Abstract
Transient receptor potential vanilloid 1 (TRPV1) is a Ca2+-permeable cation channel activated by a variety of physicochemical stimuli. The effect of hypoxia (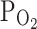 , 3%) on rat TRPV1 overexpressed in HEK293T has been studied. The basal TRPV1 current (I
TRPV1) was partly activated by hypoxia, whereas capsaicin-induced TRPV1 (I
TRPV1,Cap) was attenuated. Such changes were also suggested from hypoxia- and capsaicin-induced Ca2+ signals in TRPV1-expressing cells. Regarding plausible changes of reactive oxygen species (ROS) under hypoxia, the effects of antioxidants, vitamin C and tiron, as membrane-impermeable and -permeable, respectively, were tested. Both I
TRPV1 and I
TRPV1,Cap were increased by vitamin C, while only I
TRPV1 was slightly increased by tiron. The hypoxic inhibition of I
TRPV1,Cap was still persistent under hypoxia/vitamin C. Interestingly, hypoxia/tiron strongly inhibited both I
TRPV1 and I
TRPV1,Cap. Also, with vitamin C applied through a pipette solution, hypoxia inhibited I
TRPV1 and I
TRPV1,Cap. In contrast, hypoxia and hypoxia/tiron had no effect on the I
TRPV1 induced by acid (pH 6.2, I
TRPV1,Acid). Taken together, hypoxia partly activated TRPV1 while it decreased their sensitivity to capsaicin. Putative changes of ROS under hypoxia might underlie the side-specific effects of ROS on TRPV1: inhibitory at the extracellular and stimulatory at the intracellular side, respectively. The differential effects of hypoxia on I
TRPV1,Cap and I
TRPV1,Acid suggested that the intracellular ROS increase might attenuate the pharmacological potency of capsaicin.
, 3%) on rat TRPV1 overexpressed in HEK293T has been studied. The basal TRPV1 current (I
TRPV1) was partly activated by hypoxia, whereas capsaicin-induced TRPV1 (I
TRPV1,Cap) was attenuated. Such changes were also suggested from hypoxia- and capsaicin-induced Ca2+ signals in TRPV1-expressing cells. Regarding plausible changes of reactive oxygen species (ROS) under hypoxia, the effects of antioxidants, vitamin C and tiron, as membrane-impermeable and -permeable, respectively, were tested. Both I
TRPV1 and I
TRPV1,Cap were increased by vitamin C, while only I
TRPV1 was slightly increased by tiron. The hypoxic inhibition of I
TRPV1,Cap was still persistent under hypoxia/vitamin C. Interestingly, hypoxia/tiron strongly inhibited both I
TRPV1 and I
TRPV1,Cap. Also, with vitamin C applied through a pipette solution, hypoxia inhibited I
TRPV1 and I
TRPV1,Cap. In contrast, hypoxia and hypoxia/tiron had no effect on the I
TRPV1 induced by acid (pH 6.2, I
TRPV1,Acid). Taken together, hypoxia partly activated TRPV1 while it decreased their sensitivity to capsaicin. Putative changes of ROS under hypoxia might underlie the side-specific effects of ROS on TRPV1: inhibitory at the extracellular and stimulatory at the intracellular side, respectively. The differential effects of hypoxia on I
TRPV1,Cap and I
TRPV1,Acid suggested that the intracellular ROS increase might attenuate the pharmacological potency of capsaicin.
Keywords: TRPV1, Hypoxia, Reactive oxygen species, Capsaicin
Introduction
Transient receptor potential vanilloid 1 (TRPV1), also called capsaicin receptor, is a member of thermosensitive TRPV family (TRPV1–4) non-selective cation channels. TRPV1 is richly expressed in the C-type neurons of dorsal root ganglion (DRG) and trigeminal ganglion where TRPV1 mediates noxious stimuli into pain sensation [1–4]. The expression of TRPV1 is not limited to sensory neurons but also found in urothelial cells, keratinocytes, monocytes, endothelium, and arterial smooth muscle cells [5–9].
Owing to the relatively high permeability to Ca2+, activation of TRPV1 increases cytosolic Ca2+ concentration (Δ[Ca2+]c) which is a critical step of various intracellular signaling cascades [9, 11–13]. The increased [Ca2+]c also performs as a negative feedback mechanism for TRPV1: desensitization of TRPV1 by repetitive or sustained stimulation under physiological conditions [14–16]. In addition to capsaicin, a wide variety of physicochemical conditions are known to activate TRPV1: noxious heat (>43°C), protons (acidic pH), and endogenous lipid-derived molecules [9, 10]. Despite the large number of studies on the various conditions affecting TRPV1 activity, investigation on the influence of acute hypoxia is very rare.
Hypoxia is associated not only with tissue ischemia but also with a variety of pathological conditions such as inflammation, chronic obstructive pulmonary disease, and sleep apnea [17–19]. Because the tissue ischemia would lead to local acidosis, ischemic pain might be partly caused by pH changes in addition to various inflammatory substances. In a previous study of DRG neurons, using capsaicin- or acidic pH-induced Δ[Ca2+]c as a signal reflecting the TRPV1 activity, an effect of anoxia on TRPV1 was investigated [20]. In that study, anoxia not only increased the basal [Ca2+]c but also augmented the capsaicin-induced Δ[Ca2+]c [20]. Another recent study showed facilitation of TRPV1 activity by chronic hypoxia combined with hyperglycemia [21]. In this study, TRPV1 activity was functionally upregulated via protein kinase C (PKC)ε- and hypoxia-inducible factor-1 alpha (HIF-1α)-dependent signaling pathway, without increases in protein expression. Such results might have implications with regard to diabetic pain syndrome where hyperglycemic can be combined with inflammatory chronic hypoxia.
Although the aforementioned studies give rise to intriguing ideas about the hypoxic regulation of TRPV1, direct investigation of acute hypoxia on TRPV1 activity is still lacking. The study by Ristoiu et al. [21] is limited to chronic (>24 h) effect of hypoxia, and their measurements were performed under normoxic condition. In the study of anoxic effects on TRPV1, only Δ[Ca2+]c was measured [20]. Because [Ca2+]c is determined by various factors in addition to the Ca2+ permeable channels, one cannot exclude the influence from the Ca2+ release from intracellular stores (e.g., ER) and the altered electrical driving forces (e.g., K+ channel activity) from membrane potential. Therefore, direct measurements of electrical current under voltage clamp conditions are definitely required to precisely elucidate the effects of acute hypoxia on TRPV1 activity. Accordingly, we performed whole-cell patch clamp studies in HEK293T cells overexpressing rat TRPV1, and investigated the effects of hypoxia (3% of fractional O2 concentration) on TRPV1 activity.
Materials and methods
Cell culture and transfection
HEK293T cells were cultured in DMEM media (GIBCO, Grand Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum (GIBCO) and 1% penicillin/streptomycin (GIBCO) at 37°C in an atmosphere of 20% O2/5% CO2. One-day cultured cells were then transiently transfected with 0.5 μg of rat TRPV1 using FuGENE 6 reagent (Roche Molecular Biochemicals, Indianapolis, IN, USA) according to the manufacturer’s protocol. An amount of 0.1 μg green fluorescent protein (GFP)-expressing vector was used for cotransfection with TRPV1 to mark the cells. HEK293T cells expressing TRPV1 were detached 36–40 h after transfection and used for whole-cell patch clamp recordings.
Electrophysiological measurements
Electrophysiological meaurements were performed in the conventional whole-cell recording mode at room temperature (22–25°C). Membrane currents were measured using an Axopatch-200B patch clamp amplifier (Axon Instruments, Foster City, CA, USA). pCLAMP software v.10.2 and Digidata-1322A (Axon Instruments) were used to acquire data and apply command pulses. Transfected HEK293T cells were transferred into a bath (approximately 0.1 ml) mounted on the stage of an inverted microscope (IX50; Olympus, Osaka, Japan) and perfused with HEPES buffered normal Tyrode (NT) solution at 5 ml/min. Patch pipettes with a free-tip resistance of about 2.5–3.5 MΩ were used.
The bath solution was made hypoxic by bubbling with 100% N2 gas in a separate glass reservoir for at least 20 min before the perfusion. The reservoir was connected to the experimental chamber using oxygen-impermeable Tygon tubing (Saint-Gobain, Korea). Partial pressure of oxygen [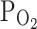 (%)] was measured in the experimental chamber using the oximeter (MI-730; Microelectrodes, Bedford City, NH, USA). Upon perfusion with N2-bubbled NT solution,
(%)] was measured in the experimental chamber using the oximeter (MI-730; Microelectrodes, Bedford City, NH, USA). Upon perfusion with N2-bubbled NT solution, 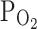 (%) of the bath solution was lowered from 21% to approximately 3%. In some cases, the perfusing solution was made moderately hypoxic by bubbling with 93% N2/7% O2 gas, which made the
(%) of the bath solution was lowered from 21% to approximately 3%. In some cases, the perfusing solution was made moderately hypoxic by bubbling with 93% N2/7% O2 gas, which made the 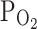 of the bath solution 8–9%.
of the bath solution 8–9%.
Fura-2 spectrofluorimetry
Ca2+ concentration [Ca2+]c was measured using the fluorescent Ca2+ indicator Fura-2 acetoxymethyl ester (Fura-2 AM). HEK293T cells expressing TRPV1 were loaded with Fura-2 AM (2 μM, 20 min, 25°C) and washed twice with fresh NT solution. The Fura-2 loaded cells were transferred into a microscope stage bath (approximately 0.1 ml) mounted on the stage of an inverted microscope (IX 70; Olympus) and perfused with HEPES buffered NT solution at 5 ml/min. Fluorescence was monitored using a Polychrome IV monochromator (TILL Photonics, Martinsried, Germany), a Cascade 650 CCD camera (Roper Scientific, Sarasota, FL, USA) and Metafluor software (Universal Imaging, Downingtown, PA, USA) at excitation wavelengths of 340 and 380 nm, and an emission wavelength of 510 nm. At the end of each experiment, Ca2+-free solution with 5 mM EGTA were applied to produce a minimum fluorescence ratio (R min; 340/380 nm). Then, 2 μM ionomycin and 10 mM CaCl2 were applied to confirm a maximum ratio of fluorescence (R max). The [Ca2+]c was calculated from the equation, [Ca2+] = K d × b × (R − R min)/(R max − R min), where K d is the dissociation constant 224 nM for Fura-2 and b is the ratio of fluorescence excitation intensities at 380 nm under Ca2+-free Ca2+-saturated conditions. The percent values of [Ca2+]c were calculated as: [Ca2+]% = [(R − R min)/(R max − R min)] × 100.
Experimental solution and chemicals
The NT bath solution used for the patch clamp was composed of 140 NaCl, 4 CsCl, 0.5 MgCl2, 1.8 CaCl2, 10 HEPES, 10 glucose and 10 sucrose (in mM) and was of pH 7.4 adjusted with NaOH. The calcium-free bath solution for some experiments included 0.5 EGTA instead of 1.8 CaCl2. To change the pH of the bath solution, 2-(N-morpholino)ethanesulfonic acid (MES)/HEPES solution was prepared by replacing HEPES with equimolar amounts of MES and HEPES (5 mM, respectively). Normal Tyrode bath solution used for Fura-2 fluorimetry was composed of 3.6 KCl, 145 NaCl, 1 MgCl2, 5 glucose and 10 HEPES (in mM), and was of pH 7.2 adjusted with NaOH. The pipette solution contained (in mM) 140 CsCl, 1 MgCl2, 3 MgATP, 10 HEPES and 5 EGTA and was titrated to pH 7.25 with CsOH. Capsaicin, capsazepine, hydrogen peroxide, ascorbic acid (vitamin C), tiron, and amiloride were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Data analysis and statistics
Data was managed and analyzed using Origin v.7.0 software (Microcal Software, Piscataway, NJ, USA). Statistical results are presented as the mean ± standard error of the mean (SEM). Paired Student’s t tests were used as appropriate to evaluate for significance, which was accepted for the P value <0.05.
Results
Partial activation of TRPV1 by hypoxia
Whole-cell patch clamp using Cs+-rich pipette solution was applied to GFP and TRPV1 co-expressed HEK293T cells. Cells were held at −20 mV and ramp-like depolarization (from −80 to 80 mV, 0.32 V/s) was repetitively applied every 10 s. The relatively depolarized holding voltage (−20 mV) was chosen to minimize the desensitization of TRPV1 upon the stimulation with capsaicin. At room temperature without specific stimuli, the current to voltage relation (I–V curve) of TRPV1-expressing cells showed outwardly rectification with reversal potential close to 0 mV, a known property of TRPV1 current (I
TRPV1). Under this control condition, changing into the hypoxic bath solution (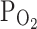 , 3%) increased the amplitudes of outwardly rectifying I
TRPV1 (n = 18; Fig. 1a). In each tested cell, full activity of TRPV1 (I
TRPV1,max) was confirmed by applying 2 μM of capsaicin, and the amplitudes of inward current at −20 mV were normalized to the capsaicin-induced I
TRPV1,max at −20 mV. The normalized amplitudes of I
TRPV1 were 7.0 ± 1.1 and 11.7 ± 2.1% in control and hypoxia, respectively (n = 18, P < 0.05; Fig. 1a, right). We also tested the effect of moderately hypoxic condition (
, 3%) increased the amplitudes of outwardly rectifying I
TRPV1 (n = 18; Fig. 1a). In each tested cell, full activity of TRPV1 (I
TRPV1,max) was confirmed by applying 2 μM of capsaicin, and the amplitudes of inward current at −20 mV were normalized to the capsaicin-induced I
TRPV1,max at −20 mV. The normalized amplitudes of I
TRPV1 were 7.0 ± 1.1 and 11.7 ± 2.1% in control and hypoxia, respectively (n = 18, P < 0.05; Fig. 1a, right). We also tested the effect of moderately hypoxic condition (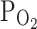 , 9%), which did not affect the basal I
TRPV1 (n = 10; Fig. 1a, right). The partial activation of TRPV1 by hypoxia was confirmed by using TRPV1 antagonist, capsazepine. The outwardly rectifying I
TRPV1 was eliminated by 5 μM capsazepine, under which hypoxia had no effect on the membrane conductance (Fig. 1b, left). Also, both the hypoxia-induced inward current and basal I
TRPV1 were abolished by 0.5 μM capsazepine (Fig. 1b, right). In GFP-only transfected HEK293T cells, the membrane conductance was significantly lower than those of TRPV1-expressed cells, and the slopes of linear I–V curves were not affected by either hypoxia or 2 μM capsaicin (Fig. 1c).
, 9%), which did not affect the basal I
TRPV1 (n = 10; Fig. 1a, right). The partial activation of TRPV1 by hypoxia was confirmed by using TRPV1 antagonist, capsazepine. The outwardly rectifying I
TRPV1 was eliminated by 5 μM capsazepine, under which hypoxia had no effect on the membrane conductance (Fig. 1b, left). Also, both the hypoxia-induced inward current and basal I
TRPV1 were abolished by 0.5 μM capsazepine (Fig. 1b, right). In GFP-only transfected HEK293T cells, the membrane conductance was significantly lower than those of TRPV1-expressed cells, and the slopes of linear I–V curves were not affected by either hypoxia or 2 μM capsaicin (Fig. 1c).
Fig. 1.
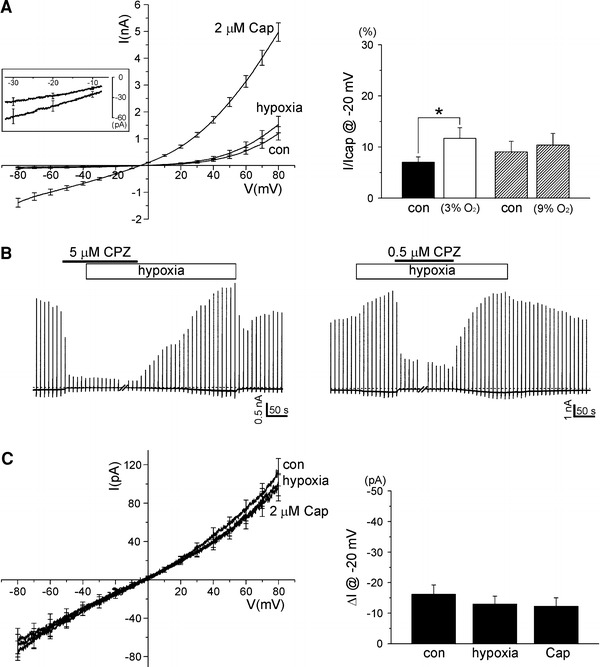
Direct effect of hypoxia on TRPV1 overexpressed in HEK293T cells. a
I–V curves were obtained by applying repetitive ramp-like pulses (from −80 to 80 mV, 0.4 V/s, 10 s interval). The representative I–V curves at each condition (control, hypoxia, 2 μM capsaicin) were averaged for comparison (n = 18). The membrane conductance was slightly increased by hypoxia (3% 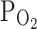 ), and maximally increased by 2 μM capsaicin (left panel). The right panel shows a summary of normalized inward currents at −20 mV and their changes by 3%
), and maximally increased by 2 μM capsaicin (left panel). The right panel shows a summary of normalized inward currents at −20 mV and their changes by 3% 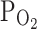 (n = 18) and 9%
(n = 18) and 9% 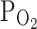 (n = 10), respectively. In each tested cell, the inward current was normalized to the maximum currents induced by 2 μM capsaicin. b Inhibition of the basal activity of TRPV1 by capsazepine. Also, the combined hypoxia had no effect on membrane conductance in the presence of capsazepine (0.5 or 5 μM). c No effect of hypoxia and capsaicin on I–V curves of GFP-only transfected cells (n = 10). Cap capsaicin, con control, CPZ capsazepine
(n = 10), respectively. In each tested cell, the inward current was normalized to the maximum currents induced by 2 μM capsaicin. b Inhibition of the basal activity of TRPV1 by capsazepine. Also, the combined hypoxia had no effect on membrane conductance in the presence of capsazepine (0.5 or 5 μM). c No effect of hypoxia and capsaicin on I–V curves of GFP-only transfected cells (n = 10). Cap capsaicin, con control, CPZ capsazepine
Inhibition of capsaicin-activated TRPV1 by hypoxia
Next, we investigated the effects of hypoxia on TRPV1 current induced by capsaicin. Because micromolar ranges of capsaicin induced very large inward currents with fast desensitization in TRPV1-overexpressed cells, 50–200 nM of capsaicin was applied at −20 mV of holding voltage. Also, ramp pulses were applied every 10 s to obtain I–V curves. To our surprise, the TRPV1 current induced by capsaicin (I
TRPV1,Cap) was decreased by hypoxia in a reversible manner (Fig. 2a). The I
TRPV1,Cap normalized to I
TRPV1,max at −20 mV was decreased to 55 ± 7.8% by hypoxia (3% 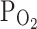 ) of the capsaicin-induced current, and reversed to 89 ± 3.7% by reoxygenation (n = 10; Fig. 2a, right). Interestingly, different from the effect on I
TRPV1, the moderate hypoxia (9%
) of the capsaicin-induced current, and reversed to 89 ± 3.7% by reoxygenation (n = 10; Fig. 2a, right). Interestingly, different from the effect on I
TRPV1, the moderate hypoxia (9% 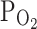 ) decreased I
TRPV1,Cap to 63 ± 9.3% (n = 7; Fig. 2a, right).
) decreased I
TRPV1,Cap to 63 ± 9.3% (n = 7; Fig. 2a, right).
Fig. 2.
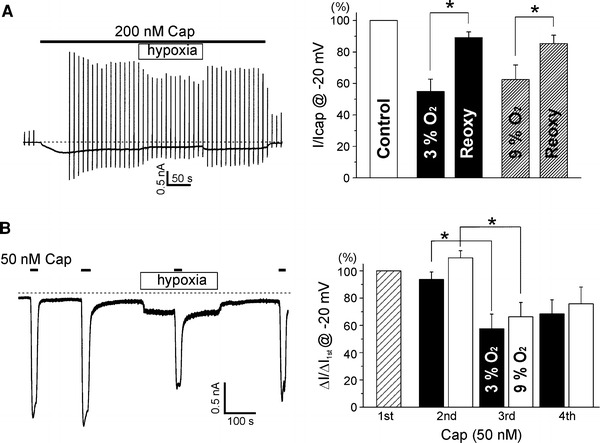
Inhibition of capsaicin-activated TRPV1 current (I
TRPV1,Cap) by hypoxia. a Representative current trace showing response to hypoxia during activation by 200 nM capsaicin. Vertical lines reflect current responses to the repetitive ramp-like pulses from −80 to 80 mV every 10 s. Right summary of inward currents at −20 mV in response to 200 nM capsaicin (normoxia, Control), capsaicin + hypoxia [3% (n = 10) or 9% (n = 7), closed bar and hatched bar, respectively], and capsaicin + reoxygenation (Reoxy). In each cell, current amplitudes were normalized to the control current induced by 200 nM capsaicin. b An exemplary current trace at −20 mV holding voltage showing the repetitive activation of TRPV1 by 50 nM capsaicin (horizontal line). Hypoxia was applied between the second and the fourth applications of capsaicin. Note that the basal current was increased by hypoxia while the amplitude of transient inward current (I
TRPV1,Cap) was decreased. Right summary of amplitudes of I
TRPV1,Cap normalized to the first response (hatched bar) and their changes by 3% 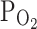 (n = 5, closed bars) and 9%
(n = 5, closed bars) and 9% 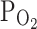 (n = 14, open bars)
(n = 14, open bars)
The inhibition of I
TRPV1,Cap was similarly observed when capsaicin was applied during hypoxia. To minimize the run-down of I
TRPV1 by repetitive application of capsaicin [6], the concentration of capsaicin was lowered to 50 nM, and the CaCl2 of the bath solution was omitted (nominal Ca2+-free NT). At −20 mV, capsaicin was applied briefly (15–20 s) four times as demonstrated in Fig. 2b. Before the third application, cells were exposed to hypoxia, and they returned to normoxia before the fourth application of capsaicin. The amplitude of I
TRPV1,Cap at −20 mV was decreased by the pretreatment with hypoxia (Fig. 2b). When normalized to the first response, the transient inward currents (I
TRPV1,Cap at −20 mV) were decreased to 57.6 ± 10.7% by hypoxia (3% 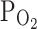 ) pretreatment, and partly reversed to 68.4 ± 10.3% by returning to normoxia (n = 5; Fig. 2b, right, closed bars). The inhibition of I
TRPV1,Cap by moderate hypoxia (9%
) pretreatment, and partly reversed to 68.4 ± 10.3% by returning to normoxia (n = 5; Fig. 2b, right, closed bars). The inhibition of I
TRPV1,Cap by moderate hypoxia (9% 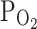 ) was also summarized as bar graphs (n = 14; Fig. 2b, right, open bars). It is notable that the partial activation of TRPV1 (I
TRPV1) was consistently observed while the response to capsaicin (I
TRPV1,Cap) was attenuated during hypoxia (Fig. 2b, left).
) was also summarized as bar graphs (n = 14; Fig. 2b, right, open bars). It is notable that the partial activation of TRPV1 (I
TRPV1) was consistently observed while the response to capsaicin (I
TRPV1,Cap) was attenuated during hypoxia (Fig. 2b, left).
No effect of hypoxia on acid-activation of TRPV1
Next, we investigated whether hypoxia affects the activation of TRPV1 by acidic pH. It has been reported that HEK293T cells express acid-sensing ion channels (ASIC) that are also activated by acidic pH [13]. Actually, both GFP-only and TRPV1-overexpressed HEK293T cells showed a transient inward current at pH 6.2, which was inhibited by 30 μM amiloride (Fig. 3a, inset). Consistent with the known property of ASIC, the amiloride-sensitive inward current spontaneously decayed to baseline at pH 6.2. In contrast, TRPV1 showed relatively constant activity under the same acidic pH, which was distinguishable from the ASIC current (Fig. 3a). Therefore, the responses of acidic pH-induced TRPV1 currents (I TRPV1,Acid) to hypoxia were analyzed at 20 s of pH 6.2 application (Fig. 3b, c). Similar to the protocol shown in Fig. 2b, pH 6.2 was applied four times where the third was applied during hypoxia; I TRPV1,Acid was not affected by hypoxia (n = 20; Fig. 3b, c).
Fig. 3.
No effect of hypoxia on acidic pH-activated TRPV1 current (I
TRPV1,Acid). a Representative current trace at −20 mV holding voltage showing the response to acidic condition (pH 6.2) in GFP-only (left) and TRPV1 + GFP-expressed (right) HEK293T cells. A transient inward current was induced by the acidic pH in both cell groups whereas a sustained inward current was additionally observed in the TRPV1-expressed cells. The pH-activated transient current was abolished by 30 μM amiloride, a blocker of ASIC (see inset). b TRPV1 was repetitively activated by acidic pH, and hypoxia (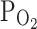 3%) was applied between the third and the fourth application of pH 6.2. c Summary of the inward currents normalized to the first response (n = 20)
3%) was applied between the third and the fourth application of pH 6.2. c Summary of the inward currents normalized to the first response (n = 20)
Effects of H2O2 and antioxidants on TRPV1
It is supposed that the production of reactive oxygen species (ROS) from NADPH oxidases (NOX) and mitochondria would be affected during hypoxia, although the direction of changes in ROS production is controversial [22]. To get a clue for the mechanisms of hypoxic regulation of TRPV1, we tested the effects of H2O2 (100 μM), vitamin C (1–2 mM, membrane impermeable ROS scavenger) and tiron (100 μM, membrane permeable ROS scavenger) on I TRPV1 and I TRPV1,Cap. Interestingly, all the three agents induced a partial activation of I TRPV1 (Figs. 4a and 5a) while their effects on I TRPV1,Cap were different (Figs. 4d and 5b, right). I TRPV1,Cap was increased by pretreatment with vitamin C (n = 14; Fig. 5b) whereas the pretreatments with H2O2 or tiron had no significant effect (n = 10 and 14; Figs. 4d and 5b, respectively).
Fig. 4.
Effects of H2O2 on TRPV1. a Representative current trace (holding voltage −20 mV) showing response to 100 μM H2O2. Vertical lines reflect current responses to the repetitive ramp-like pulses from −80 to 80 mV. Right summary of I–V curved of control and H2O2-treated conditions (n = 5). Outwardly rectifying conductance was increased by H2O2. b An exemplary current trace at −20 mV holding voltage showing the repetitive activation of TRPV1 by 50 nM capsaicin. Then, 100 μM H2O2 was applied between the second and the fourth applications of capsaicin. Right summary of the capsaicin-induced inward currents normalized to the first response. I TRPV1,Cap was not changed by H2O2 pretreatment (third bar, n = 10)
Fig. 5.
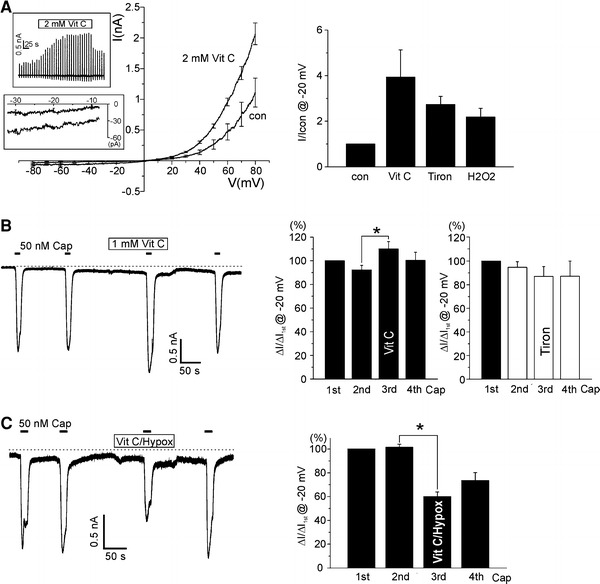
Effects of vitamin C and tiron on TRPV1 and I TRPV1,Cap. a Summary of I–V curves in response to 2 mM vitamin C in HEK293T cell expressing TRPV1 (n = 4, left panel). Current traces were obtained from the ramp-like pulses (upper inset), and the amplitudes of inward current at −20 mV were measured (lower inset, vertical expansion of I–V curve). The bar graphs in right panel shows summary of the currents measured at −20 mV and their increase by vitamin C (n = 4), tiron (100 μM, n = 7), and H2O2 (n = 5). For comparison, the amplitudes of inward current at −20 mV were normalized to the control in each cell. b Representative current trace at −20 mV holding voltage showing the repetitive activation of TRPV1 by 50 nM capsaicin (horizontal bars). 1 mM vitamin C was applied before the third stimulus of capsaicin, which augmented I TRPV1,Cap (left panel). Summary of the capsaicin-induced inward currents normalized to the first response. I TRPV1,Cap was increased by vitamin C pretreatment (n = 14, middle panel) while not changed by tiron pretreatment (n = 14, right panel). c Effects of vitamin C on the hypoxic inhibition of I TRPV1,Cap. Vitamin C was applied together with hypoxia (Vit C/Hypox) before the third application of 50 nM capsaicin. Both the increase of basal inward current and the suppression of I TRPV1,Cap by hypoxia was observed. Summary of the capsaicin-induced inward currents normalized to the first response (n = 20, right panel)
The hypoxic inhibition of I TRPV1,Cap was still observed under the pretreatment with vitamin C and tiron (n = 20 and 10; Figs. 5c and 6a, respectively). However, different from the effects of the co-treatment with vitamin C and hypoxia (vitamin C/hypoxia), the application of tiron with hypoxia (tiron/hypoxia) markedly suppressed the basal current (I TRPV1) as well as the capsaicin response (I TRPV1,Cap, n = 10). Also, recovery of I TRPV1,Cap from the inhibition by hypoxia/tiron treatment was poor (Fig. 6a). While tiron is a membrane permeable antioxidant, vitamin C is an impermeable one. Interestingly, when 1 mM vitamin C was included in the pipette solution, hypoxia inhibited I TRPV1 as well as I TRPV1,Cap (n = 7; Fig. 6b). It has also to be noted that I TRPV1,Acid was not affected even by tiron/hypoxia (n = 5; Fig. 6c).
Fig. 6.
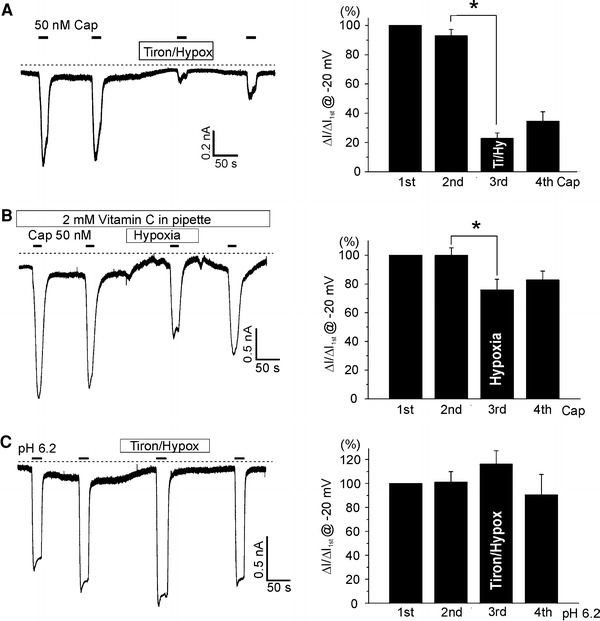
Effects of combined application of tiron and hypoxia on TRPV1, I
TRPV1,Cap and I
TRPV1,Acid. Representative current traces at −20 mV holding voltage are presented in left panels, showing the repetitive activation of TRPV1 by 50 nM capsaicin or by acidic pH (6.2). Summary of the 50 nM capsaicin-induced inward currents normalized to the first response are shown in right panels. a Combined application of tiron (100 μM) and hypoxia (3% 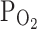 ) significantly suppressed the basal current (I
TRPV1) and I
TRPV1,Cap. b With 1 mM vitamin C in the pipette solution, hypoxia decreased I
TRPV1 as well as I
TRPV1,Cap. c Combined application of tiron (100 μM) and hypoxia (3%
) significantly suppressed the basal current (I
TRPV1) and I
TRPV1,Cap. b With 1 mM vitamin C in the pipette solution, hypoxia decreased I
TRPV1 as well as I
TRPV1,Cap. c Combined application of tiron (100 μM) and hypoxia (3% 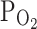 ) did not affect I
TRPV1,Acid induced by pH 6.2 while the basal current (I
TRPV1) was suppressed
) did not affect I
TRPV1,Acid induced by pH 6.2 while the basal current (I
TRPV1) was suppressed
Effects of hypoxia on [Ca2+]c in HEK293T cells expressing TRPV1
Lastly, it was examined whether the hypoxic modulations of I TRPV1 and I TRPV1,Cap are reflected as changes in [Ca2+]c. Fura-2 was loaded in HEK293T cells transfected with GFP and TRPV1. The cells showing GFP fluorescence were used for study. Consistent with the activation of TRPV1 by hypoxia alone, the basal [Ca2+]c was slightly increased by hypoxia, which was not observed in non-transfected cells (Fig. 7a, inset). Those cells showing hypoxic increase in [Ca2+]c also respond to capsaicin with large Δ[Ca2+]c. Normalized increases in fluorescence ratio [Δ(R − R min)/(R max − R min) × 100 (%)] were 5.03 ± 1.5 and 55.91 ± 4.7% for hypoxia and 1 μM capsaicin, respectively (n = 9; Fig. 7b). Repetitive application of 20 nM capsaicin induced transient Δ[Ca2+]c, and the amplitudes of transient Δ[Ca2+]c were decreased under hypoxia (Fig. 7c, d). Such results were consistent with the inhibition of I TRPV1,Cap by hypoxia.
Fig. 7.
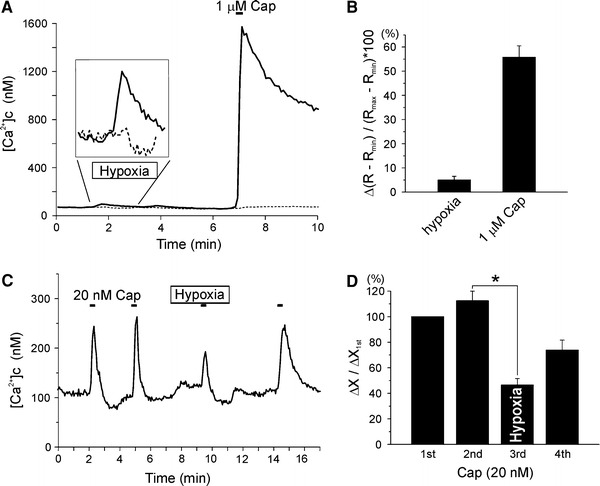
Effects of hypoxia on [Ca2+]c in TRPV1-expressing HEK293T cells. a Exemplary traces of [Ca2+]c for TRPV1-expressing (solid lines) and empty (dotted line) HEK293T cells. [Ca2+]c was increased by hypoxia in TRPV1-expressing HEK293T cells but not in empty HEK293T cells (see inset for the vertical expansion). The expression of TRPV1 was confirmed from the large increase in [Ca2+]c by 1 μM capsaicin. b Summary of normalized [Ca2+]c increase caused by hypoxia and 1 μM capsaicin (n = 9), respectively. The fluorescence ratio were normalized to the maximum R max and R min obtained by ionomycin (2 μM) and EGTA (5 mM) at the end of each experiment (see “Materials and methods”). c Representative trace of [Ca2+]c showing the transient increases in [Ca2+]c (Δ[Ca2+]c) by 20 nM capsaicin. Under hypoxia, the Δ[Ca2+]c by 20 nM capsaicin was attenuated. d Summary of Δ[Ca2+]c normalized to the first response to 20 nM capsaicin (n = 6)
Discussion
In the present study, results from the patch clamp experiment and Fura-2 spectrofluorimetry demonstrate intriguing dual effects of hypoxia on TRPV1 activity: the partial activation of TRPV1 and the attenuated response to capsaicin. Because the hypoxic increase of membrane conductance was not observed in GFP-only-expressing cells, we interpret that the effects of hypoxia is specific to TRPV1. The abolishment of hypoxic activation of TRPV1 by capsazepine also indicate the regulation of TRPV1 by hypoxia. In contrast to the hypoxic inhibition of I TRPV1,Cap, the pH-dependent activation of TRPV1 (I TRPV1,Acid) was not affected by hypoxia.
Hypoxia is a micro-environmental factor shared by various pathological inflammatory conditions. In vivo, the 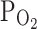 of parenchymal tissues and cells would be less than the ambient
of parenchymal tissues and cells would be less than the ambient 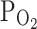 , and might be close to the moderate hypoxic conditions (9%
, and might be close to the moderate hypoxic conditions (9% 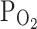 ) that we tested here. In this respect, it was noticeable that the relatively less hypoxia (9%
) that we tested here. In this respect, it was noticeable that the relatively less hypoxia (9% 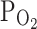 ) did not affect I
TRPV1 while still inhibiting I
TRPV1,Cap (Figs. 1a and 2a). This implies that the partial activation of I
TRPV1 might actually occur in vivo.
) did not affect I
TRPV1 while still inhibiting I
TRPV1,Cap (Figs. 1a and 2a). This implies that the partial activation of I
TRPV1 might actually occur in vivo.
At first, we assumed that hypoxia combined with agonistic conditions such as capsaicin and acidic pH would significant amplify the TRPV1 activity. Such an idea had been suggested in a previous study in which Henrich and Buckler [20] measured capsaicin-induced Δ[Ca2+]c in DRG neurons. In contrast to the results of DRG neurons, our patch clamp study showed the inhibition of I
TRPV1,Cap and no change in I
TRPV1,Acid. Although the reason(s) for such inconsistency is still unclear, there are actually several differences in the experimental conditions between the previous study and ours. While we applied hypoxia (3% 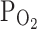 ), Henrich and Buckler [20] used anoxia or anoxia combined with glucose-free as the conditions mimicking ischemia. In addition, they used excitable cells (DRG neurons) that would presumably show complex changes in [Ca2+]c owing to the voltage-gated Ca2+ channels under the oxygen deprivation. Regarding the experimental conditions of [Ca2+]c measurement by Henrich and Buckler, one might also suspect that the anoxia might have impaired the Ca2+ removal mechanisms and/or recruited additional Ca2+ influx pathways in DRG neurons. In comparison, our study was more straightforward and showed relatively direct effects of hypoxia on TRPV1 under the voltage-clamp conditions.
), Henrich and Buckler [20] used anoxia or anoxia combined with glucose-free as the conditions mimicking ischemia. In addition, they used excitable cells (DRG neurons) that would presumably show complex changes in [Ca2+]c owing to the voltage-gated Ca2+ channels under the oxygen deprivation. Regarding the experimental conditions of [Ca2+]c measurement by Henrich and Buckler, one might also suspect that the anoxia might have impaired the Ca2+ removal mechanisms and/or recruited additional Ca2+ influx pathways in DRG neurons. In comparison, our study was more straightforward and showed relatively direct effects of hypoxia on TRPV1 under the voltage-clamp conditions.
Mechanisms of the hypoxic regulation of TRPV1
As for the mechanism, it was proposed that the changes in 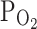 influence the oxidative conditions and the conformations of associated biomolecules. However, the responses of I
TRPV1 and I
TRPV1,Cap to the tested antioxidants were highly complex and confusing at first glance. In summary, (1) both I
TRPV1 and I
TRPV1,Cap were augmented by vitamin C, and (2) I
TRPV1 was increased while I
TRPV1,Cap was not affected by tiron or by H2O2. When combined with hypoxia, (1) I
TRPV1 was increased and I
TRPV1,Cap was inhibited by vitamin C/hypoxia, and (2) both I
TRPV1 and I
TRPV1,Cap were inhibited by tiron/hypoxia or by intracellular vitamin C/hypoxia. Although we do not yet have concrete evidence, such differential responses might be due to dual effects of intracellular and extracellular ROS and their interactions with the antioxidants having different membrane permeability. Vitamin C, when applied to the bath solution, would primarily affect the extracellular environment, whereas the membrane permeable tiron could play as an antioxidant at both sides of the plasma membrane. In addition, we hypothesize that the modulating effects of ROS on TRPV1 might be opposite: inhibition by extracellular ROS whereas a certain level of intracellular ROS is critical or supportive for TRPV1 (Fig. 8). Under hypoxia, either reduced extracellular ROS or increased intracellular ROS might partly activate TRPV1. With tiron/hypoxia or intracellular vitamin C/hypoxia, the stimulatory effects of intracellular ROS might be selectively removed, and lead to the inhibition of I
TRPV1 (Fig. 6).
influence the oxidative conditions and the conformations of associated biomolecules. However, the responses of I
TRPV1 and I
TRPV1,Cap to the tested antioxidants were highly complex and confusing at first glance. In summary, (1) both I
TRPV1 and I
TRPV1,Cap were augmented by vitamin C, and (2) I
TRPV1 was increased while I
TRPV1,Cap was not affected by tiron or by H2O2. When combined with hypoxia, (1) I
TRPV1 was increased and I
TRPV1,Cap was inhibited by vitamin C/hypoxia, and (2) both I
TRPV1 and I
TRPV1,Cap were inhibited by tiron/hypoxia or by intracellular vitamin C/hypoxia. Although we do not yet have concrete evidence, such differential responses might be due to dual effects of intracellular and extracellular ROS and their interactions with the antioxidants having different membrane permeability. Vitamin C, when applied to the bath solution, would primarily affect the extracellular environment, whereas the membrane permeable tiron could play as an antioxidant at both sides of the plasma membrane. In addition, we hypothesize that the modulating effects of ROS on TRPV1 might be opposite: inhibition by extracellular ROS whereas a certain level of intracellular ROS is critical or supportive for TRPV1 (Fig. 8). Under hypoxia, either reduced extracellular ROS or increased intracellular ROS might partly activate TRPV1. With tiron/hypoxia or intracellular vitamin C/hypoxia, the stimulatory effects of intracellular ROS might be selectively removed, and lead to the inhibition of I
TRPV1 (Fig. 6).
Fig. 8.
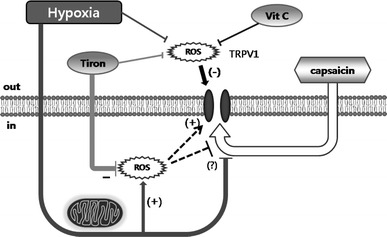
A schematic model for the O2-dependent modulation of TRPV1 activity. Hypothetical dual effects of ROS on TRPV1 at the extracellular and intracellular sides of the plasma membrane are proposed. Hypoxia might increase intracellular ROS via a putative mitochondria-dependent manner. The activation by capsaicin might be hindered by hypoxia per se or by intracellular ROS-dependent oxidation
According to the literature, the effects of H2O2 and oxidative/reducing conditions on TRPV1 are controversial [23]. Even in the same report, both reducing and oxidizing agents (e.g., dithiothreitol and diamide) showed positive effects on TRPV1, suggesting that multiple sites of TRPV1 are affected in a complex manner [23]. In comparison, our present study showed only a weak positive effect of H2O2 on I TRPV1 without change in the amplitude of I TRPV1,Cap. It has to be noted that the concentration of H2O2 (100 μM) used in this study was relatively low compared with the previous studies (10 mM). The putative side-dependent dual effects of ROS on TRPV1 might also be responsible for the indefinite results (Fig. 8).
The mechanism for the hypoxic inhibition of I TRPV1,Cap is even more shadowy than the hypoxic regulation of I TRPV1. In the present study, it has to be considered that the concentration of capsaicin to test the effects on I TRPV1,Cap was relatively low (50–200 nM). In addition, the previously known antiperoxidative property of capsaicin [24] should also be considered. Taken together, the ROS scavenging by capsaicin might have decreased an effective concentration of capsaicin near the plasma membrane under hypoxia (Fig. 8). However, the different O2-sensitivity of I TRPV1 and I TRPV1,Cap (Figs. 1a and 2a) suggested that the underlying mechanisms would not be common.
Additional intriguing result was that hypoxia had no effect on I TRPV1,Acid. Because the amino acid residues and conformations in the TRPV1 protein are differentially responsible for the pH, capsaicin, and heat [12, 16, 25], our results might suggest a specific effect of hypoxia on capsaicin binding site(s) of TRPV1. Considering the exquisite sensitivity of TRPV1 to a variety of endogenous lipid-derived molecules [26], hypoxia might alter such intrinsic regulatory mechanisms for TRPV1.
TRPV1 activation induces Ca2+ influx as well as depolarizing the inward current. Therefore, the activation of TRPV1 by hypoxia, albeit relatively small, might have physiological implication in the cells intrinsically expressing TRPV1. The hypoxic regulation of cellular functions and acute O2 sensation is often associated with intracellular Ca2+ signals [22]. The expression of TRPV1 has been suggested in vascular endothelium and pulmonary arteries where O2 sensation and contractile responses are critical issues [8, 27]. In addition, the expression of TRPV1 in the immune cells might play a role in their behavior under hypoxic inflammatory conditions, while the functional significance of TRPV1 seems different depending on the types of immune cells tested [28, 29].
Taken together, the partial activation of TRPV1 and associated Ca2+ signals might participate in the physiological responses of vascular tissues to hypoxia. However, because the acidic pH effect on I TRPV1 was not altered by acute hypoxia, the role of acute hypoxia itself in ischemic and inflammatory pain would be insignificant. The inhibition of I TRPV1,Cap suggests an intriguing possibility that hypoxia might attenuate the activation of TRPV1 by lipid-derived agonists.
Acknowledgment
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF 2009-0066749, 2011-0017370 and 2011-0001175).
References
- 1.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 2.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 3.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 4.Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 5.Hayes P, Meadows HJ, Gunthorpe MJ, Harries MH, Duckworth DM, Cairns W, Harrison DC, Clarke CE, Ellington K, Prinjha RK, Barton AJL, Medhurst AD, Smith GD, Topp S, Murdock P, Sander GJ, Terrett J, Jenkins O, Benham CD, Randall AD, Gloger IS, Davis JB. Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain. 2000;88:205–215. doi: 10.1016/S0304-3959(00)00353-5. [DOI] [PubMed] [Google Scholar]
- 6.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li WH, Lee YM, Kim JY, Kang S, Kim S, Kim KH, Park CH, Chung JH. Transient receptor potential vanilloid-1 mediates heat-shock-induced matrix metalloproteinase-1 expression in human epidermal keratinocytes. J Invest Dermatol. 2007;127:2328–2335. doi: 10.1038/sj.jid.5700880. [DOI] [PubMed] [Google Scholar]
- 8.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 9.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 10.Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gees M, Colsoul B, Nilius B (2010) The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. doi:10.1101/cshperspect.a003962 [DOI] [PMC free article] [PubMed]
- 12.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 13.Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23:183–191. doi: 10.1016/S0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- 14.Vennekens R, Owsianik G, Nilius B. Vanilloid transient receptor potential cation channels: an overview. Curr Pharm Des. 2008;14:18–31. doi: 10.2174/138161208783330763. [DOI] [PubMed] [Google Scholar]
- 15.Numazaki M, Tominaga T, Takeuchi K, Murayama N, Toyooka H, Tominaga M. Structural determinant of TRPV1 desensitization interacts with calmodulin. Proc Natl Acad Sci USA. 2003;100:8002–8006. doi: 10.1073/pnas.1337252100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuzhikandathil EV, Wang H, Szabo T, Morozova N, Blumberg PM, Oxford GS. Functional analysis of capsaicin receptor (vanilloid receptor subtype 1) multimerization and agonist responsiveness using a dominant negative mutation. J Neurosci. 2001;21:8697–8706. doi: 10.1523/JNEUROSCI.21-22-08697.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haddad JJ. Oxygen sensing and oxidant/redox-related pathways. Biochem Biophys Res Commun. 2004;316:969–977. doi: 10.1016/j.bbrc.2004.02.162. [DOI] [PubMed] [Google Scholar]
- 18.Seta KA, Spicer Z, Yuan Y, Lu G, Millhorn E (2002) Responding to hypoxia: lessons from a model cell line. Sci STKE. doi:10.1126/stke.2002.146.re11 [DOI] [PubMed]
- 19.Johnson DR, O’Connor JC, Hartman ME, Tapping RI, Freund GG. Acute hypoxia activates the neuroimmune system, which diabetes exacerbates. J Neurosci. 2007;27:1161–1166. doi: 10.1523/JNEUROSCI.4560-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrich M, Buckler KJ. Acid-evoked Ca2+ signaling in rat sensory neurons: effects of anoxia and aglycaemia. Pflugers Arch Eur J Physiol. 2009;459:159–181. doi: 10.1007/s00424-009-0715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ristoiu V, Shibasaki K, Uchida K, Zhou Y, Ton BT, Flonta M, Tominaga M. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain. 2011;152:936–945. doi: 10.1016/j.pain.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Toescu EC. Hypoxia sensing and pathways of cytosolic Ca2+ increases. Cell Calcium. 2004;36:187–199. doi: 10.1016/j.ceca.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol. 2006;70:383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 24.Kogure K, Goto S, Nishimura M, Yasumoto M, Abe K, Ohiwa C, Sassa H, Kusumi T, Terada H. Mechanism of potent antioxidative effect of capsaicin. Biochim Biophys Acta. 2002;1573:84–92. doi: 10.1016/S0304-4165(02)00335-5. [DOI] [PubMed] [Google Scholar]
- 25.Aneiros E, Cao L, Papakosta M, Stevens E, Phillips S, Grimm C. The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 2011;30:994–1002. doi: 10.1038/emboj.2011.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vriens J, Appendino G, Nilius B. Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- 27.Wang YX, Wang J, Wang C, Liu J, Shi LP, Xu M, Wang C. Functional expression of transient receptor potential vanilloid-related channels in chronically hypoxic human pulmonary arterial smooth muscle cells. J Membr Biol. 2008;223:151–159. doi: 10.1007/s00232-008-9121-9. [DOI] [PubMed] [Google Scholar]
- 28.Saunders CI, Fassett RG, Geraghty DP. Up-regulation of TRPV1 in mononuclear cells of end-stage kidney disease patients increases susceptibility to N-arachidonoyl-dopamine (NADA)-induced cell death. Biochim Biophys Acta. 2009;1792:1019–1026. doi: 10.1016/j.bbadis.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Kim KS, Shin DH, Nam JH, Park KS, Zhang YH, Kim WK, Kim SJ. Functional expression of TRPV4 cation channels in human mast cell line (HMC-1) Korean J Physiol Pharmacol. 2011;14:419–425. doi: 10.4196/kjpp.2010.14.6.419. [DOI] [PMC free article] [PubMed] [Google Scholar]


