Abstract
We aim to compare the effects of stretch on isometric tension/Ca2+ transient in the right ventricular trabeculae of control (CONT) and hypertensive (MCT, monocrotaline application) adult male and female rats. The treatment with MCT resulted in RV hypertrophy in males only. Blunted active force-length relation and substantially prolonged twitch were found in MCT-males but not MCT-females (vs same-sex CONT). Ca2+ transient was prolonged in both MCT-treated groups but extremely so in the MCT-males. The gradual stretch resulted in a distinct “bump” on Ca2+ transient decline in CONT and MCT-treated groups. The integral magnitude of the “bump” was unaffected by the treatment with MCT in males or females but was larger in males vs females. The rate of “bump” development was significantly slower in MCT-males. In conclusion, the sex-specific differences in the stretch-dependent regulation of [Ca2+]i may underlie preservation of the Frank–Starling mechanism in female rat myocardium in monocrotaline-induced pulmonary hypertension.
Keywords: Monocrotaline-induced pulmonary hypertension, Right ventricular hypertrophy, Isometric twitch, Ca2+ transient
Introduction
The strength of contraction of a cardiac muscle is highly dependent on the extent of its stretch. The strong relation between sarcomere length (SL) and developed force underlies the Frank–Starling mechanism (FSM) and involves SL-dependent actin-myosin overlap and myofilament Ca2+-sensitivity, as well as the cooperative effects in myosin cross-bridges [1–4]. The faultless action of the FSM provides an effective adaptation of ventricular contraction to increasing end-diastolic filling. In recent years, FSM has been under increasing interest as it reveals the sex-specific character of alterations in heart diseases.
Heart diseases are often accompanied by specific alteration in cytosolic Ca2+ handling which affects the strength of contraction (e.g. see reviews [5, 6]). The hearts with compensatory right ventricular hypertrophy after pulmonary hypertension have impaired systolic function [7, 8] together with altered intracellular Ca2+ homeostasis [9, 10]. Substantially slower Ca2+ transients have been found in compensatory ventricular hypertrophy and heart failure [9, 11–15], while diverse data exist about Ca2+ transient amplitude or systolic level: from severely diminished [12, 13, 16, 17] to unchanged [10, 14] or even elevated levels [16]. The impaired regulation of cytosolic Ca2+ affects stretch-induced activation of contraction; e.g., exhausted or blunted FSM has been found in failing human and canine myocardium [17–20], while others observed the preservation of this mechanism in human hearts under similar pathological conditions [21–23].
It is known that pulmonary hypertension and right ventricular hypertrophy develop differently in females vs males [24, 25]; e.g., the protective effect of sex hormones on the onset of RV hypertrophy has been proven in adult female rats [26–28]. The contractile function is preserved in pressure-overloaded left ventricles of female rats, whereas males suffer from early transition to heart failure [29, 30]. It has been shown that an adaptive reserve of myocardial contractility is higher in female vs male spontaneously hypertensive rats with compensatory hypertrophy, which correlates to the low survival rate of male rats with chronic heart failure [31].
While contractility has been shown to alter differently in male and female rats in pulmonary hypertension and RV hypertrophy, little is known about sex-specific differences in the stretch-dependent regulation of cytosolic Ca2+ in this pathological state. Such discrepancies may underlie the different effect of monocrotaline on myocardial contractility in males and females. In this study, we evaluated the stretch-dependent effects on isometric tension and Ca2+ transient in RV trabeculae of adult male and female healthy rats and rats in sustained pulmonary hypertension.
Methods
All experiments with animals conformed to the “Principles of Laboratory Animal Care” (NIH Publication No. 85-23 revised 1985) and were approved by the local Institutional Animal Care and Use Committee. Here, 20-week-old Wistar rats of both sexes were supplied from the institutional animal house and randomly divided into four groups: control male (CONT-male), control female (CONT-female), monocrotaline-treated male (MCT-male), and monocrotaline-treated female (MCT-female); n = 7 in each group. The rats of MCT groups were subjected to a single subcutaneous injection of monocrotaline-containing saline solution (2 ml/kg body weight) with a final concentration of 60 mg/kg body weight. Control rats were subjected to a single injection of an equivalent volume of MCT-free saline solution. Animals were maintained on a 12:12 h light-dark cycle and had unrestricted access to standard chow and water. MCT and CONT rats were euthanised 5 weeks after the treatment (25 weeks old).
Muscle isolation and data acquisition
Animals were anaesthetised with Zoletil® (0.02 ml/kg body weight) and euthanised by rapid cervical dislocation. The heart was quickly excised and placed in modified Krebs–Henseleit (K–H) solution containing (in mM): NaCl 118.5; KCl 4.2; MgSO4 1.2; CaCl2 2.5; glucose 11.1, bubbled with 95 %O2 + 5 %CO2 and buffered with NaHCO3 and KH2PO4 to maintain pH = 7.35. Some 2,3-butanedione monoxime (BDM, 30 mM) was added to prevent damage to muscles during isolation. Thin trabeculae were dissected from the right ventricle, attached to a force transducer and length servomotor, placed in an experimental chamber, and washed with BDM-free K-H solution.
The fluorescent indicator fura-2 in its esterified form (fura-2/AM) was used to monitor free cytosolic Ca2+. Fura-2/AM was loaded into a trabecula by continuous perfusion with oxygenated loading solution (K–H solution with 5 μM fura-2/AM and 5% w/v Pluronic F-127) for 1.5 h at 22–23 °C and 0.2 Hz pacing rate. At the end of this period, perfusion was replaced to indicator-free K–H solution and a trabecula was equilibrated for at least 60 min. Measurements were taken at 25 °C and 0.33 Hz pacing rate with electrical stimulation by rectangular impulses with 1.2-threshold amplitude and 0.5 ms duration.
Force, length and fura-2 fluorescence were simultaneously measured in a trabecula using a Muscle Research System (Scientific Instruments GmbH, Heidelberg, Germany) equipped with an inverted Axiovert 200 epifluorescent microscope (Carl Zeiss, Germany). A halogen short-arc lamp USH-103D (Ushio Inc., Japan) was used to excite fura-2 at 340 nm or 380 nm wavelengths via narrow-band filters. The emission signals were collected at a wavelength of 510 nm and converted by the hardware to the 340/380 nm ratio. The excitation and emission light passed through the 10X/0.50 FLUAR objective (Carl Zeiss, Germany). To diminish fura-2 photobleaching, emission was acquired during short periods of excitation (~30 s). Force, length and fluorescence were sampled at 10 kHz by an analogue-to-digital (A/D) and D/A data converter PCI-1716S (AdLink Technology Inc., Taiwan) using custom-made software running in a real-time environment (HyperKernel, Arc Systems Ltd., Japan) integrated with the Windows XP OS. Data were processed and analysed offline using custom-made software.
Experimental protocol and data analysis
At the end of equilibration, a trabecula was released to a slack length. Force and Ca2+ transients were simultaneously measured in the trabecula contracting in steady-state isometric conditions at this length. The trabecula was then gradually stretched for ~2–3 % of the slack length and allowed to equilibrate at a new length prior to further experimental recordings. The stretching was completed when no further increase in the amplitude of active tension was observed; this final length was assumed as L MAX.
The characteristics of tension and Ca2+ transient were measured in a trabecula under steady-state isometric conditions at different stretches (each was expressed as a relative length of 80, 85, 90, 95 % L MAX, or L MAX). Ten sequential twitches were averaged to get force and fluorescence traces at each stretch. Tension was calculated assuming the circular cross-section of a trabecula. Ca2+ transients were obtained as a ratio of fluorescence intensities emitted at 510 nm by excitation at 340 or 380 nm, without further conversion to cytosolic calcium because we were interested in stretch-related (relative) effects. The samples of the simultaneous measurement of isometric tension and non-calibrated Ca2+ transient in rat trabeculae at different stretches (indicated as a % of L MAX) are presented in Fig. 1. Rest and peak values, amplitude, time-to-peak and total duration of steady-state isometric tension and Ca2+ transient were calculated in a trabecula at each stretch.
Fig. 1.
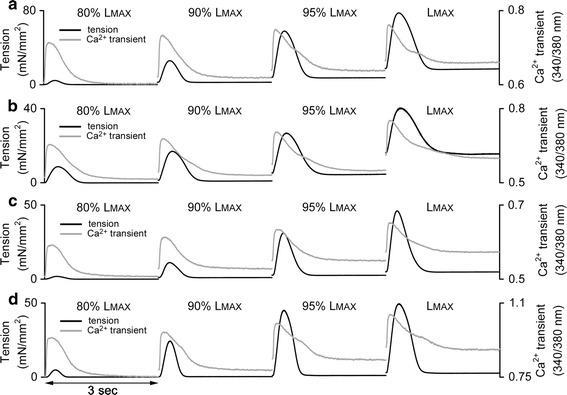
The samples of isometric tension (black lines, left Y axis) and non-calibrated Ca2+ transient (grey lines, right Y axis) measured in rat trabeculae at different stretches (indicated in a % of L MAX) under steady-state conditions. a CONT-male. b MCT-male. c CONT-female. d MCT-female
Histological assessment of endothelial proliferation of pulmonary vein
A small portion of pulmonary vein was excised and fixed in formalin before histological preparation. Fixed tissue was sliced into 5-μm-thick samples and stained by haematoxylin-eosin. The samples were analysed by a Leica DM-2000 microscope (Leica Microsystems, Germany) at 400×; images were captured and processed by the software “VideoTesT-Morphology 5.0” (VideoTesT, Saint Petersburg, Russia).
Chemicals
All chemicals were purchased from Sigma-Aldrich (USA), except fura-2/AM (Fluka Biochemika, Switzerland).
Statistics
A Mann–Whitney U test was used to evaluate differences in the mean values of individual characteristics — amplitude of tension, time-to-peak tension, Ca2+ amplitude, etc. — measured at the given stretch (expressed as a % of L MAX) and in the morphometric indices between same-sex CONT and MCT groups (i.e., CONT-male vs MCT-male, CONT-female vs MCT-female). A two-way repeated measures ANOVA was used to evaluate the combined effect of treatment (CONT vs MCT) and sex (male vs female) on the stretch-dependent changes in the measured characteristics. Mean values are given as mean ± SEM. Effects were considered significant at P < 0.05.
Results
Heart morphometry and structural changes in pulmonary vein
The morphometric indices obtained for male and female rats of the control and monocrotaline-treated groups are summarised in Table 1. A significant increase in heart weight, left ventricular weight and right ventricular weight (expressed either in absolute values or normalised to body weight) was observed in the MCT-male group compared to CONT-male. The morphometric indices in MCT-female rats did not differ from CONT-female rats. Therefore, the myocardium of female rats still lacked ventricular hypertrophy 5 weeks after the treatment with monocrotaline at a dose of 60 mg/kg body weight.
Table 1.
Morphometric indices of control and monocrotaline-treated male and female rats
| CONT-male | MCT-male | CONT-female | MCT-female | |
|---|---|---|---|---|
| Body weight (BW) (g) | 547 ± 20 | 560 ± 9 | 345 ± 10& | 338 ± 8& |
| Whole heart weight, with septum (HW) (g) | 1.32 ± 0.04 | 1.70 ± 0.04* | 0.89 ± 0.03& | 0.94 ± 0.02& |
| LV weight (LVW) (g) | 0.73 ± 0.04 | 0.89 ± 0.02* | 0.49 ± 0.02& | 0.54 ± 0.01& |
| RV weight (RVW) (g) | 0.28 ± 0.01 | 0.52 ± 0.03* | 0.20 ± 0.01& | 0.21 ± 0.01& |
| HW/BW (%) | 0.24 ± 0.01 | 0.30 ± 0.01* | 0.26 ± 0.01 | 0.28 ± 0.01 |
| LVW/HW (%) | 55.2 ± 1.5 | 52.6 ± 1.4 | 55.6 ± 1.4 | 58.0 ± 0.6& |
| RVW/HW (%) | 21.4 ± 0.4 | 30.4 ± 1.2* | 22.3 ± 0.6 | 21.9 ± 0.6& |
Data presented as mean ± SEM
Symbols indicate significant difference (at P < 0.05): * MCT-male vs CONT-male, & female vs male of corresponding group (control or MCT-treated). No significant differences were observed between MCT-females and CONT-females
BW body weight, HW whole heart weight (including septum), LVW left ventricle weight, RVW right ventricle weight
However, a similar extent of hyperplasia in endothelial cells, interstitial oedema, and inflammatory cell infiltration in the pulmonary vein was found in MCT-male and MCT-female rats (Fig. 2). We concluded that the treatment with monocrotaline was effective for inducing pulmonary hypertension in male and female rats.
Fig. 2.
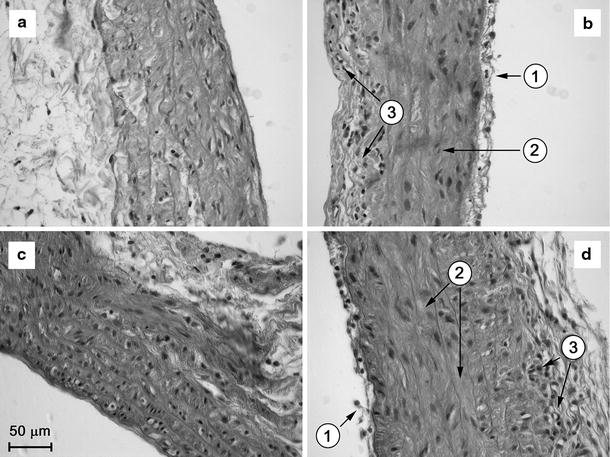
Microphotographs of transverse histological sections of pulmonary vein of control (CONT) and monocrotaline-treated (MCT) male and female rats. a CONT-male. b MCT-male. c CONT-female. d MCT-female. 1 endothelial proliferation, 2 interstitial oedema, 3 accumulation of inflammatory cells. The scale bar is common for all panels
The effect of stretch on isometric tension
The stretch-dependent changes in resting tension (developed by a trabecula in diastole) and amplitude of isometric tension (peak isometric tension minus resting tension) were examined in trabeculae of CONT and MCT-treated male and female groups. Tension was plotted against a relative length expressed as % of L MAX. The Frank–Starling mechanism was significantly blunted in MCT-male compared to CONT-male (Fig. 3a, left panel), but was highly preserved in the MCT-female group (Fig. 3a, right panel). The deficiency in amplitude of isometric tension in the MCT-treated group vs the same-sex CONT group was evaluated at each relative length (80, 85, 90, 95 % L MAX and L MAX) and the average deficiency was calculated. This average deficiency amounted to 38.8 ± 1.1 % in MCT-male rats (vs CONT-male, P < 0.05), but only to 7.5 ± 5.2 % in MCT-female rats (vs CONT-female, not significant). Male and female CONT groups were no different in their force-length relations, but this relation was significantly lowered in MCT-male vs MCT-female (P < 0.05).
Fig. 3.
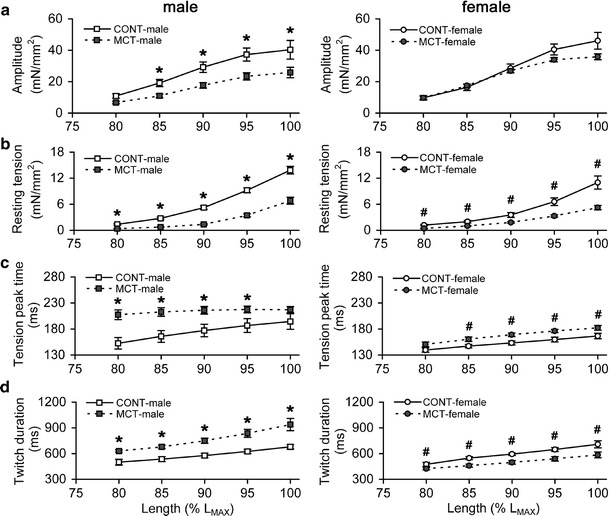
The stretch-dependent changes in tension characteristics of RV trabeculae of control and monocrotaline-treated male (left panels) and female rats (right panels). a Amplitude of isometric tension. b Resting tension. c Time-to-peak tension. d Total duration of isometric twitch. Relative length is expressed as a % of L MAX. Y axis scales are identical for males and females. Data presented as mean ± SEM. *Significant difference between CONT-male vs MCT-male at the same relative length (P < 0.05), #significant difference between CONT-female vs MCT-female at the same relative length (P < 0.05)
The resting tension-length relation was significantly shallow for MCT-male and MCT-female groups vs same-sex control rats (Fig. 3b). On average, the resting tension in MCT-male and MCT-female rats was 33.3 ± 4.5 and 45.0 ± 2.6 %, respectively, of the corresponding value in the same-sex CONT group (P < 0.05). Trabeculae from the CONT-male group developed significantly higher resting tension vs CONT-female rats (P < 0.05 for the stretches above 80 % L MAX). In contrast, no difference in resting tension-length relations was found between MCT-male and MCT-female rats.
At any relative length, the time-to-peak tension was significantly augmented by 22.9 ± 4.3 % in MCT-male and by 9.4 ± 0.5 % in MCT-female rats, respectively, over the value of same-sex control groups (Fig. 3c). The total duration of isometric twitch was higher by 31.0 ± 2.3 % in MCT-male vs CONT-male rats, but was lower by 15.4 ± 1.2 % in MCT-female vs CONT-female rats (Fig. 3d); these differences are significant at P < 0.05. Stretch caused significant increases in time-to-peak and total duration of twitch in both control groups and in the MCT-female group. In the MCT-male group, time-to-peak tension was faintly stretch-dependent (Fig. 3c, left panel), whereas total duration of isometric twitch rose with stretch to an even larger extent compared to any other group (Fig. 3d, left panel).
The effect of stretch on Ca2+ transient
The changes in free cytosolic Ca2+ in isolated RV trabeculae were monitored using the fluorescent indicator fura-2 in its cell-permeant form (fura-2/AM) and acquired as a ratio of intensities measured at 510 nm wavelength by excitation at 340/380 nm wavelengths (non-calibrated “ratio” Ca2+ transient). Due to the non-calibrated character of Ca2+ transients, we were unable to use absolute amplitudes or rest levels of the transients to compare the effects between individual groups. Rather, we evaluated stretch-dependent changes in the amplitude and rest level of non-calibrated Ca2+ transients and presented the changes as a % of the value measured at 80 % L MAX (referred to as non-stretched condition). Timing properties of Ca2+ transients were evaluated in absolute values as they are calibration-independent.
Ca2+ transient amplitude decreased with stretch in CONT-male rats while the MCT-male group demonstrated an opposite effect; both showed linear behaviour (Fig. 4a, left panel). We evaluated how much this relative change in Ca2+ transient amplitude was per stretch by 5 % L MAX. On average, each new stretch by 5 % L MAX (e.g., from 80 to 85 % L MAX, from 85 to 90 % L MAX, etc) decreased Ca2+ transient amplitude by 3.3 ± 0.1 % in CONT-male rats and increased it by 3.3 ± 0.3 % in the MCT-male group. These two groups were significantly different in the stretch-induced change in Ca2+ transient amplitude at relative lengths between 90 % L MAX and L MAX. In contrast, both female groups exhibited minor stretch-dependence of Ca2+ transient amplitude (Fig. 4a, right panel). No significant inter-sex differences in the stretch-induced modulation of Ca2+ transient amplitude were observed either in the control or MCT-treated groups.
Fig. 4.
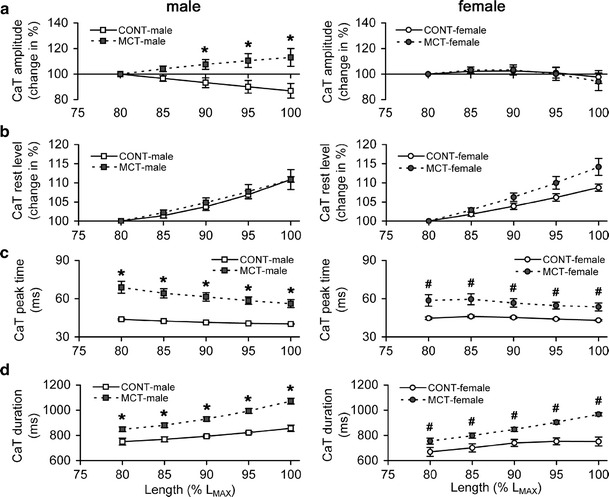
The stretch-dependent changes in the characteristics of Ca2+ transient (CaT) measured in RV trabeculae of control and monocrotaline-treated male (left panels) and female rats (right panels). a Ca2+ transient amplitude (change in % of value measured at 80 % L MAX). b Rest level of Ca2+ transient (change in % of value measured at 80 % L MAX). c Time-to-peak Ca2+ transient. d Total duration of Ca2+ transient. Relative length is expressed as a % of L MAX. Y axis scales are identical for males and females. Data presented as mean ± SEM. *Significant difference between CONT-male vs MCT-male at the same relative length (P < 0.05), #significant difference between CONT-female vs MCT-female at the same relative length (P < 0.05)
The rest level of Ca2+ transient, also expressed as a % of the value measured at 80 % L MAX (referred to as non-stretched condition), increased substantially with stretch in all groups (Fig. 4b). Each new stretch by 5 % L MAX increased the rest level of Ca2+ transient by 2.7 ± 0.5 % in CONT-male, 2.7 ± 0.2 % in MCT-male, 2.2 ± 0.2 % in CONT-female, and 3.5 ± 0.3 % in MCT-female rats (not significantly different).
The time-to-peak Ca2+ transient was significantly larger by 48.1 ± 3.0 % in MCT-male and 26.7 ± 1.4 % in MCT-female groups, respectively, over the value in the same-sex control group (Fig. 4c). Similarly, the total duration of Ca2+ transient was significantly augmented in MCT-male vs CONT-male (on average by 18.2 ± 2.2 %) and MCT-female vs CONT-female (by 18.0 ± 3.0 %), whatever relative length was tested (Fig. 4d). In all groups, the total duration of Ca2+ transient increased with stretch (as absolute or relative value, i.e. absolute increase normalised to total duration), which was significantly smaller in control vs MCT-treated groups (P < 0.05): ~25 ms or 3.2 % in CONT (males + females) vs ~55 ms or 5.8 % in MCT (males + females) per stretch by 5 % L MAX. Time-to-peak Ca2+ transient, if plotted vs relative length, was not different between the RV trabeculae of male and female rats in the control or MCT-treated groups, but the total duration of Ca2+ transient was significantly shorter in CONT-female vs CONT-male or in MCT-female vs MCT-male rats (P < 0.05).
The effect of stretch on Ca2+ transient “bump”
It has been shown that a brief delay in Ca2+ transient decline (referred to as a “bump”) is commonly observed in stretched rat cardiac muscle contracting under steady-state isometric conditions [32–34]. The consistent effect of stretch is an increase in the magnitude of the “bump”, while the overall decline of Ca2+ transient from peak to rest level remained monotonous (Fig. 5a). Figure 5b demonstrates the time profiles of the difference between an original Ca2+ transient and its monotonous decline (data from Fig. 5a). The integral magnitude of the “bump” is evaluated as an area under the corresponding time profile or, because it is equivalent, as an area between an original Ca2+ transient and its monotonous decline. Note that the time profiles in Fig. 5b have their peaks at different times (as indicated by arrows). A time of peak in the profile minus time-to-peak Ca2+ transient (that is, a peak of time profile is related to the onset of Ca2+ transient decline) is referred by us as a time-to-peak “bump”; the gap between an original Ca2+ transient and its monotonous decline is maximal at this time-to-peak “bump”. The time-to-peak “bump” is also increased by gradual stretch of a trabecula (see arrows in Fig. 5b). In this study, we evaluated stretch-dependent changes in the integral magnitude and time-to-peak of “bump” in CONT and MCT-treated groups of both sexes. The integral magnitude of “bump” was expressed as a % of total area under Ca2+ transient (assuming its monotonous decline), in order to overcome misinterpretation due to the quantitatively different amplitudes of non-calibrated Ca2+ transients, while time-to-peak “bump” was evaluated in absolute values.
Fig. 5.
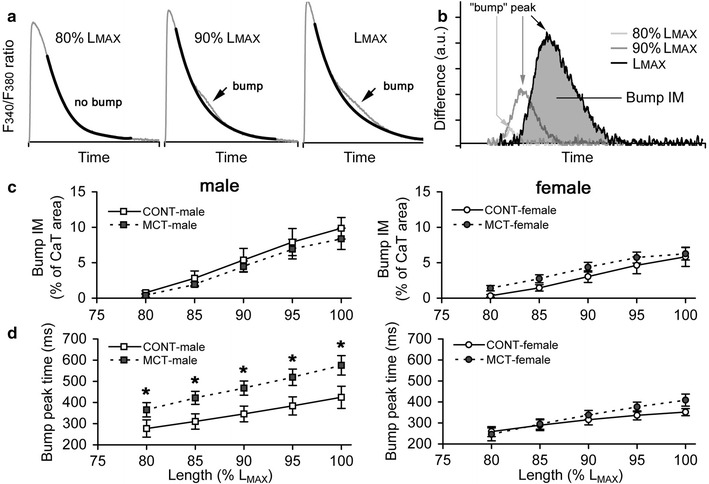
The effect of stretch on characteristics of Ca2+ transient “bump” in RV trabeculae of control and monocrotaline-treated male and female rats. a Original Ca2+ transients (grey lines) obtained in rat trabeculae at different stretches (indicated as a % of L MAX) with superimposed monotonous declines (black lines). Arrows indicate the discrepancy between the original and monotonous traces (“bump”). b Time profiles of “bump” obtained at different stretches (data from panel “a”). Arrows indicate the maximal gap between the original Ca2+ transient and its monotonous decline (time-to-peak “bump”). The time-to-peak “bump” is calculated as a time of peak of profile minus time-to-peak Ca2+ transient (i.e. time-to-peak “bump” is related to the onset of Ca2+ transient decline). The shaded area under black trace is the integral magnitude of the “bump” (bump IM). c Stretch-dependent changes in the integral magnitude of the “bump” (bump IM), expressed as a % of total area under Ca2+ transient assuming its monotonous decline (CaT), in RV trabeculae of control and MCT-treated male (left panel) and female rats (right panel). d Stretch-dependent changes in the time-to-peak “bump” in RV trabeculae of control and MCT-treated male (left panel) and female rats (right panel). Relative length is expressed as a % of L MAX. Y axis scales on panels c, d are identical for males and females. Data presented as mean ± SEM. *Significant difference between CONT-male vs MCT-male at the same relative length (P < 0.05). No differences were observed between CONT-female and MCT-female
At each stretch, the integral magnitude of “bump” was unaffected by the treatment with MCT in males or females but was approximately twofold larger in males vs females, regardless of the relative length that was tested (bump IM, Fig. 5c). The rate of “bump” development was significantly slower in MCT-treated males since the time-to-peak “bump” was 34.8 ± 0.7 % larger on average in MCT-male vs CONT-male rats (Fig. 5d, left panel). The time-to-peak “bump” values in MCT-females exceeded the values of CONT-females by 6.4 ± 3.7 % only; this was not a significant difference (Fig. 5d, right panel). Both integral magnitude and time-to-peak of “bump” are elevated with stretch, but we did not find any significant difference in this stretch-induced increase between individual groups.
Discussion
It was confirmed in this study that a single treatment with moderate dose of monocrotaline (60 mg/kg body weight) induces right ventricular hypertrophy only in male rats. The hearts of MCT-treated female rats did not exhibit RV hypertrophy within a 5-week interval. This is in agreement with earlier reports that female rats revealed less severe pulmonary hypertension compared to males [27] and therefore lacked right ventricular hypertrophy [26, 28]. In the present study, MCT-treated male and female rats experienced similar structural changes in the wall of the pulmonary vein (see Fig. 2). We conclude that the absence of stimulation of RV hypertrophy in female rats may be grounded by other factors than hypertension-induced increase in pulmonary arterial pressure.
Nevertheless, Ca2+ transients were typically slower in the MCT-treated groups of both sexes (vs same-sex control group). Of interest, Ca2+ transient was significantly slower in males vs females in monocrotaline-induced pulmonary hypertension, while in the control groups, Ca2+ transient tended to rise faster in males vs females, as shown previously [35, 36]. As a result, Ca2+ transient in the MCT-male group was extremely prolonged vs the CONT-male group: a situation which was not observed in the female groups. This finding supports the point of view that sex-specific regulation of Ca2+ transient in pulmonary hypertension and right ventricular hypertrophy is an important player in cardiac adaptation to chronic pressure overload.
The important finding of our study is that female rats under pulmonary hypertension have a longer period of stability of stretch-dependent regulation of cytosolic Ca2+ and tension development, compared with male rats. E.g., Ca2+ transient amplitude and rate of “bump” development, which at least partially underlies the Frank–Starling mechanism, were similar in control and MCT-treated female rats. Ca2+ transient peak time and total duration were moderately altered in MCT-treated females. It is not surprising, therefore, that the Frank–Starling mechanism was highly preserved in the RV myocardium of monocrotaline-treated female rats. It is also interesting that minor effects of stretch on Ca2+ transient amplitude were found in the healthy and monocrotaline-treated female rats.
In contrast, substantially blunted stretch-dependent activation of contraction and prolonged twitch were found in the RV myocardium of monocrotaline-treated male rats, very possibly due to the different effects of stretch on Ca2+ transient. Ca2+ transient amplitude consistently increased with stretch in MCT-treated male rats but decreased in control males. This substantial discrepancy may arise from the different regulation of cytosolic Ca2+ in healthy and hypertrophied myocardium. In a healthy heart, Ca2+ sensitivity of contractile proteins increases with stretch; therefore, faster and more effective Ca2+ utilisation in the force-length relation [3, 33] may lead to reduced Ca2+ transient amplitude. On the other hand, greater Ca2+ entry into a cell and higher sarcoplasmic reticulum Ca2+ uptake are found in hypertrophied myocardium [37], which elevates peak cytosolic Ca2+ [38]. A stretch-dependent increase in the utilisation of Ca2+ by troponin C (TnC) may additionally enhance Ca2+ entry and/or Ca2+ accumulation in the sarcoplasmic reticulum, thus providing the stretch-induced increase in Ca2+ transient amplitude. These [Ca2+]i regulatory pathways may operate either in coordinated or concurrent manner, however, especially in moderate ventricular hypertrophy.
The next important point of our study was to get at how stretch modifies Ca2+ decline in healthy and monocrotaline-treated male and female rats. It is known that stretch of rat cardiac muscle causes a brief deceleration of Ca2+ transient decline, referred to as a “bump” [32]. In rat ventricular myocardium, over 90 % of cytosolic Ca2+ is taken back into the sarcoplasmic reticulum (SR) and only 7 % of Ca2+ is left in a cell during relaxation [39]. Importantly, there is no evidence that Ca2+ uptake by SR is stretch-dependent in rat myocardium. Therefore, it is most likely that the “bump” reflects the release of Ca2+ from troponin C (TnC) [32, 33]. This is supported by the findings that its magnitude is highly dependent on extracellular Ca2+ levels [34] and that the application of mechano-calcium uncouplers like 2,3-butanedione monoxime inhibits the “bump” without affecting Ca2+ transient amplitude [32]. Moreover, the stretch-dependent alterations in the characteristics of the “bump” are consistent with a putative increase in Ca2+-TnC interaction in stretched muscle. In healthy myocardium, the higher the stretch, the larger amount of Ca2+ is being utilised by TnC, and the slower the release of Ca2+ from TnC is. Indeed, both integral magnitude and time-to-peak “bump” are progressively elevated with the gradual stretch of a muscle. Therefore, we hypothesise that (1) the integral magnitude of the “bump” may reflect the amount of Ca2+ released from TnC, and (2) the rate of “bump” development follows the rate of Ca2+ release from TnC. However, these measures are indirect, so further experiments are needed for validation of this hypothesis.
In the present study, we observed the “bump” on Ca2+ transient decline in all rat groups. Our novel finding is that RV myocardium of male rats (either in the control or monocrotaline-treated groups) has twofold larger integral magnitude of the “bump” and substantially slower “bump” kinetics, compared to females. This sex-specific discrepancy may serve as the key determinant in the adaptive response of the contractility of ventricular myocardium to pathological conditions. Indeed, MCT-male rats displayed slowest characteristics of the “bump” which conforms to the smallest amplitude of active tension. All other groups (CONT-male, CONT-female, and MCT-female) have highly similar “bump” kinetics, and similar force-length relations. These findings indicate that Ca2+ utilisation by TnC is greatly altered in monocrotaline-induced right ventricular hypertrophy (males), but not in pulmonary hypertension (females). An extreme prolongation of Ca2+ transients in MCT-treated males may originate from either (1) slower kinetics of Ca-TnC interaction or (2) impaired SR Ca2+ uptake due to reduced function of SERCA2a in heart failure [40], or from a combination of these processes. Additionally, various mutations in the regulatory proteins, such as troponin, have been recently revealed in the ventricular muscle of failing hearts and this pathological remodelling additionally substantiates the alteration of the Frank–Starling mechanism [6].
Our results are in line with the findings that sex affects the type of ventricular hypertrophy [41]. Stretch-dependent effects on isometric tension and Ca2+ transient in rat myocardium may be substantiated by sex-specific hormonal effects which can control (patho)physiological processes such as the development of pulmonary hypertension [26–28]. The protective effect of sex hormones on the development of right ventricular hypertrophy in female rats has also been proven, as MCT-treated adult female rats did not suffer from an increase in RV mass, in contrast to MCT-treated male rats of a similar age [26]. In contrast, MCT-treatment of bilaterally ovariectomised adult female rats led to an increase in RV weight/body weight ratio, which was comparable with male rats, although additional treatment of the oestrogen-deficient adult female rats with progesterone was protective against MCT-induced pulmonary hypertension [27]. It has also been shown that ovariectomy leads to suppression of cardiac myofilament activation in healthy female rats but increases myofilament Ca2+ sensitivity via elevated phosphorylation levels of cardiac tropomyosin in those rats under chronic angiotensin II exposure [42].
These findings and our results allow us to conclude that the RV myocardium of adult female rats is highly protected from pathological remodelling in pulmonary hypertension which preserves the Frank–Starling mechanism unaltered or just slightly blunted. The myocardium of male rats is more susceptible to this remodelling, which results in significant loss of stretch-induced activation of contraction, in part due to impaired regulation of [Ca2+]i removal from the cytosol.
Acknowledgments
The authors are grateful to Elena Mukhlynina for her valuable help in the preparation of histological samples. We thank Dr Yaroslav Kuzmenkov (“OPTEC” LLC, exclusive Distributing Partner of the Carl Zeiss AG in Russia) for his kind help with the purchase of fura-2/AM. This study was supported by the Russian Foundation for Basic Research (#13-04-00367), Integration project of Presidium UB RAS 2012-2014 (#12-C-4-1029), and Program of Presidium RAS “The integration mechanisms of molecular systems under physiological functioning” (#12-P-4-1067).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Komukai K, Kurihara S. Effect of developed tension on the time courses of Ca2+ transients and tension in twitch contraction in ferret myocardium. Cardiovasc Res. 1996;32:384–390. doi: 10.1016/0008-6363(96)00084-3. [DOI] [PubMed] [Google Scholar]
- 2.Martyn DA, Gordon AM. Influence of length on force and activation-dependent changes in troponin C structure in skinned cardiac and fast skeletal muscle. Biophys J. 2001;80(6):2798–2808. doi: 10.1016/S0006-3495(01)76247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: impact of sarcomere length. Am J Physiol Heart Circ Physiol. 2002;282(3):H1055–H1062. doi: 10.1152/ajpheart.00667.2001. [DOI] [PubMed] [Google Scholar]
- 4.de Tombe PP, Mateja RD, Tachampa K, Ait Mou Y, Farman GP, Irving TC. Myofilament length dependent activation. J Mol Cell Cardiol. 2010;48(5):851–858. doi: 10.1016/j.yjmcc.2009.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ter Keurs HEDJ. The interaction of Ca2+ with sarcomeric proteins: role in function and dysfunction of the heart. Am J Physiol Heart Circ Physiol. 2012;302(1):H38–H50. doi: 10.1152/ajpheart.00219.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobirumaki-Shimozawa F, Inoue T, Shintani SA, Oyama K, Terui T, Minamisawa S, Ishiwata S, Fukuda N. Cardiac thin filament regulation and the Frank–Starling mechanism. J Physiol Sci. 2014;64(4):221–232. doi: 10.1007/s12576-014-0314-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hessel MH, Steendijk P, den Adel B, Schutte CI, van der Laarse A. Characterization of right ventricular function after monocrotaline-induced pulmonary hypertension in the intact rat. Am J Physiol Heart Circ Physiol. 2006;291(5):H2424–H2430. doi: 10.1152/ajpheart.00369.2006. [DOI] [PubMed] [Google Scholar]
- 8.Akhavein F, St-Michel EJ, Seifert E, Rohlicek CV. Decreased left ventricular function, myocarditis, and coronary arteriolar medial thickening following monocrotaline administration in adult rats. J Appl Physiol. 2007;103(1):287–295. doi: 10.1152/japplphysiol.01509.2005. [DOI] [PubMed] [Google Scholar]
- 9.Brunner F, Wölkart G, Haleen S. Defective intracellular calcium handling in monocrotaline-induced right ventricular hypertrophy: protective effect of long-term endothelin-A receptor blockade with 2-benzo[1,3]dioxol-5-yl-3-benzyl-4-(4-methoxy-phenyl-)-4-oxobut-2-enoate-sodium (PD 155080) J Pharmacol Exp Ther. 2002;300(2):442–449. doi: 10.1124/jpet.300.2.442. [DOI] [PubMed] [Google Scholar]
- 10.Korstjens IJ, Rouws CH, van der Laarse WJ, van der Zee L, Stienen GJ. Myocardial force development and structural changes associated with monocrotaline induced cardiac hypertrophy and heart failure. J Muscle Res Cell Motil. 2002;23(1):93–102. doi: 10.1023/A:1019988815436. [DOI] [PubMed] [Google Scholar]
- 11.Maier LS, Brandes R, Pieske B, Bers DM. Effects of left ventricular hypertrophy on force and Ca2+ handling in isolated rat myocardium. Am J Physiol. 1998;274(4 Pt 2):H1361–H1370. doi: 10.1152/ajpheart.1998.274.4.H1361. [DOI] [PubMed] [Google Scholar]
- 12.Morioka S, Honda M, Ishikawa S, Ishinaga Y, Yano S, Tanaka K, Moriyama K. Changes in contractile and non-contractile proteins, intracellular Ca2+ and ultrastructures during the development of right ventricular hypertrophy and failure in rats. Jpn Circ J. 1992;56(5):469–474. doi: 10.1253/jcj.56.469. [DOI] [PubMed] [Google Scholar]
- 13.Piacentino V, 3rd, Weber CR, Chen X, Weisser-Thomas J, Margulies KB, Bers DM, Houser SR. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92(6):651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- 14.Ward ML, Pope AJ, Loiselle DS, Cannell MB. Reduced contraction strength with increased intracellular [Ca2+] in left ventricular trabeculae from failing rat hearts. J Physiol. 2003;546(Pt 2):537–550. doi: 10.1113/jphysiol.2002.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Undrovinas NA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60(4):245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ward ML, Crossman DJ, Cannell MB. Mechanisms of reduced contractility in an animal model of hypertensive heart failure. Clin Exp Pharmacol Physiol. 2011;38(10):711–716. doi: 10.1111/j.1440-1681.2011.05563.x. [DOI] [PubMed] [Google Scholar]
- 17.Brixius K, Reuter H, Bloch W, Schwinger RH. Altered hetero- and homeometric autoregulation in the terminally failing human heart. Eur J Heart Fail. 2005;7(1):29–35. doi: 10.1016/j.ejheart.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 18.Vahl CF, Timek T, Bonz A, Fuchs H, Dillman R, Hagl S. Length dependence of calcium- and force-transients in normal and failing human myocardium. J Mol Cell Cardiol. 1998;30(5):957–966. doi: 10.1006/jmcc.1998.0670. [DOI] [PubMed] [Google Scholar]
- 19.Komamura K, Shannon RP, Ihara T, Shen YT, Mirsky I, Bishop SP, Vatner SF. Exhaustion of Frank–Starling mechanism in conscious dogs with heart failure. Am J Physiol. 1993;265(4 Pt 2):H1119–H1131. doi: 10.1152/ajpheart.1993.265.4.H1119. [DOI] [PubMed] [Google Scholar]
- 20.Schwinger RH, Böhm M, Koch A, Schmidt U, Morano I, Eissner HJ, Uberfuhr P, Reichart B, Erdmann E. The failing human heart is unable to use the Frank–Starling mechanism. Circ Res. 1994;74(5):959–969. doi: 10.1161/01.RES.74.5.959. [DOI] [PubMed] [Google Scholar]
- 21.Holubarsch C, Ruf T, Goldstein DJ, Ashton RC, Nickl W, Pieske B, Pioch K, Lüdemann J, Wiesner S, Hasenfuss G, Posival H, Just H, Burkhoff D. Existence of the Frank–Starling mechanism in the failing human heart. Investigations on the organ, tissue, and sarcomere levels. Circulation. 1996;94(4):683–689. doi: 10.1161/01.CIR.94.4.683. [DOI] [PubMed] [Google Scholar]
- 22.von Lewinski D, Kockskämper J, Zhu D, Post H, Elgner A, Pieske B. Reduced stretch-induced force response in failing human myocardium caused by impaired Na(+)-contraction coupling. Circ Heart Fail. 2009;2(1):47–55. doi: 10.1161/CIRCHEARTFAILURE.108.794065. [DOI] [PubMed] [Google Scholar]
- 23.Weil J, Eschenhagen T, Hirt S, Magnussen O, Mittmann C, Remmers U, Scholz H. Preserved Frank–Starling mechanism in human end-stage heart failure. Cardiovasc Res. 1998;37(2):541–548. doi: 10.1016/S0008-6363(97)00227-7. [DOI] [PubMed] [Google Scholar]
- 24.Morey AK, Pedram A, Razandi M, Prins BA, Hu RM, Biesiada E, Levin ER. Estrogen and progesterone inhibit vascular smooth muscle proliferation. Endocrinology. 1997;138:3330–3339. doi: 10.1210/endo.138.8.5354. [DOI] [PubMed] [Google Scholar]
- 25.English KM, Jones RD, Jones TH, Morice AH, Channer KS. Gender differences in the vasomotor effects of different steroid hormones in rat pulmonary and coronary arteries. Hormone Metab Res. 2001;33:645–652. doi: 10.1055/s-2001-18689. [DOI] [PubMed] [Google Scholar]
- 26.Ahn BH, Park HK, Cho HG, Lee HA, Lee YM, Yang EK, Lee WJ. Estrogen and enalapril attenuate the development of right ventricular hypertrophy induced by monocrotaline in ovariectomized rats. J Korean Med Sci. 2003;18(5):641–648. doi: 10.3346/jkms.2003.18.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tofovic PS, Zhang X, Petrusevska G. Progesterone inhibits vascular remodeling and attenuates monocrotaline-induced pulmonary hypertension in estrogen-deficient rats. Prilozi. 2009;30(1):25–44. [PubMed] [Google Scholar]
- 28.Bal E, Ilgin S, Atli O, Ergum B, Sirmagul B. The effects of gender difference on monocrotaline-induced pulmonary hypertension in rats. Hum Exp Toxicol. 2013;32(7):766–774. doi: 10.1177/0960327113477874. [DOI] [PubMed] [Google Scholar]
- 29.Douglas PS, Katz SE, Weinberg EO, Chen MH, Bishop SP, Lorell BH. Hypertrophic remodeling: gender differences in the early response to left ventricular pressure overload. J Am Coll Cardiol. 1998;32(4):1118–1125. doi: 10.1016/S0735-1097(98)00347-7. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg EO, Thienelt CD, Katz SE, Bartunek J, Tajima M, Rohrbach S, Douglas PS, Lorell BH (1999) Gender differences in molecular remodeling in pressure overload hypertrophy. J Am Coll Cardiol 34(1):264–273 [DOI] [PubMed]
- 31.Tamura T, Said S, Gerdes AM. Gender-related differences in myocyte remodeling in progression to heart failure. Hypertension. 1999;33(2):676–680. doi: 10.1161/01.HYP.33.2.676. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Patterson MF, Morgan DL, Julian FJ. Basis for late rise in fura 2 R signal reporting [Ca2+]i during relaxation in intact rat ventricular trabeculae. Am J Physiol. 1998;274:C1273–C1282. doi: 10.1152/ajpcell.1998.274.5.C1273. [DOI] [PubMed] [Google Scholar]
- 33.Kentish JC, Wrzosek A. Changes in force and cytosolic Ca2+ concentration after length changes in isolated rat ventricular trabeculae. J Physiol. 1998;506(2):431–444. doi: 10.1111/j.1469-7793.1998.431bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lookin O, Protsenko Y. Preload-induced changes in isometric tension and [Ca2+]i in rat myocardium. Cent Eur J Biol. 2011;6(5):730–742. doi: 10.2478/s11535-011-0052-6. [DOI] [Google Scholar]
- 35.Leblanc N, Chartier D, Gosselin H, Rouleau JL. Age and gender differences in excitation-contraction coupling of the rat ventricle. J Physiol. 1998;511(Pt 2):533–548. doi: 10.1111/j.1469-7793.1998.533bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrell SR, Ross JL, Howlett SE. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2010;299(1):H36–H45. doi: 10.1152/ajpheart.00299.2010. [DOI] [PubMed] [Google Scholar]
- 37.Malhotra A, Penpargkul S, Schaible T, Scheuer J. Contractile proteins and sarcoplasmic reticulum in physiologic cardiac hypertrophy. Am J Physiol. 1981;241(2):H263–H267. doi: 10.1152/ajpheart.1981.241.2.H263. [DOI] [PubMed] [Google Scholar]
- 38.Chen-Izu Y, Chen L, Bányász T, McCulle SL, Norton B, Scharf SM, Agarwal A, Patwardhan A, Izu LT, Balke CW. Hypertension-induced remodeling of cardiac excitation-contraction coupling in ventricular myocytes occurs prior to hypertrophy development. Am J Physiol Heart Circ Physiol. 2007;293(6):H3301–H3310. doi: 10.1152/ajpheart.00259.2007. [DOI] [PubMed] [Google Scholar]
- 39.Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87(4):275–281. doi: 10.1161/01.RES.87.4.275. [DOI] [PubMed] [Google Scholar]
- 40.Hu ST, Shen YF, Liu GS, Lei CH, Tang Y, Wang JF, Yang YJ. Altered intracellular Ca2+ regulation in chronic rat heart failure. J Physiol Sci. 2010;60(2):85–94. doi: 10.1007/s12576-009-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leinwand LA. Sex is a potent modifier of the cardiovascular system. J Clin Invest. 2003;112(3):302–307. doi: 10.1172/JCI200319429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandit S, Woranush W, Wattanapermpool J, Bupha-Intr T. Significant role of female sex hormones in cardiac myofilament activation in angiotensin II-mediated hypertensive rats. J Physiol Sci. 2014;64(4):269–277. doi: 10.1007/s12576-014-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


