Abstract
The purpose of this study was to examine whether elevation of muscle temperature per se might be a stimulatory factor to increase muscle glucose uptake. Heat stimulation to rat hindlimbs increased glucose uptake measured in vivo in the extensor digitorum longus (EDL) and soleus muscles with a significant increase in muscle temperature. This thermal effect was observed again when glucose uptake was measured in vitro in both isolated muscles immediately after the heat stimulation in vivo. When heat stimulation was imposed on isolated EDL muscles, glucose uptake was facilitated in proportion to the increase in muscle temperature. The heat stimulation led to a significant amplification in the phosphorylation of AMP-activated protein kinase (AMPK) and Akt, and treatment with compound C, wortmannin, or LY294002 partially blocked the thermal effect on muscle glucose uptake. We provide evidence that elevation of muscle temperature per se can directly stimulate muscle glucose uptake and that this thermal effect is compound C-, wortmannin-, and LY294002-inhibitable.
Keywords: Rat, Akt, Muscle temperature, AMPK, PI3K
Introduction
Skeletal muscle is considered to be the most important tissue in glucose homeostasis. Insulin is one of the major stimulants that can induce muscle glucose uptake. This induction of glucose uptake is achieved through a phosphatidylinositol 3-kinase (PI3K)-dependent translocation of glucose transporter (GLUT) 4 from an intracellular storage site to both the plasma membrane and the transverse tubules [1]. Insulin activates PI3K, which subsequently activates Akt, known to be critical for insulin-stimulated GLUT4 translocation. A 160-kDa Akt substrate, TBC1D4 (also known as AS160) links insulin signaling to GLUT4 translocation [2]. TBC1D4 is a Rab-GTPase-activating protein (GAP) that in the basal state retains GLUT4 in an intracellular compartment through the activity of its Rab-GAP domain. Phosphorylation of TBC1D4 by Akt is thought to relieve the GAP-mediated inhibition and lead to an increase in GLUT4 translocation.
Muscle contraction is also a major inducer of glucose uptake. Like insulin, muscle contraction initiates GLUT4 translocation; however, in contrast to insulin, muscle contraction stimulates glucose uptake via a PI3K-independent pathway [1]. Although the molecular mechanisms underlying PI3K-independent glucose uptake are not fully understood, there has been intense investigation into the role of AMP-activated protein kinase (AMPK) in this process [1, 3]. The active form of the AMPK phosphorylates a paralog of TBC1D4, TBC1D1, in an action that may be critical for the induction of GLUT4 translocation [2]. In addition to AMPK, several other molecules have also emerged as potential regulators of PI3K-independent glucose uptake. These include p38 mitogen-activated protein kinase (MAPK), calcium/calmodulin-dependent protein kinase (CaMK) II, and nitric oxide (NO) synthase (NOS) [1, 4, 5].
Interestingly, it was shown that acute heat stimulation to various cell types by increasing incubation temperature stimulated some of the molecules involved in glucose uptake. For instance, heat stimulation activated AMPK [6–8] and p38 MAPK [8] in rat hepatocytes [6], skeletal muscle-derived C2C12 [7], and skeletal muscle-derived L6 [8] cells. Temperature sensitivity of Akt activation was also observed in L6 cells [8] as well as in the murine mammary breast carcinoma cell line, NF9006, and the human colon adenocarcinoma cell line, SW480 [9]. These results led to the hypothesis that elevation of muscle temperature per se might stimulate muscle glucose uptake through some glucose uptake-associated molecule activations. This hypothesis is not in agreement with previous observations where no stimulatory effect on glucose uptake was observed in skeletal muscle-derived L6 cells exposed to acute heat stimulation [8]. However, cultured myotubes, which resemble fetal muscle, are minimally responsive to insulin stimulation of glucose uptake and are not a suitable model for studying regulation of glucose uptake in skeletal muscle [10]. Therefore, the finding obtained from cultured myotubes cannot be extrapolated, or assumed to have relevance, to skeletal muscle.
Here, we reexamined whether elevation of muscle temperature per se might be a stimulatory factor to increase muscle glucose uptake in rat skeletal muscle. To elevate muscle temperature artificially, heat stimulation was imposed on rat hindlimbs in vivo and isolated muscles in vitro. The thermal impact on muscle signal transduction pathways and its contribution to the glucose uptake were also investigated.
Materials and methods
Treatment of animals
This research was approved by the Animal Studies Committee of Niigata University of Health and Welfare. Male Wistar rats were obtained from CLEA Japan (Tokyo, Japan). Rats were housed individually at constant room temperature (23 ± 1 °C) in a 12-h light (08:00–20:00 h)/12-h dark cycle and were provided standard laboratory chow and water ad libitum. Rats weighing ~250 and ~60 g were used in experiments after being fasted for 3 h.
Heat stimulation in vivo
Rats weighing ~250 and ~60 g were anesthetized continuously by volatile anesthetic isoflurane (MK-A110D; Muromachi Kikai, Tokyo, Japan) according to the manufacturer’s instructions. Under anesthesia, rats were placed on a board floating in temperature-controlled water set at either 36 or 42 °C. Both rat hindlimbs were immersed in the water for 30 min through a hole in the board. Three min before the end of heat stimulation, blood was obtained from a tail vein, and blood glucose and lactate levels were measured immediately by hand-held analyzers as described below (see Blood parameters). Blood was also introduced into heparin-treated capillaries to measure plasma insulin concentrations. Immediately after the cessation of heat stimulation, the temperatures of extensor digitorum longus (EDL) and soleus muscles were measured in situ as described below (see Muscle temperature).
Glucose uptake in vivo
For surgical operation, we used bigger rats weighing ~250 g in this experiment, instead of rats weighing ~60 g used in other experiments in the present study. Four days before the experiment day, rats were anesthetized by an intraperitoneal injection of sodium pentobarbital (50 mg/kg body weight), and the right jugular vein was cannulated. On the day of the experiment, rat hindlimbs underwent heat stimulation as described above. Glucose uptake in vivo was measured with glucose analog 2-deoxyglucose (2DG) as described [11] with minor modifications. After 2DG transport into the cells, 2DG is phosphorylated by hexokinase (HK), and produces 2DG-6-P (2DG6P) which is not further metabolized. Because 2DG6P is an extremely weak inhibitor of HK [12], 2DG6P accumulation reflects glucose uptake. Ten min before the end of heat stimulation, 2DG (3 mmol/kg body weight) was injected through a cannula. Immediately after the cessation of heat stimulation, EDL and soleus muscles were removed, and then clamp-frozen with liquid nitrogen. The intracellular accumulation of 2DG6P was measured fluorometrically as described below (see Muscle metabolites).
Glucose uptake in vitro
Extensor digitorum longus and soleus muscles of control rats (without any treatment) or those muscles of rats that underwent heat stimulation in vivo were used for measurement of glucose uptake in vitro according to the method as described [11–14] with some modifications. Isolated muscles of rats weighing ~60 g were incubated for 10 min at 36, 38, 40, or 42 °C in 4 ml of Krebs-Hensleit buffer (KHB) containing 8 mM 2DG, 32 mM mannitol, and 0.1 % bovine serum albumin (BSA) either with or without 10 mU/ml human insulin. After the incubation, the muscles were blotted, and then clamp-frozen in liquid nitrogen. The intracellular accumulation of 2DG6P was measured fluorometrically. In parallel experiments, isolated muscles were incubated for 10 min at 36, 38, 40, or 42 °C in 4 ml of KHB containing 8 mM glucose, 32 mM mannitol and 0.1 % BSA. After the incubation, muscle temperature was measured in vitro as described below (see Muscle temperature). During all incubation periods in the present study, the incubation flasks were gassed continuously with 95 % O2–5 % CO2.
Time-course experiments in vitro
To further characterize the thermal effect on glucose uptake, we used a time-course approach as shown in the incubation schedules in Fig. 3a. Isolated EDL muscles of rats weighing ~60 g were pre-incubated for 20 min according to the schedules in incubation 1(10 min at 36 °C + 10 min at 36 °C), incubation 2 (10 min at 36 °C + 10 min at 36 °C), incubation 3 (10 min at 36 °C + 10 min at 42 °C), incubation 4 (10 min at 42 °C + 10 min at 36 °C), or incubation 5 (10 min at 42 °C + 10 min at 36 °C) in 4 ml of KHB containing 8 mM glucose, 32 mM mannitol, and 0.1 % BSA. After the pre-incubation, muscles were then incubated with 2DG for 10 min at either 36 °C (incubations 1, 3, 4) or 42 °C (incubations 2, 5) as described above.
Fig. 3.
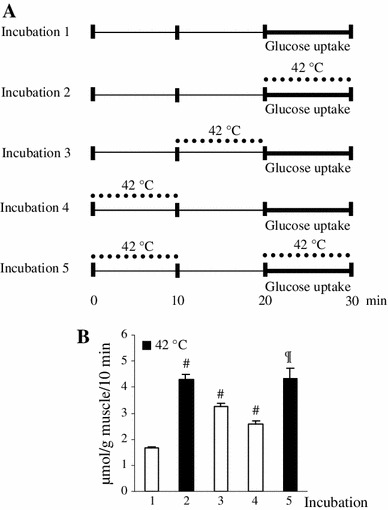
Time course for glucose uptake in vitro in response to heat stimulation, cessation of stimulation, and re-stimulation in vitro. Incubation schedules for the time-course experiment are shown in a. Isolated extensor digitorum longus muscles of rats weighing ~60 g were pre-incubated without 2-deoxyglucose (2DG) for 20 min according to the schedules in incubation 1 (10 min at 36 °C + 10 min at 36 °C), incubation 2 (10 min at 36 °C + 10 min at 36 °C), incubation 3 (10 min at 36 °C + 10 min at 42 °C), incubation 4 (10 min at 42 °C + 10 min at 36 °C), or incubation 5 (10 min at 42 °C + 10 min at 36 °C). After the pre-incubation, muscles were then incubated with 2DG for 10 min at either 36 °C (incubations 1, 3, 4) or 42 °C (incubation 2, 5) (b). Data are expressed as the mean ± SE (n = 5–7). # p < 0.05 as compared to the control (incubation 1).¶ p < 0.05 as compared to the incubations 1, 3, and 4
Inhibitor experiments in vitro
We also measured the effect of heat stimulation on glucose uptake in the presence of various pharmacological inhibitors. Isolated EDL muscles of rats weighing ~60 g were pre-incubated for 30 min at 36 °C in 4 ml of KHB containing 8 mM glucose, 32 mM mannitol, and 0.1 % BSA either with or without 100 nM wortmannin (a PI3K inhibitor) in 0.01 % DMSO, 10 μM LY294002 (a PI3K inhibitor) in 0.1 % DMSO, 100 μM N G-monomethyl-l-arginine (L-NMMA; a nitric oxide inhibitor), 10 μM KN-93 (a CaMKII inhibitor), 10 μM compound C (an AMPK inhibitor) in 0.1 % DMSO, or 10 μM SB203580 (a p38 MAPK inhibitor) in 0.1 % DMSO. After the pre-incubation, muscles were then incubated with 2DG for 10 min at 42 °C as described above. The inhibitors were also present in the medium during the glucose uptake measurements. Vehicle controls included 0.1 % DMSO where appropriate. Wortmannin was from Sigma-Aldrich (St. Louis, MO), and the other compounds were from Merck (Darmstadt, Germany).
Muscle temperature
To evaluate muscle temperature in situ, we measured a muscle’s surface temperature in situ by using the product DS103 with a direct thermo-sensor probe (Tateyamakagaku, Toyama, Japan). In our preliminary experiment, we noticed that the thermo-sensor probe was not suitable for measuring the temperature of isolated skeletal muscle because touching the probe to the surface of the isolated muscle rapidly decreased the muscle’s temperature. To minimize thermal loss, we used an infrared thermometer (FLIR i5; FLIR Systems, Seattle, WA) to measure muscle temperature in vitro. The delta increase was calculated by subtracting the temperature measured after 36 °C-stimulation (as a control) from the temperature measured after each heat stimulation (38, 40, or 42 °C).
Muscle metabolites
Extensor digitorum longus and soleus muscles were homogenized in 0.3 M perchloric acid (PCA), and the extracts were used to measure glycogen by the amyloglucosidase method [15]. The remaining PCA extracts were centrifuged at 1,000×g for 10 min at 4 °C. After centrifugation, the supernatant was collected and neutralized by the addition of 2 M KOH, followed by fluorometric measurements of ATP, phosphocreatine (PCr), lactate, glucose-6-phosphate (G6P), and 2DG6P [16].
Blood parameters
Blood glucose and lactate levels were measured by using the products Glutest Every (Sanwa Kagaku Kenkyusho, Nagoya, Japan) and Lactate Pro (Arkray, Kyoto, Japan), respectively. Plasma insulin concentrations were measured by enzyme immunoassay kits (Morinaga Institute of Biological Science, Yokohama, Japan).
Immunoblot analysis
Isolated EDL muscles of rats weighing ~60 g were incubated for 10 min at either 36 or 42 °C in 4 ml of KHB containing 8 mM glucose, 32 mM mannitol and 0.1 % BSA, and then clamp-frozen in liquid nitrogen. The muscles were homogenized in ice-cold buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl, 10 % glycerol, 1 % Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 10 mM Na4P2O7, 100 mM NaF, 2 mM Na3VO4, 2 mM PMSF, aprotinin (10 μg/ml), and leupeptin (10 μg/ml) [13, 14]. The homogenates were rotated end-over-end at 4 °C for 60 min, and centrifuged at 10,000×g for 10 min at 4 °C. Aliquots of the supernatants were used for the immunoblot analysis.
Briefly, supernatants were electrophoretically separated by SDS-PAGE and transferred to PVDF membranes. The membranes were incubated overnight with antibodies against p-AMPK (Thr172), p-p38 MAPK (Thr180/Tyr182), p-Akt (Thr308), p-Akt (Ser473), p-TBC1D1 (Ser700), p-TBC1D4 (Thr642), GLUT4, or glyceraldehyde-3-phosphate dehydrogenase (GAPDH) at 4 °C, followed by incubation for 90 min with HRP-conjugated anti–rabbit IgG. Antibodies against GLUT4 and GAPDH were from Biogenesis (Poole, UK) and Sigma-Aldrich (St. Louis, MO), respectively. Other antibodies were from Cell Signaling Technology (Beverly, MA). Immunoreactive bands were visualized by enhanced chemiluminescence reagent (GE Healthcare Japan, Hino, Japan), and quantified using NIH Image.
Statistics
Values are expressed as the mean ± SE. Differences among multiple groups were determined using a one-way analysis of variance (ANOVA) followed by Tukey–Kramer test. When two mean values were compared, analysis was performed using an unpaired t test. A value of p < 0.05 was considered significant.
Results
Skeletal muscle fibers are classically divided into either type I (slow-twitch, oxidative) or type II (fast-twitch, glycolytic). Under resting conditions, the temperatures of the EDL (rich in type II fibers) and soleus (rich in type I fibers) muscles were 32.9 ± 0.1 and 34.0 ± 0.1 °C in rats weighing ~250 g, and 33.3 ± 0.2 and 35.0 ± 0.2 °C in rats weighing ~60 g, respectively.
To elevate muscle temperature, rat hindlimbs were immersed in temperature-controlled water set at either 36 or 42 °C for 30 min. Although the EDL and soleus muscles are anatomically located deep within the hindlimb, applying 42 °C-heat stimulation to the exterior of the leg successfully increased the temperature of both muscles compared to stimulation with 36 °C in the muscles of rats weighing ~250 g (Fig. 1a) and ~60 g (Fig. 2c).
Fig. 1.
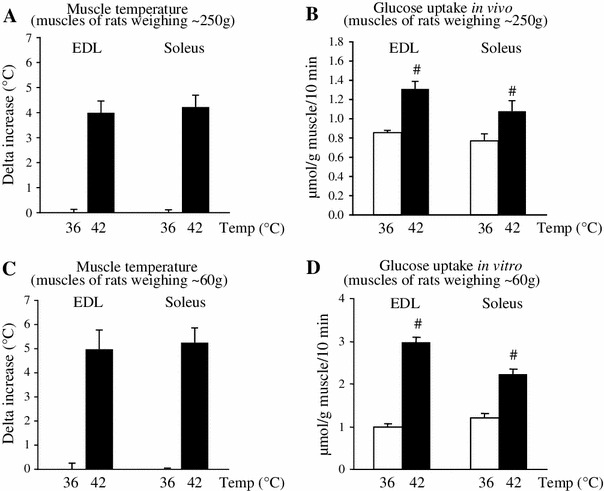
Heat stimulation in vivo facilitates muscle glucose uptake in vivo and in vitro. Under anesthesia, the hindlimbs of rats weighing ~250 and ~60 g were immersed for 30 min in temperature-controlled water set at either 36 or 42 °C. For measurement of the in vivo thermal effect on muscle glucose uptake in vivo (b), 2-deoxyglucose (2DG) was injected to rats weighing ~250 g through a cannula 10 min before the end of heat stimulation. Immediately after the cessation of heat stimulation, the temperatures of extensor digitorum longus (EDL) and soleus muscles were measured in situ (a), and then both muscles were snap-frozen. For measurement of the in vivo thermal effect on muscle glucose uptake in vitro (d), the temperatures of EDL and soleus muscles of rats weighing ~60 g were measured in situ immediately after the cessation of heat stimulation (c), and then both muscles were removed and were incubated with 2DG for 10 min at 36 °C. Data are expressed as the mean ± SE (n = 3 for a, n = 6 for b, n = 4–5 for c, n = 6 for d). # p < 0.05 as compared to the control (36 °C). Temp temperature
Fig. 2.
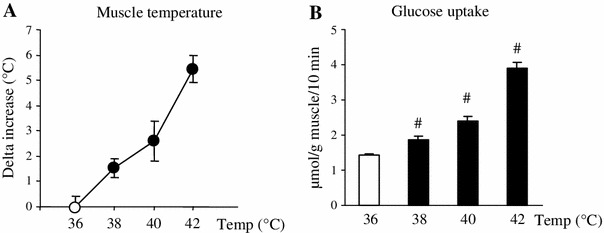
Elevation of muscle temperature per se directly facilitates muscle glucose uptake in vitro. Isolated extensor digitorum longus muscles of rats weighing ~60 g were incubated with 2-deoxyglucose for 10 min at 36, 38, 40, or 42 °C for measurements of muscle temperature (a) and glucose uptake (b). Data are expressed as the mean ± SE (n = 3 for a, n = 5 for b). # p < 0.05 as compared to the control (36 °C). Temp temperature
During the heat stimulation in vivo, the glucose uptake measured in vivo was significantly increased in the EDL and soleus muscles (Fig. 1b). This thermal effect was observed again when glucose uptake was measured in vitro in both isolated muscles immediately after the heat stimulation in vivo (Fig. 1d).
When blood parameters were measured immediately after the heat stimulation in vivo, blood glucose levels were comparable between groups, while higher blood lactate levels were observed in heat-stimulated rats (Table 1). We also found that heat stimulation in vivo significantly increased plasma insulin concentrations (Table 1).
Table 1.
Effect of heat stimulation on blood parameters
| Temperature (°C) | 36 | 42 |
|---|---|---|
| Glucose (mg/dl) | 147 ± 5 | 141 ± 4 |
| Insulin (ng/ml) | 0.34 ± 0.10 | 2.91 ± 0.24# |
| Lactate (mM) | 2.4 ± 0.2 | 4.2 ± 0.2# |
Data are expressed as mean ± SE (n = 6)
# p < 0.05 vs. 36 °C
In order to exclude any systemic interference, we further examined the effect of heat stimulation in vitro on glucose uptake in vitro. When isolated EDL muscles were incubated in water set at various controlled temperatures, muscle temperature increased with water temperature (Fig. 2a). At 38 °C-heat stimulation, only 2 °C above the control temperature (36 °C), there was a detectable stimulatory effect of heat on glucose uptake (Fig. 2b). As water and muscle temperatures increased, glucose uptake gradually rose until at 42 °C it reached a threefold increase over the control (Fig. 2b).
To examine whether the thermal effect on glucose uptake represented a controlled physiological response, rather than an uncontrolled inflow of glucose due to cellular damage (e.g., loss of membrane integrity), we measured changes in glucose uptake in vitro by EDL muscles in response to heat stimulation in vitro applied at timed intervals (Fig. 3). Compared to glucose uptake measured during heat stimulation (incubation 2 in Fig. 3a: 4.3 ± 0.2 μmol/g muscle/10 min), the increased glucose uptake was rapidly decreased to 77 % immediately after the cessation of heat stimulation (incubation 3 in Fig. 3a: 3.3 ± 0.1 μmol/g muscle/10 min) and 60 % 10 min after the stimulation (incubation 4 in Fig. 3a: 2.6 ± 0.1 μmol/g muscle/10 min). Re-stimulation again increased glucose uptake to the level observed in the first heat stimulation (incubation 5 in Fig. 3a: 4.3 ± 0.4 μmol/g muscle/10 min). These tightly controlled responses of glucose uptake to heat stimulation suggest that the thermal effect on glucose uptake is a specific cellular response to temperature.
Table 2 summarizes the effect of heat stimulation on EDL muscle metabolites. Muscle glycogen was significantly decreased at 40 and 42 °C as compared to the control temperature of 36 °C. This facilitated glycogenolysis was accompanied by an accumulation of G6P and lactate levels, showing promotion of glycolysis. Furthermore, gradual reductions in ATP and PCr levels were observed as heat stimulation increased.
Table 2.
Effect of heat stimulation on muscle metabolites in extensor digitorum longus muscles
| Temperature (°C) | 36 | 38 | 40 | 42 |
|---|---|---|---|---|
| μmol/g muscle | ||||
| Glucose-6-phosphate | 0.09 ± 0.01 | 0.25 ± 0.02# | 0.46 ± 0.04# | 0.92 ± 0.05# |
| Glycogen | 15.7 ± 0.5 | 14.7 ± 0.4 | 11.8 ± 0.5# | 9.8 ± 0.4# |
| Lactate | 6.2 ± 0.6 | 9.5 ± 0.5# | 13.4 ± 0.6# | 18.6 ± 0.9# |
| ATP | 7.0 ± 0.1 | 6.9 ± 0.1 | 6.2 ± 0.3# | 5.3 ± 0.3# |
| Phosphocreatine | 30.1 ± 1.0 | 26.6 ± 0.7# | 23.5 ± 1.0# | 18.9 ± 0.9# |
Data are expressed as mean ± SE (n = 7–9)
# p < 0.05 vs. 36 °C
Figure 4 shows the effect of heat stimulation on muscle signal transduction pathways associated with glucose uptake. Immunoblot analysis revealed that 42 °C-heat stimulation significantly amplified the phosphorylation of AMPK (Thr172), Akt (Thr308), and p38 MAPK (Thr180/Tyr182). Kinase target residues Ser700 in TBC1D1 and Thr642 in TBC1D4, thought to be phosphorylated by AMPK and Akt, respectively, were also increasingly phosphorylated after heat stimulation. We also noted a significant rise in Akt (Ser473) phosphorylation in response to heat stimulation, whereas GLUT4 and GAPDH protein levels did not change (data not shown).
Fig. 4.
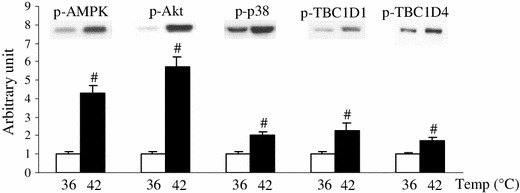
Elevation of muscle temperature has direct impacts on muscle signal transduction pathways associated with glucose uptake. Isolated extensor digitorum longus muscles of rats weighing ~60 g were incubated without 2-deoxyglucose for 10 min at either 36 or 42 °C. Phosphorylation of signaling molecules was detected by immunoblot analysis with antibodies against p-AMPK (Thr172), p-Akt (Thr308), p-p38 MAPK (Thr180/Tyr182), p-TBC1D1 (Ser700), and p-TBC1D4 (Thr642). A representative blot is shown above each bar. Data are expressed as the mean ± SE (n = 4–8). # p < 0.05 as compared to the control (36 °C). Temp temperature
In parallel experiments, the effects of pharmacological inhibitors of PI3K-independent pathways on heat stimulation-induced glucose uptake were examined in EDL muscles (Fig. 5). In agreement with the immunoblotting results, compound C, an AMPK inhibitor, partially but significantly inhibited heat stimulation-induced glucose uptake. By contrast, neither SB203580 (a p38 MAPK inhibitor), KN-93 (a CaMKII inhibitor), nor L-NMMA (a NOS inhibitor) affected the thermal response.
Fig. 5.
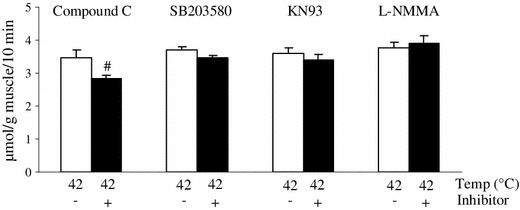
AMPK inhibitor partially blocked the heat stimulation-induced muscle glucose uptake in vitro. Isolated extensor digitorum longus muscles of rats weighing ~60 g were pre-incubated without 2-deoxyglucose (2DG) for 30 min at 36 °C either with or without 10 μM compound C (an AMPK inhibitor), 10 μM SB203580 (a p38 MAPK inhibitor), 10 μM KN-93 (a CaMKII inhibitor), or 100 μM NG-monomethyl-l-arginine (L-NMMA; a nitric oxide inhibitor), and then were incubated with 2DG for 10 min at 42 °C. Data are expressed as the mean ± SE (n = 5–8). # p < 0.05 as compared to the control (without the inhibitor). Temp temperature
If heat stimulation-induced glucose uptake occurs through only PI3K-independent pathways, it would be expected that the thermal effect and the maximal effect of insulin together on glucose uptake would be additive [12]. However, when the two effects were measured in combination, we observed only a partially additive result (Fig. 6a), suggesting that the mechanisms may partially overlap. Given that heat stimulation increased Akt phosphorylation, we expected that wortmannin, a PI3K inhibitor might inhibit heat stimulation-induced glucose uptake. In accord with this hypothesis, the thermal effect on glucose uptake was partially inhibited in the presence of wortmannin (Fig. 6b). Treatment with LY294002, another PI3K inhibitor, also partially inhibited the thermal effect on glucose uptake (Fig. 6b).
Fig. 6.
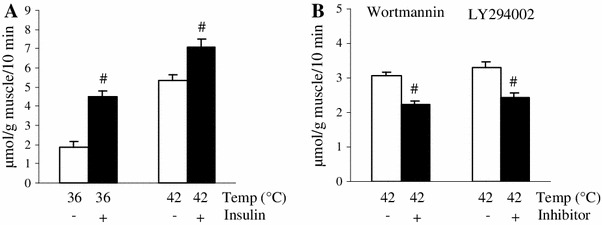
PI3K inhibitor partially blocked the heat stimulation-induced muscle glucose uptake in vitro. Isolated extensor digitorum longus (EDL) muscles of rats weighing ~60 g were incubated with 2-deoxyglucose (2DG) for 10 min at either 36 or 42 °C in the presence or absence of 10 mU/ml insulin (a). Isolated EDL muscles were pre-incubated without 2DG for 30 min at 36 °C either with or without 100 nM wortmannin or 10 μM LY294002, and then were incubated with 2DG for 10 min at 42 °C. Data are expressed as the mean ± SE (n = 6–9). # p < 0.05 as compared to the control (without the inhibitor). Temp temperature
Discussion
The glucose analog 2DG has been used for measurement of muscle glucose uptake/transport activity. In contrast to the phosphorylated product 2DG6P, which is an extremely weak inhibitor of HK, G6P is known to severely inhibit HK [12]. Under conditions where accumulations of glucose transport-inhibitory substrates, including intracellular G6P, are present, the phosphorylation step by HK as well as the glucose transport step across the cell membrane could be a rate-limiting step for 2DG6P accumulation. The 2DG6P accumulation measured under these conditions is defined as “glucose uptake”, not “glucose transport activity”. In the present study, we measured 2DG6P accumulation in the presence of G6P accumulation. Therefore, we have used the term “glucose uptake”. In the balance between the inhibitory effect of HK by G6P accumulation and the stimulatory effect of HK by the Q10 effect, we found a significant increase in 2DG6P accumulation in the heat-stimulated muscles measured in vivo and in vitro. The present results confirm that elevation of muscle temperature per se can directly stimulate glucose uptake in skeletal muscle.
Our results showed that the cellular regulation of glucose uptake in response to the elevation of muscle temperature was highly sensitive, differentiating temperature differences as small as 2 °C, and precisely tunes to the changes of muscle temperature. Also, this effect was observed regardless of the composition of muscle fiber types. Although the physiological role of the thermal effect is not clear, it could be possible to expect a role in the facilitation of muscle glucose uptake in certain physiological situations, such as physical exercise and sepsis where body/muscle temperature is increased. In the present study, despite the thermal effect on muscle glucose uptake in vivo, we did not observe a hypoglycemic effect in vivo during heat stimulation. This may be due to the promotion of hepatic glucose output, as reported [17].
We demonstrated here that elevation of muscle temperature has direct and acute impacts on signal transduction pathways associated with glucose uptake in intact skeletal muscle. However, because of the complexity of skeletal muscle, i.e., the numerous kinases and phosphatases located in downstream or parallel pathways, all of which would be exposed to higher temperature during heat stimulation, the activation of glucose uptake-associated molecules does not necessarily confirm that these molecules were functionally linked to changes in glucose uptake.
In the present study, we found a stimulatory effect of heat stimulation on AMPK kinase and TBC1D1, and an inhibitory effect of compound C on glucose uptake. These results suggest a role of the AMPK-dependent pathway for the thermal effect on muscle glucose uptake. The precise mechanisms by which heat brought about the activation of AMPK are not clear, but allosteric regulation via a reduction in ATP, PCr, and glycogen levels may have contributed [1, 3].The compound C has been frequently used as an inhibitor of AMPK, however, it also has inhibitory effects on a number of protein kinases [18]. Although the physiological role of these kinases other than AMPK for the muscle glucose transport system is not defined, the result with compound C needs to be interpreted with caution.
One of most accepted concepts regarding glucose uptake is that muscle contraction in vitro induces glucose uptake through a PI3K-independent pathway. This concept is largely based on evidence that wortmannin does not inhibit glucose uptake induced by muscle contraction in vitro [19–22]. In contrast to these data obtained in vitro, Wojtaszewski et al. [23] used a perfusion technique to measure glucose uptake in vivo and reported that 1 μM wortmannin inhibited 50 % of glucose uptake induced by in situ muscle contraction. The concentration of wortmannin used in that study was relatively high, but neither 1 μM [22], nor 2 μM wortmannin [21] inhibited glucose uptake induced by muscle contraction in vitro.
These results might be interpreted to mean that glucose uptake induced by contractile activity in vivo, but not in vitro, contains a component that is inhibitable by wortmannin. Importantly, we noticed in the present study that heat stimulation-induced glucose uptake was partially inhibited by wortmannin. An inhibitory effect of LY294002, another PI3K inhibitor, on the thermal effect on glucose uptake was also observed. Given that in situ muscle contraction/physical exercise (but not muscle contraction in vitro) increased muscle temperature (unpublished observations), a plausible interpretation is that the thermal effect on glucose uptake may occur also through a PI3K-dependent pathway as well as an AMPK-dependent pathway.
Another possible interpretation is that wortmannin and LY294002 blunted the thermal effect by inhibiting mammalian target of rapamycin (mTOR) rather than PI3K inhibition. This is based on previous studies showing that muscle-specific deletion of rictor (rapamycin-insensitive companion of mTOR), a component of mTORcomlex 2, have impaired insulin-stimulated glucose uptake [24] and that wortmannin and LY294002 can prevent mTOR activity as a direct action [25].The use of more specific inhibitors or gene-modulating animals will be necessary to clarify the wortmannin- and LY294002-sensitive mechanisms for the thermal effect on glucose uptake.
It is possible that increased oxidant activity generated in response to heat stimulation [26] could impact insulin signaling and glucose uptake. Skeletal muscle constitutively produces reactive nitrogen species (RNS) and reactive oxygen species (ROS) [27]. NO, an RNS, stimulates GLUT4 translocation and glucose uptake in skeletal muscle [19, 28], and NOS activity is temperature-dependent [29]. One study [19], but not another [28], showed that NO-induced glucose uptake was partially inhibited by wortmannin. However, our experiment using an NOS inhibitor demonstrated that there was not a link between NO and heat stimulation-induced glucose uptake.
Another oxidant, hydrogen peroxide (H2O2), a major ROS, can also induce muscle glucose uptake [30–32]. It was reported that the incubation of isolated EDL muscles with ~0.6 mM H2O2 increased glucose uptake and Akt phosphorylation without AMPK activation [31], and that incubation with higher concentrations of H2O2 (1–3 mM) began to activate AMPK [32], suggesting that Akt is more sensitive than AMPK to ROS. Nevertheless, ROS contribution to the thermal effects observed in the present study are still unclear because AMPK seems to be more sensitive than Akt in response to heat stimulation. In our preliminary experiment, we observed that 38 °C-heat stimulation in vitro significantly increased AMPK phosphorylation but Akt phosphorylation was not statistically increased compared to those in 36 °C-heat stimulation.
We also measured the thermal effect on signal transduction pathways and the effects of inhibitors on glucose uptake only in EDL muscles (rich in type II fibers). Thus, we cannot rule out the possibility that we might have obtained different results using soleus muscles (rich in type I fibers). Along this line, experiments using soleus muscle from Akt2 −/− mice showed that lack of Akt activation had no effect on glucose uptake induced by treadmill running exercise where the increase in muscle temperature is expected [33]. However, that study measured glucose uptake in vitro 15 min after the cessation of exercise, presumably enough time for the thermal effect to be lost. Therefore, the molecular mechanisms underlying the thermal effect on glucose uptake in type I fiber-rich muscles remain to be examined.
Moon et al. [8] examined the effect of acute 45 °C-heat stimulation on glucose uptake measured with 2DG in skeletal muscle-derived L6 cells, and they found that glucose uptake was not affected, although they observed a significant increase in AMPK and Akt phosphorylation. Although we cannot explain this discrepancy between results, our results are partially supported by a previous study by Holloszy et al. [34]. In that study, they found by using frog skeletal muscle that glucose transport activity measured by 3-O-methyl-glucose (3MG), another glucose analog, was higher when measured at 19 °C compared to 0 °C [34]. However, the range of temperature they used is not in a physiological range observed in mammalian skeletal muscle. In addition, the glucose transport system in amphibian muscle seems markedly different from mammalian muscle, because Holloszy et al. [34] also reported that the maximal effect of contraction and insulin on glucose transport in frog muscles was not additive.
We have demonstrated herein that elevation of muscle temperature in a physiological range stimulates glucose uptake in rat skeletal muscle. We showed that: (1) elevation of muscle temperature per se is a stimulatory factor to increase muscle glucose uptake, (2) the thermal effect on glucose uptake is very rapid and sensitive. Furthermore, (3) elevation of muscle temperature per se stimulates AMPK and Akt, and (4) the thermal effect on muscle glucose uptake is inhibitable by compound C, wortmannin, and LY294002.
Acknowledgments
This work was supported by a grant-in-aid for young scientists (B) No. 23700787 from the Japan Society for the Promotion of Science (JSPS), a 38th research grant of Japan Health & Research Institute, and a grant-in-aid for advanced research from Niigata University of Health and Welfare, 2010 and 2011.
Conflict of interest
None of the authors have any conflicts of interest associated with this study.
References
- 1.Jessen N, Goodyear LJ. Contraction signaling to glucose transport in skeletal muscle. J ApplPhysiol. 2005;99:330–337. doi: 10.1152/japplphysiol.00175.2005. [DOI] [PubMed] [Google Scholar]
- 2.Sakamoto K, Holman GD. Emerging role for AS160/TBC1D4 and TBC1D1 in the regulation of GLUT4 traffic. Am J physiol Endocrinol Metab. 2008;295:E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jensen TE, Wojtaszewski JFP, Richter EA. AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient. Acta Physiol. 2009;196:155–174. doi: 10.1111/j.1748-1716.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- 4.Richter EA, Nielsen JN, Jørgensen SB, Frøsig C, Birk JB, Wojtaszewski JFP. Exercise signaling to glucose transport in skeletal muscle. Proc Nutr Soc. 2004;63:211–216. doi: 10.1079/PNS2004343. [DOI] [PubMed] [Google Scholar]
- 5.Merry TL, McConell GK. Skeletal muscle glucose uptake during exercise: a focus on reactive oxygen species and nitric oxide signaling. IUBMB Life. 2009;61:479–484. doi: 10.1002/iub.179. [DOI] [PubMed] [Google Scholar]
- 6.Corton JM, Gillespie JG, Hardie DG. Role of the AMP-activated protein kinase in the cellular stress response. Curr Biol. 1994;4:315–324. doi: 10.1016/S0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- 7.Liu CT, Brooks GA. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J Appl Physiol. 2012;112:354–361. doi: 10.1152/japplphysiol.00989.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moon B, Duddy N, Ragolia L, Gegum N. Stimulation of glycogen synthesis by heat shock in L6 skeletal-muscle cells: regulatory role of site-specific phosphorylation of glycogen-associated protein phosphatase 1. Biochem J. 2003;371:857–866. doi: 10.1042/BJ20021644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oehler-Jänne C, von Bueren AO, Vuong V, Hollenstein A, Grotzer MA, Pruschy M. Temperature sensitivity of phospho-Ser(473)-PKB/AKT. Biochem Biophys Res Commun. 2008;375:399–404. doi: 10.1016/j.bbrc.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 10.Holloszy JO, Hansen PA. Regulation of glucose transport into skeletal muscle. Rev Physiol Biochem Pharmacol. 1996;128:99–193. doi: 10.1007/3-540-61343-9_8. [DOI] [PubMed] [Google Scholar]
- 11.Ueyama A, Sato T, Yoshida H, Magata K, Koga N. Nonradioisotope assay of glucose uptake activity in rat skeletal muscle using enzymatic measurement of 2-deoxyglucose-6-phosphate in vitro and in vivo. Biol Signals Recept. 2000;9:267–274. doi: 10.1159/000014649. [DOI] [PubMed] [Google Scholar]
- 12.Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–985. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- 13.Koshinaka K, Sano A, Howlett KF, Yamazaki T, Sasaki M, Sakamoto K, Kawanaka K. Effect of high-intensity intermittent swimming on postexercise insulin sensitivity in rat epitrochlearis muscle. Metabolism. 2008;57:749–756. doi: 10.1016/j.metabol.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Sano A, Koshinaka K, Abe N, Morifuji M, Koga J, Kawasaki A, Kawanaka K. The effect of high-intensity intermittent swimming on post-exercise glycogen supercompensation in rat skeletal muscle. J Physiol Sci. 2012;62:1–9. doi: 10.1007/s12576-011-0170-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passonneau JV, Lauderdale VR. A comparison of three methods of glycogen measurements in tissue. Anal Biochem. 1974;60:405–412. doi: 10.1016/0003-2697(74)90248-6. [DOI] [PubMed] [Google Scholar]
- 16.Passonneau JV, Lowry OH. Enzymatic analysis. A practical guide. Totowa: Humana; 1993. [Google Scholar]
- 17.Hargreaves M, Angus D, Howlett K, Conus NM, Febbraio M. Effect of heat stress on glucose kinetics during exercise. J Appl Physiol. 1996;81:1594–1597. doi: 10.1152/jappl.1996.81.4.1594. [DOI] [PubMed] [Google Scholar]
- 18.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem. 2007;J408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higaki Y, Hirshman MF, Fujii N, Goodyear LJ. Nitric oxide increases glucose uptake through a mechanism that is distinct from the insulin and contraction pathways in rat skeletal muscle. Diabetes. 2001;50:241–247. doi: 10.2337/diabetes.50.2.241. [DOI] [PubMed] [Google Scholar]
- 20.Funai K, Cartee GD. Inhibition of contraction-stimulated AMP-activated protein kinase inhibits contraction-stimulated increases in PAS-AS160 in rat skeletal muscle. Diabetes. 2009;58:1096–1104. doi: 10.2337/db08-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AD, Hansen PA, Holloszy JO. Wortmannin inhibits insulin-stimulated but not contraction-stimulated glucose transport activity in skeletal muscle. FEBS Lett. 1995;361:51–54. doi: 10.1016/0014-5793(95)00147-2. [DOI] [PubMed] [Google Scholar]
- 22.Yeh JI, Gulve EA, Rameh L, Birnbaum MJ. The effect of wortmannin on rat skeletal muscle. J Biol Chem. 1995;270:2107–2111. doi: 10.1074/jbc.270.44.26558. [DOI] [PubMed] [Google Scholar]
- 23.Wojtaszewski JFP, Laustsen JL, Derave W, Richter EA. Hypoxia and contractions do not utilize the same signaling mechanism in stimulating skeletal muscle glucose transport. Biochim Biophys Acta. 1998;1380:396–404. doi: 10.1016/S0304-4165(98)00011-7. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Harris TE, Keller SR, Choi KM, Magnuson MA, Lawrence JC., Jr Muscle-specific deletion of rictor impairs insulin-stimulated glucose transport and enhances basal glycogen synthase activity. Mol Cell Biol. 2008;28:61–70. doi: 10.1128/MCB.01405-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunn GJ, Williams J, Sabers C, Wiederrecht G, Lawrence JC, Jr, Abraham RT. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin and LY294002. EMBO J. 1996;15:5256–5267. [PMC free article] [PubMed] [Google Scholar]
- 26.Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level, and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–R705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- 27.Murrant C, Reid M. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech. 2001;55:236–248. doi: 10.1002/jemt.1173. [DOI] [PubMed] [Google Scholar]
- 28.Etgen GJ, Jr, Fryburg DA, Gibbs EM. Nitric oxide stimulates skeletal muscle glucose transport through a calcium/contraction- and phosphatidylinositol-3-kinase-independent pathway. Diabetes. 1997;46:1915–1919. doi: 10.2337/diab.46.11.1915. [DOI] [PubMed] [Google Scholar]
- 29.Venturini G, Colasanti M, Fioravanti E, Bianchini A, Ascenzi P. Direct effect of temperature on the catalytic activity of nitric oxide synthases types I, II, and III. Nitric Oxide. 1999;3:375–382. doi: 10.1006/niox.1999.0250. [DOI] [PubMed] [Google Scholar]
- 30.Cartee GD, Holloszy JO. Exercise increases susceptibility of muscle glucose transport to activation by various stimuli. Am J physiol Endocrinol Metab. 1990;258:E390–E393. doi: 10.1152/ajpendo.1990.258.2.E390. [DOI] [PubMed] [Google Scholar]
- 31.Higaki Y, Mikami T, Fujii N, Hirshman MF, Koyama K, Seino T, Tanaka K, Goodyear LJ. Oxidative stress stimulates skeletal muscle glucose uptake through a phosphatidylinositol 3-kinase-dependent pathway. Am J physiol Endocrinol Metab. 2008;294:E889–E897. doi: 10.1152/ajpendo.00150.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyoda T, Hayashi T, Miyamoto L, Yonemitsu S, Nakano M, Tanaka S, Ebihara K, Masuzaki H, Hosoda K, Inoue G, Otaka A, Sato K, Fushiki T, Nakao K. Possible involvement of the a1 isoform of 5′AMP-activated protein kinase in oxidative stress-stimulated glucose transport in skeletal muscle. Am J physiol Endocrinol Metab. 2004;287:E166–E173. doi: 10.1152/ajpendo.00487.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sakamoto K, Arnolds DE, Fujii N, Kramer HF, Hirshman MF, Goodyear LJ. Role of Akt2 in contraction-stimulated cell signaling and glucose uptake in skeletal muscle. Am J physiol Endocrinol Metab. 2006;291:E1031–E1037. doi: 10.1152/ajpendo.00204.2006. [DOI] [PubMed] [Google Scholar]
- 34.Holloszy JO, Narahara HT. Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J Biol Chem. 1965;9:3493–3500. [PubMed] [Google Scholar]


