fungal infection is not well understood. Previous studies have demonstrated that sickness behavior induced by LPS is mediated by prostaglandins [5]. Behavioral changes in the burrowing and open field tests following LPS treatment have been shown to be related to prostaglandin E2 in the brain; however, these effects were not reversed in animals treated with dexamethasone, despite the suppression of cytokines, COX expression, and prostaglandin E2 production [5, 30, 31].
The endocrine system also plays an important role in maintaining homeostasis in response to infection. Infectious and inflammatory processes trigger the activation of the HPA axis and sickness behavior [17, 30, 31]. Consistent with this phenomenon, our results demonstrated that corticosterone levels were significantly increased 2 h after zymosan administration, indicating increased HPA axis activity and an augmentation of the allostatic load. This increase in corticosterone levels coincided with the most significant differences in behavioral parameters.
Microorganism infection stimulates the host’s immune system and triggers inflammation, with an initial release of TNF-α and IL-1 [32]. These cytokines can reach the hypothalamus in regions where the blood–brain barrier is ineffective and bind to specific receptors [33], causing the production of prostaglandins via the COX pathway [12]. A previous study demonstrated that zymosan administration induces TNF-α release [34]; however, zymosan particles also trigger a series of signaling events that activate the Syk and Src families of tyrosine kinases [10]. Both routes converge to activate phospholipase Cγ and, through the generation of diacylglycerol, activate protein kinase C and mitogen-activated protein kinase (MAPK) cascades. Phosphorylation by MAPK and Ca2+-driven translocation of cytosolic phospholipase A2 stimulates arachidonic acid release from cell phospholipids, which is converted to prostaglandins via COX enzymes. The activation of IκB kinases via myeloid differentiation primary response gene 88 is another significant factor that may explain COX-2 induction by zymosan [10]. Overall, our findings support the hypothesis that zymosan induced sickness behavior involves the COX pathway, as COX inhibitors attenuated the behavioral changes induced by zymosan.
The association between activation of the immune system and mood disorders has been reported by several studies; accumulating data suggest that inflammatory responses play an important role in the pathophysiology of depression. Furthermore, peripheral application of zymosan similar to LPS [35] serves as a model for major depression.
Acknowledgments
We would like to thank Dr. Lucila L. K. Elias and Dr. José Antunes-Rodrigues from the University of São Paulo for assistance with the hormonal measurements. We also acknowledge the Brazilian National Council for Scientific and Technical Development (CNPq; #300977/2013-1) and the Research Support Foundation of Minas Gerais (FAPEMIG; #APQ-00041-15) for financial support.
Compliance with ethical standards
Ethical approval
All experiments were conducted according to the Declaration of Helsinki regulations addressing the welfare of experimental animals and were approved by the Ethics Committee of the Federal University of Alfenas (#360/2011).
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- 1.Cremeanssmith J, Newberry B. Zymosan: induction of sickness behavior and interaction with lipopolysaccharide. Physiol Behav. 2003;80:177–184. doi: 10.1016/j.physbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hubschle T, Rafalzik S, Gerstberger R, Roth J. Induction of fever and sickness behavior in telemetrically monitored rats during systemic inflammation induced by zymosan. J Anim Vet Adv. 2007;6:569–575. [Google Scholar]
- 4.McCusker RH, Kelley KW. Immune-neural connections: how the immune system’s response to infectious agents influences behavior. J Exp Biol. 2013;216:84–98. doi: 10.1242/jeb.073411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Paiva VN, Lima SN, Fernandes MM, Soncini R, Andrade CA, Giusti-Paiva A. Prostaglandins mediate depressive-like behaviour induced by endotoxin in mice. Behav Brain Res. 2010;215:146–151. doi: 10.1016/j.bbr.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Kelley KW, Bluthé RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17:112–118. doi: 10.1016/S0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Buisman-Pijlman F, Hutchinson MR. Toll-like receptor 4: innate immune regulator of neuroimmune and neuroendocrine interactions in stress and major depressive disorder. Front Neurosci. 2014;8:309. doi: 10.3389/fnins.2014.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbull AV, Rivier CL. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol Rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Volman TJ, Hendriks T, Goris RJ. Zymosan-induced generalized inflammation: experimental studies into mechanisms leading to multiple organ dysfunction syndrome. Shock. 2005;23:291–297. doi: 10.1097/01.shk.0000155350.95435.28. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez Y, Valera I, Municio C, Hugo E, Padrón F, Blanco L, Rodríguez M, Fernández N, Crespo MS (2010) Eicosanoids in the innate immune response: TLR and non-TLR routes. In: Parker A (ed) Mediators of inflammation, vol. 2010. p 14 [DOI] [PMC free article] [PubMed]
- 11.Bastos-Pereira AL, Fraga D, Ott D, Simm B, Murgott J, Roth J, Zampronio AR. Involvement of brain cytokines in zymosan-induced febrile response. J Appl Physiol. 2014;116:1220–1229. doi: 10.1152/japplphysiol.01278.2013. [DOI] [PubMed] [Google Scholar]
- 12.Engblom D, Ek M, Saha S, Ericsson-Dahlstrand A, Jakobsson PJ, Blomqvist A. Prostaglandins as inflammatory messengers across the blood-brain barrier. J Mol Med. 2002;80:5–15. doi: 10.1007/s00109-001-0289-z. [DOI] [PubMed] [Google Scholar]
- 13.Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81:688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–162. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rorato R, Menezes AM, Giusti-Paiva A, de Castro M, Antunes-Rodrigues J, Elias LL. Prostaglandin mediates endotoxaemia-induced hypophagia by activation of pro-opiomelanocortin and corticotrophin-releasing factor neurons in rats. Exp Physiol. 2009;94:371–379. doi: 10.1113/expphysiol.2008.045435. [DOI] [PubMed] [Google Scholar]
- 16.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacody Ther. 1977;229:327–336. [PubMed] [Google Scholar]
- 17.Ribeiro DE, Maiolini VM, Soncini R, Antunes-Rodrigues J, Elias LL, Vilela FC, Giusti-Paiva A. Inhibition of nitric oxide synthase accentuates endotoxin-induced sickness behavior in mice. Pharmacol Biochem Behav. 2013;103:535–540. doi: 10.1016/j.pbb.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Haba R, Shintani N, Onaka Y, Wang H, Takenaga R, Hayata A, Baba A, Hashimoto H. Lipopolysaccharide affects exploratory behaviors toward novel objects by impairing cognition and/or motivation in mice: possible role of activation of the central amygdala. Behav Brain Res. 2012;228:423–431. doi: 10.1016/j.bbr.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 19.Ohgi Y, Futamura T, Kikuchi T, Hashimoto K. Effects of antidepressants on alternations in serum cytokines and depressive-like behavior in mice after lipopolysaccharide administration. Pharmacol Biochem Behav. 2013;103:853–859. doi: 10.1016/j.pbb.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Tahara Y, Aoyama S, Shibata S (2016) The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci 1–10. doi:10.1007/s12576-016-0450-7 [DOI] [PMC free article] [PubMed]
- 21.Antunes-Rodrigues J, de Castro M, Elias LL, Valença MM, McCann SM. Neuroendocrine control of body fluid metabolism. Physiol Rev. 2004;84:169–208. doi: 10.1152/physrev.00017.2003. [DOI] [PubMed] [Google Scholar]
- 22.Karatsoreos IN, McEwen BS. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn Sci. 2011;15:576–584. doi: 10.1016/j.tics.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 23.McEwen BS. Mood disorders and allostatic load. Biol Psychiatry. 2003;54:200–207. doi: 10.1016/S0006-3223(03)00177-X. [DOI] [PubMed] [Google Scholar]
- 24.McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 25.Flower RJ, Rothwell NJ. Lipocortin-1: cellular mechanisms and clinical relevance. Trends Pharmacol Sci. 1994;15:71–76. doi: 10.1016/0165-6147(94)90281-X. [DOI] [PubMed] [Google Scholar]
- 26.Gerke V, Moss SE. Annexins: from structure to function. Physiol Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 27.Abraham SM, Lawrence T, Kleiman A, Warden P, Medghalchi M, Tuckermann J, Saklatvala J, Clark AR. Anti-inflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J Exp Med. 2006;203:1883–1889. doi: 10.1084/jem.20060336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goulding NJ, Euzger HS, Butt SK, Perretti M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm Res. 1998;47(Suppl 3):S158–S165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- 29.King EM, Holden NS, Gong W, Rider CF, Newton R. Inhibition of NF-kappaB-dependent transcription by MKP-1: transcriptional repression by glucocorticoids occurring via p38 MAPK. J Biol Chem. 2009;284:26803–26815. doi: 10.1074/jbc.M109.028381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teeling JL, Cunningham C, Newman TA, Perry VH. The effect of non-steroidal anti-inflammatory agents on behavioural changes and cytokine production following systemic inflammation: implications for a role of COX-1. Brain Behav Immun. 2010;24:409–419. doi: 10.1016/j.bbi.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero LM, Dickens MJ, Cyr NE. The reactive scope model—a new model integrating homeostasis, allostasis, and stress. Horm Behav. 2009;55:375–389. doi: 10.1016/j.yhbeh.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Teeling JL, Felton LM, Deacon RM, Cunningham C, Rawlins JN, Perry VH. Sub-pyrogenic systemic inflammation impacts on brain and behavior, independent of cytokines. Brain Behav Immun. 2007;21:836–850. doi: 10.1016/j.bbi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Bondeson J, Browne KA, Brennan FM, Foxwell BM, Feldmann M. Selective regulation of cytokine induction by adenoviral gene transfer of IkappaBalpha into human macrophages: lipopolysaccharide-induced, but not zymosan-induced, proinflammatory cytokines are inhibited, but IL-10 is nuclear factor-kappaB independent. J Immunol. 1999;162:2939–2945. [PubMed] [Google Scholar]
- 34.Cao C, Matsumura K, Shirakawa N, Maeda M, Jikihara I, Kobayashi S, Watanabe Y. Pyrogenic cytokines injected into the rat cerebral ventricle induce cyclooxygenase-2 in brain endothelial cells and also upregulate their receptors. Eur J Neurosci. 2001;13:1781–1790. doi: 10.1046/j.0953-816x.2001.01551.x. [DOI] [PubMed] [Google Scholar]
- 35.Sanguedolce MV, Capo C, Bongrand P, Mege JL. Zymosan-stimulated tumor necrosis factor-alpha production by human monocytes. Down-modulation by phorbol ester. J Immunol. 1992;148:2229–2236. [PubMed] [Google Scholar]


