Abstract
We investigated age-induced changes in mRNA expression profiles of sex-steroidogenic enzymes and sex-steroid receptors in 3-, 12-, and 24-month-old male rat brain subregions [cerebral cortex (CC), hypothalamus (Hy) and cerebellum (CL)]. In many cases, the expression levels of mRNA decreased with age for androgen synthesis enzyme systems, including Cyp17a1, Hsd17b and Srd5a in the CC and CL, but not in the Hy. Estradiol synthase Cyp19a1 did not show age-induced decline in the Hy, and nearly no expression of Cyp19a1 was observed in the CC and CL over 3–24 m. Androgen receptor Ar increased in the Hy but decreased in the CC with age. Estrogen receptor Esr1 increased in the CC and Hy, and did not change in the CL with age. Esr2 did not change in the CC and Hy, but decreased in the CL with age. As a comparison, age-induced changes of brain-derived neurotrophic factor mRNA were also investigated.
Electronic supplementary material
The online version of this article (doi:10.1007/s12576-015-0363-x) contains supplementary material, which is available to authorized users.
Keywords: Aging, Brain, Cortex, Hypothalamus, Cerebellum, Steroidogenesis, Steroid receptor
Introduction
Many studies have been accumulated for aging effects on the cognitive impairment or dysfunction of the reproductive system [2, 17]. A decline in sex steroids, especially 17β-estradiol (E2), has been thought to be one of the most important factors involved in age-related neural dysfunction, since E2 significantly decreases in plasma upon menopause, which elicits cognitive decline and dysfunction of the hypothalamus–pituitary–gonadal axis [31]. The relationship between neural function and E2 has been extensively investigated, including the modulatory effect of E2 on synaptic plasticity [4, 18, 21], and the molecular mechanism of the beneficial effect of E2 replacement therapy by using rodents and primates [6, 25]. Recently, studies on the relationship between androgen decline and age-related neural and reproductive dysfunction have also been performed in male mice [22].
These aging effects had been attributed to the decline in plasma E2 and testosterone (T), which are synthesized in the ovary and testis [10, 31]. Importantly, recent studies demonstrate that young adult male and female rat hippocampal neurons synthesize estrogen and androgen whose levels are higher than those in the plasma (Fig. S1) [7, 9, 11, 13, 15]. Therefore, age-related changes in brain sex-steroid synthesis should be investigated, since brain derived sex steroids may be more effective than gonadal sex steroids on synaptic plasticity with age.
Here, we investigated age-induced changes in the mRNA level of steroidogenic enzymes and steroid receptors in the cerebral cortex (CC), hypothalamus (Hy), and cerebellum (CL), in which endogenous steroidogenesis has not been extensively investigated in adult and aged animals. We compared young adult (3-month-old, 3 m), middle-aged (12-month-old, 12 m) and aged (24-month-old, 24 m) male rats. We observed that aging induced not only monotonous decrease but also increase (often in Hy), and no change in mRNAs, depending on individual mRNAs and difference in regions.
Materials and methods
Animals
Male Wistar rats of 3, 12 and 24 m were used in the current study. We used normal aged rats in which the brains did not have tumor-like structures including abnormal pituitary and hypophyseal adenomas. Animals were housed in the animal facility of the Tokyo Metropolitan Institute of Gerontology, under a 12 h-light/dark cycle and were allowed ad libitum access to food and water. All experiments were performed with the approval of the Committee for Animal Research of the University of Tokyo.
Total RNA isolation
Rats were deeply anesthetized and were decapitated. The CC, Hy, CL and hippocampus (Hi) were quickly removed and immersed in ice-cold artificial cerebrospinal fluid (pH 7.4, 290 mOsm), and stored in liquid nitrogen until use. Total RNA was extracted using an SV Total RNA Isolation System (Promega, USA) according to the manufacturer’s instructions. Total RNA was then treated with Recombinant DNase I (RNase-free DNase I; Takara, Japan), and then purified. The concentration was quantified by absorption at 260 and 280 nm.
Primer design
Since the expression of steroidogenic enzymes and steroid receptors is extremely low in the brain, PCR primers with high sensitivity and specificity were carefully designed for precise analysis (Table 1) [15]. We designed the primers considering Gibbs energy (ΔG) as follows: (1) ΔG of the whole primer [ΔG (whole)] was calculated with a nearest-neighbor model [1], and set to be below the mean value for all of the primer candidates (ΔG av) to obtain good stability in primer-target interaction. (2) ΔG of the five 3′-terminal bases of the primer [ΔG (5base)] was set to be higher than ΔG av for improved specificity. Consequently, ΔG of 5′-terminal bases was set to be less than the ΔG av, resulting not only in the improved stability in 5′-terminal interaction but also in the improved DNA polymerase recognition. Two (forward and reverse) primers were designed on separate exons and primers were designed to avoid ‘PCR debris’ including primer dimers.
Table 1.
Primers and PCR conditions
| Enzyme/protein name | Gene name | Accession no. | Direction | Primer sequence (5′–3′) | Product length [bp] | T a (°C) | PCR cycles | Exponential amplification |
|---|---|---|---|---|---|---|---|---|
| StAR | Star | NM_031558 | Forward | 5′-CTGGTGGGGCCCCGAGACTT-3′ | 360 | 60 | 32 | 28–34 |
| Reverse | 5′-CAATGGCGTGCAGGTAGATGTGGT-3′ | |||||||
| TSPO | Tspo | NM_012515 | Forward | 5′-GGCTATGGTTCCCTTGGGTCTCTACAC-3′ | 362 | 63 | 28 | 26–30 |
| Reverse | 5′-AGGCCCAATGGTCATGAAAGCAGGAT-3′ | |||||||
| P450(17α) | Cyp17a1 | NM_012753 | Forward | 5′-TGGGGCGGGCATAGAGACAACT-3′ | 477 | 62 | 33 | 30–36 |
| Reverse | 5′-AGCAAGGCCGTGAAGACAAAGAGC-3′ | |||||||
| 17β-HSD1 | Hsd17b1 | NM_012851 | Forward | 5′-ACTCCGGGCGTGTGCTGGTGA-3′ | 517 | 65 | 30 | 28–32 |
| Reverse | 5′-GGCGTGTCTGGATCCCCTGAAACTT-3′ | |||||||
| 17β-HSD3 | Hsd17b3 | NM_054007 | Forward | 5′-CTCCCCAACCTGCTCCCAAGTCATTT-3′ | 408 | 65 | 31 | 29–33 |
| Reverse | 5′-AGCAAGGCAGCCACAGGTTTCAGC-3′ | |||||||
| 5α-reductase1 | Srd5a1 | NM_017070 | Forward | 5′-ACCGCGTCCTGCTGGCTATGTTT-3′ | 318 | 63 | 25 | 23–27 |
| Reverse | 5′-GGCCTCCCCTGGGTATCTTGTATCC-3′ | |||||||
| 5α-reductase2 | Srd5a2 | NM_022711 | Forward | 5′-AGGTGGCTTGTTTACGTATGTCTCTG-3′ | 453 | 57 | 32 | 29–35 |
| Reverse | 5′-GGCCTCTGTGAAGCTCCAAAAG-3′ | |||||||
| P450arom | Cyp19a1 | NM_017085 | Forward | 5′-CTGATCATGGGCCTCCTCCTG-3′ | 276 | 58 | 32 | 30–34 |
| Reverse | 5′-CCCACGCTTGCTGCCGAATCT-3′ | |||||||
| AR | Ar | NM_012502 | Forward | 5′-CAACTTTCCGCTCGCTCTGTC-3′ | 536 | 56 | 26 | 24–28 |
| Reverse | 5′-TCTGGGGTGGGAAGTAATAGTCG-3′ | |||||||
| ERα | Esr1 | NM_012689 | Forward | 5′-GCCGGCTGCGCAAGTGTTACG-3′ | 467 | 68 | 28 | 26–30 |
| Reverse | 5′-GGAGCGCCAGACCAGACCAATCA-3′ | |||||||
| ERβ | Esr2 | NM_012754 | Forward | 5′-GCAAACCAGGAGGCAGAAAGTAGC-3′ | 591 | 58 | 28 | 26–30 |
| Reverse | 5′-AAGTGGGCAAGGAGACAGAAAGTAAGTA-3′ | |||||||
| BDNF | Bdnf | NM_001270630 | Forward | 5′-AGGGGCATAGACAAAAGGCACTG-3′ | 403 | 59 | 23 | 21–25 |
| Reverse | 5′-AACGGCAACAAACCACAACATTAT-3′ | |||||||
| GAPDH | Gapdh | NM_017008 | Forward | 5′-TATGACTCTACCCACGGCAAGTTCAA-3′ | 830 | 60 | 18 | 16–24 |
| Reverse | 5′-ACCACCCTGTTGCTGTAGCCATATTCAT-3′ |
Exponential Amplification: PCR cycle numbers for the exponential amplification phase, obtained by using the 3 m hippocampal cDNA templates
bp Base pair, T a annealing temperature
RT-PCR
RT-PCR was performed essentially as described elsewhere [15]. The sequences of oligonucleotide primers, the number of PCR cycles, and the PCR cycle numbers for exponential amplification phase, used in RT-PCR, are shown in Table 1. For RT, total RNA was reverse-transcribed into first-strand cDNA using an oligo(dT) primer. The reaction solution (25 μl) contained 10 μg of total RNA, 1 × RT buffer, 1 mM dNTP mixture, 2 μg oligo(dT)15 (Promega, USA), 40 U RNasin Plus (Promega), and 200 U RTase (Toyobo, Japan). The reaction was carried out at 42 °C for 60 min, and stopped by heating at 75 °C for 15 min. cDNA was treated with 4 U RNase H (Takara bio, Japan) at 37 °C for 30 min, and stored at −20 °C until use.
PCR was performed in 25 μl of PCR mixture comprising cDNA corresponding to 100 ng of total RNA, 1 × PCR buffer, 0.2 M dNTP mixture, 0.2 μM forward and reverse primers, and 0.63 U Blend Taq polymerase (Toyobo, Japan). PCR was performed with cycle reactions at 95 °C for 30 s, 56–68 °C for 20 s, and 72 °C for 30 s, with an initial denaturing at 95 °C for 2 min and a final elongation at 72 °C for 5 min. The PCR products were applied to 2 % agarose gels. Gels were stained with ethidium bromide (EtBr) and visualized under UV light. Fluorescence images were recorded with Printgraph (ATTO, Japan). For quantitative estimation, images of the bands were analyzed using the Image J software (National Institutes of Health, Bethesda, MD). In all the cases, we first plotted amplification curves in order to obtain the exponential amplification phase of the PCR plot.
DNA sequencing
PCR products were extracted from agarose gels using a Wizard SV Gel and PCR Clean-up System (Promega) and cloned into pGEM-T-Easy vectors (Promega, USA). Sequencing reactions were performed using a BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, USA). Signals were detected using an ABI PRISM 3130 Genetic Analyzer (Applied Biosystems, USA). In all the expression analyses, the sequence identity between PCR products and target sequences was confirmed with DNA sequencing.
Comparison of the mRNA levels for different enzymes and receptors
We used the comparison method for mRNA levels of different enzymes/receptors obtained by using different primers [15]. Relative abundance of different genes was estimated by adopting the expression level of glyceraldehyde-3-phosphate dehydrogenase mRNA (Gapdh) as an internal standard. Optical density value in each band was divided by the number of (1 + e)c, where c is a PCR cycle number and e is an amplification efficacy obtained from the PCR amplification curves in the exponential amplification phase. For more theoretical details, see supplementary materials.
Statistical analysis
Data are expressed as mean±SEM. The mRNA levels at different ages were compared by one-way ANOVA followed by Tukey’s post hoc test when significant differences were found. A p < 0.05 was considered to be statistically significant.
Results
Age-induced change in expression levels of mRNAs encoding sex-steroidogenic enzymes and sex-steroid receptors
We performed the PCR analysis in three brain subregions, including the CC, Hy and CL for male rats, which were aged 3 months (3 m), 12 months (12 m), and 24 months (24 m). In the following the italic name (e.g., Gapdh) means the gene name of a protein (e.g., GAPDH). The expression levels of Gapdh mRNA did not show a significant age-related change over 3–24 m (Fig. 1). The Gapdh levels were not different among these three brain subregions, within experimental error. Therefore, the expression levels of the respective enzymes and receptors can be normalized by Gapdh. Furthermore, the level of each enzyme and receptor was normalized by enzymes/receptors levels in 3 m Hi, which were set to be 100 %.
Fig. 1.
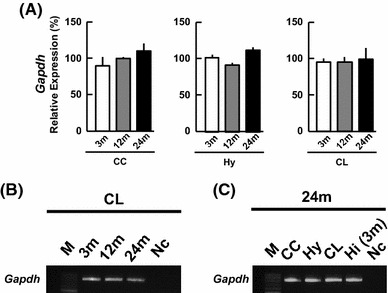
mRNA expression of Gapdh (internal control) in the cerebral cortex (CC), hypothalamus (Hy), and cerebellum (CL) at 3, 12 and 24 m. a The vertical axis indicates the expression level for Gapdh calculated from the intensity of ethidium bromide bands. The expression level of each mRNA was normalized by 3 m hippocampus (Hi) which is set to be 100 %. Each value is mean ± SEM. Data are taken from duplicate determinations for each brain subregion of 4 rats. b A typical Gapdh PCR image for the CL. From left to right, 100-bp DNA ladder (M), 3, 12, 24 m, the sample without template cDNA as negative control (Nc). c A typical Gapdh PCR image at 24 m. From left to right, 100-bp DNA ladder (M), CC, Hy, CL, 3 m Hi (positive control), negative control (Nc). The PCR products are visualized with ethidium bromide
Although the patterns of age-related changes of mRNAs are complex, we could categorize them into four types: (1) monotonous decrease-type (2) monotonous increase-type (3) no change-type, and (4) up and down-type.
Enzymes for androgen/estrogen synthesis
Cytochrome P450(17α) [Cyp17a1]
P450(17α) converts pregnenolone (PREG) to dehydroepiandrosterone (DHEA), or/and progesterone (PROG) to androstenedione (ADione). Cyp17a1 expression level in the CC monotonously decreased with age, from 279 % (3 m) to 163 % (12 m), and 62 % (24 m) (Fig. 2a). In the Hy, the Cyp17a1 level did not alter significantly with age. In the CL, the mRNA level at 24 m was lower than that at 3 m.
Fig. 2.
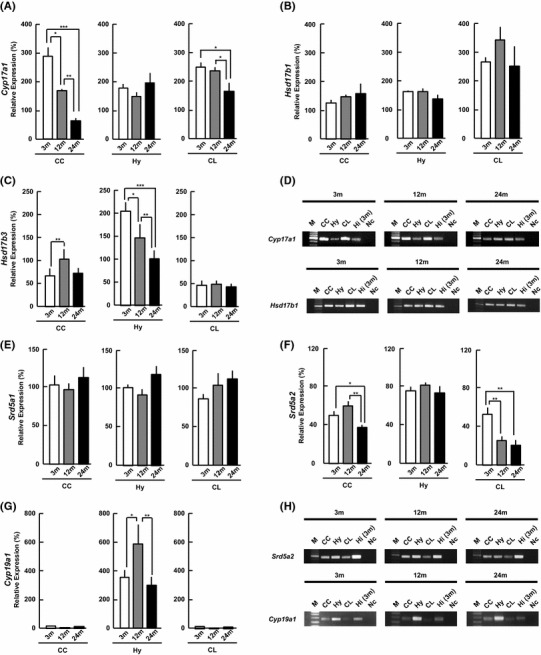
mRNA expression of the steroidogenic enzymes [a Cyp17a1, b Hsd17b1, c Hsd17b3, e Srd5a1, f Srd5a2, g Cyp19a1] at 3, 12, and 24 m. The vertical axis indicates the expression level for each enzyme calculated from the intensity of ethidium bromide bands. The expression level of each mRNA was normalized with Gapdh and the corresponding mRNA expression in 3 m Hi, as described in Methods. Each value is mean ± SEM. Statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001. Data are taken from duplicate determinations for each brain subregion of 4 rats. d, h Typical PCR images for (d) Cyp17a1 and Hsd17b1, and (h) Srd5a2 and Cyp19a1. In each panel, from left to right, 100-bp DNA ladder (M), CC, Hy, CL, 3 m Hi (positive control), the sample without template cDNA (negative control, Nc)
17β-hydroxysteroid dehydrogenase (17β-HSD) type 1 [Hsd17b1] and type 3 [Hsd17b3]
17β-HSDs (1 and 3) convert DHEA to androstenediol (ADiol), androstenedione (ADione) to T, or estrone E1 to E2. The Hsd17b1 levels did not significantly change with age in the three subregions (Fig. 2b).
On the other hand, the expression level of Hsd17b3 in the Hy monotonously decreased with age from 202 % (3 m) to 100 % (24 m) (Fig. 2c). The Hsd17b3 level in the CC showed up-and-down change with age. In the CL, the Hsd17b3 level did not show age-related change.
5α-reductase 1 [Srd5a1] and 5α-reductase 2 [Srd5a2]
5α-reductases (1 and 2) convert T to dihydrotestosterone (DHT). The Srd5a1 mRNA expression levels did not significantly change with aging in all three subregions (Fig. 2e).
On the other hand, the Srd5a2 expression level decreased with age, from 50 % (3 m) to 37 % (24 m). The Srd5a2 level in the CL showed age-related monotonous decrease from 3 to 24 m as follows: 52 % (3 m), 26 % (12 m) and 21 % (24 m) (Fig. 2f). However, the Srd5a2 level in the Hy did not show a significant change with age.
Cytochrome P450aromatase (P450arom) [Cyp19a1]
P450arom converts T to E2. Interestingl, in the CC and CL, the expression level of Cyp19a1 was nearly negligible (below 5 %), over 3, 12 and 24 m, suggesting almost no estradiol synthesis (Fig. 2g). On the other hand, the Hy had a high Cyp19a1 expression level and showed up-and-down change with aging as follows: 340 % (3 m), 563 % (12 m), and 288 % (24 m).
Comparison of enzymes between three subregions of 24 m brain
The expression level of Cyp17a1 in the CC was lower than that in the Hy and CL (Fig. 2a). The expression level of Hsd17b1 in the CL was approximately 2-fold that in the CC and Hy (Fig. 2b). Srd5a1 was nearly the same in three brain subregions (Fig. 2e). The relative abundance of Hsd17b3 (Fig. 2c) and Srd5a2 (Fig. 2f) mRNAs were in the following order: Hy > CC ≥ CL. The Cyp19a1 levels in the CC and CL were very low (Fig. 2g).
Receptors for androgen and estrogen
Androgen receptor (AR) [Ar]
Interestingly, in the Hy, the Ar level increased monotonously with age, from 120 % (3 m), to 145 % (12 m) and 151 % (24 m) (Fig. 3a). The level of Ar in the CC decreased from 3 to 24 m. The Ar level in the CL did not change with age.
Fig. 3.
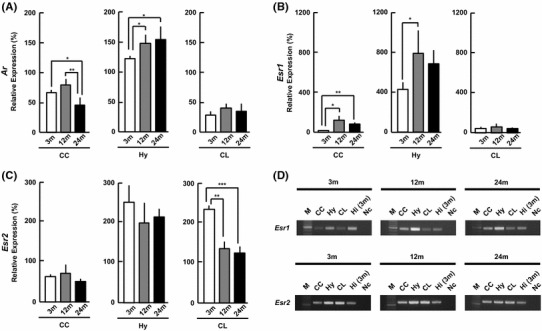
mRNA expression of sex-steroid receptors [a Ar, b Esr1, c Esr2] at 3, 12, and 24 m. The vertical axis indicates the expression level for each receptor calculated from the intensity of ethidium bromide bands. Each value is mean±SEM. Statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001. Data are taken from duplicate determinations for each brain subregion of 4 rats. d Typical PCR images for Esr1 and Esr2. In each panel, from left to right, 100-bp DNA ladder (M), CC, Hy, CL, 3 m Hi (positive control), no template DNA (negative control, Nc). The normalization of each mRNA was performed in the same manner as Fig. 2
Estrogen receptor ERα [Esr1] and ERβ [Esr2]
The Esr1 expression level in the CC and Hy increased from 3 to 12 m and 24 m. In the CL, Esr1 did not change with age (Fig. 3b).
The Esr2 expression levels in the CC and Hy did not change with age, but Esr2 in the CL monotonously decreased with age (Fig. 3c).
Comparison of sex-steroid receptors between three subregions of 24 m brain
The mRNA levels of Ar (Fig. 3a), Esr1 (Fig. 3b) and Esr2 (Fig. 3c) were higher in the Hy than in the CC and CL. The Ar level in the Hy was approximately 4-fold that in the CC and CL. The Esr1 level in the Hy was approximately 10- to 15-fold that in the CC and CL.
Cholesterol transport proteins
Steroidogenic acute regulatory protein (StAR) and translocator protein (TSPO) transport cholesterol to the mitochondrial inner membrane from the cytoplasm.
StAR [Star]
The Star expression level did not change in the CC (Fig. 4a). The Star levels showed down-and-up change with age in the Hy and CL.
Fig. 4.
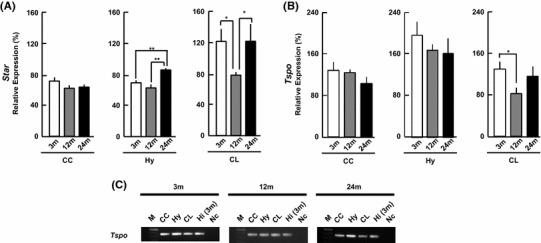
mRNA expression of Star and Tspo in 3, 12, and 24 m. a, b The vertical axis indicates the expression level for each enzyme calculated from the intensity of ethidium bromide bands. Each value is mean±SEM. Statistical significance, *p < 0.05, **p < 0.01. Data are taken from duplicate determinations for each brain subregion of 4 rats. c Typical PCR images for Tspo. In each panel, from left to right, 100-bp DNA ladder (M), CC, Hy, CL, Hi, no template DNA (negative control, Nc). The normalization of each mRNA was performed in the same manner as Fig. 2
TSPO [Tspo]
The Tspo level did not show age-related change in the CC and Hy (Fig. 4b). On the other hand, the Tspo level showed down-and-up change in the CL.
Comparison of transport proteins between three subregions of 24 m brain
The expression level of Star was CL > Hy > CC (Fig. 4a). The Tspo expression level in the Hy was 2- to 3-fold higher than that in the CC and CL (Fig. 4b).
Brain-derived neurotrophic factor [Bdnf]
Rat Bdnf mRNAs contain at least nine splice variants. To detect all splice variants, we designed the Bdnf primers in the common region. The Bdnf expression level decreased from 3 to 24 m in the CC, but increased in the Hy and did not significantly change in the CL (Fig. 5). The relative abundance of Bdnf mRNAs in 24 m brain subregions was in the following order: Hy ~ CL > CC (Fig. 5).
Fig. 5.
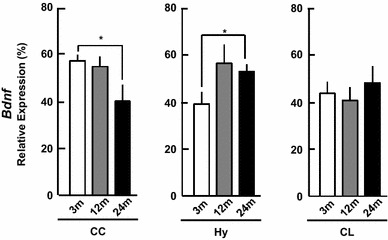
mRNA expression of Bdnf in 3, 12 and 24 m. The vertical axis indicates the expression level calculated from the intensity of ethidium bromide bands. Each value is mean ± SEM. Statistical significance, *p < 0.05. Data are taken from duplicate determinations for each brain subregion of 4 rats. The normalization of each mRNA was performed in the same manner as Fig. 2
Comparison of all different enzymes and receptors in 24, 12, 3 m brains
We compared relative expression levels of all enzymes and receptors, examined in the current study (Fig. 6). Since Cyp19a1 expression was the lowest among all the enzymes and receptors, we normalized by setting Cyp19a1 in 3 m Hi to be 100 %. Hippocampal Cyp19a1 was chosen as the standard, since this kind of comparison was already demonstrated for 3 m Hi elsewhere [15].
Fig. 6.
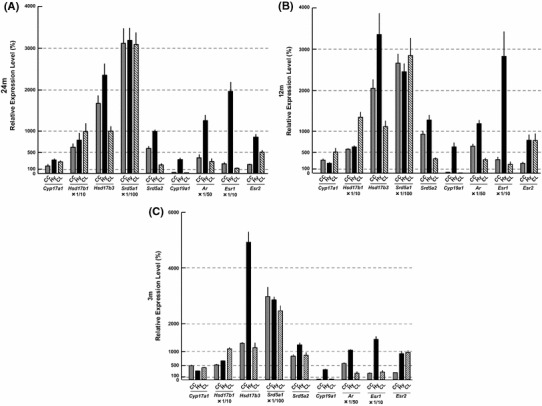
Comparison of expression levels of mRNAs between different enzymes and receptors in (a) 24 m, (b) 12 m and (c) 3 m brains. The comparison was performed using the method as described in Materials and methods. The normalization of each mRNA was performed by setting Cyp19a1 of 3 m Hi as 100 %. Data are also normalized by Gapdh. The vertical scale is different for each enzyme/receptor. For comparison, the vertical scale should amplify by 100-fold for Srd5a1; by 50-fold for Ar; by 10-fold for Hsd17b1 and Esr1; by 1-fold for Cyp17a1, Hsd17b3, Srd5a2, Cyp19a1 and Esr2. The data at 3 m was modified from Kimoto et al. [15]
Several interesting characteristics can be seen for 24 m in Fig. 6. Hsd17b1 was higher (by 3- to 10-fold) than Hsd17b3. Srd5a1 was much higher (by 300- to 1,500-fold) than Srd5a2. Esr1 was higher than Esr2, by ~10-fold in the CC, by ~20-fold in the Hy and by ~2-fold in the CL. In the CC and CL, enzymes for T synthesis were expressed at relatively high levels, but Cyp19a1 for E2 synthesis was extremely low, suggesting almost no estradiol synthesis in the CC and CL. As a comparison, relative expression levels of all the enzymes and receptors in 12 and 3 m are also shown.
Discussion
Only a few investigations have been accumulated about age-related changes in sex-steroidogenic enzymes and sex-steroid receptors in the CC, Hy and CL, although many earlier investigations have been performed for age-induced changes in glutamate receptors and postsynaptic density protein 95 (PSD-95) in the CC, Hy, CL and the hippocampus [5, 24]. In addition, information for normal aging has not been sufficiently accumulated, compared with investigations for Alzheimer’s disease.
In the current study, we demonstrated age-related changes of mRNA expressional levels of sex-steroidogenic enzymes/receptors in important brain subregions (CC, Hy, and CL). The age-induced changes of mRNAs are complex, but can be categorized into four patterns: (1) monotonous decrease, (2) monotonous increase, (3) no change, and (4) up and down change.
Androgen synthesis systems
In many cases, the expression levels of mRNA decreased with age for androgen synthesis enzyme systems, including Cyp17a1 and Srd5a2 in the CC and CL. On the other hand, no decrease with age was observed for Hsd17b(1,3) and Srd5a1 in the CC and CL. The expression level of Srd5a1 was the highest among all steroidogenic enzymes, and much higher than Srd5a2. The catalytic activity of Srd5a2 is, however, much higher (~100-fold) than Srd5a1, as judged from its Km value for substrates T or PROG [20]. Taken together, these results suggest that androgen synthesis activity in aged CC and CL may moderately decrease.
In the CC, Cyp17a1 decreased to approximately 35 % and Srd5a2 decreased to approximately 70 % at 24 m, therefore, the capacity of DHT synthesis might decrease to roughly 25 % (0.35 × 0.7 = 0.25) with age. In the CL, Cyp17a1 decreased to approximately 65 % and Srd5a2 decreased to approximately 40 % at 24 m, therefore, the capacity of DHT synthesis might decrease to roughly 25 % (0.65 × 0.4 = 0.26) with age. In the Hy, only Hsd17b3 level decreased to approximately 50 % at 24 m, but Cyp17a1, Hsd17b1 and Srd5a(1, 2) did not show age-related change. Therefore, the capacity of DHT synthesis might decrease to roughly 50 % with age.
Only a few studies have been published on steroidogenic enzymes in the brains of aged animals [16, 29]. In the prefrontal cortex, Hy and Hi, mRNAs of 3BHSD1/2, 17BHSD(1,3,5) are expressed in middle-aged macaque monkeys (approximately 10 years old) [29]. The level of 17BHSD(1,3) was, however, extremely low. When the CL and Hi of Alzheimer’s disease patients and normal aging humans were compared, no significant differences were observed for StAR, CYP11A1, HSD3B2, HSD17B(1,3,5) and SRD5A2 [16].
Only a few studies have been published for StAR expression [27]. In 24 m rat Hi, StAR protein immunoreactivity was higher than that in 2 m Hi. These results may not be different the current observation that Star mRNA level did not decrease with age in the CC, Hy and CL (Fig. 4a).
Estrogen synthesis enzyme P450arom
The capacity of E2 synthesis in the Hy may not considerably decrease with age, because Cyp19a1 did not decrease, and only Hsd17b3 decreased with age in the Hy. Interestingly, in the CC and CL, almost no expression of Cyp19a1 was observed over 3–24 m, implying that the activity of estrogen synthesis in the CC and CL would be very weak. The CC and CL of adult and aged rats might need E2 supply from other regions, including the Hy or Hi, via diffusion/penetration of E2, since the Hi and Hy could have relatively high activity of E2 synthesis [8]. Penetration of low level E2 (~0.02 nM) from blood plasma may not be sufficient in the male brain. On the other hand, E2 may be sufficient in the CL in the neonatal stage, since Cyp19a1 is significantly expressed in the CL at the neonatal stage [26].
Androgen and estrogen receptors
Interestingly, the expression level of Ar in the Hy monotonously increased with age (Fig. 3). This may be consistent with the previous study in which the number and density of AR-positive cells in the Hy increased during aging [31]. On the other hand, the Ar level in the CC decreased and that in the CL did not change (Fig. 3). These results may be somewhat different from earlier works that show no change of Ar mRNA level in rat CC and Hy [14].
Age-induced moderate increase in Esr1 (ERα mRNA) in the Hy was observed (Fig. 3). Age-related change of ERα mRNA has been examined in subregions of the Hy [30]. Esr1 levels do not change in the medial preoptic nucleus, arcuate nucleus, and ventromedial nucleus, but Esr1 decreases in the periventricular preoptic nucleus [30].
The expression level of Esr2 (ERβ mRNA) did not change in the CC and Hy with age, but decreased in the CL (Fig. 3). An earlier study also implies no change of Esr2 in the CC and Hy, and decrease in the CL with age [32]. Age-related change of Esr2 has been examined in subregions of the Hy [30]. Esr2 does not change with age in the paraventricular nucleus, but Esr2 decreases in the periventricular preoptic nucleus, medial preoptic nucleus, and paraventricular nucleus.
Although the expression level of Esr1 was low in the CC and CL, compared with the Hy, moderate expression of Esr2 in these regions may support sufficient function of (ERα+ERβ) in the aged state (Fig. 6).
Enzymes and receptors in the Hy often show no decline or increase with age
With aging of rat Hy, mRNAs of P450arom, 5α-reductase, and ERβ do not decrease [32], and AR and ERα increase, by going from 3 to 24 m. Since the sealing of the blood–brain barrier in the Hy is much looser than that in other brain regions, including the CC and CL, the decline in plasma sex steroids may induce resistance against the tendency of age-induced decrease in receptors and steroidogenic enzymes in the Hy. In the Hy, the number of ERα-immunoreactive cells and AR-immunoreactive cells increase with age [31]. These age-resistant phenomena might occur through the hypothalamus–pituitary–gonadal axis during the aging processes, due to strong interactions between plasma sex steroids and the Hy.
Neurotrophic factors
Neurotrophic factors as well as sex steroids play important roles in neuroprotection and maintenance of neural activity. In the Hy and CL, Bdnf did not decrease (Fig. 5), therefore age-dependent decrease in sex-steroidogenesis may play an important role in the impairment or deficiency of neural functions with age. The CC may be most sensitive to aging, since both sex-steroidogenic enzymes and Bdnf decreased with age (current study).
There have been many earlier reports concerning BDNF protein and mRNA. In rat CC, BDNF protein shows a decrease [12] or no change [23] with age. In the Hy and CL, BDNF protein and mRNA levels do not change with age, which is consistent with the current observation (Fig. 5) [3, 12, 23, 28].
Comparison between semi-quantitative PCR and real-time PCR
We can obtain essentially the same information from the current semi-quantitative PCR method [7, 9, 15] and real-time PCR [19]. In order to perform normalization, both methods need to choose a good standard housekeeping gene, which must not change with age in addition to region-independent expression. The real-time PCR also cannot avoid these processes. We observed that Gapdh satisfied these criteria. Since the expression level of mRNAs for steroidogenic enzymes is extremely low [7, 9, 15], the primer design is most important. The primer design (with specificity and selectivity) is dependent on the free energy calculation. Our semi-quantitative PCR methods have been better-optimized (from much experience) than real-time PCR (with less experience). Since we can choose the PCR cycle number within the exponential amplification phase, we can achieve essentially the same results with the semi-quantitative PCR method and the real-time PCR method.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Figure S1 Hippocampal steroid metabolism pathways and enzymes/receptors examined in the current study. Names of steroids are denoted by boxed character
Footnotes
Arisa Munetomo and Yasushi Hojo contributed equally to the present work.
References
- 1.Breslauer KJ, Frank R, Blocker H, Marky LA. Predicting DNA duplex stability from the base sequence. Proc Natl Acad Sci USA. 1986;83:3746–3750. doi: 10.1073/pnas.83.11.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Hardy MP, Huhtaniemi I, Zirkin BR. Age-related decreased Leydig cell testosterone production in the brown Norway rat. J Androl. 1994;15:551–557. [PubMed] [Google Scholar]
- 3.Ewa BS, Beata L, Ilona K, Dariusz S, Janusz M. Brain derived neurotrophic factor (BDNF) containing neurons in the hypothalamic paraventricular and supraoptic nuclei of juvenile and middle-aged rats after chronic stress. Int J Dev Neurosci Off J Int Soc Develop Neurosci. 2012;30:139–146. doi: 10.1016/j.ijdevneu.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Foy MR, Xu J, Xie X, Brinton RD, Thompson RF, Berger TW. 17beta-estradiol enhances NMDA receptor-mediated EPSPs and long-term potentiation. J Neurophysiol. 1999;81:925–929. doi: 10.1152/jn.1999.81.2.925. [DOI] [PubMed] [Google Scholar]
- 5.Gore AC, Oung T, Woller MJ. Age-related changes in hypothalamic gonadotropin-releasing hormone and N-methyl-D-aspartate receptor gene expression, and their regulation by oestrogen, in the female rat. J Neuroendocrinol. 2002;14:300–309. doi: 10.1046/j.1365-2826.2002.00777.x. [DOI] [PubMed] [Google Scholar]
- 6.Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci Off J Soc Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hojo Y, Hattori T, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WGM, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017 alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hojo Y, Higo S, Ishii H, Ooishi Y, Mukai H, Murakami G, Kominami T, Kimoto T, Honma S, Poirier D, Kawato S. Comparison between hippocampus-synthesized and circulation-derived sex steroids in the hippocampus. Endocrinology. 2009;150:5106–5112. doi: 10.1210/en.2009-0305. [DOI] [PubMed] [Google Scholar]
- 9.Hojo Y, Murakami G, Mukai H, Higo S, Hatanaka Y, Ogiue-Ikeda M, Ishii H, Kimoto T, Kawato S. Estrogen synthesis in the brain—role in synaptic plasticity and memory. Mol Cell Endocrinol. 2008;290:31–43. doi: 10.1016/j.mce.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Janmaat S, Akwa Y, Doulazmi M, Bakouche J, Gautheron V, Liere P, Eychenne B, Pianos A, Luiten P, Groothuis T, Baulieu EE, Mariani J, Sherrard RM, Frederic F. Age-related Purkinje cell death is steroid dependent: rORalpha haplo-insufficiency impairs plasma and cerebellar steroids and Purkinje cell survival. Age (Dordr) 2011;33:565–578. doi: 10.1007/s11357-010-9203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato A, Hojo Y, Higo S, Komatsuzaki Y, Murakami G, Yoshino H, Uebayashi M, Kawato S. Female hippocampal estrogens have a significant correlation with cyclic fluctuation of hippocampal spines. Front Neural Circuits. 2013;7:149. doi: 10.3389/fncir.2013.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh-Semba R, Semba R, Takeuchi IK, Kato K. Age-related changes in levels of brain-derived neurotrophic factor in selected brain regions of rats, normal mice and senescence-accelerated mice: a comparison to those of nerve growth factor and neurotrophin-3. Neurosci Res. 1998;31:227–234. doi: 10.1016/S0168-0102(98)00040-6. [DOI] [PubMed] [Google Scholar]
- 13.Kawato S, Hojo Y, Kimoto T. Histological and metabolism analysis of P450 expression in the brain. Methods Enzymol. 2002;357:241–249. doi: 10.1016/S0076-6879(02)57682-5. [DOI] [PubMed] [Google Scholar]
- 14.Kerr JE, Allore RJ, Beck SG, Handa RJ. Distribution and hormonal regulation of androgen receptor (AR) and AR messenger ribonucleic acid in the rat hippocampus. Endocrinology. 1995;136:3213–3221. doi: 10.1210/endo.136.8.7628354. [DOI] [PubMed] [Google Scholar]
- 15.Kimoto T, Ishii H, Higo S, Hojo Y, Kawato S. Semicomprehensive analysis of the postnatal age-related changes in the mRNA expression of sex steroidogenic enzymes and sex steroid receptors in the male rat hippocampus. Endocrinology. 2010;151:5795–5806. doi: 10.1210/en.2010-0581. [DOI] [PubMed] [Google Scholar]
- 16.MacKenzie SM, Dewar D, Stewart W, Fraser R, Connell JM, Davies E. The transcription of steroidogenic genes in the human cerebellum and hippocampus: a comparative survey of normal and Alzheimerʼs tissue. J endocrinol. 2008;196:123–130. doi: 10.1677/JOE-07-0427. [DOI] [PubMed] [Google Scholar]
- 17.Midzak AS, Chen H, Papadopoulos V, Zirkin BR. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol Cell Endocrinol. 2009;299:23–31. doi: 10.1016/j.mce.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 18.Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WGM, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- 19.Munetsuna E, Hojo Y, Hattori M, Ishii H, Kawato S, Ishida A, Kominami SAJ, Yamazaki T. Retinoic acid stimulates 17 beta-estradiol and testosterone synthesis in rat hippocampal slice cultures. Endocrinology. 2009;150:4260–4269. doi: 10.1210/en.2008-1644. [DOI] [PubMed] [Google Scholar]
- 20.Normington K, Russell DW. Tissue distribution and kinetic characteristics of rat steroid 5 alpha-reductase isozymes. Evidence for distinct physiological functions. J Biol Chem. 1992;267:19548–19554. [PubMed] [Google Scholar]
- 21.Ooishi Y, Mukai H, Hojo Y, Murakami G, Hasegawa Y, Shindo T, Morrison JH, Kimoto T, Kawato S. Estradiol rapidly rescues synaptic transmission from corticosterone-induced suppression via synaptic/extranuclear steroid receptors in the hippocampus. Cereb Cortex. 2012;22:926–936. doi: 10.1093/cercor/bhr164. [DOI] [PubMed] [Google Scholar]
- 22.Ota H, Akishita M, Akiyoshi T, Kahyo T, Setou M, Ogawa S, Iijima K, Eto M, Ouchi Y. Testosterone deficiency accelerates neuronal and vascular aging of SAMP8 mice: protective role of eNOS and SIRT1. PLoS One. 2012;7:e29598. doi: 10.1371/journal.pone.0029598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perovic M, Tesic V, Mladenovic Djordjevic A, Smiljanic K, Loncarevic-Vasiljkovic N, Ruzdijic S, Kanazir S. BDNF transcripts, proBDNF and proNGF, in the cortex and hippocampus throughout the life span of the rat. Age (Dordr) 2013;35:2057–2070. doi: 10.1007/s11357-012-9495-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pires RS, Real CC, Folador TS, Tellini NR, Torrao AS, Britto LR. Differential response of AMPA and NMDA glutamate receptors of Purkinje cells to aging of the chicken cerebellum. Neurosci Lett. 2010;478:146–149. doi: 10.1016/j.neulet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci Off J Soc Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sakamoto H, Mezaki Y, Shikimi H, Ukena K, Tsutsui K. Dendritic growth and spine formation in response to estrogen in the developing Purkinje cell. Endocrinology. 2003;144:4466–4477. doi: 10.1210/en.2003-0307. [DOI] [PubMed] [Google Scholar]
- 27.Sierra A, Lavaque E, Perez-Martin M, Azcoitia I, Hales DB, Garcia-Segura LM. Steroidogenic acute regulatory protein in the rat brain: cellular distribution, developmental regulation and overexpression after injury. Eur J Neurosci. 2003;18:1458–1467. doi: 10.1046/j.1460-9568.2003.02872.x. [DOI] [PubMed] [Google Scholar]
- 28.Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Sorwell KG, Kohama SG, Urbanski HF. Perimenopausal regulation of steroidogenesis in the nonhuman primate. Neurobiol Aging. 2012;33(1487):e1413–e1481. doi: 10.1016/j.neurobiolaging.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/S0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- 31.Wu D, Lin G, Gore AC. Age-related changes in hypothalamic androgen receptor and estrogen receptor alpha in male rats. J Comp Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi-Shima N, Yuri K. Age-related changes in the expression of ER-beta mRNA in the female rat brain. Brain Res. 2007;1155:34–41. doi: 10.1016/j.brainres.2007.04.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Hippocampal steroid metabolism pathways and enzymes/receptors examined in the current study. Names of steroids are denoted by boxed character


