Obtaining ethics committee approval before commencing a biomedical research study is mandatory as per the present guidelines. For investigators, it is also essential to find out whether their ethics committee is registered with the Central Drugs Standard Control Organization (CDSCO), or else that committee doesn't have a stand in issuing permission letters for any clinical trial. Similarly, for approving biomedical research other than clinical trials, the committee is to be registered with the Department of Health Research (DHR). Thus, we would like to highlight the needs and the process of registration of the ethics committee in DHR as well as CDSCO. Institutional Ethics Committees (IECs) are the custodians of the rights, safety, and well-being of research participants. The IEC also looks into the interests of the investigators. The committee functions independently, has members from both the medical fraternity and the non-medical community, and reviews the research proposals from the institute. However, even such committees require monitoring, accountability, and transparency in their constitution and functioning.[1]
Why Do We Need to Register the Institutional Ethics Committee?
Registering the IEC ensures the quality of conduct and performance of functions of the IEC, which in turn favors ethically supervised and sound clinical trials and other biomedical research.
Registration of IECs reviewing biomedical and health research has been made mandatory by the Ministry of Health and Family Welfare, Government of India. The New Drugs and Clinical Trial Rules 2019, Chapter IV, “Ethics Committee for Biomedical and Health Research” states that all ethics committees reviewing biomedical research should be registered with the authority designated by the Central Government in the Ministry of Health and Family Welfare, DHR.[2] An office for Ethics Committee Registration has been set up under DHR for coordinating the IEC registrations. This office works in close liaison with DHR and Indian Council of Medical Research (ICMR).[3]
Ethics Committees looking into clinical trials, bioavailability, and bioequivalence studies shall additionally apply to the Central Licensing Authority, CDSCO, Ministry of Health and Family Welfare, Govt. of India.[2]
Where to Register IEC?
The DHR (Naitik) portal (https://naitik.gov.in/)
The CDSCO (SUGAM) portal (https://cdscoonline.gov.in/).
Who Registers?
An applicant who registers with the IEC can be the Member Secretary of the IEC. He should have an undertaking from the Head of the Institute who authorizes him in writing to register in CDSCO/DHR. He should also have access to the email of the IEC.
How to Register IEC?
-
1. Steps to be followed to register on the DHR portal:
Applicant registration using a username (IEC email ID) and password. Using an IEC email account ID (not personal email) is necessary here.
Login to the portal using your username and password.
-
Applicant details, organization details, and member secretary of IEC details:
Details of EC: For example, ethics committee type (independent or institutional), name of ethics committee, name of applicant as appears in Aadhar card, date of birth, nationality, email ID, father or spouse's name, and mobile number.
Details of the organization: Name, type (government or private), address, and contact number.
Details of the member secretary: Name, email ID, date of birth, and spouse/father's name.
Documents to be submitted online: Scanned copies/e-copies of identity proof (Aadhar card of applicant), address proof of the institute, ink signed and stamped “undertaking” or justification document where the Head of the Institute authorizes the Member Secretary to register in DHR. All the documents should be in PDF or JPG format.
The hard copies of the documents submitted online, that is, self-attested copy of identity proof (Aadhar), a self-attested copy of address proof of the institute, and an ink signed and stamped original undertaking, are to be sent by post to the DHR, New Delhi. The online documents submitted should be the same as those sent by post.
The DHR receives the documents both online and offline, verifies them, and sends the username and password to access the portal.
Following this, the secretary has to upload the next set of documents (Standard Operating Procedure (SOP) of the IEC, curriculum vitae (CV), and Good Clinical Practice certificates of its members).
-
The applicant then uploads the following documents in the DHR portal:
Application for registration (upload a signed copy of covering letter/application for registration of the ethics committee)
Authority under which the ethics committee has been constituted (uploads the authority letter from the authorized person of hospital/institute on the Institutes' letterhead. The authority letter from the appellate authority head of the institution will state, for example, “I/we hereby authorize the formation of the EC and is constituted under my/our authority. The committee will function independently in decision-making process and will function according to the New Drugs and Clinical Trials Rules 2019 and ICMR National Ethical Guidelines. The institute will provide infrastructure, administrative, and financial support for the functioning of the Ethics Committee.”
The latest version of the SOP of the IEC. Individual page numbers of the SOP subtopics are to be mentioned while uploading [details in Table 1].
Bio-data/CV of the members in the format given in the DHR portal.[6] The bio-data contains details of name, full working address, telephone number, email id, present affiliation, affiliation status with the host institute, qualification, role in the IEC, reasons for suitability of the member in the designated role, previous experience in ethics committees, relevant ethics training, and relevant publications [Table 2].[6]
Training certificates of members (should reflect training in the National Ethical Guidelines 2017, Good Clinical Practice, and applicable regulations in India) and the agenda of the training can be uploaded along with the trainer's name and details.
Details if the committee has been audited or inspected before (upload relevant documents; if not, upload justification)
Before beginning to upload, one may refer to the checklist items for registration given by DHR.[4]
Once the SOP, CV, and training of members are uploaded, an autogenerated ‘Undertaking by the Ethics Committee’ appears in the Naitik portal (in the Action tab). This undertaking contains the full name, address, and title of the chairperson of the IEC; the name and address of the IEC; the names, email ID, phone numbers, mailing addresses, qualifications with specialization, and designations of all the members of the IEC. The downloaded undertaking by the committee is signed by both the chairperson and member secretary of the IEC and uploaded to the Naitik portal.
Another auto-generated document, ‘Form CT-01’, is to be submitted to the Naitik portal. This document contains the details of the applicant and institution and is to be signed by the applicant and uploaded in the Naitik portal.
The DHR looks into all the documents and the IEC constitution and raises queries, if any. The applicant is notified by mail, and the corrections are to be uploaded to the Naitik portal.
If changes are made to the IEC member constitution and details, the undertaking is to be downloaded again, re-signed by the chairperson and member secretary, and uploaded.
Table 1.
Details required in SOP of IEC
| Details required in the SOP of IEC[4] | |
|---|---|
| 1. | Membership requirements of the ethics committee |
| 2. | The terms of reference, roles, and responsibilities of the committee members |
| 3. | Conditions of appointment of the IEC members and quorum requirements |
| 4. | Procedure for resignation, replacement, or removal of members |
| 5. | The standard operating procedures to be followed by the committee in general |
| 6. | Standard operating procedures to be followed by the committee for vulnerable populations |
| 7. | Policy regarding training for new and existing committee members, along with standard operating procedures |
| 8. | Policy to monitor or prevent the conflict of interest along with standard operating procedures |
For designing the SOP, one may look into the National Ethical Guidelines for Biomedical and Health Research involving Human Participants, ICMR, 2017[5]
Table 2.
Practical tips about the composition of IEC
| 1. | Preferably, 50% of the ethics committee members should be non-affiliated or from outside the institution.[2] |
| 2. | The total number of members in an EC should preferably be between 7 and 15.[2] |
| 3. | The head of the institution should appoint all EC members, including the chairperson. The chairperson should be a non-affiliated member, whereas the member secretary should be affiliated with the institute.[2] |
| 4. | Constitution of the Ethics Committee for Clinical Trials[2] The Ethics Committee should have a minimum of seven members from medical, non-medical, scientific and non-scientific areas with at least (i) One layperson; (ii) One woman member; (iii) One legal expert; (iv) One independent member (such as a social scientist or representative of a non-governmental organization, a philosopher or ethicist, or theologian). |
| 5. | The committee should include at least one member whose primary area of interest or specialization is nonscientific and at least one member who is independent of the institution. |
| 6. | The medical scientists and clinicians on the ethics committee should have post graduate qualifications and adequate experience in their respective areas of specialization. |
| 7. |
Quorum requirements[2]
|
| 8. | Independent Ethics Committee[3] Researchers who have no institutional attachments can apply for ethics committee approval from Independent Ethics Committees (Ind EC). The Ind EC should be a registered legal entity. The individuals who govern the Ind EC should not be members of the proposed EC, and will oversee and monitor the functioning of the Ind EC. |
A flowchart of the above mentioned steps is given in Flowchart 1.
Flowchart 1.
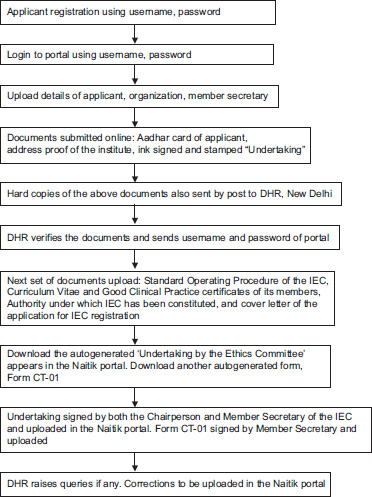
Flowchart showing the steps of registration of the IEC in DHR
-
2. CDSCO portal
The procedure, steps, and documents remain the same as those of the DHR portal.
How Long Does the Registration Remain and When Does Re-Registration Need to be Done?
If all the documents, SOP, CVs, IEC constitution, and training certificates, are in their proper place and the queries have been addressed, the DHR will issue a provisional registration certificate. The provisional registration remains valid for two years, and the final registration granted by DHR shall remain valid for a period of five years from the date of its issue unless suspended or cancelled by the designated authority at DHR.[2]
Registration in CDSCO is valid for 5 years from the date of issue of the registration certificate.[2]
Re-registration or renewal of the IEC shall be applied 90 days prior to the expiry of the registration.[2] If there are no changes in the IEC, the applicant renders a certificate indicating there are no changes. Otherwise, a fresh set of documents is to be submitted. This applies for both DHR and CDSCO registrations.
If there are any changes to the composition of the registered IEC (within its five years of being registered), they should be informed to the DHR and CDSCO.[2]
The IEC should be competent and independent in its functioning. Registering the IEC with DHR and/or CDSCO further increases its authenticity and competency.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors acknowledge the enthusiasm of all the study participants.
References
- 1.Das MK, Singh D. Ethics committee registration and re-registration with the regulatory authority in India. Natl Med J India. 2019;32:157–60. doi: 10.4103/0970-258X.278682. [DOI] [PubMed] [Google Scholar]
- 2.NewDrugs_CTRules_2019. [Last accessed on 2023 Feb 24];Gazette notification. Available from: https://cdsco.gov.in/opencms/export/sites/CDSCO_WEB/Pdf-documents/NewDrugs_CTRules_2019.pdf . [Google Scholar]
- 3.Department of Health Research Registration of Ethics Committees reviewing Biomedical and Health Research. [Last accessed on 2023 Feb 24]; Available from: https://naitik.gov.in/DHR/Homepage#about_content . [Google Scholar]
- 4.Checklist for Ethics Committee Registration for Biomedical and Health Research. [Last accessed on 2023 Feb 24]; Available from: https://naitik.gov.in/DHR/resources/app_srv/DHR/global/pdf/downloads/Checklist_22Feb22.pdf . [Google Scholar]
- 5.National Ethical Guidelines for Biomedical and Health Research involving Human Participants. [Last accessed on 2023 Feb 24];Indian Council of Medical Research. 2017 Available from: https://main.icmr.nic.in/sites/default/files/guidelines/ICMR_Ethical_Guidelines_2017.pdf . [Google Scholar]
- 6.Format for bio-data. [Last accessed on 2023 Feb 24];CV_22Feb22. Available from: https://naitik.gov.in/DHR/resources/app_srv/DHR/global/pdf/downloads/CV_22Feb22.pdf . [Google Scholar]


