Abstract
The administration of kainic acid (KA) causes seizures and produces neurodegeneration in hippocampal CA3 pyramidal cells. The present study investigated a possible role of acupuncture in reducing hippocampal cell death and inflammatory events, using a mouse model of kainic acid-induced epilepsy. Male C57BL/6 mice received acupuncture treatments at acupoint HT8 or in the tail area bilaterally once a day for 2 days and again immediately after an intraperitoneal injection of KA (30 mg/kg). HT8 is located on the palmar surface of the forelimbs, between the fourth and fifth metacarpal bones. Twenty-four hours after the KA injection, neuronal cell survival, the activations of microglia and astrocytes, and mRNA expression of two proinflammatory cytokines, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α), were measured in the hippocampus. Acupuncture stimulation at HT8, but not in the tail area, significantly reduced the KA-induced seizure, neuron death, microglial and astrocyte activations, and IL-1β mRNA expression in the hippocampus. The acupuncture stimulation also decreased the mRNA expression of TNF-α, but it was not significant. These results indicate that acupuncture at HT8 can inhibit hippocampal cell death and suppress KA-induced inflammatory events, suggesting a possible role for acupuncture in the treatment of epilepsy.
Keywords: Acupuncture, Kainic acid, Microglia, Astrocyte, Interleukin-1β, Epilepsy
Introduction
Epilepsy is one of the most common serious brain disorders and affects people of all ages. Although various antiepileptic drugs are used in the treatment of epilepsy, they are only effective in 60–70 % of the patient population. Moreover, they do not modify the disease process, but merely suppress epileptic symptoms such as seizures [1, 2].
Temporal lobe epilepsy is the most common type of epilepsy in the adult population, and is frequently associated with the loss of neurons from the hippocampus and other brain regions [3]. Kainic acid (KA) is a neuroexcitatory amino acid for the induction of seizures. The administration of KA causes seizures and produces neurodegeneration in dentate hilar cells and hippocampal CA3 pyramidal cells, similar to that observed in the human epileptic hippocampus [4–6]. Therefore, the KA-induced epilepsy animal model has been widely used as an experimental model of human temporal lobe epilepsy [7].
KA administration causes hippocampal cell death via various pathways. KA induces excessive amounts of glutamate in the hippocampus, leading to neuronal apoptosis and necrosis [8]. KA also increases the creation of reactive oxidative species and reactive nitrogen species, which plays an important role in the neuronal damage [9]. Moreover, KA administration increased the activations of microglia and astrocytes in the hippocampus [10–12]. Microglial cells are specialized macrophages in the central nervous system that are capable of immune defense, and astrocytes are the most numerous glia cells in central nervous system [13]. Their activations contribute to KA-induced neuronal cell death through a sustained inflammatory response [6] and the induction of inflammatory mediators such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) [14].
Acupuncture has been used to alleviate a variety of neurological disorders, including epilepsy [15], and a good deal of evidence supports its neuroprotective effect against KA toxicity. In animal models, acupuncture or electro-acupuncture stimulation has been shown to reduce neuronal cell death induced by KA; this effect may be attributable to the neuroprotection of hippocampal neurons and the regulation of GABA-mediated signaling via increased glutamate decarboxylase [16, 17]. In addition, some studies in a bronchial asthma rat model [18] and in arthritis animal models [19–21] suggest that acupuncture has an anti-inflammatory effect, possibly as a result of cytokine suppression. Moreover, acupuncture has been shown to suppress microglial activation in spinal cord [22] and cytokines, and IL-1β in spinal cord [23] and colon [24]. These anti-inflammatory effects have also been demonstrated in the brains of mice with MPTP-induced Parkinson’s disease [25, 26], as well as in arthritis animal models. Therefore, acupuncture has the potential to suppress KA-induced inflammatory events in the hippocampus, but the mechanism is still unclear.
Our previous study showed that acupuncture stimulation at HT8 can suppress the KA-induced hippocampal cell death via increasing glutamate decarboxylase [16]. In the present study, we investigated whether acupuncture can reduce hippocampal cell death, microglial activation, and inflammatory cytokine mRNA levels associated with epilepsy.
Methods
Animals and grouping
Male C57BL/6 mice (8 weeks old, weighing 20–23 g; Orientbio, Korea) were housed at room temperature (22 ± 3 °C) under a standard 12-h light/dark cycle (lights on at 0700 hours) with unlimited access to food and water. The animals were handled in accordance with the current guidelines established in the NIH Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1985), and all efforts were made to minimize animal suffering and reduce the number of animals used. The mice were randomly assigned to four groups: the saline group (n = 12), which was injected with normal saline and did not receive acupuncture stimulation; the KA group (n = 18), which was injected with KA (Sigma, St. Louis, MO, USA) and did not receive acupuncture stimulation; the KA + HT8 group (n = 16), which was injected with KA and received acupuncture stimulation bilaterally at acupoint HT8; and the KA + NA group (n = 18), which was injected with KA and received acupuncture stimulation bilaterally in the tail as the control group of acupuncture stimulation.
Acupuncture stimulation and kainic acid injection
Between 1000 and 1030 hours, the mice in the KA + HT8 group were lightly immobilized by grasping the loose skin behind the ears with thumb and forefinger of an asistant, and acupuncture needles (0.18 × 8 mm; Dongbang Acupuncture, Korea) were inserted at the bilateral HT8 acupoints. HT8 is located on the palmar surface of the forelimbs, between the fourth and fifth metacarpal bones. The position of the acupoint in mice corresponds anatomically to its location in humans [27]. Acupoints in the heart meridian (HT) have been used to treat psychopathic and neurological disorders such as epilepsy [19]. Among these acupoints, HT8 (Shaofu) is traditionally known as a representative acupoint for balancing homeostasis by regulating the excitatory and inhibitory functions in the body [28]. The needle was inserted to a depth of 1 mm and was turned at a rate of two spins and counter-spins per second for 30 s, with removal immediately afterward. The entire stimulation lasted 60 s. The mice in the saline and KA groups were also immobilized in a similar fashion for 60 s. The stimulation was repeated three times: once a day for 2 days prior to KA injection, and then immediately after KA injection. The procedure for acupuncture stimulation in the KA + NA group was same as that in the KA + HT8 group, except that the acupuncture points were 5 mm distal to the lateral side of the tail base where there are no acupoints. The animals of the saline and KA groups were also immobilized in a similar fashion for 60 s. KA (30 mg/kg of free base; Sigma) was injected intraperitoneally with a BD Ultra-fine II insulin syringe (Becton, Dickson, Franklin Lakes, NJ, USA) just before the last acupuncture stimulation. The mice in the saline group were injected with normal saline instead of KA.
Quantification of behavioral seizures
After acupuncture stimulation or immobilization for 60 s following the saline or KA injection, we directly observed the behavioral changes in the mice for 120 min, to determine the severity of the seizure activity. The mice were evaluated on a previously established six-point seizure scale [29]: stage 1 immobility; stage 2 forelimb and/or tail extension, rigid posture; stage 3 repetitive movements, head bobbing; stage 4 rearing and falling; stage 5 continuous rearing and falling; and stage 6 severe tonic–clonic seizures.
Immunohistochemistry
Twenty-four hours after the KA injection, the mice (n = 6 from each group) were perfused with 4 % paraformaldehyde dissolved in 0.1 M phosphate buffer (PB). The brains were removed from the cranium, post-fixed for a day, washed in 0.1 M PB, and immersed in a 30 % sucrose solution for storage at 4 °C prior to sectioning. Frozen sections (40 μm) were cut using a cryostat.
Hippocampal sections (−1.82 to −2.06 mm from bregma) from each animal were stained. To identify degenerating neurons in the hippocampus, cresyl violet staining was performed. The sections were mounted on silane-coated slides, air-dried, and incubated for 1 min in a 1 % solution of cresyl violet. Next, the sections were washed thoroughly in cold tap water, rinsed briefly in 1 % acetic acid solution for 10 s, dehydrated by immersion in ascending grades of alcohol, cleared with xylene, and coverslipped using mounting medium. The severity and extent of neurodegeneration in the CA3 area of the hippocampus were assessed using Duan’s method [30] and scored quantitatively: 0, normal; 1, <10 % pyknotic neurons in CA3; 2, 11–40 % pyknotic neurons in CA3; 3, >40 % pyknotic neurons in CA3; 4, slight (<10 %) neuron loss in CA3; 5, moderate (11–40 %) neuron loss in CA3; and 6, severe (>40 %) neuron loss in CA3 area.
Sections were also immunohistochemically processed to detect microglia in the mouse hippocampus. Briefly, the sections were incubated with CD11b (AbD Serotec, Oxford, UK) or GFAP (Cell Signaling Technology, Beverly, MA, USA) primary antibodies diluted 1:1,000 for 24 h at 4 °C. After washing in 0.05 M phosphate-buffered saline (PBS), the sections were incubated with biotinylated anti-rat IgG (Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature, followed by incubation with ABC reagent (Vector Laboratories) for 1 h at room temperature. Then, the sections were washed in PBS, incubated with 0.02 % diaminobenzidine (DAB) and 0.003 % hydrogen peroxide in 0.1 M Tris–HCl-buffered saline (pH 7.5) for 5 min, rinsed with PBS, mounted on gelatin-coated slides, air-dried, dehydrated, and coverslipped. The slides were observed under a bright-field BX51 microscope (Olympus, Japan) and photographed using a DP70 camera (Olympus). The number of CD11b or GFAP positive cell bodies in the CA3 was counted on each section and analyzed using an image analyzer (Optimas v.6.5; Media Cybernetics, MD, USA). The cell count is expressed as the mean number of cells per mm2.
IL-1β and TNF-α gene expressions measured by quantitative real-time PCR
Twenty-four hours after KA injection, six mice from each group were killed, and the whole hippocampus was immediately extracted from each. Real-time PCR was performed to quantify the amounts of IL-1β and TNF-α mRNA in the hippocampi. The PCR reaction mixture contained 5 mM MgCl2, 2 μL of SYBR Green 1, 1 μL of hippocampal cDNA, and a pair of specific primers in a total volume of 20 μL. Specific primer pairs were used separately to amplify IL-1β, TNF-α, and ß-actin, as an endogenous control. Real-time PCR was carried out in capillary glass tubes in a Light Cycler 1.5 instrument (Roche Diagnostics, Basel, Switzerland). The amplification program consisted of 10 min at 95 °C, followed by 40 cycles of 95 °C for 10 s, 63 °C (IL-1β) or 61 °C (TNF-α and ß-actin) for 5 s, and 72 °C for 10 s. After amplification, the samples underwent a melting curve analysis consisting of 1 cycle of 95 °C for 0 s, 63 °C (IL-1β and TNF-α) or 61 °C (ß-actin) for 15 s, and 95 °C for 10 s, at the step acquisition mode. A negative control was included in each run to assess primer specificity and possible contamination. The threshold cycle (Ct) was taken to be the PCR cycle at which the reporter fluorescence could be detected above baseline. The expression level was obtained from a logarithmic plot of the fluorescence signal above background noise. The values for IL-1β and TNF-α were normalized to that for ß-actin.
Statistical analysis
All data are expressed as the mean ± SEM. Data were analyzed by one-way ANOVA with the Neuman–Keuls post hoc test, and differences were considered statistically significant at P < 0.05.
Results
KA-induced epileptic seizure
All the KA injected subjects began seizures within 20 min and showed the most severe seizure degrees within 50 min. With acupuncture stimulation, the severity of seizure in the KA + HT8 group was lower than those in the KA group and the survival ratio in the KA + HT8 group was greater than those in the KA group during 24 h. The mice in the KA + NA group also displayed lesser severity of seizure and KA-induced death comparing to those in the KA group, but showed lesser effects compared to those in the KA + HT8 group (Table 1).
Table 1.
Classification of seizure parameters in C57BL/6 mice after KA administration
| Group | Maximum seizure parameters, the number of mice | |||
|---|---|---|---|---|
| Repetitive movements (Stage 3) | Rearing and falling (Stage 4) | Severe seizure (Stages 5, 6) | Death (Stage 7) | |
| KA (n = 18) | 0 | 3 | 9 | 6 |
| KA + HT8 (n = 16) | 4 | 7 | 1 | 4 |
| KA + NA (n = 18) | 2 | 5 | 5 | 6 |
KA-induced neuronal death in CA3 of the hippocampus
The KA-induced cell death in the CA3 was assessed using cresyl violet staining. Twenty-four hours after KA administration, the CA3 pathological score in the KA group was 3.9 ± 0.34, which means that pyramidal layers had been destroyed. The CA3 score in the KA + HT8 group (2.5 ± 0.37) was significantly less than that in the KA group (P < 0.05), whereas the score in the KA + NA group was not significantly different from that in the KA group. Thus, acupuncture stimulation only at HT8 significantly suppressed KA-induced neuronal destruction (Fig. 1).
Fig. 1.
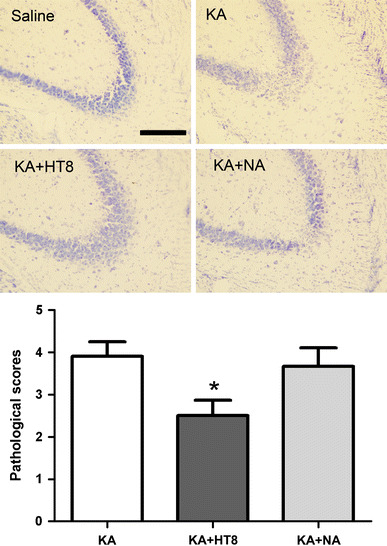
Neuronal cell death in hippocampal CA3 region after kainic acid (KA) administration. Saline saline injected control group; KA KA injected group; KA + HT8 acupuncture at HT8 with KA injection group; KA + NA acupuncture at tail with KA injection group. Scale bar 100 μm. All data are presented as the mean ± SEM. *P < 0.05 versus KA group
Microglial activation in CA3 of the hippocampus
The number of CD11b-positive cells was significantly higher in the KA group (168.9 ± 19.36 cells/mm2; P < 0.001) compared with the saline group. However, the number was significantly lower in the KA + HT8 group (61.78 ± 20.86 cells/mm2) compared with that in the KA group (P < 0.01). Although the number in the KA + NA group (119.3 ± 22.37 cells/mm2) was also lower than that in the KA group, the difference was not significant (Fig. 2).
Fig. 2.
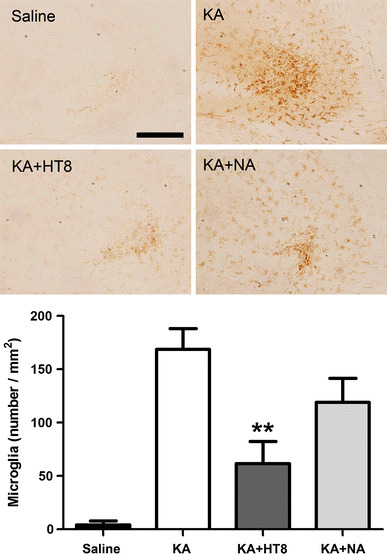
The expressions of CD11b-positive cells in hippocampal CA3 region after kainic acid (KA) administration. Saline saline injected control group; KA KA injected group; KA + HT8 acupuncture at HT8 with KA injection group; KA + NA acupuncture at tail with KA injection group. Scale bar 100 μm. All data are presented as the mean ± SEM. **P < 0.01 versus KA group
Astrocyte activation in CA3 of the hippocampus
The number of GFAP-positive cells was significantly higher in the KA group (238.6 ± 32.83 cells/mm2; P < 0.001) compared with the saline group. Compared with the number of GFAP-positive cells in the KA group, the number was significantly lower in the KA + HT8 group (72.8 ± 18.26 cells/mm2; P < 0.01). Although the number in the KA + NA group (206.7 ± 45.60 cells/mm2) was also lower than that in the KA group, the difference was not significant (Fig. 3). Thus, acupuncture stimulation at HT8 significantly suppressed the activations of microglia and astrocyte.
Fig. 3.
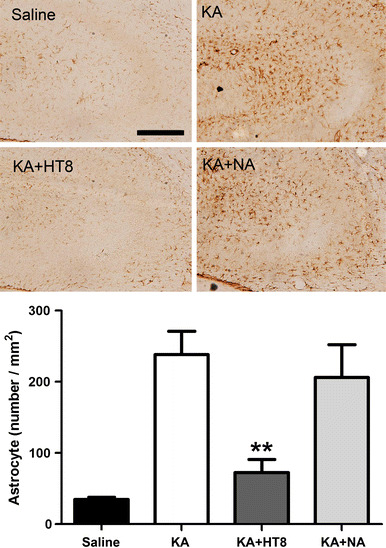
The expressions of GFAP-positive cells in hippocampal CA3 region after kainic acid (KA) administration. Saline saline injected control group; KA KA injected group; KA + HT8 acupuncture at HT8 with KA injection group; KA + NA acupuncture at tail with KA injection group. Scale bar 100 μm. All data are presented as the mean ± SEM. **P < 0.01 versus KA group
IL-1β and TNF-α gene expression in CA3 of the hippocampus
In the KA group, IL-1β gene expression was significantly increased (388.0 ± 66.05 %; P < 0.05) compared with that in the saline group (100.0 ± 26.37 %). However, the level of IL-1β gene expression was significantly lower in the KA + HT8 group (136.9 ± 54.47 %) compared with that in the KA group (P < 0.05). Although IL-1β gene expression in the KA + NA group (275.4 ± 90.68 %) was also lower than that in the KA group, the difference was not significant (Fig. 4a). A similar trend was seen for TNF-α gene expression, but there were no statistically significant difference (P = 0.0798, Fig. 4b).
Fig. 4.
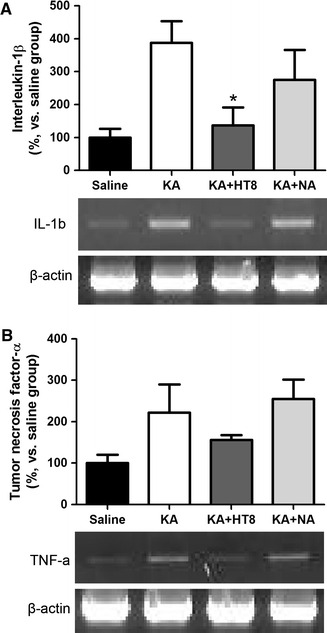
The expressions of the interleukin-1β (a) and the tumor necrosis factor-α (b) genes in the mouse hippocampus after kainic acid administration. Saline saline injected control group; KA KA injected group; KA + HT8 acupuncture at HT8 with KA injection group; KA + NA acupuncture at tail with KA injection group. All data are presented as the mean ± SEM. *P < 0.05 versus KA group
Discussion
The present results demonstrate that acupuncture stimulation at acupoint HT8 can reduce KA-induced hippocampal cell death, microglial activation, and IL-1β gene expression in the mouse hippocampus. Given that acupuncture stimulation at tail base (non-acupoint) fails to suppress the KA-induced seizure and hippocampal changes, these neuroprotective and anti-inflammatory effects are innate characteristics of the stimulation at acupoint HT8.
Inflammatory events play a major role in the pathological processes of epilepsy and seizure-induced brain damage. After status epilepticus, microglia and astrocyte are activated [10–12], leading to the upregulation of cytokine and chemokine expression [31]. These events occur in epilepsy patients and animal epilepsy models. KA, an excitotoxin, causes epileptic seizures, activates microglia and astrocyte [10–12], and produces neurodegeneration of dentate hilar cells and hippocampal CA3 pyramidal cells [4–6]. The inhibition of microglial activation has been shown to reduce excitotoxic spinal cord neuronal cell death [32]. We evaluated neuronal cell death by cresyl violet staining and assessed microglia and astrocyte activations, and only the KA + HT8 group had significantly fewer activated microglia and astrocytes than the KA group. Thus, considering that acupuncture stimulation at HT8 (KA + HT8 group), but not at the tail area (KA + NA group), and suppressed KA-induced neuronal destruction and microglial activations in the hippocampus, a reduction in microglial activation can account for the neuroprotective effect of acupuncture stimulation in the present study.
IL-1β is a primary mediator of KA-induced excitability of hippocampal neurons [33] and plays a central role in KA-induced neurodegeneration [34]. We evaluated the expression of IL-1β by real-time PCR. The expression of IL-1β in the hippocampus was significantly lower in the KA + HT8 group than in the KA group, but IL-1β expression in the KA + NA group did not differ significantly from that in the KA group. These results indicate that the neuroprotective effect of acupuncture stimulation at HT8 results from reduced IL-1β expression owing to the suppression of microglial activation.
TNF-α is a cytokine that can cause cell death, and it is known to increase in hippocampi after KA administration [35]. But Järvelä et al. [36] reported that IL-1β mRNA expression in hippocampi increased 4 h after KA injection and remained significantly higher than in the control group at 24 h, but TNF-α mRNA expression was not maintained significantly at 24 h, although it also increased 4 h after KA injection. In this study, the mRNA expression of TNF-α showed similar trend to that of IL-1β, but there were no statistically significant difference (P = 0.0798) 24 h after KA administration. We supposed that the negative statistic result of TNF-α mRNA expression was due to the failure of maintaining significant TNF-α mRNA increase to 24 h after KA administration, but more rigorous study is needed for clarifying our supposition.
In this study, acupuncture stimulation at HT8 suppressed KA-induced neuronal cell death, microglial activation, and IL-1β gene expression, suggesting that its neuroprotective effects may be mediated by the suppression of KA-induced inflammatory events. However, the possibility that acupuncture may mitigate KA-induced seizures via other mechanisms remains. Furthermore, this study investigated the effects of acupuncture on KA-induced status epilepticus only, and not on chronic epilepsy or spontaneous recurrent seizures. Despite these limitations, our findings may provide important clues for understanding the mechanism of acupuncture in epilepsy intervention.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (No. 2005-0049404).
Contributor Information
Chang Shik Yin, Phone: +82-2-9614989, FAX: +82-2-9664237, Email: acuyin@khu.ac.kr.
Hi-Joon Park, Phone: +82-2-9599435, FAX: +82-2-9664237, Email: acufind@khu.ac.kr.
References
- 1.Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- 2.Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/S1474-4422(02)00073-X. [DOI] [PubMed] [Google Scholar]
- 3.Sperk G, Drexel M, Pirker S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia. 2009;50(Suppl 12):29–31. doi: 10.1111/j.1528-1167.2009.02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akahoshi N, Murashima YL, Himi T, Ishizaki Y, Ishii I. Increased expression of the lysosomal protease cathepsin S in hippocampal microglia following kainate-induced seizures. Neurosci Lett. 2007;429:136–141. doi: 10.1016/j.neulet.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ari Y, Cossart R. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 2000;23:580–587. doi: 10.1016/S0166-2236(00)01659-3. [DOI] [PubMed] [Google Scholar]
- 6.Vezzani A. Innate immunity and inflammation in temporal lobe epilepsy: new emphasis on the role of complement activation. Epilepsy Curr. 2008;8:75–77. doi: 10.1111/j.1535-7511.2008.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadler JV. Kainic acid as a tool for the study of temporal lobe epilepsy. Life Sci. 1981;29:2031–2042. doi: 10.1016/0024-3205(81)90659-7. [DOI] [PubMed] [Google Scholar]
- 8.Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ueda Y, Yokoyama H, Nakajima A, Tokumaru J, Doi T, Mitsuyama Y. Glutamate excess and free radical formation during and following kainic acid-induced status epilepticus. Exp Brain Res. 2002;147:219–226. doi: 10.1007/s00221-002-1224-4. [DOI] [PubMed] [Google Scholar]
- 10.Kralic JE, Ledergerber DA, Fritschy JM. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur J Neurosci. 2005;22:1916–1927. doi: 10.1111/j.1460-9568.2005.04386.x. [DOI] [PubMed] [Google Scholar]
- 11.Rappold PM, Lynd-Balta E, Joseph SA. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089:171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 12.Taniwaki Y, Kato M, Araki T, Kobayashi T. Microglial activation by epileptic activities through the propagation pathway of kainic acid-induced hippocampal seizures in the rat. Neurosci Lett. 1996;217:29–32. doi: 10.1016/0304-3940(96)13062-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang XM, Zhu J. Kainic acid-induced neurotoxicity: targeting glial responses and glia-derived cytokines. Curr Neuropharmacol. 2011;9:388–398. doi: 10.2174/157015911795596540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ. Role of microglial IKKbeta in kainic acid-induced hippocampal neuronal cell death. Brain. 2008;131:3019–3033. doi: 10.1093/brain/awn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H, Park HJ, Park J, Kim MJ, Hong M, Yang J, Choi S. Acupuncture application for neurological disorders. Neurol Res. 2007;29(Suppl 1):S49–S54. doi: 10.1179/016164107X172211. [DOI] [PubMed] [Google Scholar]
- 16.Kim ST, Jeon S, Park HJ, Hong MS, Jeong WB, Kim JH, Kim Y, Lee HJ, Chung JH. Acupuncture inhibits kainic acid-induced hippocampal cell death in mice. J Physiol Sci. 2008;58:31–38. doi: 10.2170/physiolsci.RP010907. [DOI] [PubMed] [Google Scholar]
- 17.Guo J, Liu J, Fu W, Ma W, Xu Z, Yuan M, Song J, Hu J. The effect of electroacupuncture on spontaneous recurrent seizure and expression of GAD(67) mRNA in dentate gyrus in a rat model of epilepsy. Brain Res. 2008;1188:165–172. doi: 10.1016/j.brainres.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Carneiro ER, Carneiro CR, Castro MA, Yamamura Y, Silveira VL. Effect of electroacupuncture on bronchial asthma induced by ovalbumin in rats. J Altern Complement Med. 2005;11:127–134. doi: 10.1089/acm.2005.11.127. [DOI] [PubMed] [Google Scholar]
- 19.Yim YK, Lee H, Hong KE, Kim YI, Lee BR, Son CG, Kim JE. Electro-acupuncture at acupoint ST36 reduces inflammation and regulates immune activity in collagen-induced arthritic mice. Evid Based Complement Altern Med. 2007;4:51–57. doi: 10.1093/ecam/nel054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HW, Uh DK, Yoon SY, Roh DH, Kwon YB, Han HJ, Lee HJ, Beitz AJ, Lee JH. Low-frequency electroacupuncture suppresses carrageenan-induced paw inflammation in mice via sympathetic post-ganglionic neurons, while high-frequency EA suppression is mediated by the sympathoadrenal medullary axis. Brain Res Bull. 2008;75:698–705. doi: 10.1016/j.brainresbull.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Chae Y, Hong MS, Kim GH, Hahm DH, Park HJ, Ha E, Kim MJ, Yang J, Lee H. Protein array analysis of cytokine levels on the action of acupuncture in carrageenan-induced inflammation. Neurol Res. 2007;29(Suppl 1):S55–S58. doi: 10.1179/016164107X172365. [DOI] [PubMed] [Google Scholar]
- 22.Shan S, Qi-Liang MY, Hong C, Tingting L, Mei H, Haili P, Yan-Qing W, Zhi-Qi Z, Yu-Qiu Z. Is functional state of spinal microglia involved in the anti-allodynic and anti-hyperalgesic effects of electroacupuncture in rat model of monoarthritis? Neurobiol Dis. 2007;26:558–568. doi: 10.1016/j.nbd.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang RX, Liu B, Qiao JT, Wang L, Ren K, Berman BM, Lao L. Electroacupuncture suppresses spinal expression of neurokinin-1 receptors induced by persistent inflammation in rats. Neurosci Lett. 2005;384:339–343. doi: 10.1016/j.neulet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Zhang H, Chen CT, Yang QY, Liao WF, Chen PG. Effects of electroacupuncture at Shangjuxu (ST 37) on interleukin-1beta and interleukin-4 in the ulcerative colitis model rats. J Tradit Chin Med. 2009;29:60–63. doi: 10.1016/S0254-6272(09)60033-9. [DOI] [PubMed] [Google Scholar]
- 25.Kang JM, Park HJ, Choi YG, Choe IH, Park JH, Kim YS, Lim S. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Res. 2007;1131:211–219. doi: 10.1016/j.brainres.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 26.Liu XY, Zhou HF, Pan YL, Liang XB, Niu DB, Xue B, Li FQ, He QH, Wang XH, Wang XM. Electro-acupuncture stimulation protects dopaminergic neurons from inflammation-mediated damage in medial forebrain bundle-transected rats. Exp Neurol. 2004;189:189–196. doi: 10.1016/j.expneurol.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 27.Yin CS, Jeong HS, Park HJ, Baik Y, Yoon MH, Choi CB, Koh HG. A proposed transpositional acupoint system in a mouse and rat model. Res Vet Sci. 2008;84:159–165. doi: 10.1016/j.rvsc.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Lee H, Lee JY, Kim YJ, Kim S, Yin C, Khil JH, Kwon K, Choi SM, Park HJ. Acupuncture for symptom management of rheumatoid arthritis: a pilot study. Clin Rheumatol. 2008;27:641–645. doi: 10.1007/s10067-007-0819-3. [DOI] [PubMed] [Google Scholar]
- 29.Schauwecker PE, Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc Natl Acad Sci USA. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duan RS, Chen Z, Dou YC, Concha Quezada H, Nennesmo I, Adem A, Winblad B, Zhu J. Apolipoprotein E deficiency increased microglial activation/CCR3 expression and hippocampal damage in kainic acid exposed mice. Exp Neurol. 2006;202:373–380. doi: 10.1016/j.expneurol.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Turrin NP, Rivest S. Innate immune reaction in response to seizures: implications for the neuropathology associated with epilepsy. Neurobiol Dis. 2004;16:321–334. doi: 10.1016/j.nbd.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 32.Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng H, Zhu W, Zhao H, Wang X, Wang W, Li Z. Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: crucial role of interleukin-1beta. Neuroimmunomodulation. 2010;17:31–38. doi: 10.1159/000243083. [DOI] [PubMed] [Google Scholar]
- 34.Oprica M, Eriksson C, Schultzberg M. Inflammatory mechanisms associated with brain damage induced by kainic acid with special reference to the interleukin-1 system. J Cell Mol Med. 2003;7:127–140. doi: 10.1111/j.1582-4934.2003.tb00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Y, Lim CM, Kim SW, Park JY, Seo JS, Han PL, Yoon SH, Lee JK. Fluoxetine attenuates kainic acid-induced neuronal cell death in the mouse hippocampus. Brain Res. 2009;1281:108–116. doi: 10.1016/j.brainres.2009.04.053. [DOI] [PubMed] [Google Scholar]
- 36.Järvelä JT, Lopez-Picon FR, Plysjuk A, Ruohonen S, Holopainen IE. Temporal profiles of age-dependent changes in cytokine mRNA expression and glial cell activation after status epilepticus in postnatal rat hippocampus. J Neuroinflamm. 2011;8:29. doi: 10.1186/1742-2094-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]


