Abstract
Men have shorter rate-corrected QT intervals (QTc) than women, especially at the period of adolescence or later. The aim of this study was to elucidate the long-term effects of testosterone on cardiac excitability parameters including electrocardiogram (ECG) and potassium channel current. Testosterone shortened QT intervals in ECG in castrated male rats, not immediately after, but on day 2 or later. Expression of Kv7.1 (KCNQ1) mRNA was significantly upregulated by testosterone in cardiomyocytes of male and female rats. Short-term application of testosterone was without effect on delayed rectifier potassium channel current (IKs), whereas IKs was significantly increased in cardiomyocytes treated with dihydrotestosterone for 24 h, which was mimicked by isoproterenol (24 h). Gene-selective inhibitors of a transcription factor SP1, mithramycin, abolished the effects of testosterone on Kv7.1. Testosterone increases Kv7.1-IKs possibly through a pathway related to a transcription factor SP1, suggesting a genomic effect of testosterone as an active factor for cardiac excitability.
Electronic supplementary material
The online version of this article (10.1007/s12576-017-0590-4) contains supplementary material, which is available to authorized users.
Keywords: Testosterone, Electrocardiogram, QT interval, Potassium channel, Kv7.1
Introduction
Clinical studies have identified striking differences between men and women in the incidence of many different cardiovascular diseases including arrhythmias. Torsades de pointes (TdP) arrhythmia is a potentially fatal form of polymorphic ventricular tachycardia that typically occurs in a setting of prolonged QT interval duration measured from electrocardiogram (ECG) recordings [1–4]. Female gender is an independent risk factor for developing drug-related TdP [5]. Evidence from clinical studies suggest that the gender-dependent differences in rate-corrected QT intervals (QTc) may be primarily due to the impact of male sex steroid hormones [6–8] because QTc intervals of prepubertal boys and girls are similar to those found in adult women, whereas postpubertal males generally have shorter QTc intervals. A clinical study demonstrated that the correlated JT interval (JTc) in the castrated male group were significantly longer than those in the normal male group and were similar to those found in the normal female group [9]; JT interval is defined as QT—QRS duration. Also, exogenous testosterone has been shown to affect JTc intervals. Testosterone replacement therapy in the castrated male group produced shortening of JTc similar to that observed in normal males. These previous findings suggest that male sex steroid hormones can influence cardiac ventricular repolarization, and may be responsible for the shorter QT intervals observed in adult men compared to those found in adult women.
The QT interval in ECG includes the duration of ventricular depolarization and repolarization, and corresponds to the action potential duration in ventricular cardiomyocytes [5, 8]. Repolarization in mammalian ventricular muscle is controlled by a fine balance of several inward and outward transmembrane ionic currents and electrogenic pump mechanisms. Among them, voltage-gated potassium (K+) (Kv) channel currents largely influence the amplitudes and durations of cardiac action potentials [6] and, in most cells, two classes of Kv currents have been distinguished: transient outward K+ currents (Ito) and delayed rectifier K+ currents (IKr, IKs); the rapidly (IKr) and slowly (IKs) activating delayed rectifier potassium channels. In addition to Kv currents, the inwardly rectifying K+ (Kir) current (IK1) plays a pivotal role in myocardial action potential repolarization. In this context, we hypothesized that testosterone could produce these effects on ECG via modifying the K+ currents. In the present study, we investigate the effects of testosterone on ECG parameters and expression of K+ channels. In addition, current densities of IKs are evaluated in ventricular cardiomyocytes isolated from neonatal rats by short-term (5 min) and long-term (24 h) application of testosterone.
Methods
Animals
Adult female and male Wistar rats (250–300 g) were housed for a week at the study site utilizing a 12-h light/dark cycle with a testosterone-free diet and water provided ad libitum. All rats were purchased from Kyudo Co., Ltd. (Fukuoka, Japan). Animal care and use were approved by an institutional committee. The animals were maintained according to National Institute of Health and international guidelines (NIH publication No. 85-23, revised 1996).
Surgeries
Briefly, animals were anesthetized with pentobarbital sodium (40–50 mg/kg, i.p.) before implanting a telemetric ECG radio transmitter, 25 × 15 × 9 mm in size and 7 g in weight (TA11CTA-F40, Data Sciences International, St. Paul, MN), into the subcutaneous pouch on the ventral side of the animal. The paired wire electrodes for the pericardial bipolar leads (apex-base leads) were placed under the skin of the dorsal and ventral thorax. ECG data were set to perform at a sample rate of 5 kHz for 5 min every 30 min. In castration surgery, a midline ventral skin incision is made in the scrotum. The testis, vas deferens, fatty tissue and attached epidermis were gently pulled out from the incision. The blood vessels supplying the testis were ligated and the testis was removed. Then the procedure was repeated on the opposite side.
Preparation of neonatal rat cardiomyocytes and cell culture
Neonatal cardiomyocytes were prepared from 1- to 3-day-old Wistar rats using established techniques as described previously [10, 11], with some modification. Briefly, hearts were removed from neonatal rats and the ventricles were minced into 1-mm3 pieces in ADS buffer (mM) (116 NaCl, 20 HEPES, 12.5 NaH2PO4, 5.4 glucose, 5.4 KCl, 0.83 MgSO4; pH 7.35 by NaOH) and digested 2–3 times with 0.05% collagenase type II (Worthington Biochemical Corporation, Lakewood, NJ, USA) in ADS buffer at 37 °C for 10 min. The dispersed cells from each digestion were combined and purified by centrifugation (1500 rpm, 3 min). The cells were cultured in Dulbecco’s modified Eagle’s medium D6046 (DMEM, Sigma-Aldrich) containing 10% fetal bovine serum (FBS). The cells were pre-plated at 37 °C in a 95% O2 incubator for 1 h to remove cardiac fibroblasts as much as possible. The cardiomyocytes were maintained at 37 °C under 5% CO2 in DMEM for 24 h, with or without dihydrotestosterone (DHT), supplemented with 5% fetal bovine serum. In some experiments, isoproterenol (ISO), a PKA inhibitor H89 or an Sp1 inhibitor mithramycin (see below) were included in the culture medium.
Electrophysiology
Whole-cell voltage-clamp experiments were performed as described previously [10], with some modification. Macroscopic IKs was recorded in the conventional whole-cell patch-clamp technique using an EPC-9 amplifier (HEKA Elektronik, Lambrecht, Germany). IKs was recorded from the holding potential (VHP) of − 60 mV followed by various test potentials. IKs was assessed at the terminal phase of test pulses to minimize the interference of other K+ channel currents. The external solution for measuring whole-cell IKs contained (in mM) 2 MgCl2, 20 HEPES, 11.1 glucose, 140 N-methyl-d-glucamine (pH was adjusted to 7.35 with HCl). The patch pipette solution was filled with a solution of the following composition (in mM) 140 KCl, 1 MgCl2, 5 MgATP, 10 HEPES, 3 CaCl2 and 10 EGTA (pH was adjusted to 7.2 with KOH).
Quantitative RT-PCR
The total RNA was extracted from the cardiomyocytes using TRIzol reagent (Life Technologies Inc., Gaithersburg, MD). The cDNA was synthesized from 1 µg of total RNA using a Transcriptor First Strand cDNA Synthesis Kit (Roche Molecular System Inc., Almeda, CA). The real-time PCR was performed using 2 µl cDNA template from each RT reaction in a 20-µl PCR mix on a Light Cycler 2.0 (Roche) and Light Cycler 96 (Roche), using SYBR green (Roche) as a detection reagent. Primer pairs are indicated in Supplemental Table 1; Kv7.1, minK, Kv11.1, MiRP1, Kv4.2, Kv4.3, KChIP2, Kir2.1, CREB and SP1 were with primers in Table 1A and LightCycler® Capillaries (Roche) in most studies except data in Fig. 5, whereas Kv7.1, CREB and SP1 were with primers in Supplemental Table 1B and LightCycler® 480 Multiwell Plate 96 (Roche) in the study in Fig. 5. The size of the PCR products was confirmed using a 2% agarose ethidium bromide-stained gel under UV irradiation. Samples were normalized against the housekeeping gene, β-actin, and results were reported for each sample relative to the control. To knock down gene expression, we used siRNA and infected rat neonatal cardiomyocytes according to the manufacturer’s instructions. mRNA abundance was assayed using qRT-PCR.
Fig. 5.
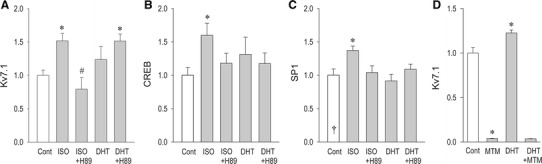
Modulation of the expression of Kv7.1, CREB and Sp1 by dihydrotestosterone (DHT) or isoproterenol (ISO). Cardiomyocytes were incubated with DHT (3 nM) or ISO (0.1 µM) for 24 h in the presence or absence of a PKA inhibitor H89 (1 µM) to assess the expression of Kv7.1 (a), CREB (b), and Sp1 (c). Modulation of Kv7.1 expression by DHT (3 nM) was assessed with or without an Sp1 specific inhibitor mithramycin (50 nM) (d). Data are expressed as the mean ± SEM. *p < 0.05 vs. control, #p < 0.05 vs ISO
Western blot
Isolated rat hearts were homogenized on ice with lysis buffer [Tris–HCl (pH 7.4) 10 mM, NaCl 150 mM, TritonX-100 1.0%, sodium deoxycholate 0.5%, SDS 0.1%, 2-mercaptoethanol 0.0007%]. The homogenate was centrifuged at 13,000 rpm at 4 °C for 4 min. The supernatant was collected and protein was diluted in Laemmli buffer with subsequent incubation at 97 °C for 3 min. For each sample, 20 μg of protein extracts were subjected to 10% SDS-PAGE. After electrophoresis, the proteins were transferred to a nitrocellulose membrane (BIO-RAD). After blocking with 5% milk in Tris-buffered NaCl with 0.1% Tween 20, the membrane was incubated with rabbit anti-KvLQT1 primary antibody (1:2000, Alomone), anti-Kv4.2 primary antibody (1:200, Alomone), anti-Kir2.1 primary antibody (1:200, Alomone), and anti-GAPDH primary antibody (1:1000, Santa Cruz Biotechnology, Texas, USA) in 5% skim milk at 4 °C overnight. This was followed by 1-h incubation with sheep anti-rabbit IgG secondary antibody conjugated with HRP (1:2000, American Qualex Antibodies, California, USA) at 4 °C. The signal was visualized using an ECL Plus detection system (GE Healthcare, Buckinghamshire, UK). The intensity of the background and each band were determined using GelDoc XR Plus with Image Lab Software (BIO-RAD).
Drugs
Testosterone was diluted in 2-hydroxypropyl-β-cyclodextrin as previously described [12], and intermittently injected into the peritoneal cavity. For continuous dosing, testosterone was dissolved in ethanol at a concentration of 8.4 mg/ml and 2 ml was loaded into an osmotic pump, according to specifications (ALZET). Testosterone propionate supplementation was accomplished by intraperitoneal injection at a dose of 2.5 mg/kg as previously described [13]. Ethyloleate was used as a vehicle for testosterone propionate treatment. For the cultured cells, dihydrotestosterone (DHT) was prepared as a 100-mM stock solution in ethanol, dissolved in ultrapure water, and dissolved in bath solution to achieve a final concentration described in the following. The final concentration of ethanol was under 0.01%. A specific Sp1 inhibitor, mithramycin, 1-mM stock solution, was prepared with methanol and diluted with MilliQ water to make a 10-μM stock solution, diluted to the desired concentration using D6046 medium just before use. To knock down gene expression, we used siRNA (CREB-1 siren (r): sc-72030, Control siRNA-B: sc-44230), OPTI-MEM® I and Lipofectamine® RNAiMAX to infect rat neonatal cardiomyocytes. All the chemicals were purchased from Wako Pure Chemicals and Industries Ltd., (Tokyo, Japan), Gibco by Life Technologies (Waltham, Massachusetts, USA), SANTA CRUZ BIOTECHNOLOGY (Dallas, Texas, USA) or Sigma Chemical Co. (St. Louis, MO., USA).
Statistical analysis
Data were acquired using computer software (Pulse/PulseFit, V.8.11, HEKA Elektronik), and all curve fittings and figures were made on Sigma Plot (version 10; SPSS, Inc., Chicago, IL, USA). All results are presented as mean ± SEM, with the number of observations indicated by (n). Values were analyzed by Student’s t-test or ANOVA, with post hoc analyses (Tukey–Kramer or Dunnett), where appropriate. Non-normal data were analyzed by Man-Whitney rank-sum test or Steel–Dwass test (BellCurve Ver.2.00, Social Survey Research Information Co., Japan). Differences were considered significant at p < 0.05.
Additional information
See Methods in the online-only Data Supplement for a detailed description of quantitative RT-PCR DNA microarray, and real-time PCR.
Results
Effect of testosterone on ECG
ECG recordings from the castrated rats before and after application of testosterone were performed for up to 14 days. After the subcutaneous injection of testosterone (1000 µg/kg/day), the mean QT intervals were significantly shortened, not immediately after, but day 2 or later after the treatment (Fig. 1b). No significant changes of RR intervals, PR time, or QRS time were recorded up to the observation period of 7 days. The representative ECG recordings show that QT interval was shortened, whereas p wave and QRS complex were unchanged (Fig. 1a, inset).
Fig. 1.

Effects of testosterone supplementation on ECG and ECG parameters in castrated male rats. a Representative time series changes in RR intervals and QT intervals by intraperitoneally administered testosterone (1000 µg/kg/day), and ECG records at the time indicated (a–c). A sample of ECG on day 0 and day 13 are shown in inset. b ECG parameters of RR interval (ms), PR time (ms), QRS time (ms) and QT interval (ms) on day 0 (control), day 1 and day 7. Data are presented as the mean value ± SEM. *p < 0.05 vs day 0
Changes in K+ channel expression by castration and testosterone supplements
In order to clarify the effect of castration and the long-term effect of testosterone on QT interval shortening, we performed real-time RT-PCR analyses to examine ion channel expression focusing on K+ channels and related proteins in cardiomyocytes (Fig. 2). Kv7.1, also known as KCNQ1 (KvLQT1), was significantly downregulated by 39% by castration in ventricular myocytes. Importantly, Kv7.1-mRNA expression was significantly upregulated by testosterone supplementation. However, other K+ channels and their related subunits including Kv4.2 (Ito) and Kir2.1 (IK1) were unchanged by testosterone. Testosterone increased Kv7.1 also in the female hearts (Supplemental Figures 1, 2). Upregulation of Kv7.1-channel expression was confirmed by the measurement of protein levels (Fig. 3). Consistent with mRNA expression in Fig. 2, Kv4.2 and Kir2.1 proteins were unchanged by testosterone treatment for 2 weeks.
Fig. 2.

Reduction of Kv7.1 by castration and its upregulation by testosterone. Changes in K channel and K channel-related genes were assessed by qRT-PCR in ventricular cardiomyocytes from castrated male rats: IKs (Kv7.1, minK), IKr (Kv11.1, MiRP1), Ito (Kv4.2, Kv4.3, KChIP2) and IK1 (Kir2.1). Testosterone was intraperitoneally administered (1000 µg/kg) once a day for 2 weeks from day 7 after castration. Data are presented as the mean value ± SEM (n = 6). *< 0.05 vs castration
Fig. 3.

Changes in K channel expression (protein) by testosterone. Expression of Kv7.1 (a), Kv4.2 (b), and Kir2.1 (c) as assessed by western blot analysis. Testosterone was intraperitoneally administered (1000 µg/kg) once a day for 2 weeks from day 7 after castration. Data were expressed as mean ± SEM (n = 4). *p < 0.05 vs control (castration)
Long-term testosterone treatment increases IKs density in cardiomyocytes
Various pathophysiological conditions of the heart modify expression of ion channels in cardiac and vascular myocytes. We decided to evaluate the short-term and long-term effects of dihydrotestosterone (DHT), an active metabolite of testosterone, on IKs in cardiomyocytes, in vitro. To observe the effect, we used an IKr inhibitor (E-4031) and an Ito inhibitor (4-aminopyridine, 4-AP) in the bath solution to minimize the interference of IKr and Ito on the measurement of IKs. Amplitudes of IKs assessed at the end of test pulses were not altered during the acute application of 3 nM DHT (Fig. 4a, c), indicating no direct acute action of DHT on IKs in cardiomyocytes. Thereafter, the chronic or long-term effect of DHT on IKs was investigated. To examine the chronic effect of DHT on cardiac IKs, cultured neonatal rat ventricular cardiomyocytes were treated with 3 nM DHT for 24 h. A long-term application of DHT significantly increased IKs by 63 ± 5%, assessed at the potential of 60 mV. Importantly, current–voltage relationships for IKs were unchanged by the long-term treatment of DHT, which indicates that DHT does not affect the kinetics of IKs after long-term application.
Fig. 4.

Short-term (5 min) and long-term (24 h) effect of dihydrotestosterone (DHT) on IKs. Representative IKs traces before (control) and 5 min after DHT (3 nM) treatment (a). IKs was activated by depolarizing voltage steps indicated in inset. E-4031 (5 µM) and 4AP (4 mM) were given to the bath solution to minimize the interference of IKr and Ito, respectively. Representative IKs traces from ventricular cardiomyocytes cultured for 24 h without DHT (vehicle) and with 3 nM DHT (b). Group data of current (I)–voltage (V) relationship of IKs for the short-term effect (c) and long-term effect of DHT (d). Data are presented as the mean value ± SEM (n = 6). *p < 0.01, **p < 0.05, p = 0.052 vs vehicle
Mechanism and transcriptional upregulation of Kv7.1
A comparison of the effect of DHT and an adrenergic receptor agonist isoproterenol (ISO) on the expression of Kv7.1 was performed as shown in Fig. 5 (also in Supplemental Figure 5). Both ISO and DHT upregulated Kv7.1 in cardiomyocytes when applied for 24 h. Although an inhibitor of a protein kinase A (H89) significantly reduced the effect of ISO on Kv7.1, upregulation of Kv7.1 by DHT was unaffected by H89 (Fig. 5a). The cAMP response element (CRE) binding protein (CREB) is a nuclear transcription factor known to play important physiological and pathophysiological roles in a wide range of organs including the heart [14]. As expected, ISO highly increased the expression of CREB mRNA, whereas DHT did not modify the expression. In silico prediction of transcription factor-binding sites of Kv7.1 suggests a possible interaction between a transcription factor SP1 and this ion channel (Supplemental Figure 3). As shown in Fig. 6c, however, expression of SP1 mRNA was upregulated by ISO but not by DHT. A gene-selective inhibitor of a transcription factor SP1, mithramycin, abolished the effect of dihydrotestosterone on Kv7.1. Further experiments indicate that silencing CREB through targeting small interfering RNA (siRNA) inhibits Kv7.1 upregulation by ISO but not by dihydrotestosterone (Fig. 6). These results are consistent with our data in Fig. 5, supporting the idea that ISO upregulates Kv7.1 depending on CREB, and that testosterone/DHT works independently of CREB to upregulate Kv7.1 expression.
Fig. 6.

CREB-dependent and independent upregulation of Kv7.1 by DHT and isoproterenol (ISO). Expression of Kv7.1 mRNA in cardiomyocytes was assessed after silencing CREB; CREB siRNA and scrambled siRNA (Nega siRNA) were used. Ventricular cardiomyocytes were cultured with 3 nM DHT (a) or 0.1 µM ISO (b) for 24 h with or without CREB siRNA. Data are expressed as mean ± SEM (n = 5 or 6). *p < 0.05 vs Nega siRNA (DHP, ISO)
Discussion
Testosterone and QT intervals
Testosterone is of importance for the regulation of growth, differentiation, and function in a wide array of target tissues, including those in the cardiovascular system [15–21]. It is well established that men have shorter QT intervals than women based on many clinical and animal studies. However, whether this difference is due to a prolonged QT interval in women or a shortened QT interval in men is still not fully understood, although a role for sex hormones is strongly suggested. There are extensive clinical and animal data describing actions of sex hormones on ECG and action potentials in cardiomyocytes; sex steroids may have genomic and non-DNA binding-dependent actions on cardiomyocytes [8, 22–24]. The electrical events underlying the QT interval correspond with the phases of action potential generation. At the cellular level, upregulation of outward currents (delayed rectifier potassium channel currents and inward-rectifier potassium channel current) and downregulation of l-type calcium channel current may provide a plausible mechanism for shortening of QT intervals. Genomic steroid effects are often considered to result in alteration in cellular activity after several hours or even days [25, 26]. This latency is believed to be due to the multiple steps required from the time of entry of the steroid hormones into the cell until changes in transcriptional activity occur. In this context, upregulation of Kv7.1-IKs by testosterone as a long-term effect in this study is consistent with other reports in the following respects: (1) men’s QT intervals start to shorten during teenage years [7, 27, 28], (2) castration prolongs QT intervals in humans and in male dogs [9, 29, 30], (3) there is a significant negative correlation between the QTc interval and total testosterone concentrations in men with hypogonadism [31], and (4) testosterone replacement therapy decreases QT intervals in men with hypogonadism [31]. This evidence strongly suggests that testosterone could be the main determinant of gender-related differences in QT intervals [32].
Despite the overwhelming evidence of genomic impact of testosterone on the cardiovascular system, it is also well recognized that testosterone can act via mechanisms independent of the ligand-dependent transactivation of nuclear receptors. This is known as “non-genomic” signaling, which typically occurs within a short time frame. Androgen receptor is expressed in many cell types including cardiomyocytes [33, 34], although males have significantly higher levels of androgen receptor mRNA than females, which suggests that effects of testosterone could be more amplified in male hearts than in female hearts. However, these differences might be caused by androgens by themselves, because the difference becomes significant at the age of adolescence and later. Regarding the age-dependent augmentation of androgen receptor, acute and long-term effects of androgens on neonatal heart and adult heart may not be identical. Because our in vitro studies were all performed using neonatal cardiomyocytes, long-term effects of androgens which require classical “canonical” receptors may include potential quantitative disputes when compared with those in adult heart. Regarding non-genomic actions of testosterone, testosterone-induced modification of electrophysiological features of cardiomyocytes must occur in a time span not long enough to allow gene transcription, usually seconds to minutes. From this point of view, androgen effects which require hours to days in revealing the actions are likely to be the result of genomic actions. In in vivo application of testosterone, QT time was prolonged not immediately after, but several days later, after application of testosterone (Fig. 1), which indicates that changes in ECG parameters do not occur in a time span of seconds or minutes. It is also important to point out that action potential duration was significantly longer in female than in male myocytes without any sex hormones in rabbits [35] and in rats [8]. These results are consistent with a finding that cardiomyocytes derived from male rats and female rats are substantially different, independently of the acute presence/absence of sex hormones. Despite this evidence, we are not opposed to the non-genomic actions of androgens on the membrane currents of cardiomyocytes. In our study we could not detect any short-term effect of testosterone on IKs, probably because our electrophysiological methods are not suitable for the evaluation of cellular signal pathways including akt/NO activation which is postulated as a key non-genomic signaling pathway caused by testosterone in the cardiovascular system [33].
Transcriptional regulation of Kv7.1
An obvious question is how testosterone upregulates Kv7.1 as a long-term effect. Despite the importance of Kv7.1 in the repolarization of cardiomyocytes, and in the pathogenesis of the hereditary long QT syndrome (LQT1), very little is known yet about the mechanisms responsible for transcriptional regulation of Kv7.1 expression. Although we have successfully demonstrated that isoproterenol and testosterone upregulate Kv7.1 in adult and neonatal cardiomyocytes, and have postulated a possible signaling pathway for the regulation of Kv7.1 (Fig. 7), we still need to identify region(s) of the Kv7.1 gene that are important for the functional binding of the transcription factor(s), such as by Sp1. In general, regulation of transcription is a complex set of events controlled by DNA sequences positioned in proximity to the genes (promoters) and by elements acting at a distance (enhancers). Promoters and enhancers that activate polymerase II transcribed mRNA genes are formed by a combined piece made of short sequences recognized by sequence-specific regulators. One common feature of the promoter regions of Kv7.1 genes is that they are GC rich; there exist multiple Sp1 consensus binding sites in the proximal promoter regions of the Kv7.1 gene (Supplemental Figure 3). In the KCNQ family, KCNQ2 and KCNQ3, genes coding Kv7.2 and Kv7.3, respectively, are important K+ channels for regulating neuronal activity. Of note, KCNQ1, KCNQ2 and KCNQ3 contain evolutionally conserved promoter regions (Supplemental Figure 3) [36]. We also note that rat and human Sp1 contain a consensus binding sequence for CREB in in silico prediction (Supplemental Figure 4). Importantly, functional bindings of Sp1 to KCNQ2 and KCNQ3 have been identified [36], suggesting that KCNQ1 may also be regulated by Sp1 as postulated by this study. In conclusion, the results of this study have elucidated that testosterone upregulates Kv7.1-IKs through a pathway related to a transcription factor SP1, suggesting a genomic effect of testosterone as an active factor for cardiac excitability, which may responsible, at least in part, for gender-dependent difference in ECG morphology and also a potential arrhythmogenic substrate in males.
Fig. 7.

Schematic illustration for Kv7.1 transcriptional regulation by testosterone and β-adrenoceptor stimulation. DHT dihydrotestosterone, AR androgen receptor, AC adenylate cyclase, β-AR β-adrenoceptor, PKA protein kinase A
Study limitations
Although the present study provides novel evidence that testosterone is a prominent factor for Kv7.1-IKs channel expression causing QT abbreviation, we could not exclude other mechanisms for gender-dependent differences in QT intervals. Also, we are uncertain whether testosterone exerts non-genomic actions on ECG through the experimental protocol in this study. Our data are not in agreement with those reported in guinea pig cardiomyocytes: Zhang et al. showed that prolonged β-adrenergic stimulation decreased the density of IKs, ICa.L and the inward-rectifier K+ channel current (IK1). The reasons why there are different effects of isoproterenol ionic currents are unknown. Since sustained β-adrenergic stimulation increased ICa.L in rabbit cardiomyocytes [37], species differences may partially explain the underlying mechanisms. Because the genetic differences in ion channel molecular structures between human and rat are obvious, as shown in Supplemental Figure 3, further investigation is clearly warranted concerning the role of testosterone on human ECG. Experiments with human induced pluripotent stem cells to substitute for human cardiomyocytes [38] would also be needed in the future, before drawing the final conclusions.
Conclusions
Testosterone shortened QT intervals in rats as a long-term effect, possibly through the genomic effect, and the effect was mediated by the upregulation of Kv7.1-IKs. Isoproterenol increased Kv7.1 expression through a transcription pathway CREB-Sp1, whereas testosterone increased Kv7.1 independently of CREB-mediated transcription.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Abbreviations
- DHT
Dihydrotestosterone
- TdP
Torsades de pointes
- QTc
Corrected QT intervals
- JTc
Correlated JT interval
- Kv
Voltage-gated potassium channel
- Kir
Inwardly rectifying potassium channel
- KCNQ1
Potassium voltage-gated channel subfamily Q member 1
- IK1
Anomalous inwardly rectifying potassium current
- Ito
Transient outward potassium currents
- IKr
Rapidly activating delayed rectifier potassium currents
- IKs
Slowly activating delayed rectifier potassium current
- ISO
Isoproterenol
- CREB
cAMP response element binding protein
- Sp1
Specificity protein 1
Sources of funding
This work was supported in part by JSPS KAKENHI number 25460292 (K.O.) from the Japan Society for the Promotion of Science, Tokyo.
Compliance with ethical standards
Conflict of interest
All authors have declared that no conflict of interest exists.
References
- 1.Roden DM, Spooner PM. Inherited long QT syndromes: a paradigm for understanding arrhythmogenesis. J Cardiovasc Electrophysiol. 1999;10:1664–1683. doi: 10.1111/j.1540-8167.1999.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 2.Tamargo J. Drug-induced torsade de pointes: from molecular biology to bedside. Jpn J Pharmacol. 2000;83:1–19. doi: 10.1254/jjp.83.1. [DOI] [PubMed] [Google Scholar]
- 3.Itoh H, Dochi K, Shimizu W, Denjoy I, Ohno S, Aiba T, et al. A common mutation of long QT syndrome type 1 in Japan. Circ J. 2015;79:2026–2030. doi: 10.1253/circj.CJ-15-0342. [DOI] [PubMed] [Google Scholar]
- 4.Sumitomo N. Clinical features of long QT syndrome in children. Circ J. 2016;80:598–600. doi: 10.1253/circj.CJ-16-0046. [DOI] [PubMed] [Google Scholar]
- 5.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.1993.03510210076031. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann MH, Timothy KW, Frankovich D, Fromm BS, Keating M, Locati EH, et al. Age-gender influence on the rate-corrected QT interval and the QT-heart rate relation in families with genotypically characterized long QT syndrome. J Am Coll Cardiol. 1997;29:93–99. doi: 10.1016/S0735-1097(96)00454-8. [DOI] [PubMed] [Google Scholar]
- 7.Rautaharju PM, Zhou SH, Wong S, Calhoun HP, Berenson GS, Prineas R, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 8.Argenziano M, Tiscornia G, Moretta R, Casal L, Potilinski C, Amorena C, Gras EG. Arrhythmogenic effect of androgens on the rat heart. J Physiol Sci. 2017;67:217–225. doi: 10.1007/s12576-016-0459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, et al. Sex differences on the electrocardiographic pattern of cardiac repolarization: possible role of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Morishima M, Zheng M, Uchino T, Mannen K, Takahashi A, et al. Transcription factors Csx/Nkx2.5 and GATA4 distinctly regulate expression of Ca2+ channels in neonatal rat heart. J Mol Cell Cardiol. 2007;42:1045–1053. doi: 10.1016/j.yjmcc.2007.03.905. [DOI] [PubMed] [Google Scholar]
- 11.Ueba H, Brines M, Yamin M, Umemoto T, Ako J, Momomura S, et al. Cardioprotection by a nonerythropoietic, tissue-protective peptide mimicking the 3D structure of erythropoietin. Proc Natl Acad Sci USA. 2010;107:14357–14362. doi: 10.1073/pnas.1003019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor GT, Weiss J, Pitha J. Testosterone in a cyclodextrin-containing formulation: behavioral and physiological effect of episode-like pulses in rats. Pharm Res. 1989;6:641–646. doi: 10.1023/A:1015922019038. [DOI] [PubMed] [Google Scholar]
- 13.Witayavanitkul N, Woranush W, Bupha-Intr T, Wattanapermpool J. Testosterone regulates cardiac contractile activation by modulating SERCA but not NCX activity. Am J Physiol Heart Circ Physiol. 2013;304:H465–H472. doi: 10.1152/ajpheart.00555.2012. [DOI] [PubMed] [Google Scholar]
- 14.Schulte JS, Seidl MD, Nunes F, Freese C, Schneider M, Schmitz W, et al. CREB critically regulates action potential shape and duration in the adult mouse ventricle. Am J Physiol Heart Circ Physiol. 2012;302:H1998–H2007. doi: 10.1152/ajpheart.00057.2011. [DOI] [PubMed] [Google Scholar]
- 15.Suzuki E, Eda-Fujiwara H, Satoh R, Saito R, Miyamoto T. The effect of androgen on the retention of extinction memory after conditioned taste aversion in mice. J Physiol Sci. 2013;63:171–181. doi: 10.1007/s12576-013-0258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dela Cruz C, Pereira OC. Prenatal testosterone supplementation alters puberty onset, aggressive behavior, and partner preference in adult male rats. J Physiol Sci. 2012;62:123–131. doi: 10.1007/s12576-011-0190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitaoka Y, Machida M, Takemasa T, Hatta H. Expression of monocarboxylate transporter (MCT) 1 and MCT4 in overloaded mice plantaris muscle. J Physiol Sci. 2011;61:467–472. doi: 10.1007/s12576-011-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujisawa T, Shinohara K. Sex differences in the recognition of emotional prosody in late childhood and adolescence. J Physiol Sci. 2011;61:429–435. doi: 10.1007/s12576-011-0156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jelodar G, Khaksar Z, Pourahmadi M. Endocrine profile and testicular histomorphometry in adult rat offspring of diabetic mothers. J Physiol Sci. 2009;59:377–382. doi: 10.1007/s12576-009-0045-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kłapcińska B, Jagsz S, Sadowska-Krepa E, Górski J, Kempa K, Langfort J. Effects of castration and testosterone replacement on the antioxidant defense system in rat left ventricle. J Physiol Sci. 2008;58:173–177. doi: 10.2170/physiolsci.RP002208. [DOI] [PubMed] [Google Scholar]
- 21.Goto K, Takahashi K, Yamamoto M, Takamatsu K. Hormone and recovery responses to resistance exercise with slow movement. J Physiol Sci. 2008;58:7–14. doi: 10.2170/physiolsci.RP003107. [DOI] [PubMed] [Google Scholar]
- 22.Drici MD, Burklow TR, Haridasse V, Glazer RI, Woosley RL. Sex hormones prolong the QT interval and downregulate potassium channel expression in the rabbit heart. Circulation. 1996;94:1471–1474. doi: 10.1161/01.CIR.94.6.1471. [DOI] [PubMed] [Google Scholar]
- 23.Trépanier-Boulay V, St-Michel C, Tremblay A, Fiset C. Gender-based differences in cardiac repolarization in mouse ventricle. Circ Res. 2001;89:437–444. doi: 10.1161/hh1701.095644. [DOI] [PubMed] [Google Scholar]
- 24.Bai CX, Kurokawa J, Tamagawa M, Nakaya H, Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112:1701–1710. doi: 10.1161/CIRCULATIONAHA.104.523217. [DOI] [PubMed] [Google Scholar]
- 25.Akazawa H. Mechanisms of cardiovascular homeostasis and pathophysiology—from gene expression, signal transduction to cellular communication. Circ J. 2015;79:2529–2536. doi: 10.1253/circj.CJ-15-0818. [DOI] [PubMed] [Google Scholar]
- 26.Krystien VL, Christian W, Arthur AW. Catecholaminergic polymorphic ventricular tachycardia. Circ J. 2016;80:1285–1291. doi: 10.1253/circj.CJ-16-0326. [DOI] [PubMed] [Google Scholar]
- 27.Pham TV, Rosen MR. Sex, hormones, and repolarization. Cardiovasc Res. 2002;53:740–751. doi: 10.1016/S0008-6363(01)00429-1. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa M, Takahashi N, Watanabe M, Ichinose M, Nobe S, Yonemochi H, et al. Gender differences in ventricular repolarization: terminal T wave interval was shorter in women than in men. Pacing Clin Electrophysiol. 2003;26:59–64. doi: 10.1046/j.1460-9592.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 29.Bidoggia H, Maciel JP, Capalozza N, Mosca S, Blaksley EJ, Valverde E, et al. Sex-dependent electrocardiographic pattern of cardiac repolarization. Heart J. 2000;140:430–436. doi: 10.1067/mhj.2000.108510. [DOI] [PubMed] [Google Scholar]
- 30.Fülöp L, Bányász T, Szabó G, Tóth IB, Bíró T, Lôrincz I, et al. Effects of sex hormones on ECG parameters and expression of cardiac ion channels in dogs. Acta Physiol. 2006;188:163–171. doi: 10.1111/j.1748-1716.2006.01618.x. [DOI] [PubMed] [Google Scholar]
- 31.Charbit B, Christin-Maître S, Démolis JL, Soustre E, Young J, Funck-Brentano C. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol. 2009;103:887–890. doi: 10.1016/j.amjcard.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 32.James AF, Choisy SC, Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007;94:265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Lucas-Herald A, Alves-Lopes R, Montezano AC, Ahmed SF, Touyz RM. Genomic and non-genomic effects of androgens in the cardiovascular system: clinical implications. Clin Sci. 2017;131:1405–1418. doi: 10.1042/CS20170090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Wu S, Ruan Y, Hong L, Xing X, Lai W. Testosterone suppresses oxidative stress via androgen receptor-independent pathway in murine cardiomyocytes. Mol Med Rep. 2011;4:1183–1188. doi: 10.3892/mmr.2011.539. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y, Ai X, Oster RA, Bers DM, Pogwizd SM. Sex differences in repolarization and slow delayed rectifier potassium current and their regulation by sympathetic stimulation in rabbits. Pflugers Arch. 2013;465:805–818. doi: 10.1007/s00424-012-1193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mucha M, Ooi L, Linley JE, Mordaka P, Dalle C, Robertson B, et al. Transcriptional control of KCNQ channel genes and the regulation of neuronal excitability. J Neurosci. 2010;30:13235–13245. doi: 10.1523/JNEUROSCI.1981-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akuzawa-Tateyama M, Tateyama M, Ochi R. Sustained β-adrenergic stimulation increased l-type Ca2+ channel expression in cultured quiescent ventricular myocytes. J Physiol Sci. 2006;56:165–172. doi: 10.2170/physiolsci.RP001406. [DOI] [PubMed] [Google Scholar]
- 38.Noseda M, Abreu-Paiva M, Schneider MD. The quest for the adult cardiac stem cell. Circ J. 2015;79:1422–1430. doi: 10.1253/circj.CJ-15-0557. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


