Abstract
We examined the adaptation of plantar flexor muscles of female rats to 6 weeks (5 days/week) of lengthening contractions. After repeated lengthening contractions, a decrease in myofiber area of gastrocnemius medialis (26%) was accompanied by an increase in extracellular matrix (ECM) (42%) and collagen content (30.9%) without changes in muscle mass. Decrease in myofiber area (13%) and muscle mass of soleus (19%) was associated with increased collagen content (28%) and ECM (15%). Relative number of soleus myofibers stained for fast myosin increased by 26%. For plantaris, increases in collagen content (32.3%), percent ECM (17%), and myofiber area (6%) were recorded. We also observed (1) increases (3.3%) in the collagen content of the Achilles tendon, (2) no change in the crosslink content of any of the tissues tested, and (3) no difference in the force-frequency relationship of the plantar flexor muscles. Substantial decreases in myofiber areas with increases in muscle connective tissue by 6 weeks of repeated lengthening contractions did not appear to result in isometric force loss.
Keywords: Training, Myofiber area, Collagen, Force transmission
Introduction
If the load is sufficient, resistance training results in increases in muscle force (strength) and myofiber areas (hypertrophy) in both animals and humans, providing excellent models for the mechanistic study of muscle enlargement. However, some resistance training programs have been reported to lead to decreased performance known as overtraining. Few animal models have been developed to study training programs that might lead to decreased performance, but some studies have reported a lack of growth from resistance training in rats. For example, after 10 weeks of concentric contractions (192 repetitions) performed every 3rd or 4th day, the gastrocnemius muscle of rats did not enlarge [1]. No increase in mass of rat gastrocnemius was also noted for isovelocity (concentric/eccentric) training at 24°/s but not 12°/s under the same high-loading conditions [2]. In contrast, progressive resistance exercise (i.e., weight training) for 16 weeks produced larger gastrocnemius muscles in rats whereas the same training regimen without weights did not. Thus, there appears to be a training spectrum where load, repetition number, and contraction duration (tension-time integral) interact differently to produce muscle adaptations that are not always hypertrophic (e.g., increased strength without enlargement).
Increased strength without any change in muscle size might result if the transmission of force was improved with training [3] by connective tissue proliferation and/or remodeling. It has been proposed that resistance training might result in additional connective tissue attachments along the length of myofibers that would decrease the effective length of the muscle fibers but increase the isometric force [4]. We have shown that collagen struts can form in the endomysium between myofibers from repeated lengthening contractions [5] but did not evaluate any functional parameters. Because of their connective tissue sheath, broken muscle fibers retain the ability to transmit force [6], illustrating the importance of nonmyofiber components in force transmission. Therefore, alterations in connective tissue content and organization might be able to compensate partially for decreased myofiber areas and maintain isometric force during certain conditions of over-use or muscle disease so that functional impairment could be prevented or delayed.
We designed a 6-week training program using repeated lengthening contractions to study muscle fiber and nonfiber changes in all three plantar flexor muscles of the rat together with functional measurements of the isometric force-frequency relationship. Based on observations in our lab, a continuum from adequate stimulus for increased muscle strength and hypertrophy to decreased function and fibrosis from repeated lengthening contractions exists depending on repetition number, rest interval, and frequency of exposure. The current study was undertaken to examine a number of possible adaptations to repeated lengthening contractions including muscle fiber size and number, ECM, collagen and collagen crosslink content, isometric force-frequency relationship, and—for the soleus muscle—changes in the relative number of fast fibers.
Methods
Female Sprague-Dawley rats (4–5 months) were used for all experiments. The study complied with Animal Welfare Act P.L. 99-158 and DHHS Guidelines governing the care and use of laboratory animals. All experimental procedures were approved by and followed the guidelines of the West Virginia University Animal Care and Use Committee (WVU-ACUC #9809-02). Rats had access to water and laboratory chow ad libitum and were housed in animal facilities maintained at 21°C with a 12:12 h light:dark cycle (lights on 06:00 a.m.–06:00 p.m.). Experiments were designed to examine isometric force and muscle-specific adaptations after exposure to repeated lengthening contractions, daily for 6 weeks, except weekends.
Training with lengthening contractions
For each training session, rats were anesthetized and trained using our manual training program [7] with slight modifications. Electrodes for electrical field stimulation were inserted subcutaneously and positioned bilaterally along the surface of the plantar flexor muscles of the left hindlimb. The stimulation site was subcutaneous, and the electrodes were placed parallel to the leg muscles only for as long as the stimulation was delivered each day. Electrical field stimulation with 0.2 ms pulses at 70 Hz and 40 V was used. With the rat lying on its back, the left foot was held by the investigator (WTS) between thumb and index finger at a starting ankle position of 140°. Following onset of stimulation, the ankle was rotated to 40° by pushing at the sole of the foot (i.e., stretching the activated muscles producing lengthening contractions) and returned to the starting position (i.e., muscles shorten producing concentric contractions) with little resistance. Each training session consisted of 5 bouts of 10 stretch-shorten cycles (time required for 10 repetitions was about 4–5 s) with a rest period of 30 s between bouts. In between the training sessions, the rats were returned to normal cage activity. During the 6 weeks of training, rat plantar flexor muscles performed a total of 1,500 stretch-shorten cycles. A group of untrained female Sprague-Dawley rats served as controls.
Isometric force measurements
Isometric force measurements were taken 48 h after the last training bout using a different protocol. Rats were anesthetized with sodium pentobarbital (75 mg/kg, i.p.), and additional doses were administered as needed. Force measurements of the plantar flexor muscle–tendon complex of the left hindlimb [training (n = 10), control rats (n = 10)] were performed using direct stimulation of the tibial nerve. Details on the dissection procedure for nerve cuff placement, animal positioning, and force recording device are described elsewhere [8, 9]. In short, during muscle activation by nerve stimulation, the left foot of the rat was kept firmly positioned on an aluminum plate by two cross-bars. Below the aluminum plate was a Z-11/5 kg load cell (HBM, Marlboro, MA, USA). Force of the plantar flexor muscle–tendon complex was measured as a reaction force under the sole of the rat’s foot [8, 9]. Knee and ankle angle were held at 90°. For each rat, the maximum voltage for muscle force production with a stimulation frequency of 80 Hz and a pulse duration of 200 μs was determined (mean ± SE: 5.6 ± 0.3 V).
Force-frequency measurements were performed using isometric contractions at 5, 10, 20, 40, 60, 80, 100, and 120 Hz with stimulation times of 1,500 ms for 5–20 Hz, 900 ms for 40 Hz, and 600 ms for 60–120 Hz stimulations. Each contraction was performed twice at each frequency. Rest periods between isometric contractions were 1–2 min.
Tissue sampling and preparation
Immediately following the testing for the force-frequency relationship, rats were euthanized with an overdose of sodium pentobarbital. Plantar flexor muscles (i.e., soleus, plantaris, and gastrocnemius muscles) and Achilles tendons of left and right hindlimb were dissected, trimmed of fat, and weighed. In each group, left and right adrenal glands were pooled and wet weights measured. Achilles tendon and mid-belly samples from the individual plantar flexors were taken and frozen on corks using isopentane cooled by liquid nitrogen. For morphometric analysis, 10 μm frozen sections were collected on glass slides. Sampling, handling of tissue, and details on methods used for measuring collagen content [hydroxyproline (HYP)] and the mature, nonreducible cross-link pyridinoline (HP) from sectioned material were described by Miller et al. [10]. HYP and HP were determined by high-performance liquid chromatography and quantified relative to known amounts of type I collagen and HP, respectively. Collagen content was expressed as micrograms per milligram of dry weight using purified collagen as the standard. Collagen cross-links were expressed as moles of HP per mole of collagen.
Morphometric analysis
For morphometric analysis, the extracellular protein fibronectin was used to visualize the noncontractile tissue [11] and serve as a boundary marker for myofibers, similarly to methods reported previously [12]. Fibronectin was visualized using indirect immunohistochemistry applied to sectioned muscle samples. After a 200× dilution was prepared in phosphate-buffered saline, pH 7.4 (PBS), the primary antibody, rabbit anti-fibronectin IgG (AB 1942, Chemicon International, Temecula, CA), was applied to the slides, incubated for 30 min, and rinsed in PBS. The secondary antibody, FITC-labeled donkey anti-rabbit IgG (AP 182R, Chemicon International, Temecula, CA), was diluted 100× and applied for 30 min. The slides were rinsed in PBS, a drop of glycerin/PBS solution was applied, and a coverslip placed on the slide. Four muscle samples were used from each group.
For determination of the relative number of fast fibers in the soleus muscles, a double-labeled technique was used. In addition to the fibronectin staining procedure above, mouse anti-fast myosin IgG (M-4276, Sigma Chemical, St. Louis, MO), and goat anti-mouse IgG Cy3 (PA43002, Amersham Biosciences, Piscataway, NJ) were diluted 20× and applied for 30 min each. The use of concentrated antibodies allowed the fast-myosin fibers (red) to be visualized using the FITC filters (green). This technique allowed discrimination of individual fibers that were close together. Four pictures were taken from each muscle sample representing a medial, lateral, dorsal, and ventral area of the muscle. Color photographs (35 mm slides) were taken at 10× primary magnification and each contained about 200 fibers for analysis.
The four 35 mm slides for each muscle were scanned using a Nikon LS-1000 35 mm film scanner into Adobe PhotoShop 6.0 (Adobe Systems, San Jose, CA). The images were measured using image analysis software (Image Pro Plus 4.5, Media Cybernetics, Silver Spring, MD) for myofiber areas and percent noncontractile content using a custom calibration sample. Attempts were made to exclude arteries and veins from the measurement of noncontractile content. This exclusion reduced the noncontractile material in some samples. For calculation of the relative frequency distribution of fiber areas, all areas below 50 μm2 were excluded from analysis. For fiber counting, the microscope slides were placed under an Olympus Provis AX70 microscope, and images were captured using Microbrightfield software. The images containing an entire cross-section of the muscles were subsequently imported into Adobe Photoshop. Using a “layer” feature, a dot was placed on each myofiber. The images containing only dots were saved as individual files and counted using Image Pro to yield total fiber counts. To count the number of fast myosin fibers in the soleus, the scanned images were imported into Photoshop, and spots were placed on the stained fibers using a “layer.” The “layer” was saved as a separate image file, and the spots were counted using the image analysis software. Another “layer” was created to contain spots for the remaining unstained myofibers. The percentage of fast fibers was calculated from these counts. For statistical analysis, the mean values for all the fibers in each muscle and the percent noncontractile material in four 35 mm slides from each muscle sample were used [13].
Statistical analysis
Weights of individual plantar flexor muscles were not different between the right hindlimb of trained and control rats (Student t test, P < 0.05). Therefore, paired Student t tests were used to test for differences between left and right hindlimb of trained rats for (1) weights of the individual plantar flexor muscles, (2) collagen content in individual plantar flexor muscles and Achilles tendon, and (3) collagen cross-link content in individual plantar flexor muscles and Achilles tendon. Unpaired Student t tests were used to test for differences between trained rats and control rats for (1) adrenal gland weight and (2) isometric force at an ankle position of 90°. The morphometric data were entered into an analysis program (Prism 3.0, GraphPAD Software, San Diego, CA), where means, standard errors, Student t tests, and frequency distributions were performed. Data are presented as mean ± SE. Differences were accepted as significant at P < 0.05.
Results
Daily resistance training for 6 weeks using repeated lengthening contractions of electrically activated rat plantar flexor muscles did not result in an increase in isometric force, although there was a trend toward increased force at higher stimulation frequencies (Fig. 1). The absence of a difference in isometric force at any of the stimulation frequencies tested revealed that muscle injury was not present at the time of testing.
Fig. 1.
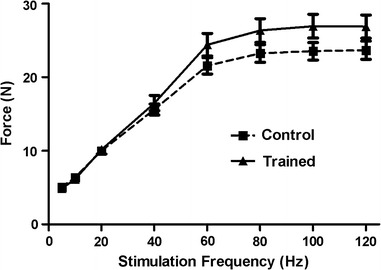
Relationship between stimulation frequency and isometric force of the rat plantar flexor muscles for control rats and for rats trained with repeated lengthening contractions
Muscle mass
Because daily exercise for 6 weeks would stress the rats, the effect of stress on the trained rats was assessed by weighing the adrenal glands. The adrenal glands from the trained rats were 17% larger than control rats (Table 1) providing indirect evidence for an increased stress reaction. The weights of the soleus, plantaris, and gastrocnemius muscles of the right (untrained) hind-limbs were similar in both trained and control rats (Table 1). In contrast, after training of the plantar flexor muscles (i.e., the muscles of the left hind-limb), the mass of the soleus muscle decreased by 19% (Table 1), but the masses of the plantaris and gastrocnemius muscles were unchanged (Table 1).
Table 1.
Body and tissue weights from control rats and rats trained with lengthening contractions
| Control | Training | |||
|---|---|---|---|---|
| Body wt (g) | 271 ± 3 | 266 ± 3 | ||
| Adrenal wt (mg) | 62 ± 1 | 72 ± 3* | ||
| Control | Training | |||
|---|---|---|---|---|
| Right hindlimb | Left hindlimb | Right hindlimb | Left hindlimb | |
| Soleus wt (mg) | 134 ± 3 | 130 ± 3 | 137 ± 2 | 116 ± 3* |
| Plantaris wt (mg) | 377 ± 6 | 366 ± 10 | 375 ± 7 | 370 ± 7 |
| Gastrocnemius wt (mg) | 1,671 ± 23 | 1,691 ± 48 | 1,692 ± 29 | 1,627 ± 30 |
*P < 0.05 (significant difference between control and trained rats, Student’s t test)
Contractile components
Muscle mass is the sum of the contractile and noncontractile components, so each was assessed using morphometric analysis. Figure 2 is an illustration of soleus (a, b), stained for fast myosin and fibronectin, and gastrocnemius medialis (c, d) to quantify myofiber areas. In the gastrocnemius medialis and soleus muscles of trained rats, myofiber areas decreased by 26 and 13%, respectively. A small but significant increase in myofiber area by 6% was observed for the plantaris muscles (Table 2). Very small fibers were present in the soleus and gastrocnemius medialis muscles but not present in the plantaris muscles (Fig. 3). If muscle fiber branching resulted in response to training, small muscle fibers and increased ECM would result because each fiber has its own connective tissue sheath.
Fig. 2.
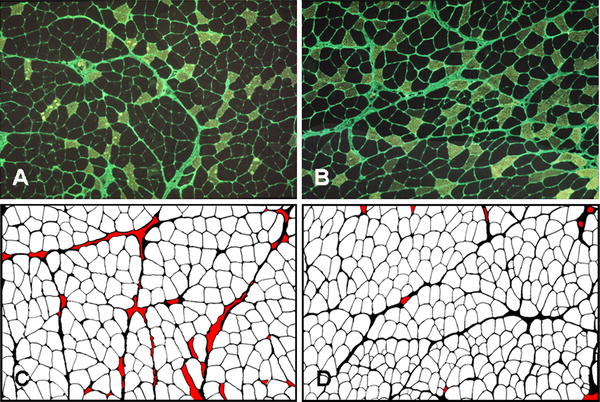
Immunohistochemical localization of fast myosin and fibronectin in soleus muscles from control (a) and trained (b) rats. Fibronectin staining was used for image analysis of muscles; the medial gastrocnemius muscles are illustrated from control (c) and trained (d) rats. Red areas represent areas not included in the measurements due to tears in the tissue or the presence of large blood vessels. Primary magnification 25×
Table 2.
Measured variables in control and trained rats
| Soleus | Plantaris | Medial gastrocnemius | ||||
|---|---|---|---|---|---|---|
| Control | Trained | Control | Trained | Control | Trained | |
| Fiber area (μm2) | 3,039 ± 95 | 2,653 ± 143* | 3,026 ± 88 | 3,191 ± 112* | 3,842 ± 154 | 2,840 ± 189* |
| ECM content (%) | 15.1 ± 1.0 | 17.4 ± 0.7† | 13.3 ± 0.6 | 15.5 ± 0.6* | 12.3 ± 0.4 | 17.5 ± 1.1* |
| Collagen content (μg/mg dry wt) | 26.9 ± 1.5 | 33.9 ± 3.5* | 18.6 ± 1.6 | 24.7 ± 0.9* | 10.0 ± 0.5 | 13.1 ± 1.2* |
| Collagen crosslinks (mol HP/mol collagen) | 0.27 ± 0.01 | 0.28 ± 0.02 | 0.24 ± 0.01 | 0.25 ± 0.01 | 0.35 ± 0.03 | 0.32 ± 0.02 |
ECM Extra-cellular matrix, HP pyridinoline
*P < 0.05, † P = 0.06 (Significant difference between control rats and rats trained with lengthening contractions, Student’s t test)
Fig. 3.
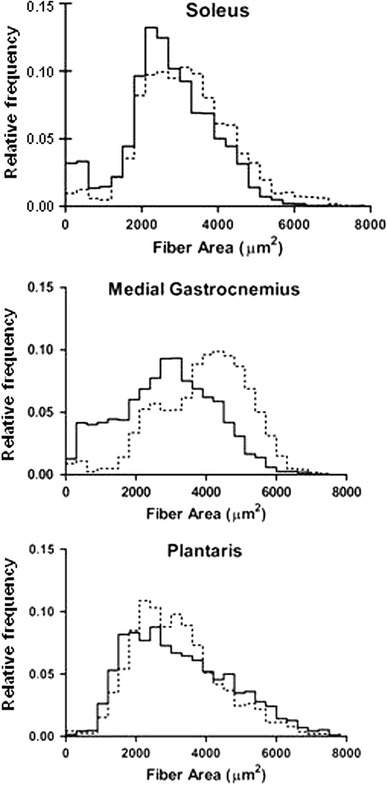
Frequency distribution histograms of soleus, gastrocnemius medialis, and plantaris muscles after 6 weeks of repeated lengthening contractions (5 days/week). Solid lines Trained muscles, dashed lines control muscles
In soleus muscles from trained rats, the relative number of fast fibers, identified by antibody localization, increased by 26% from 17.4 ± 0.9 to 22.0 ± 1.5%. Overall, the contractile component of the plantar flexor muscles was decreased after 6 weeks of training with lengthening contractions.
Noncontractile components
The noncontractile components were assessed by morphometric and chemical analysis. The relative area of ECM increased in gastrocnemius medialis by 42% and plantaris by 17%; for the increase in ECM of the soleus muscle (increase of 15%) there was a trend to reach significance (P = 0.06) (Table 2). In contrast, HPLC analysis revealed significant increases in collagen content in all plantar flexor muscles: soleus by 27.6%, plantaris by 32.3%, and gastrocnemius medialis by 30.9% (Table 2) as well as for the Achilles tendon by 3.3%. No changes in collagen cross-links (Table 2) were detected in any of the tissues.
Discussion
Substantial decrease in myofiber areas and increase in muscle connective tissue by 6 weeks of repeated lengthening contractions did not result in a loss of isometric force as would be expected from overtraining. An increase in muscle mass is a common outcome from resistance training programs in rats given sufficient rest to prevent overtraining. Overtraining is recognized as a decrease in performance following large training volumes and/or high intensity programs [14]. Although daily training using maximum lengthening contractions would be considered a high intensity training program [15], no decrement in performance, as assessed by maximum isometric force measurements, was observed for the plantar flexor muscles of rats. Instead a small but insignificant increase in maximum isometric force (14%) was recorded. The lack of change in isometric force (strength) occurred together with a decrease in myofiber area of the gastrocnemius medialis and soleus muscles with a slight increase in plantaris muscles (Table 2); only the soleus muscle actually lost muscle mass. Although the relative frequency distribution of soleus muscle indicates a decrease in relatively large (i.e., fast) muscle fibers, the relative increase in fast fibers in the soleus may be explained by a combined decrease in area of slow and fast fibers in the trained soleus. Gastrocnemius medialis and plantaris muscle, both considered fast-twitch and bi-articular, showed opposite responses for changes in myofiber areas. Thus, the effects of our training program were muscle-specific and not necessarily fiber-type related. Furthermore, observations in plantaris muscle of a decrease in muscle mass seem to be in contradiction with an increase in muscle fiber area. However, without measurement of the total number of muscle fibers in each muscle and assessment of muscle length, further interpretation is impossible. However, since the gastrocnemius muscle is responsible for 80% [16, 17] of the isometric force of the plantar flexor muscles, a decrease in myofiber area of about 26% for the gastrocnemius muscle would be expected to decrease the isometric force recorded after resistance training unless fiber branching or hyperplasia was present.
Although increases in fast fibers have also been reported for rat soleus muscles following chronic overload [11], the increase in the number of fast fibers as identified by myosin antibody localization in the rat soleus muscles following resistance training was an unexpected finding. Fast fibers are known to increase in soleus muscles of rats following hind-limb unloading [18, 19], spinal cord injury and hypothyroidism—all conditions of muscle atrophy. Recently, an increase in the ratio of fast to slow fibers in the rat soleus has also been reported following clenbuterol-induced hypertrophy [20], but, in single fibers from the soleus, multiple isoforms were expressed [21].
A transition from slow to fast isoforms normally occurs during regeneration following muscle injury and might account for the increased number of fast fibers. Although injury and regeneration commonly follow a single bout of unaccustomed lengthening contractions in the rat soleus muscle [7], protection seems to occur when the training continues, as markers for injury disappear including the absence of fibronectin positive myofibers in the present study. Using the presence of developmental myosin-positive myofibers to identify muscle regeneration in the soleus of resistance-trained rats in our study, only very small fibers were positive, and these probably represent activated satellite cells known to respond to resistance training. Although activation of satellite cells is essential to initiate muscle regeneration (for a review see [22]), a relationship between those cells and fiber branching and/or multiple slow and fast isoforms in single muscle fibers is not clear. Thus, slow to fast fiber shifts in the soleus muscle of trained rats appeared to result from the daily training regimen. However, we did not examine the tissue for expression of multiple forms within a single fiber. Therefore, it is possible that the small muscle fibers represent regenerated muscle fibers.
Daily training results in diminished recovery time and may be a risk factor for overtraining [23, 24]. The importance of recovery time for muscle enlargement was recognized [25]. When training with lengthening contractions occurred every other day for 16 weeks, muscle enlargement was not observed. However, if 3 days of rest occurred between the exercise sessions, muscle growth occurred from the same training regimen [25]. In contrast, training every other day for 8 weeks using 4 bouts of 10 repetitions of shortening/lengthening contractions led to hypertrophy only if the angular velocity of ankle rotation was very slow (12°/s) [2]. These studies illustrate that muscle enlargement in response to resistance training is highly complex. Furthermore, muscle enlargement is not required for increases in isometric force (strength) in rats. In spite of a lack of muscle enlargement from a 5-week isometric training program, isometric force increased for the plantar flexor muscles [26].
For force to be transmitted to the skeleton, connective tissue is required in the form of tendon and fascial connections. In our trained rats, the Achilles tendon contained more collagen per unit dry weight, implying that the collagen fibril density increased in order to accommodate the muscle force, hence a stronger tendon. Although the effects of endurance training on muscle [27–29] and tendon [30, 31] collagens have been studied, tendon adaptations to repeated lengthening contractions have not been studied except when used to produce tendinosis in rats [32]. To produce tendinosis, 1 h of 1,800 repetitions 3 days a week for up to 4 weeks was used [32]. In our study, there was no evidence of pathology or overt inflammation in the Achilles tendons from the repeated lengthening contractions but increased collagen content was recorded. The increase in collagen content could have resulted from a decrease in noncollagen components (e.g., proteoglycans) of the tendon. Further studies are needed to define the noncollagen changes that occurred. However, since collagen cross-link content did not increase in the Achilles tendons of trained rats, tendon stiffness would not be expected to change even with an increase in collagen content. Interestingly, however, evidence of a decrease in joint stiffness was observed in male rats trained with lengthening contractions over a 20-day period [33].
In humans, resistance training increased the total amount of connective tissue in skeletal muscle but in proportion to the myofiber enlargement [34]. In our studies of rat plantar flexor muscles, increased ECM and the formation of collagen struts between myofibers were reported following lengthening contractions. These collagen connections could serve to increase the lateral transmission of force from the sarcomeres to the tendon. For the anterior tibial compartment of the rat, extramuscular myofascial force transmission has been reported [35, 36]. Therefore, the presence of increased collagen content of muscles trained with lengthening contractions could have offset any decrease in force production by reduced myofiber area. Interestingly, greater expression of transforming growth factor-β1, a collagen-inducing growth factor, was shown after 4 days of such training [37].
In summary, daily training with lengthening contraction for 6 weeks did not result in overtraining as isometric force production was maintained, if not increased, in spite of a decrease in myofiber areas of the soleus and gastrocnemius muscles. Indirect evidence for an increase in specific tension due to an increased proportion of connective tissue offsetting the decrease in myofiber area was supported by an increase in ECM area and collagen content. Increased collagen content of the Achilles tendon provided evidence that tendons may become stronger as well. Finally, an unexpected shift from slow to fast fiber types in the soleus muscle was observed following training. Perhaps as more slow fibers become transformed to fast fibers, the soleus muscle may become more fatigable.
Acknowledgments
The authors thank Dr. Cheryl Smith for her technical assistance and Dr. William Kraemer for his comments on the criteria for overtraining. This work was supported in part by Cooperative Agreement No. R01-OH02918-09 from NIOSH and the Centers for Disease Control and Prevention to W.T.S. The content and opinions in this paper are solely the responsibility of the authors and do not necessarily represent the official view of the National Institute of Occupational Safety and Health, Centers for Disease Control and Prevention.
References
- 1.Wong TS, Booth FW. Protein metabolism in rat gastrocnemius muscle after stimulated chronic concentric exercise. J Appl Physiol. 1990;69:1709–1717. doi: 10.1152/jappl.1990.69.5.1709. [DOI] [PubMed] [Google Scholar]
- 2.Caiozzo VJ, Ma E, McCue SA, Smith E, Herrick RE, Baldwin KM. A new animal model for modulating myosin isoform expression by altered mechanical activity. J Appl Physiol. 1992;73:1432–1440. doi: 10.1152/jappl.1992.73.4.1432. [DOI] [PubMed] [Google Scholar]
- 3.Enoka RM. Muscle strength and its development. New perspectives. Sports Med. 1988;6:146–168. doi: 10.2165/00007256-198806030-00003. [DOI] [PubMed] [Google Scholar]
- 4.Jones DA, Rutherford OM, Parker DF. Physiological changes in skeletal muscle as a result of strength training. Q J Exp Physiol. 1989;74:233–256. doi: 10.1113/expphysiol.1989.sp003268. [DOI] [PubMed] [Google Scholar]
- 5.Stauber WT, Knack KK, Miller GR, Grimmett JG. Fibrosis and intercellular collagen connections from four weeks of muscle strains. Muscle Nerve. 1996;19:423–430. doi: 10.1002/mus.880190402. [DOI] [PubMed] [Google Scholar]
- 6.Street SF. Lateral transmission of tension in frog myofibers: a myofibrillar network and transverse cytoskeletal connections are possible transmitters. J Cell Physiol. 1983;114:346–364. doi: 10.1002/jcp.1041140314. [DOI] [PubMed] [Google Scholar]
- 7.Stauber WT, Fritz VK, Vogelbach DW, Dahlmann B. Characterization of muscles injured by forced lengthening. I. Cellular infiltrates. Med Sci Sports Exerc. 1988;20:345–353. doi: 10.1249/00005768-198808000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Cutlip RG, Stauber WT, Willison RH, McIntosh TA, Means KH. Dynamometer for rat plantar flexor muscles in vivo. Med Biol Eng Comput. 1997;35:540–543. doi: 10.1007/BF02525537. [DOI] [PubMed] [Google Scholar]
- 9.Willems ME, Stauber WT. Isometric and concentric performance of electrically stimulated ankle plantar flexor muscles in intact rat. Exp Physiol. 1999;84:379–389. doi: 10.1017/S0958067099017844. [DOI] [PubMed] [Google Scholar]
- 10.Miller GR, Smith CA, Stauber WT. Determination of fibrosis from cryostat sections using high performance liquid chromatography: skeletal muscle. Histochem J. 1999;31:89–94. doi: 10.1023/A:1003592710740. [DOI] [PubMed] [Google Scholar]
- 11.Stauber WT, Smith CA. Cellular responses in exertion-induced skeletal muscle injury. Mol Cell Biochem. 1998;179:189–196. doi: 10.1023/A:1006832509488. [DOI] [PubMed] [Google Scholar]
- 12.Miller GR, Stauber WT. Use of computer-assisted analysis for myofiber size measurements of rat soleus muscles from photographed images. J Histochem Cytochem. 1994;42:377–382. doi: 10.1177/42.3.8308255. [DOI] [PubMed] [Google Scholar]
- 13.Stauber WT, Smith CA, Miller GR, Stauber FD. Recovery from 6 weeks of repeated strain injury to rat soleus muscles. Muscle Nerve. 2000;23:1819–1825. doi: 10.1002/1097-4598(200012)23:12<1819::AID-MUS4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Fry AC, Kraemer WJ. Resistance exercise overtraining and overreaching. Neuroendocrine responses. Sports Med. 1997;23:106–129. doi: 10.2165/00007256-199723020-00004. [DOI] [PubMed] [Google Scholar]
- 15.Fry AC, Kraemer WJ, van Borselen F, Lynch JM, Marsit JL, Roy EP, Triplett NT, Knuttgen HG. Performance decrements with high-intensity resistance exercise overtraining. Med Sci Sports Exerc. 1994;26:1165–1173. [PubMed] [Google Scholar]
- 16.Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- 17.Woittiez RD, Baan GC, Huijing PA, Rozendal RH. Functional characteristics of the calf muscles of the rat. J Morphol. 1985;184:375–387. doi: 10.1002/jmor.1051840311. [DOI] [PubMed] [Google Scholar]
- 18.Bigard AX, Boehm E, Veksler V, Mateo P, Anflous K, Ventura-Clapier R. Muscle unloading induces slow to fast transitions in myofibrillar but not mitochondrial properties. Relevance to skeletal muscle abnormalities in heart failure. J Mol Cell Cardiol. 1998;30:2391–2401. doi: 10.1006/jmcc.1998.0798. [DOI] [PubMed] [Google Scholar]
- 19.Caiozzo VJ, Baker MJ, McCue SA, Baldwin KM. Single-fiber and whole muscle analyses of MHC isoform plasticity: interaction between T3 and unloading. Am J Physiol. 1997;273:C944–C952. doi: 10.1152/ajpcell.1997.273.3.C944. [DOI] [PubMed] [Google Scholar]
- 20.Zeman RJ, Ludemann R, Easton TG, Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a beta 2-receptor agonist. Am J Physiol. 1988;254:E726–E732. doi: 10.1152/ajpendo.1988.254.6.E726. [DOI] [PubMed] [Google Scholar]
- 21.Oishi Y, Imoto K, Ogata T, Taniguchi K, Matsumoto H, Roy RR. Clenbuterol induces expression of multiple myosin heavy chain isoforms in rat soleus fibres. Acta Physiol Scand. 2002;176:311–318. doi: 10.1046/j.1365-201X.2002.01036.x. [DOI] [PubMed] [Google Scholar]
- 22.Chargé SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 23.Petibois C, Cazorla G, Poortmans JR, Deleris G. Biochemical aspects of overtraining in endurance sports: a review. Sports Med. 2002;32:867–878. doi: 10.2165/00007256-200232130-00005. [DOI] [PubMed] [Google Scholar]
- 24.Smith LL. Cytokine hypothesis of overtraining: a physiological adaptation to excessive stress? Med Sci Sports Exerc. 2000;32:317–331. doi: 10.1097/00005768-200002000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Wong TS, Booth FW. Protein metabolism in rat tibialis anterior muscle after stimulated chronic eccentric exercise. J Appl Physiol. 1990;69:1718–1724. doi: 10.1152/jappl.1990.69.5.1718. [DOI] [PubMed] [Google Scholar]
- 26.Lac G, Cavalie H. A rat model of progressive isometric strength training. Arch Physiol Biochem. 1999;107:144–151. doi: 10.1076/apab.107.2.144.4337. [DOI] [PubMed] [Google Scholar]
- 27.Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat skeletal muscle following downhill running. Pflügers Arch. 1999;437:857–864. doi: 10.1007/s004240050855. [DOI] [PubMed] [Google Scholar]
- 28.Kovanen V, Suominen H, Heikkinen E. Connective tissue of “fast” and “slow” skeletal muscle in rats–effects of endurance training. Acta Physiol Scand. 1980;108:173–180. doi: 10.1111/j.1748-1716.1980.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 29.Kovanen V, Suominen H, Heikkinen E. Mechanical properties of fast and slow skeletal muscle with special reference to collagen and endurance training. J Biomech. 1984;17:725–735. doi: 10.1016/0021-9290(84)90103-9. [DOI] [PubMed] [Google Scholar]
- 30.Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tipton CM, Matthes RD, Maynard JA, Carey RA. The influence of physical activity on ligaments and tendons. Med Sci Sports. 1975;7:165–175. [PubMed] [Google Scholar]
- 32.Messner K, Wei Y, Andersson B, Gillquist J, Rasanen T. Rat model of Achilles tendon disorder. A pilot study. Cells Tissues Organs. 1999;165:30–39. doi: 10.1159/000016671. [DOI] [PubMed] [Google Scholar]
- 33.Ochi E, Nakazato K, Ishii N. Effects of eccentric exercise on joint stiffness and muscle connectin (titin) isoform in the rat hindlimb. J Physiol Sci. 2007;57:1–6. doi: 10.2170/physiolsci.RP008806. [DOI] [PubMed] [Google Scholar]
- 34.MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol. 1984;57:1399–1403. doi: 10.1152/jappl.1984.57.5.1399. [DOI] [PubMed] [Google Scholar]
- 35.Huijing PA, Baan GC. Extramuscular myofascial force transmission within the rat anterior tibial compartment: proximo-distal differences in muscle force. Acta Physiol Scand. 2001;173:297–311. doi: 10.1046/j.1365-201X.2001.00911.x. [DOI] [PubMed] [Google Scholar]
- 36.Huijing PA, Baan GC. Myofascial force transmission causes interaction between adjacent muscles and connective tissue: effects of blunt dissection and compartmental fasciotomy on length force characteristics of rat extensor digitorum longus muscle. Arch Physiol Biochem. 2001;109:97–109. doi: 10.1076/apab.109.2.97.4269. [DOI] [PubMed] [Google Scholar]
- 37.Heinemeier KM, Olesen JL, Haddad F, Langberg H, Kjaer M, Baldwin KM, Schjerling P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J Physiol. 2007;582:1303–1316. doi: 10.1113/jphysiol.2007.127639. [DOI] [PMC free article] [PubMed] [Google Scholar]


