Abstract
The olfactory bulb receives cholinergic basal forebrain input, as does the neocortex; however, the in vivo physiological functions regarding the release of extracellular acetylcholine and regulation of regional blood flow in the olfactory bulb are unclear. We used in vivo microdialysis to measure the extracellular acetylcholine levels in the olfactory bulb of urethane-anesthetized rats. Focal chemical stimulation by microinjection of l-glutamate into the horizontal limb of the diagonal band of Broca (HDB) in the basal forebrain, which is the main source of cholinergic input to the olfactory bulb, increased extracellular acetylcholine release in the ipsilateral olfactory bulb. When the regional cerebral blood flow was measured using laser speckle contrast imaging, the focal chemical stimulation of the HDB did not significantly alter the blood flow in the olfactory bulb, while increases were observed in the neocortex. Our results suggest a functional difference between the olfactory bulb and neocortex regarding cerebral blood flow regulation through the release of acetylcholine by cholinergic basal forebrain input.
Keywords: Cholinergic system, Horizontal limb of the diagonal band of Broca, Laser speckle contrast imaging, Microdialysis, Rat
Introduction
It is well established that patients with Alzheimer’s disease exhibit olfactory dysfunction as well as impairments of memory and other cognitive function [1–3]. The intracerebral cholinergic system originating in the basal forebrain and projecting to the neocortex, hippocampus, and olfactory bulb contributes to cognition, memory, and olfactory functions, respectively [4–6]. In addition to these roles, our research group has demonstrated in rats that the activation of the basal forebrain cholinergic system projecting into the neocortex and hippocampus releases acetylcholine (ACh), resulting in vasodilation and an increase in the regional blood flow in these regions [7–11].
The nucleus basalis of Meynert (NBM) of the basal forebrain provides a major source of cholinergic projection into the neocortex [12, 13]. Focal stimulation of the NBM, either chemically or electrically, produces an increase in ACh release in the neocortex [9], which causes a widespread increase in regional blood flow within the neocortices [7, 14]. Cholinergic neurons projecting their fibers into the hippocampus are detected in the septal complex (medial septal nucleus [MS] and vertical limb of the diagonal band of Broca [VDB]) [12, 15]. Moreover, focal stimulation of the septal complex, chemically or electrically, increases ACh release in the hippocampus, and thus increases regional blood flow in the hippocampus [8, 14]. The increased response of regional blood flow both in the neocortex and the hippocampus evoked by the activation of the basal forebrain neurons is due to cholinergic vasodilation, because such responses are blocked by cholinergic receptor antagonists [7, 8].
The olfactory bulb receives cholinergic axonal projections from nerve cells originating in the nucleus of the horizontal limb of the diagonal band of Broca (HDB) [12, 16]. Moreover, 80–90% of the neurons originating in the NBM/SI (substantia innominate) and projecting to the cortex, 35–45% of neurons originating in the MS/VDB and projecting to the hippocampus, and 10–20% of neurons originating in the HDB and projecting to the olfactory bulb are cholinergic (choline acetyltransferase [ChAT] positive) [12]. Cholinergic neurons in the basal forebrain exhibit degeneration in patients with Alzheimer’s disease [17] and in healthy aged people [18, 19]. Due to the small number of nerve cells, the cholinergic system originating in the HDB and projecting to the olfactory bulb might lose their function earlier than the septo-hippocampal or NBM-cortical cholinergic systems during the aging process or progression of Alzheimer’s disease. However, the physiological function of the cholinergic system originating in the HDB and projecting to the olfactory bulb has not been well studied. Considering the previous findings concerning the vasodilative neural system for regional blood flow in the neocortex and hippocampus, there is a possibility that the cholinergic fibers originating in the HDB and projecting into the olfactory bulb act as intracerebral vasodilators for blood vessels of the olfactory bulb via the release of ACh. However, no studies have addressed this question to date. Thus, the present study aimed to clarify whether the cholinergic nerve fibers originating in the HDB and projecting into the olfactory bulb release ACh and contribute to the neural regulation of blood flow in the olfactory bulb.
The first portion of the present study was carried out to determine whether the extracellular ACh release in the olfactory bulb is increased by focal stimulation of the HDB. There have been no reports that have measured the extracellular release of ACh in the olfactory bulb. In the present study, we applied an in vivo microdialysis technique which has been used in our previous studies to measure the extracellular ACh release in the neocortex [11] and the hippocampus [20].
The second portion of the present study was carried out to determine whether the regional blood flow in the olfactory bulb is increased by focal stimulation of the HDB. Laser Doppler flowmetry is commonly used to measure the level of regional blood flow in a particular area of the brain, including the olfactory bulb [21, 22]. Laser speckle flowmetry is advantageous for assessing two-dimensional blood flow in broad regions of the brain, and has been recently used in animal experiments [23]. Using laser speckle flowmetry, our recent study successfully observed a vasodilative blood flow response in the neocortex evoked by NBM activation or by somatic afferent stimulation in both rats [24] and mice [25]. In the present study, we used both laser Doppler flowmetry and laser speckle flowmetry to measure the level of blood flow in the olfactory bulb. Using laser speckle flowmetry, the level of blood flow in the frontal and parietal cortices was also measured, together with that in the olfactory bulb.
Materials and methods
The experiments were performed on 22 male adult Wistar rats (body weight, 290–430 g; 4–8 months old), bred at the Tokyo Metropolitan Institute of Gerontology. The study was conducted with the approval and in accordance with the guidelines for animal experimentation prepared by the Animal Care and Use Committee of Tokyo Metropolitan Institute of Gerontology.
General surgery and anesthesia
The rats were anesthetized with urethane (1.1 g/kg, i.p.). Respiration was maintained by means of an artificial respirator (model 683, Harvard, USA) through a tracheal cannula. The end-tidal CO2 concentration was maintained at 3.0–4.0% by monitoring with a respiratory gas monitor (Microcap, Oridion Medical, Jerusalem, Israel). The arterial blood pressure was measured through a catheter inserted into a femoral artery with a pressure transducer (TP-400T, Nihon Kohden, Tokyo). Body temperature was measured rectally and continuously using a thermistor, and maintained at approximately 37.5°C by means of a body temperature control system (ATB-1100, Nihon Kohden). The depth of anesthesia was adjusted by additional urethane doses (100 mg/kg, i.v. via a catheter inserted into a femoral vein) when necessary and by monitoring body movement, blood pressure stability and respiratory movement.
Measurement of ACh release in the olfactory bulb
Extracellular ACh in the olfactory bulb was collected via a microdialysis technique in 10 rats (Fig. 1a). The procedures for the microdialysis technique and ACh measurements were the same as that of our previous study involving the rat parietal cortex [11, 26]. The animals were mounted on a stereotaxic instrument (SR-5, Narisige) in a prone position, and the olfactory bulb was exposed by removing a portion of the skull and dura. A microdialysis probe (outer diameter, 0.5 mm; length of perfusion, 3 mm; CMA/12, CMA/Microdialysis AB, Sweden) was stereotaxically implanted into the granule cell layer of the right olfactory bulb to a depth of 3.5 mm from the brain surface at AP = +7.5, L = +0.5 (Fig. 1b) according to Paxinos and Watson’s atlas [27], as described by El-Etri et al. [28]. In one rat, after the level of ACh release in the right olfactory bulb was measured, ACh release in the left olfactory bulb was also measured. The microdialysis probe was perfused at a speed of 2 μl/min with an artificial cerebral spinal fluid (aCSF) containing (in mM) NaCl (122.7), KCl (2.4), CaCl2 (1.5), MgCl2 (1.1), NaHCO3 (27.5), KH2PO4 (0.6), Na2SO4 (0.5), glucose (6), and the acetylcholinesterase inhibitor physostigmine (5 μM). The aCSF was bubbled with 95% O2/5% CO2 to adjust the pH to 7.4. The recovery rate of ACh of the microdialysis probe in vitro at room temperature was usually 14–16%. The perfused fluid was collected every 3 min in a sample cup kept on ice. The perfusate in each sample (6 μl) was mixed with 6 μl (120 fmol) of isopropylhomocholine, an internal standard, dissolved in aCSF. ACh was measured by high-performance liquid chromatography (HPLC) using an electrochemical detector (HTEC-500, Eicom, Kyoto) (Fig. 1c). The mobile phase, consisting of 50 mM KHCO3, 400 mg/l sodium 1- decanesulfonate (Tokyo Kasei Kogyo, Japan), and 50 mg/l EDTA·2Na, was pumped at a rate of 150 μl/min through a microbore separation column (AC-GEL, 2 × 150 mm). The ACh was converted to hydrogen peroxide, and betaine by immobilized acetylcholinesterase and choline oxidase packed into a column (AC-ENZYMEPAKII, 1.0 × 4 mm). Both the separation and enzyme columns were maintained at 33°C. Hydrogen peroxide was measured using an electrochemical detector, and ACh was calculated by measuring hydrogen peroxide. The platinum working electrode was held at 0.45 V vs Ag/AgCl.
Fig. 1.
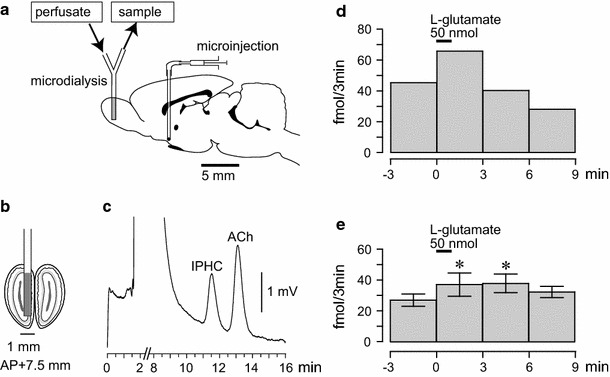
Effects of focal chemical stimulation of the HDB on extracellular ACh release in the olfactory bulb ipsilateral to the stimulation. a–c Diagram of the experiment demonstrating the microdialysis probe in the olfactory bulb (a and b), microinjection needle in the HDB, and the chromatogram of a 10-μl mixed solution containing the sample and internal standard (c isopropylhomocholine: IPHC). The ACh and IPHC peaks represent 68 fmol and 100 fmol measured by electrochemical detector after separation by HPLC. d, e The effects of focal chemical stimulation of the unilateral HDB on extracellular ACh release in the olfactory bulb. The level of ACh release in the perfusate every 3 min, measured by a microdialysis technique, is plotted on the ordinate. The onset of HDB stimulation is expressed as time zero on the abscissa. The upper horizontal bar indicates a microinjection of l-glutamate (50 nmol in 50 nl) into the HDB for 1 min. d Sample response in one rat. e Summary of the responses (n = 6). In each rat, one to two trials were summarized. Each column and vertical bar represent the mean ± SEM. * p < 0.05; significantly different from the pre-stimulus control values (−3 to 0 min) using a one-way repeated-measures ANOVA followed by a Dunnett’s multiple comparison test
Measurement of regional blood flow in the olfactory bulb and cortex
The animals were mounted on a stereotaxic instrument (SR-5R-HT, Narishige) in a prone position. In eight rats, the regional blood flow in the olfactory bulb and cortex (frontal and parietal cortices) was measured via laser speckle contrast imaging. Following a craniotomy, the surface of the brain was covered with mineral oil. The laser speckle contrast imaging device was then fixed, and the zoom was adjusted to cover the dorsal surface of the brain from the most anterior part of the olfactory bulb to the middle of the parietal cortex (Fig. 2a), according to the methods described previously [24, 25]. Laser speckle contrast imaging was performed with a Moor full-field perfusion imaging device consisting of an infrared laser diode (785 nm wavelength) and a CCD camera (Moor Instruments, Devon, U.K.). The viewing field covered approximately 108 mm2 (12 × 9 mm) with a matrix of 152 × 113 pixels, providing an approximate resolution of 79 µm per pixel. The images were sampled at 25 Hz with an exposure time of 4 ms. On-line averaging generated one mean image every 30 s. These images were acquired continuously throughout the entire trial, providing 30 images over a period of 15 min.
Fig. 2.
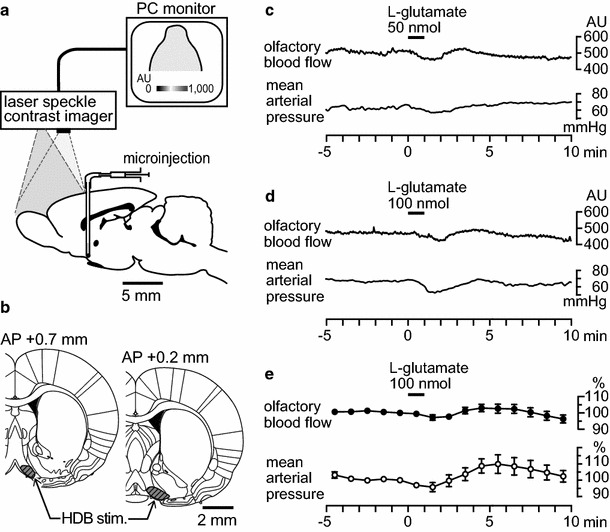
Effects of focal chemical stimulation of the HDB on blood flow in the olfactory bulb ipsilateral to the stimulation. a Diagram of the experiment demonstrating the measurement of cerebral blood flow using a laser speckle contrast imager, and microinjection needle in the HDB. b The HDB area is indicated by the shaded portion. c, d Sample recordings of the olfactory blood flow and mean arterial pressure following a microinjection of l-glutamate (c 50 nmol in 50 nl, d 100 nmol in 100 nl) into the HDB in one rat. e Summary of responses (n = 8). Each point and vertical bar represents a mean ± SEM. Slight changes in the olfactory blood flow and mean arterial pressure were not statistically significant
For the analysis of spatial changes in blood flow, the acquired images were further averaged over 1-min time bins, leading to an average of 15 images. The baseline image just before HDB stimulation was then subtracted from the other images to assess the relative blood flow changes. To quantify the temporal blood flow changes (in arbitrary units), time courses were extracted in three regions of interest (ROI) (Fig. 3a), including the olfactory bulb (AP = +6.4–7.6 mm from bregma, L = 0.7–1.5 mm to the midline), frontal (AP = +2.0–3.2 mm, L = 1.0–2.2 mm), and parietal (AP = −0.5 to −1.7 mm, L = 2.0–3.2 mm) cortices [27, 29], ipsilateral to the side of HDB stimulation.
Fig. 3.
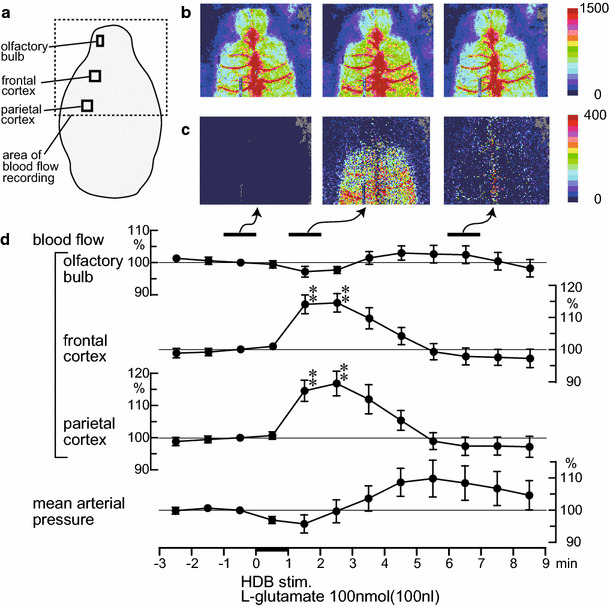
Spatiotemporal changes in the regional cerebral blood flow following a microinjection of l-glutamate (100 nmol in 100 nl) into the HDB. a Area of blood flow recorded by a laser speckle contrast imager is indicated by the dotted line. b,c Sample recording of the regional cerebral blood flow in one rat. b Averaged signal over selected periods of 1 min. c Differential signal changes obtained from b when subtracting the baseline (−1 to 0 min) signal from subsequent images. d Percentage signal changes in the olfactory bulb, frontal, and parietal cortices ipsilateral to the HDB stimulation (data extracted from the ROIs indicated by the black squares in a) and mean arterial pressure averaged every 1 min (n = 8). Each point and vertical bar represent a mean ± SEM. ** p < 0.01; significantly different from the pre-stimulus control values (−1 to 0 min) using a one-way repeated-measures ANOVA followed by a Dunnett’s multiple comparison test
In four rats, the blood flow in the olfactory bulb was measured using laser Doppler flowmetry as described previously [21, 22]. The recording probe (diameter, 0.8 mm) of the laser Doppler flowmeter (ALF 21D, Advance, Tokyo, Japan) was placed in contact with the dorsal surface of the unilateral olfactory bulb following a partial craniotomy.
Stimulation of the HDB
The unilateral HDB (AP = + 0.7, L = 1.0, V = 8.8 or AP = +0.2, L = 1.4, V = 8.8, Paxinos and Watson’s atlas [27]), was focally stimulated, either chemically or electrically (Fig. 2b). For chemical stimulation of the HDB, a small hole was made in the skull using a dental drill and an injection needle (31 gauge) connected to a 0.5-μl microsyringe (#86250, Hamilton, USA) and Teflon tubing was inserted into the HDB ipsilateral to the olfactory bulb where the ACh or blood flow was measured. In the experiment involving the recording of blood flow using laser speckle contrast imaging, either side of the HDB was stimulated. Through the injection needle, 50–100 nmol of sodium l-glutamate in 50–100 nl (Wako Pure Chemicals, Osaka, Japan) was microinjected for 1 min into the HDB to specifically stimulate the cell bodies of the neurons, but not the nearby nerve fibers. This amount of l-glutamate is adequate to activate basal forebrain cholinergic neurons in the NBM [7, 9] or septal complex [8]. In the ACh release measurement experiment, a dose of l-glutamate (50 nmol/50 nl) was injected twice into the HDB in each rat. For the blood flow measurement experiment, two doses of l-glutamate (50 nmol/50 nl and 100 nmol/100 nl) were injected in this order into the HDB. The minimum interval between the injections was 16 min. At the end of the experiment, 50–100 nl of pontamine sky blue dye was administered to visualize the injection site. Rats were killed by injection of an overdose of pentobarbital. The brains were removed, and histological verification of the tip position of the needle was carried out using frozen coronal brain sections, cut to a thickness of 50 μm.
For the electrical stimulation of the HDB, a coaxial metal electrode with an outer diameter of 0.3 mm was stereotaxically inserted into the HDB, ipsilateral to the olfactory bulb where the ACh or blood flow was measured. Focal electrical stimulation of the HDB was performed using a stimulator (SEN-3301, Nihon Kohden, Tokyo) and stimulus isolation unit (SS-202 J, Nihon Kohden). The parameters for the electric stimulation consisted of a 0.5-ms duration and a 50-Hz frequency for 30 s or 3 min. The stimulus intensity varied from 100–200 μA. Histological verification of the tip position of the stimulation electrode was performed after experiment completion on frozen transverse brain sections.
Statistical analysis
All values are presented as the mean ± SEM. Statistical comparisons were carried out by means of a one-way repeated-measures ANOVA followed by a Dunnett’s multiple comparison test, or paired t-test. A P value < 0.05 was considered to be statistically significant.
Results
Effects of HDB stimulation on ACh release in the olfactory bulb
Extracellular ACh release in the olfactory bulb under resting conditions was 38 ± 6 fmol/3 min (n = 10). In each animal, the basal level of ACh release was stable throughout the experiments.
Figure 1d demonstrates a sample response of the extracellular ACh release in the ipsilateral olfactory bulb following a microinjection of l-glutamate (50 nmol in 50 nl) for 1 min into the unilateral HDB. In the rat, the ACh release under resting conditions was 45 fmol/3 min, which increased to 66 fmol/3 min during the first 3 min following a microinjection of l-glutamate into the HDB. The ACh release then returned to the pre-stimulus control levels for the next 3 min.
Figure 1e summarizes the responses of the extracellular ACh release in the olfactory bulb following a microinjection of l-glutamate (50 nmol in 50 nl) tested in six rats. Extracellular ACh release was significantly increased following a microinjection of l-glutamate into the HDB (F 4,20 = 3.0, p < 0.05, assessed by one-way repeated-measures ANOVA). The increase response of ACh release was significant for the first 3 min following the microinjection and the next 3 min, and then it returned to the pre-stimulus control level.
Focal electrical stimulation of the HDB at intensities of 100 and 200 μA produced an increase in the extracellular ACh release in the olfactory bulb during the stimulation in a stimulus intensity-dependent manner. Following the stimulation at 100 μA and 200 μA, ACh release was significantly increased from 35 ± 10 fmol/3 min to 54 ± 14 fmol/3 min [t(4) = 3.9, p < 0.05, assessed by paired t-test], and from 30 ± 8 fmol/3 min to 68 ± 19 fmol/3 min [t(4) = 11.9, p < 0.001, assessed by paired t-test], respectively (n = 5 rats).
Effects of HDB stimulation on regional blood flow in the olfactory bulb
Figure 2c and d present the sample responses of the blood flow in the ipsilateral olfactory bulb and mean arterial pressure following microinjections of l-glutamate (50 nmol in 50 nl and 100 nmol in 100 nl) for 1 min into the unilateral HDB. Figure 2e summarizes the blood flow responses in the olfactory bulb and mean arterial pressure following chemical stimulation of the HDB tested in eight rats. The olfactory blood flow remained unchanged following a microinjection of l-glutamate into the HDB, irrespective of the l-glutamate dose. The mean arterial pressure was marginally decreased following chemical stimulation of the HDB; however, the response was not statistically significant (Fig. 2e).
Focal electrical stimulation of the HDB at intensities of 100 μA and 200 μA produced no consistent responses in blood flow within the olfactory bulb in the four rats that were tested.
Spatio-temporal changes in cerebral blood flow following HDB stimulation
Figure 3b and c represent the laser speckle flow images in a rat before and after HDB stimulation with a microinjection of l-glutamate (100 nmol/100 nl) for 1 min. Following the HDB stimulation, the blood flow in the olfactory bulb was not affected. In contrast, HDB stimulation produced an increase in blood flow within widespread areas of the cerebral cortex (frontal and parietal cortices) 1–2 min after the stimulation (Fig. 3b, c). The increased blood flow in the cerebral cortex then returned to the pre-stimulus control level 6–7 min after the stimulation (Fig. 3b, c). The averaged responses of the blood flow in the olfactory bulb, frontal, and parietal cortices ipsilateral to the site of HDB stimulation, and that of the mean arterial pressure in eight rats is shown in Fig. 3d. Moreover, the olfactory blood flow and mean arterial pressure exhibited no obvious changes following HDB stimulation. The blood flow in the frontal and parietal cortices significantly increased following HDB stimulation (frontal cortex: F 14,98 = 5.6, p < 0.0001, parietal cortex: F 14,98 = 5.9, p < 0.0001). This blood flow increase response in the frontal and parietal cortices was significant at 1–3 min after starting the l-glutamate microinjection. The increased blood flow in the frontal and parietal cortices gradually returned to the pre-stimulus control levels in approximately 3 min (Fig. 3d). The magnitudes of increased blood flow in the frontal and parietal cortices at 2–3 min after the start of the l-glutamate microinjection were 114 ± 3% and 117 ± 4% of the pre-stimulus control values, respectively (Fig. 4).
Fig. 4.
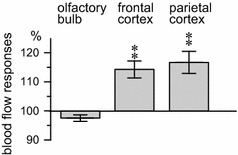
Differences in the blood flow response between the olfactory bulb and cerebral cortices (frontal and parietal cortices) by focal chemical stimulation of the HDB. Percent changes in the blood flow for each area 1–2 min after the onset of the l-glutamate injection are presented (n = 8). Statistical comparisons for each measure are reported in Fig. 3 and associated text
Discussion
This study is the first to demonstrate that the focal stimulation of HDB, either chemically or electrically, increases extracellular ACh release in the olfactory bulb ipsilateral to the stimulation, using an in vivo microdialysis method in rats. Furthermore, the increased ACh release in the olfactory bulb produced by activation of the HDB was found to have little vasodilatory action in the olfactory bulb.
In the mammalian brain, intracerebral cholinergic neurons originating in the basal forebrain project their fibers to the neocortex, hippocampus and olfactory bulb [12, 30]. It has been reported that the stimulation of the NBM (the major source of cholinergic projection to the neocortex) and the septal complex (the major source of cholinergic projection to the hippocampus) increases extracellular ACh release in the neocortex and hippocampus, respectively, in rats [8, 9, 11]. Histological studies have shown that the olfactory bulb receives cholinergic axonal projections from nerve cells originating in the HDB [12, 16]. However, there have been no reports directly measuring the extracellular release of ACh in the olfactory bulb and its response to HDB activation in vivo. The present study successfully measured the level of extracellular ACh release in the olfactory bulb using an in vivo microdialysis method in anesthetized rats. In addition, we showed that the focal stimulation of HDB, either chemically or electrically, produces a significant increase in ACh release in the olfactory bulb ipsilateral to the stimulation. Our present study clearly demonstrated that the cholinergic input to the olfactory bulb originating in the HDB functions to release ACh in the olfactory bulb, similar to the cholinergic input to the neocortex and hippocampus originating in the NBM and septal complex, respectively (Fig. 5).
Fig. 5.
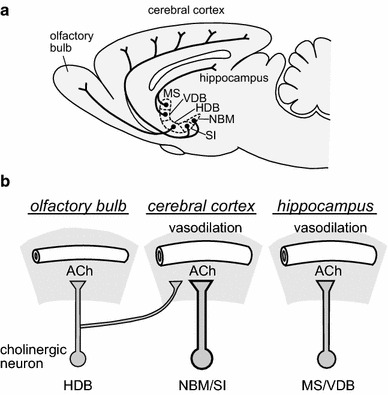
Summary of the basal forebrain cholinergic vasodilative system. a Diagram showing the cholinergic neurons in the basal forebrain and their fiber projections (modified from Sato et al. [10] based on Rye et al. [12]). b Comparative schema of ACh release and vasodilation in the three cholinergic pathways of the HDB-olfactory bulb, NBM/SI-cerebral cortex, and MS/VDB-hippocampus. HDB horizontal limb of the diagonal band of Broca, NBM nucleus basalis of Meynert; SI substantia innominate; MS medial septal nucleus; VDB vertical limb of the diagonal band of Broca
Previous studies have found that the activation of cholinergic fibers originating in the NBM and septal complex in the basal forebrain and projecting to the neocortex and hippocampus increases the regional blood flow in the neocortex and the hippocampus through the release of ACh [7–9, 31]. These regional blood flow responses have been demonstrated to involve the activation of both muscarinic and nicotinic ACh receptors in the neocortex [7], as well as the activation of the nicotinic ACh receptors in the hippocampus [8]. Based on this evidence, we expected that the activation of the cholinergic pathway originating in the HDB and extending into the olfactory bulb might also have a vasodilatory function in the olfactory bulb. Contrary to our expectations, the present results revealed that focal chemical stimulation of the HDB does not significantly influence the regional blood flow in the olfactory bulb; however, such stimulation significantly increases the regional blood flow in the neocortex (frontal and parietal cortices where the blood flow was measured). Histological studies have found that the neurons originating in the HDB project their fibers into both the olfactory bulb, as well as a portion of the neocortex [16, 32]. Therefore, this HDB-neocortical projection may mediate the vasodilatory response in the neocortex (Fig. 5). The present results regarding the increased cortical blood flow in response to HDB stimulation implies that the HDB is adequately activated following stimulation. Therefore, our results suggest that cholinergic input to the olfactory bulb from the HDB may not contribute to the regulation of blood flow in the olfactory bulb.
Our previous studies showed that the stimulation of nicotinic ACh receptors via an intravenous injection of nicotine increases the regional blood flow in the neocortex, hippocampus, and olfactory bulb [21, 33, 34]. In addition, the threshold dose of nicotine producing vasodilation was much higher in the olfactory bulb (100 μg/kg) than in the neocortex (3 μg/kg) or hippocampus (30 μg/kg). In the neocortex, nicotinic ACh receptors in the brain parenchyma were suggested to mediate the nicotine-induced vasodilation. This is because the blood flow response was not influenced by a blood–brain barrier (BBB)-impermeable nicotinic ACh receptor antagonist, hexamethonium, but was abolished by a BBB-permeable nicotinic AChR receptor antagonist [34]. In contrast, hexamethonium ablated the blood flow response in the olfactory bulb, indicating that in the olfactory bulb, nicotinic ACh receptors outside of the BBB, presumably located in the extracranial nerves innervating arteries serving the olfactory bulb, mediate the nicotine-induced vasodilation [21]. These data also suggest that basal forebrain cholinergic fibers projecting into the olfactory bulb are less functional for vasodilatory effects.
Concerning the reason for absence of vasodilatory role in the HDB cholinergic inputs to the olfactory bulb, distribution density of cholinergic receptor subtypes may be related. The subtype of nicotinic ACh receptors that contribute to cholinergic vasodilation in the neocortex was found to be the alpha-4 beta-2 subtype [35]. Distribution of nicotinic and muscarinic ACh receptors as well as cholinergic innervation have been detected in the olfactory bulb [36, 37]. However, a hybridization histochemical study which examined the distribution of neuronal nicotinic ACh receptor subtypes revealed that alpha-4 and beta-2 mRNAs were expressed in all layers of the neocortex (isocortex) while those were not detected in most layers of the olfactory bulb [38]. Lower distribution density of alpha-4 beta-2 subtype nicotinic ACh receptors in the olfactory bulb may be a reason for absence of a vasodilatory role in cholinergic innervation. Another reason may be related to the distribution pattern of perivascular cholinergic terminals originating in the basal forebrain neurons. Both the fronto-parietal and the perirhinal cortices receive cholinergic inputs from the basal forebrain neurons. However, when studied at the ultrastructural level in the fronto-parietal and perirhinal cortices, perivascular nerve terminals were located significantly closer to microvessels in the fronto-parietal than in the perirhinal cortex [39]. These differences are in agreement with physiological blood flow studies showing that NBM stimulation increases blood flow in the fronto-parietal cortices, but not in the perirhinal cortex [40]. The cholinergic nerve terminals from the basal forebrain neurons reaching microvessels may be fewer in the olfactory bulb, and this possibility should be examined in future studies. Rat strain differences do not contribute to the reason for absence of a vasodilatory role in the HDB cholinergic inputs to the olfactory bulb, because we used the Wistar strain of rats in the present study as well as the previous reports [7, 8]. The physiological role of cholinergic input to the olfactory bulb arising from the basal forebrain may be primarily in modulation of olfactory sensory processing [5, 41], rather than in blood flow regulation in the olfactory bulb.
Cholinergic neurons in the basal forebrain exhibit degeneration in patients with Alzheimer’s disease [17] and in healthy aged people [18, 19]. Histological studies demonstrated that 80–90% of neurons originating in the NBM/SI and projecting to the cortex, 35–45% of the neurons originating in the MS/VDB and projecting to the hippocampus, and 10–20% of the neurons originating in the HDB and projecting to the olfactory bulb are cholinergic (ChAT-positive) [12]. We speculate that the small number of the cholinergic neurons originating in the HDB and projecting to the olfactory bulb might lose their function earlier than those of the septo-hippocampal or NBM-cortical cholinergic system during the aging process or progression of Alzheimer’s disease. Further study will be necessary to clarify this possibility. In addition, the absence of a vasodilatory role in the HDB cholinergic inputs to the olfactory bulb, demonstrated in the present study, may contribute to the age-related decreases in the volume of the olfactory bulb [42, 43] and olfactory sensitivity [3, 44], because of less protection by blood flow supply to olfactory bulb neurons. Cholinergic vasodilatory regulation of blood flow in the neocortex and the hippocampus may be important for maintaining cognition and memory functions [45, 46]. Individual variability of the degree of degeneration in the basal forebrain cholinergic neurons in Alzheimer’s disease and in aged people may cause variability of blood flow in the neocortex and hippocampus in those patients.
In conclusion, the present study demonstrated that the activation of the HDB in the basal forebrain produces an increase in ACh release in the olfactory bulb. The increased ACh was less functional regarding blood flow regulation in the olfactory bulb, which is in contrast to that displayed in the neocortex and hippocampus (Fig. 5).
Acknowledgements
This work was supported by JSPS KAKENHI (Grant Number JP15K08225 to S.U.) and by the Smoking Research Foundation.
Author contributions
Both authors contributed to the conception and design of the research, performed experiments and analyzed data, and interpreted the results of the experiments. S.U. drafted the manuscript; both authors edited and revised the manuscript, and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Djordjevic J, Jones-Gotman M, De Sousa K, Chertkow H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2008;29:693–706. doi: 10.1016/j.neurobiolaging.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 3.Doty RL, Kamath V. The influences of age on olfaction: a review. Front Psychol. 2014 doi: 10.3389/fpsyg.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballinger EC, Ananth M, Talmage DA, Role LW. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron. 2016;91:1199–1218. doi: 10.1016/j.neuron.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Souza RD, Vijayaraghavan S. Paying attention to smell: cholinergic signaling in the olfactory bulb. Front Synaptic Neurosci. 2014 doi: 10.3389/fnsyn.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Micheau J, Marighetto A. Acetylcholine and memory: a long, complex and chaotic but still living relationship. Behav Brain Res. 2011;221:424–429. doi: 10.1016/j.bbr.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 7.Biesold D, Inanami O, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases cerebral cortical blood flow in rats. Neurosci Lett. 1989;98:39–44. doi: 10.1016/0304-3940(89)90370-4. [DOI] [PubMed] [Google Scholar]
- 8.Cao W-H, Inanami O, Sato A, Sato Y. Stimulation of the septal complex increases local cerebral blood flow in the hippocampus in anesthetized rats. Neurosci Lett. 1989;107:135–140. doi: 10.1016/0304-3940(89)90805-7. [DOI] [PubMed] [Google Scholar]
- 9.Kurosawa M, Sato A, Sato Y. Stimulation of the nucleus basalis of Meynert increases acetylcholine release in the cerebral cortex in rats. Neurosci Lett. 1989;98:45–50. doi: 10.1016/0304-3940(89)90371-6. [DOI] [PubMed] [Google Scholar]
- 10.Sato A, Sato Y. Regulation of regional cerebral blood flow by cholinergic fibers originating in the basal forebrain. Neurosci Res. 1992;14:242–274. doi: 10.1016/0168-0102(92)90071-J. [DOI] [PubMed] [Google Scholar]
- 11.Uchida S, Hotta H, Misawa H, Kawashima K. Sustained subcutaneous infusion of nicotine enhances cholinergic vasodilation in the cerebral cortex induced by stimulation of the nucleus basalis of Meynert in rats. Eur J Pharmacol. 2011;654:235–240. doi: 10.1016/j.ejphar.2010.12.029. [DOI] [PubMed] [Google Scholar]
- 12.Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience. 1984;13:627–643. doi: 10.1016/0306-4522(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 13.Wenk H, Bigl V, Meyer U. Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res. 1980;2:295–316. doi: 10.1016/0165-0173(80)90011-9. [DOI] [PubMed] [Google Scholar]
- 14.Adachi T, Inanami O, Ohno K, Sato A. Responses of regional cerebral blood flow following focal electrical stimulation of the nucleus basalis of Meynert and the medial septum using the [14C]iodoantipyrine method in rats. Neurosci Lett. 1990;112:263–268. doi: 10.1016/0304-3940(90)90214-T. [DOI] [PubMed] [Google Scholar]
- 15.Milner TA, Amaral DG. Evidence for a ventral septal projection to the hippocampal formation of the rat. Exp Brain Res. 1984;55:579–585. doi: 10.1007/BF00235290. [DOI] [PubMed] [Google Scholar]
- 16.Záborszky L, Carlsen J, Brashear HR, Heimer L. Cholinergic and GABAergic afferents to the olfactory bulb in the rat with special emphasis on the projection neurons in the nucleus of the horizontal limb of the diagonal band. J Comp Neurol. 1986;243:488–509. doi: 10.1002/cne.902430405. [DOI] [PubMed] [Google Scholar]
- 17.Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, DeLong MR. Alzheimer’s disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- 18.Grothe M, Heinsen H, Teipel S. Longitudinal measures of cholinergic forebrain atrophy in the transition from healthy aging to Alzheimer’s disease. Neurobiol Aging. 2013;34:1210–1220. doi: 10.1016/j.neurobiolaging.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer’s disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–745. doi: 10.1212/WNL.34.6.741. [DOI] [PubMed] [Google Scholar]
- 20.Uchida S, Suzuki A, Kagitani F, Hotta H. Responses of acetylcholine release and regional blood flow in the hippocampus during walking in aged rats. J Physiol Sci. 2006;56:253–257. doi: 10.2170/physiolsci.SC001706. [DOI] [PubMed] [Google Scholar]
- 21.Shiba K, Machida T, Uchida S, Hotta H. Effects of nicotine on regional blood flow in the olfactory bulb in rats. Eur J Pharmacol. 2006;546:148–151. doi: 10.1016/j.ejphar.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 22.Shiba K, Machida T, Uchida S, Hotta H. Sympathetic neural regulation of olfactory bulb blood flow in adult and aged rats. Auton Neurosci. 2009;147:75–79. doi: 10.1016/j.autneu.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21:195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Piché M, Uchida S, Hara S, Aikawa Y, Hotta H. Modulation of somatosensory-evoked cortical blood flow changes by GABAergic inhibition of the nucleus basalis of Meynert in urethane-anaesthetized rats. J Physiol. 2010;588:2163–2171. doi: 10.1113/jphysiol.2010.187633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hotta H, Uchida S, Kagitani F, Maruyama N. Control of cerebral cortical blood flow by stimulation of basal forebrain cholinergic areas in mice. J Physiol Sci. 2011;61:201–209. doi: 10.1007/s12576-011-0139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida S, Hotta H, Misawa H, Kawashima K. The missing link between long-term stimulation of nicotinic receptors and the increases of acetylcholine release and vasodilation in the cerebral cortex of aged rats. J Physiol Sci. 2013;63:95–101. doi: 10.1007/s12576-012-0239-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Compact 6th edition. Amsterdam: Academic Press; 2009. [Google Scholar]
- 28.El-Etri MM, Ennis M, Griff ER, Shipley MT. Evidence for cholinergic regulation of basal norepinephrine release in the rat olfactory bulb. Neuroscience. 1999;93:611–617. doi: 10.1016/S0306-4522(99)00169-4. [DOI] [PubMed] [Google Scholar]
- 29.Zilles K. The cortex of the rat. Berlin: Springer; 1985. [Google Scholar]
- 30.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6) Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 31.Sato A, Sato Y, Uchida S. Activation of the intracerebral cholinergic nerve fibers originating in the basal forebrain increases regional cerebral blood flow in the rat’s cortex and hippocampus. Neurosci Lett. 2004;361:90–93. doi: 10.1016/j.neulet.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Luiten PG, Gaykema RP, Traber J, Spencer DG., Jr Cortical projection patterns of magnocellular basal nucleus subdivisions as revealed by anterogradely transported Phaseolus vulgaris leucoagglutinin. Brain Res. 1987;413:229–250. doi: 10.1016/0006-8993(87)91014-6. [DOI] [PubMed] [Google Scholar]
- 33.Kagitani F, Uchida S, Hotta H, Sato A. Effects of nicotine on blood flow and delayed neuronal death following intermittent transient ischemia in rat hippocampus. Jpn J Physiol. 2000;50:585–595. doi: 10.2170/jjphysiol.50.585. [DOI] [PubMed] [Google Scholar]
- 34.Uchida S, Kagitani F, Nakayama H, Sato A. Effect of stimulation of nicotinic cholinergic receptors on cortical cerebral blood flow and changes in the effect during aging in anesthetized rats. Neurosci Lett. 1997;228:203–206. doi: 10.1016/S0304-3940(97)00401-1. [DOI] [PubMed] [Google Scholar]
- 35.Uchida S, Hotta H, Kawashima K. Long-term nicotine treatment reduces cerebral cortical vasodilation mediated by alpha4beta2-like nicotinic acetylcholine receptors in rats. Eur J Pharmacol. 2009;609:100–104. doi: 10.1016/j.ejphar.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Durand M, Coronas V, Jourdan F, Quirion R. Developmental and aging aspects of the cholinergic innervation of the olfactory bulb. Int J Dev Neurosci. 1998;16:777–785. doi: 10.1016/S0736-5748(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 37.Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 38.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, Swanson LW. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 39.Vaucher E, Hamel E. Cholinergic basal forebrain neurons project to cortical microvessels in the rat: electron microscopic study with anterogradely transported Phaseolus vulgaris leucoagglutinin and choline acetyltransferase immunocytochemistry. J Neurosci. 1995;15:7427–7441. doi: 10.1523/JNEUROSCI.15-11-07427.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaucher E, Borredon J, Bonvento G, Seylaz J, Lacombe P. Autoradiographic evidence for flow-metabolism uncoupling during stimulation of the nucleus basalis of Meynert in the conscious rat. J Cereb Blood Flow Metab. 1997;17:686–694. doi: 10.1097/00004647-199706000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Devore S, Linster C. Noradrenergic and cholinergic modulation of olfactory bulb sensory processing. Front Behav Neurosci. 2012 doi: 10.3389/fnbeh.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yousem DM, Geckle RJ, Bilker WB, Doty RL. Olfactory bulb and tract and temporal lobe volumes. Normative data across decades. Ann N Y Acad Sci. 1998;855:546–555. doi: 10.1111/j.1749-6632.1998.tb10624.x. [DOI] [PubMed] [Google Scholar]
- 43.Hinds JW, McNelly NA. Aging of the rat olfactory bulb: growth and atrophy of constituent layers and changes in size and number of mitral cells. J Comp Neurol. 1977;72:345–367. doi: 10.1002/cne.901710304. [DOI] [PubMed] [Google Scholar]
- 44.Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol Behav. 2004;81:435–442. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 45.Ogoh S. Relationship between cognitive function and regulation of cerebral blood flow. J Physiol Sci. 2017;67:345–351. doi: 10.1007/s12576-017-0525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ushijima Y, Okuyama C, Mori S, Nakamura T, Kubota T, Nishimura T. Relationship between cognitive function and regional cerebral blood flow in Alzheimer’s disease. Nucl Med Commun. 2002;23:779–784. doi: 10.1097/00006231-200208000-00012. [DOI] [PubMed] [Google Scholar]


