Abstract
Expression of the dnaKJ and groESL1 heat shock operons of Bradyrhizobium japonicum depends on a ς32-like transcription factor. Three such factors (RpoH1, RpoH2, and RpoH3) have previously been identified in this organism. We report here that they direct transcription from some but not all ς32-type promoters when the respective rpoH genes are expressed in Escherichia coli. All three RpoH factors were purified as soluble C-terminally histidine-tagged proteins, although the bulk of overproduced RpoH3 was insoluble. The purified proteins were recognized by an anti-E. coli ς32 serum. While RpoH1 and RpoH2 productively interacted with E. coli core RNA polymerase and produced E. coli groE transcript in vitro, RpoH3 was unable to do so. B. japonicum core RNA polymerase was prepared and reconstituted with the RpoH proteins. Again, RpoH1 and RpoH2 were active, and they initiated transcription at the B. japonicum groESL1 and dnaKJ promoters. In all cases, the in vitro start site was shown to be identical to the start site determined in vivo. Promoter competition experiments revealed that the B. japonicum dnaKJ and groESL1 promoters were suboptimal for transcription by RpoH1- or RpoH2-containing RNA polymerase from B. japonicum. In a mixture of different templates, the E. coli groESL promoter was preferred over any other promoter. Differences were observed in the specificities of both sigma factors toward B. japonicum rpoH-dependent promoters. We conclude that the primary function of RpoH2 is to supply the cell with DnaKJ under normal growth conditions whereas RpoH1 is responsible mainly for increasing the level of GroESL1 after a heat shock.
The ς subunit confers promoter recognition ability upon bacterial RNA polymerases. Sigma factors specifically interact with the catalytic, four-subunit core RNA polymerase (α2ββ′). Only after assembly with ς to form the so-called holo-RNA polymerase can the enzyme recognize promoter sequences and initiate transcription accurately. Upon initiation, the ς factor is released and the core enzyme elongates the RNA chain (reviewed in reference 9).
Bacteria often contain multiple sigma factors (for reviews, see references 8, 10, and 11). Under normal growth conditions, transcription of most genes in Escherichia coli is initiated by RNA polymerase containing the major sigma factor ς70 (also called ςD). Alternative sigma factors coordinate transcription of distinct subsets of coregulated genes which, for example, are required for nitrogen assimilation (ς54 [ςN]), for flagellum gene expression (ς28 [ςF]), during stationary phase (ς38 [ςS]), or under conditions of heat stress (either ς32 [ςH] or ς24 [ςE], depending on whether the stress signal is elicited in the cytoplasm or the periplasm) (17).
We are studying the regulation of heat shock genes in Bradyrhizobium japonicum, the nitrogen-fixing root nodule symbiont of the soybean plant. This organism uses three different strategies to control the expression of heat shock proteins (Hsps) at elevated temperatures. Two mechanisms rely on conserved DNA elements (CIRCE and ROSE) which are located immediately downstream of the transcription start sites of some heat shock operons (2, 18, 20). In both cases, it is likely that a putative repressor protein binds to these sites and prevents transcription under normal growth conditions. Regulation of a third class of heat shock genes is mediated by ς32-like factors (2, 16). B. japonicum is the first known organism which contains an rpoH gene family coding for three different ς32-like factors (RpoH1, RpoH2, and RpoH3) (18, 19). Notably, these proteins differ not only in their primary sequence (the number of identical amino acids varies between 49 and 66%) but also in their significance to survival and in the way they are regulated. RpoH2 appeared to be crucial for survival under all conditions because several attempts to disrupt the corresponding gene failed. By contrast, RpoH1 and RpoH3 could be deleted individually. Even the rpoH1-rpoH3 double mutant had no obvious growth defect under various conditions.
All three RpoH proteins were capable of producing high levels of β-galactosidase from a groE-lacZ fusion in a ς32-deficient E. coli strain (19). In fact, rpoH1 had originally been discovered by searching for this phenotype in a complementation approach (18). A puzzling observation was made when the abilities of the RpoH factors to complement the temperature-sensitive phenotype of the rpoH mutant were compared. Only RpoH2 reached a similar complementation capacity to E. coli ς32 in allowing the strain to grow at otherwise restrictive temperatures (19). While RpoH1 still permitted growth at intermediate temperatures, RpoH3 almost completely failed. Taken together, these results could be taken as a hint of a somewhat overlapping but also divergent promoter specificity of the three RpoH factors of B. japonicum.
In the present study, we set out to systematically test the promoter specificities of the RpoH factors. To complete our previous studies, we checked which Hsps were produced in each complemented E. coli strain. We were particularly interested in establishing an in vitro transcription system to approach this question. By the successful use of purified components, we show that RpoH1 and RpoH2 indeed favor different promoters.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
E. coli cells were grown in Luria-Bertani (LB) medium (15) supplemented with ampicillin (200 μg/ml) or kanamycin (30 μg/ml) if required. E. coli A7448 (4, 30) was grown at 28°C; the routine growth temperature for other E. coli strains was 37°C. B. japonicum was grown aerobically at 28°C in PSY medium (22) supplemented with 0.1% (wt/vol) arabinose and 100 μg of spectinomycin per ml.
Plasmid constructions.
Recombinant DNA techniques were performed by standard methods (23). The individual rpoH genes were amplified from plasmids containing these genes (18, 19) by PCR with Vent DNA polymerase as specified by the manufacturer (New England Biolabs, Beverly, Mass.). The oligonucleotides used as primers were designed so that they introduced a NdeI recognition site overlapping the start codon of each gene and either a XhoI site immediately downstream of the 3′ ends of rpoH1 and rpoH2 or a NotI site at the equivalent position of rpoH3. The PCR-generated fragments were cut with NdeI-XhoI or NdeI-NotI and ligated into pET24b(+) (Novagen, ams Biotechnology, Lugano, Switzerland) digested with the same enzymes. The resulting plasmids, pRJ5086, pRJ5101, and pRJ5102, encoded C-terminally histidine-tagged RpoH1, RpoH2, and RpoH3 proteins, respectively. The vector-encoded amino acids leucine and glutamic acid were introduced between the carboxy-terminal amino acid of RpoH1 or RpoH2 and the histidine tag. A stretch of five amino acids (AAALE) was introduced at the corresponding position of RpoH3-His6.
Plasmids used as transcription templates were based on pRJ9519, which contains the B. japonicum rrn terminator. It derives from pRJ9601 (3) from which the rrn promoter and an internal EcoRI restriction site upstream of the terminator have been deleted. Plasmid pRJ5099 carries a 0.9-kb EcoRI-BglII fragment containing the B. japonicum dnaKJ promoter region (16). The B. japonicum groESL1 template pRJ5134 contains a BglII-EcoRV-digested 0.4-kb PCR fragment originating from pRJ8067 (2). While the BglII site was present in the sequence upstream of groESL1, the EcoRV site was introduced into the groES1 gene by the PCR primer (Sig106, 5′-GGCTTGATATCGATCGGGATC-3′ [recognition site underlined]). Plasmid pEC5162 bears a 0.4-kb E. coli groESL promoter fragment that was PCR amplified from pEC8182 (kindly provided by H.-M. Fischer, Zürich, Switzerland), a subclone of pND5 (12). EcoRI restriction sites at either end of the fragment were created by the PCR primers (Sig122, 5′-GCGAATTCCCTGGGCCAGCCC-3′; Sig123, TCGAATTCTTTACGCTTGACG-3′ [EcoRI sites underlined]).
The correct nucleotide sequences of all PCR-amplified fragments introduced into pET24b(+) or the transcription vector pRJ9519 were confirmed by sequencing. It revealed for pEC5162 that in this particular case the EcoRI site in pRJ9519, which was assumed to be destroyed (see above), was present and had been cut by EcoRI, resulting in a product that was approximately 100 bp shorter than expected.
Overproduction and purification of RpoH-His6 proteins.
Freshly transformed E. coli BL21/pLys cells (26) carrying pRJ5086, pRJ5101, or pRJ5102 were used for overproduction of RpoH proteins. When the cells had reached an optical density at 600 nm of 0.7 at 28°C, production of the recombinant proteins was induced by the addition of 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After 2 h, the cells were harvested and disrupted in a French pressure cell, and proteins were purified from the soluble fraction of a 500-ml cell culture (RpoH1 and RpoH2) or 2,000-ml culture (RpoH3). The extracts were loaded onto a 1.5-ml Ni-nitrilotriacetic acid-agarose column (Qiagen, Hilden, Germany), and chromatography was performed as specified in the pET System Manual (Novagen). Protein fractions were dialyzed against storage buffer (20 mM Tris-HCl [pH 8.0], 200 mM NaCl, 50% glycerol) and then stored at −20°C.
Purification of B. japonicum RNA polymerase core enzyme.
RNA polymerase was prepared as described previously (3). Core RNA polymerase was separated from holoenzyme on a Resource Q column (6 ml; Pharmacia, Uppsala, Sweden). Up to 1.4 mg of RNA polymerase was loaded onto the column equilibrated with T50GED (50 mM Tris-HCl [pH 8.0], 10% glycerol, 1 mM EDTA, 0.1 mM dithiothreitol) containing 0.1 M NaCl. After the column was washed with the same buffer, bound protein was eluted with 170 ml of a linear 0.1 to 0.325 M NaCl gradient. Peak fractions which were devoid of sigma factor (judged by sodium dodecyl sulfate [SDS]-polyacrylamide gel electrophoresis [12% polyacrylamide]) were pooled, dialyzed against T10GED buffer containing 0.02 M NaCl and 50% (vol/vol) glycerol, and concentrated by ultrafiltration.
Western blot (immunoblot) analysis.
Crude extracts of E. coli cells were prepared, separated on SDS–12% polyacrylamide gels, and transferred to nitrocellulose membranes as described previously (18). The following antisera were used: anti-E. coli ς32, DnaK, and GrpE sera (B. Bukau, Freiburg, Germany; 3,000-fold dilutions [7]); anti-E. coli GroEL serum (Epicentre Technologies, Madison, Wis.; 20,000-fold dilution); and anti-E. coli IbpA serum, which recognizes the small Hsps IbpA and IbpB (A. Easton, St. Louis, Mo.; 1,500-fold dilution [1]). The primary rabbit antibodies were detected with a chemiluminescence Western blotting kit (Boehringer GmbH, Mannheim, Germany).
In vitro transcription.
Multiple-round transcription assays with RNA polymerase holoenzyme were carried out in a volume of 20 μl under standard conditions as described previously (3). RNA polymerase core enzyme (1.15 μg of B. japonicum enzyme or 1 U of E. coli enzyme [Boehringer Mannheim] per assay) was reconstituted with RpoH factors (0.1 μg per assay; molar ratio of core RNA polymerase and RpoH protein, 1:1) for 20 min at 4°C in a volume of 10 μl containing 40 mM Tris-HCl (pH 8.0), 10 mM MgCl2, 0.1 mM EDTA, 0.1 mM dithiothreitol, 150 mM KCl, 0.4 mM K3PO4, and 0.2 mg of bovine serum albumin per ml. The reaction was started by adding the reconstituted enzyme to a 10-μl sample containing the same components as the reconstitution buffer plus 1 mM of each nucleoside triphosphate, 1 μCi of [α-32P]UTP (800 Ci/mmol; DuPont, Bad Homburg, Germany), 1 U of RNase inhibitor per μl, and 20 nM DNA template (final concentrations).
For single-round transcription experiments, the DNA template was added to the reconstitution sample before the remaining components together with 100 ng of heparin per ml (final concentration) were added. The template concentration in promoter competition experiments was 10 nM for each plasmid.
Suitable RNA size markers were synthesized in vitro with T7 or T3 RNA polymerase with linearized pBluescript-based plasmids as templates.
Transcript mapping.
RNA isolation and primer extension analysis was performed as described elsewhere (2). The start site of transcripts synthesized in vitro was determined as described previously (3). The oligonucleotides 702 (2) and DnaK12 (16) were used to determine the start sites of in vitro-synthesized B. japonicum groESL1 or dnaK transcripts. Oligonucleotide Sig123, which was used to amplify the promoter region, was also used to determine the in vivo and in vitro start site of the E. coli groESL operon.
RESULTS
Plasmid-encoded RpoH proteins of B. japonicum selectively transcribe heat shock promoters in E. coli.
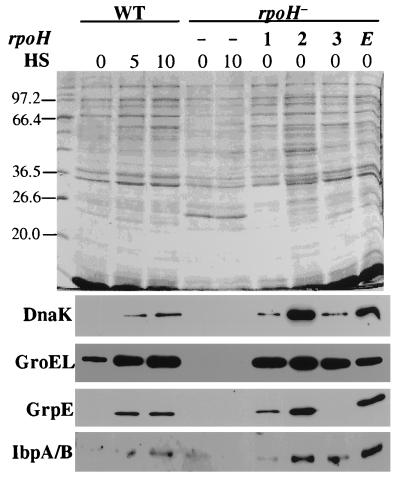
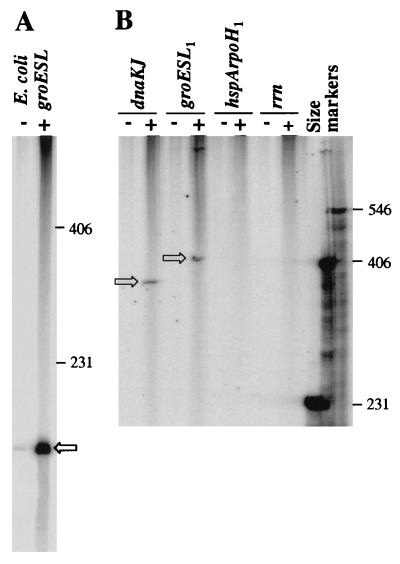
We have previously described that all three RpoH proteins of B. japonicum were functionally active in a ς32-negative E. coli reporter strain which contains a chromosomally integrated E. coli groE-lacZ fusion (19). This strain produced high β-galactosidase activity when it was complemented with any of the three plasmids harboring an rpoH gene under the control of the lac promoter. We now monitored the ability of these complemented strains to produce heat shock proteins in E. coli. To this end, we performed immunoblot analyses with antibodies raised against different Hsps. Extracts from normally grown and heat-shocked cells of E. coli MC4100 and the ς32-negative strain containing pUC18 without insert served as controls. The typical heat-inducible synthesis of the Hsps GroEL, DnaK, GrpE, and IbpA plus IbpB was observed in the wild type but not in the mutant (Fig. 1). As expected from the activation of the groE-lacZ fusion, all strains complemented by rpoH produced high levels of GroEL. However, the amounts of other Hsps in these strains were quite different. While E. coli ς32 and B. japonicum RpoH2 produced comparable, high levels of DnaK, GrpE, and small Hsps, RpoH1 and in particular RpoH3 produced only small or undetectable amounts of DnaK and GrpE. This result indicated that the sigma factors conferred different promoter specificities upon E. coli core RNA polymerase.
FIG. 1.
Detection of heat shock proteins produced by E. coli MC4100 (WT) and E. coli A7448 (rpoH−). E. coli A7448 transformed with pUC18 (−), pRJ5000 (rpoH1, indicated as 1), pRJ5002 (rpoH2, indicated as 2), pRJ5040 (rpoH3, indicated as 3), or pEC5007 (E. coli rpoH, indicated as E) was grown in the presence of 0.5 mM IPTG. Crude cell extracts were prepared from cells grown at 28°C (0) or heat-shocked cells (heat shock [HS] from 28 to 43°C for 5 or 10 min as indicated) and separated on a SDS–12% polyacrylamide gel. A Coomassie blue-stained gel is shown at the top. The apparent molecular masses (in kilodaltons) of relevant marker proteins are indicated to the left of the gel. Similar gels with suitably diluted samples (1/5, 1/10, 1/3, and 1/15 of the amounts loaded onto the stained gel) were used for immunodetection of the Hsps DnaK, GroEL, GrpE, and IbpA plus IbpB, respectively.
The B. japonicum RpoH factors use the same in vivo start site as E. coli ς32.
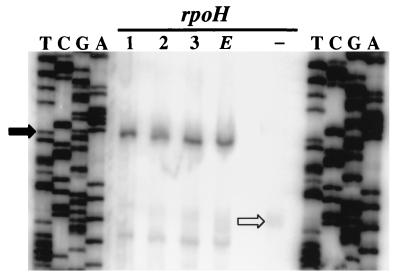
Since all four RpoH factors synthesized GroEL protein efficiently, we analyzed whether the heterologous proteins initiated transcription at the same position as E. coli ς32. Total RNA was isolated from each complemented strain, and the 5′ end of the groESL transcript was determined by primer extension. This strain contains two groESL promoter regions: the original operon and a chromosomally integrated groE-lacZ fusion. Transcripts of both operons were detected by the primer used in these experiments. The transcripts formed by all four RpoH proteins start at the identical position, which corresponds to the previously described ς32-dependent site (Fig. 2) (5). Small amounts of a shorter product probably represent prematurely terminated reverse transcripts. RNA from the mutant containing only the vector resulted in a faint signal that represents transcripts originating from the ς70-dependent promoter (Fig. 2) (30). This transcript was hardly observed with RNA from the rpoH+ strains, presumably because the abundant RpoH proteins prevented transcription from the ς70 promoter by promoter occlusion. It has been shown that this promoter is very weak and virtually inactive in rpoH+ strains (14, 30).
FIG. 2.
Primer extension analysis of groESL transcripts synthesized in E. coli A7448. Reverse transcripts were obtained with 15 μg of total RNA isolated from the strain complemented with B. japonicum rpoH1 (lane 1), rpoH2 (lane 2), or rpoH3 (lane 3) or E. coli rpoH (lane E) and 30 μg of RNA from the same strain transformed with pUC18 (lane −). Oligonucleotide Sig123 was used as the primer for the primer extension and corresponding sequencing reactions (lanes T, C, G, and A). The transcription start site used by the ς32-like proteins is indicated by a solid arrow; the open arrow marks the transcript initiated at the ς70-dependent promoter.
Overexpression and purification of histidine-tagged RpoH1, RpoH2, and RpoH3.
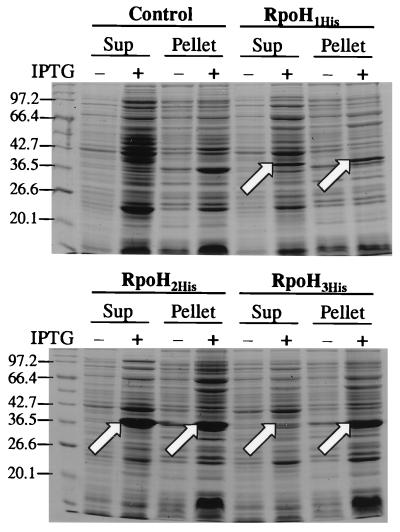
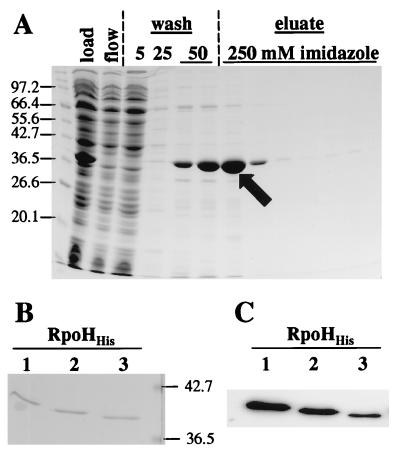
Knowing that amino-terminally or carboxy-terminally histidine-tagged ς32 of E. coli is active in vivo and in vitro (7), we constructed plasmids to produce the B. japonicum RpoH proteins with a histidine tag attached to their C termini. Crude cell extracts containing these proteins were prepared and separated into pellet and supernatant fractions (Fig. 3). Surprisingly, the overproduction efficiency and the solubility of the RpoH proteins varied over a wide range. RpoH2His and RpoH3His were overproduced to the highest levels. However, more than 95% of RpoH3His was found in the pellet fraction, indicating that the majority of this protein was insoluble. By contrast, approximately 50% of overproduced RpoH1His and RpoH2His remained in the supernatant. All three proteins were purified from the soluble supernatant fraction by nickel-nitrilotriacetic acid chromatography. The proteins exhibited different binding affinities to the column resin. While some RpoH2His had already eluted during the wash step with an imidazole concentration of 50 mM, RpoH1His and RpoH3His were still retained at this concentration (data not shown). The purification profile for RpoH2His (Fig. 4A) serves as an example to show that the nickel affinity chromatography technique allowed efficient purification of histidine-tagged B. japonicum RpoH proteins.
FIG. 3.
Solubility of overproduced B. japonicum RpoH proteins. The supernatant (Sup) and pellet (Pellet) fraction of crude cell extracts prepared before (−) or after (+) IPTG induction were separated by centrifugation at 15,000 rpm (SS-34 rotor) at 4°C for 30 min. Control cells contain the vector pET24b(+) without insert. RpoH1His, RpoH2His, and RpoH3His were produced from plasmids pRJ5086, pRJ5101, and pRJ5102, respectively. After electrophoresis in an SDS–12% polyacrylamide gel, the proteins were stained with Coomassie blue. The apparent molecular masses (in kilodaltons) of marker proteins are indicated to the left of the gel. Arrows point to the overproduced RpoHHis protein.
FIG. 4.
Purification of RpoH2His and immunodetection of B. japonicum RpoHHis proteins. (A) Aliquots of the RpoH2His supernatant fraction which was loaded onto the column (load), the flowthrough (flow), the different wash (wash), and the elution (eluate) fractions were separated on an SDS–12% polyacrylamide gel, and the proteins were stained with Coomassie blue. The imidazole concentration in the wash and elution buffers is indicated. The apparent molecular masses (in kilodaltons) of the marker proteins are indicated to the left of the gel. The arrow points to the protein fraction which was subsequently used for in vitro experiments. (B) Coomassie blue-stained SDS–12% polyacrylamide gel of 100 ng of each purified B. japonicum RpoHHis protein. The apparent molecular masses of two marker proteins are indicated to the right of the gel. (C) Immunodetection of purified B. japonicum RpoH proteins with anti-E. coli ς32 serum. For each protein, a 5-ng sample was subjected to Western blot analysis.
In previous work, we showed that RpoH1 or RpoH2 produced in E. coli A7448 was detectable by an anti-E. coli ς32 serum in an immunoblot (18, 19) but that RpoH3 did not consistently react with the antiserum. By using the purified His-tagged proteins (Fig. 4B), we could now show that this serum also recognizes RpoH3His but clearly does so less well than it recognizes the other proteins (Fig. 4C).
Multiple-round in vitro transcription assays.
In a first attempt to test whether the purified RpoH proteins were transcriptionally active in vitro, we simulated the situation in the E. coli rpoH mutant in which the proteins had been shown to function in vivo. E. coli core RNA polymerase was reconstituted with the individual RpoHHis proteins, and the ability to transcribe the E. coli groESL promoter was monitored in a multiple-round transcription assay. The core enzyme alone produced small amounts of transcript (Fig. 5A), most probably as a result of some residual ς32 protein in the preparation that was detectable by Western blot analysis (data not shown). Transcription was stimulated by the addition of RpoH1His and in particular by RpoH2His. RpoH3His appeared to be inactive because the reconstituted enzyme did not produce more transcript than core RNA polymerase alone. Since RpoH3 was active at the E. coli groESL promoter in vivo (compare Fig. 1) we conclude that the purified protein was in an inactive state, probably because the small portion of soluble protein was in an inactive conformation. Transcriptional activity of RpoH1 and RpoH2 required the presence of RNA polymerase because the sigma factors alone were unable to synthesize transcript. As positive controls, we used E. coli and B. japonicum holo-RNA polymerases, both of which contain significant amounts of RpoH protein as revealed by Western blot analysis (data not shown). The transcripts produced by these enzymes were indistinguishable from the ones formed by the reconstituted enzymes, indicating that transcription had been initiated at the same position (Fig. 5A).
FIG. 5.
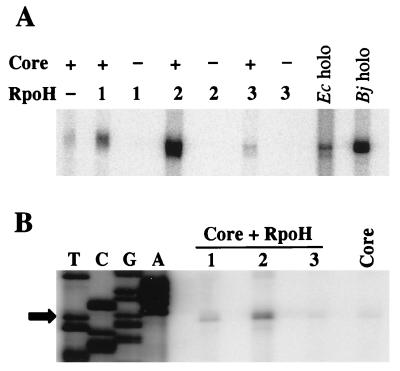
Transcription of the E. coli groESL promoter by E. coli RNA polymerase core enzyme and B. japonicum RpoH factors. (A) The enzyme combinations tested are indicated. Core, E. coli RNA polymerase core enzyme; RpoH, purified RpoHHis protein; Ec holo, E. coli RNA polymerase holoenzyme; Bj holo, B. japonicum RNA polymerase holoenzyme. (B) Primer extension analysis of in vitro-synthesized RNA. The enzyme with which the transcript was generated is indicated. The primer extension and sequencing reactions (TCGA) were performed with oligonucleotide Sig123. The transcription start site is depicted by an arrow.
To confirm that the reconstituted enzymes use the same start site as in vivo, we determined the 5′ end of in vitro-synthesized RNA by primer extension analysis (Fig. 5B). It was found to be identical to the start site of the ς32-dependent groESL (5) (compare Fig. 2). The presence of comparable faint signals produced by RNA polymerase core enzyme and RpoH3-containing enzyme again reflected the notion that the latter was not active.
Next we tested a physiologically more relevant combination by reconstituting B. japonicum core RNA polymerase with the purified RpoH factors and transcribing the B. japonicum groESL1 and dnaKJ promoters, which are dependent on a ς32-type factor in vivo (2, 16). Again, RpoH3His did not stimulate transcription (data not shown), but transcription from both promoters was initiated when RNA polymerase had been reconstituted with RpoH1His or RpoH2His (Fig. 6A and B). The groESL1 and dnaKJ transcripts are of the expected lengths (401 and 358 nucleotides, respectively), as judged from the RNA size marker. To demonstrate that these transcripts had been initiated at the same site as in vivo, in vitro-synthesized RNA was subjected to primer extension analysis. The determined 5′ ends of the groESL1 and dnaKJ transcripts (Fig. 6C and D, respectively) were mapped to the same positions as described previously (2, 16).
FIG. 6.
Transcription of the B. japonicum groESL1 promoter and the dnaKJ promoter by B. japonicum RNA polymerase reconstituted with purified RpoH factors. (A) Transcription of the B. japonicum groESL1 promoter. The enzyme combinations tested are indicated. Core, B. japonicum RNA polymerase core enzyme; RpoH, purified RpoHHis protein. The arrow points to an RNA size marker of 406 nucleotides. (B) Transcription of the B. japonicum dnaKJ promoter. The enzyme combinations are as indicated in panel A. (C) Primer extension analysis of in vitro-synthesized groESL1 transcript. Oligonucleotide 702 was used for the sequencing (TCGA) and primer extension reaction. The triangle points to the start site, which has been determined in vivo (2). The origin of small amounts of slower-migrating bands is not known because corresponding products were not observed after in vitro transcription. (D) Primer extension analysis of in vitro-synthesized B. japonicum dnaKJ transcript. Oligonucleotide DnaK12 was used for the sequencing (TCGA) and primer extension reaction. The 5′ end of the reverse transcript (indicated by a triangle) corresponds to the major start site found in vivo (16).
Single-round in vitro transcription assays.
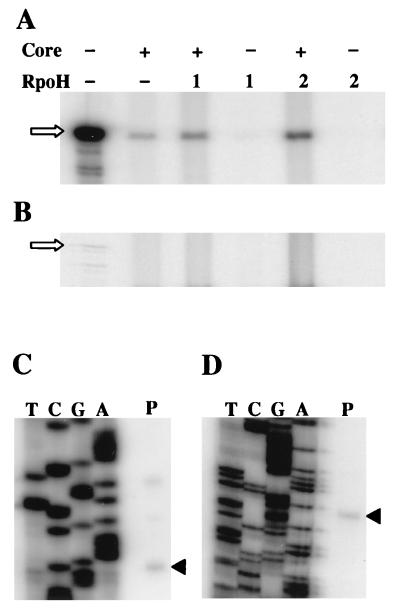
To gain more insight into possible promoter specificities of RpoH1 and RpoH2, it was important to establish a single-round transcription system. B. japonicum core RNA polymerase was reconstituted with RpoH2His, and transcription at five different promoters was assayed under conditions which allowed for the production of only one transcript by each preformed RNA polymerase-template complex. First we tested the E. coli groESL promoter, which was transcribed very well by this enzyme combination (Fig. 7A). The B. japonicum dnaKJ and groESL1 promoters were also transcribed specifically (Fig. 7B). As a control, we examined the hspA rpoH1 and rrn promoters, which are expected to be transcribed by B. japonicum ς80, the ς70 homolog (3, 18). The corresponding products would be 625 and 240 nucleotides long, respectively. Neither promoter was transcribed by the RNA polymerase containing RpoH2His, indicating that this sigma factor specifically recognizes ς32-like promoters.
FIG. 7.
Single-round transcription of different promoters by B. japonicum core RNA polymerase reconstituted with RpoH2His. The E. coli (A) or B. japonicum (B) promoters which were transcribed are indicated. Experiments were performed with core RNA polymerase alone (−) and the same enzyme reconstituted with RpoH2His (+). Arrows point to the transcripts; the position and length (in nucleotides) of RNA size markers are indicated to the right.
Promoter competition experiments.
To further investigate the promoter specificities of RpoH1 and RpoH2, we performed experiments in which an equimolar mix of different templates was provided for transcription. The first mix contained a composite of all five promoters which had been tested individually in the last experiment. B. japonicum core RNA polymerase was reconstituted with RpoH1His and RpoH2His, and transcription was assayed under single-round conditions (Fig. 8). As suggested by the experiment in Fig. 7, RNA polymerase containing RpoH2His preferentially transcribed from the E. coli groESL promoter. (The preference for the E. coli promoter may even be underestimated, because the E. coli groESL transcript contains 50 uracils that can be radiolabeled whereas the B. japonicum dnaKJ and groESL1 transcripts contain 81 and 80 uracils, respectively.) Transcription from the B. japonicum ς32-type promoters was much lower with the dnaKJ promoter, yielding slightly more transcript than the groESL1 promoter. RNA polymerase containing RpoH1His also transcribed the E. coli groESL promoter to the highest level. Small amounts of the groESL1 transcript and very little dnaKJ transcript were obtained, which implies that RpoH1 and RpoH2 have different promoter specificities. The hspA rpoH1 and rrn promoters that were also present in the mixture were not transcribed by the reconstituted enzymes.
FIG. 8.
Single-round promoter competition experiment with B. japonicum core RNA polymerase reconstituted with RpoH1His or RpoH2His. The E. coli groESL promoter and the B. japonicum dnaKJ, groESL1, hspA rpoH1, and rrn promoters were provided at equimolar concentrations (10 nM each; the template concentration is not limiting in this assay). The transcripts obtained are indicated.
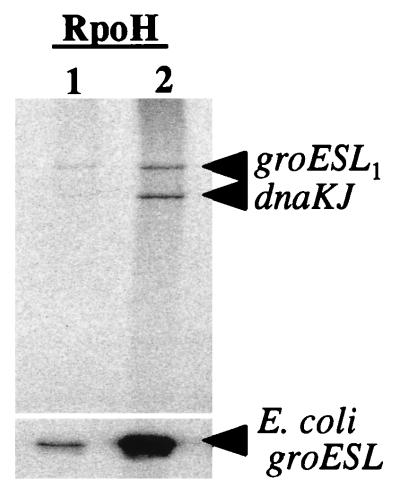
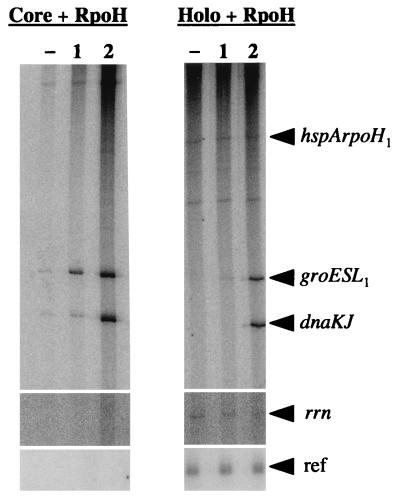
After this initial comparative assessment of the promoter specificities conferred by the different RpoH factors, we performed another template competition experiment in which only B. japonicum promoters were provided. Not only B. japonicum core but also holo-RNA polymerase was mixed with the individual purified RpoH factors. The experiment with holoenzyme showed that the added sigma factors can associate with core enzyme present in this preparation (Fig. 9). As expected, the ς80 holoenzyme produced some hspA rpoH1 and rrn transcript and also an 80-nucleotide reference transcript that is encoded by each vector carrying one of the promoter fragments (3). These three transcripts were not obtained when core polymerase was reconstituted with RpoH proteins. The experiments with core enzyme and with holoenzyme confirmed the different promoter specificities of RpoH1 and RpoH2. While the latter enzyme transcribed the groESL1 and dnaKJ promoter well and the dnaKJ promoter slightly better than groESL1, the RpoH1-containing RNA polymerase produced less transcript and favored the groESL1 promoter (Fig. 9; see also Fig. 8).
FIG. 9.
Single-round promoter competition experiment with B. japonicum RNA polymerase core enzyme and holoenzyme reconstituted with purified RpoH proteins. The B. japonicum dnaKJ, groESL1, hspA rpoH1, and rrn promoters were provided at equimolar concentrations (10 nM each). The corresponding transcripts are indicated. The vector-encoded reference transcript (ref) was produced only in the presence of the holoenzyme.
DISCUSSION
Different promoter specificities of B. japonicum RpoH factors.
After the previous identification of three disparately regulated rpoH genes in B. japonicum (18, 19), we were left with the question of why this organism uses a gene family for functions that in other bacteria (e.g., E. coli) are accomplished by a single rpoH gene. In principle, an organism can use two different strategies to achieve coordinate regulation of a gene under different environmental conditions: (i) The gene can be put under the control of different promoters, or (ii) several copies of this gene may be present, each having a distinctly regulated promoter. A paradigm for the differential regulation of reiterated genes is the groESL multigene family of B. japonicum. The expression of five groESL operons appears to be controlled by four mechanisms, two of which do not contribute to chaperonin production under heat shock conditions (low constitutive expression of groESL2 and NifA-dependent expression of groESL3) (6). Heat-induced expression of groESL genes depends on two separate mechanisms, namely, RpoH factor(s) for groESL1 and CIRCE (controlling inverted repeat of chaperone expression) (31) for groESL4 and groESL5 (2). The rpoH genes of B. japonicum were also defined as a disparately regulated gene family. Transcription of rpoH1, for instance, is heat inducible by a novel mechanism (18, 20), whereas rpoH2 is transcribed constitutively from a ς70-type promoter under normal growth conditions and induced from a ςE-type promoter under severe heat shock conditions (19).
However, the option to differentially regulate each member does not seem to be the only reason for the presence of an rpoH multigene family in B. japonicum. The situation becomes more complex because the individual rpoH genes are not functionally equivalent and have (at least to some extent) different promoter specificities in vivo and in vitro. The finding that all RpoH proteins were capable of synthesizing GroEL in an E. coli reporter strain agrees well with their previously shown activation of a groE-lacZ fusion. The limited capacity of RpoH1 and RpoH3 to produce other Hsps, especially GrpE, provides a possible answer to why these proteins were insufficient in complementing the temperature-sensitive phenotype of an E. coli ς32 mutant (19).
The promoter selectivity of RpoH1 and RpoH2 was further corroborated by in vitro studies. Purified RpoH3His protein appeared to be inactive because it showed very little (if any) activity at all promoters tested. RpoH3His also was much less soluble than RpoH1His and RpoH2His. Although the small fraction of soluble protein was purified from the crude supernatant, it may still be in an aggregated and transcription-incompetent form. By contrast, RpoH1His and, in particular, RpoH2His were highly active in transcribing ς32-dependent promoters. Much to our surprise, the endogenous B. japonicum groESL1 and dnaKJ promoters were transcribed less well than the heterologous E. coli groESL promoter. This raises the question why the known ς32-type promoters of B. japonicum contain a suboptimal promoter sequence. It is particularly puzzling because a heat shock promoter specific for the alpha subdivision of the proteobacteria (to which B. japonicum belongs) was proposed on the basis of a comparison of groESL and dnaKJ promoters from different members of this subdivision (16, 24). The consensus sequence differs from the typical ς32-dependent heat shock promoter of E. coli at several positions. However, a comparative functional analysis of heat shock promoters from the alpha and gamma proteobacteria has not been performed yet. Our first attempt in this direction implies that the heat shock promoters of the alpha proteobacteria are not necessarily optimized for recognition by the endogenous sigma factors. In keeping with this finding are the results of a mutational analysis of the ς32-dependent P1 promoter of the Caulobacter crescentus rpoH gene, which is autoregulated (29). This promoter also is not optimal, and its activity can be increased substantially by a particular point mutation in the −35 region. Generally, an efficient expression of heat shock genes under normal growth conditions is not required and may even be deleterious. It has been reported for E. coli that cells overproducing Hsps are sick (27). Thus, rather weak promoters may have evolved to reduce expression. After a heat shock, the abundant RpoH protein (in B. japonicum mainly RpoH1) may override the weak promoter activities.
The purified RpoH factors directed transcription exclusively from ς32-type promoters. A similar strict promoter dependence was also described for the corresponding enzymes of E. coli and C. crescentus (5, 28) with one notable exception: the P1 promoter of rrnB, one of seven rrn operons in E. coli, can be transcribed from RNA polymerase containing either ς70 or ς32 (21). This is thought to be important for ribosome synthesis at high temperatures. Neither the in vitro transcription performed in this work nor the earlier characterization of the rrn operon of B. japonicum (3, 13) gave any indication that a similar situation applies here. This is particularly surprising because B. japonicum contains only a single rRNA operon.
Function of RpoH1 and RpoH2 in B. japonicum.
On the basis of results obtained previously it was proposed that RpoH2 is essential for the synthesis of ς32-dependent proteins under normal growth conditions whereas RpoH1 provides their synthesis under stress conditions (19). The function of RpoH3 remains obscure. Combining all data known at present, we arrive at the following, refined model.
(i) Normal growth conditions.
Although the rpoH2 gene is transcribed to a considerable extent under these conditions, very little RpoH protein is detectable in cell extracts (18, 19). The corresponding protein is probably unstable during normal growth, as is E. coli ς32 (25). The observation that neither the rpoH2 nor the dnaK gene could be deleted had already suggested a direct dependence of the DnaK chaperone, which is required under all conditions, on this sigma factor (16, 19). The in vitro transcription data confirm that RpoH2 directs the transcription of the dnaKJ promoter efficiently. The groESL1 promoter could also be transcribed in vitro, but this does not seem to play a role in vivo because the groESL1 transcript is almost undetectable under normal growth conditions (2).
(ii) Heat shock conditions.
A temperature upshift greatly stimulates the production of RpoH1 in B. japonicum (18, 19). The parallel induction of the groESL1 transcript (2) indicated that it occurred as a result of an increase in RpoH1 production. The in vitro activity of RpoH1 supports this notion. By comparison, the dnaKJ promoter was weakly transcribed, which raises the question how heat induction of DnaK is accomplished. Since maximal induction of this protein is already reached a few minutes after a temperature upshift (16), it is highly possible that RpoH2 is responsible for this increase. The RpoH2 level in the cell increases in the initial phase after a heat shock before it rapidly decreases (19). Under continuous stress conditions, the weak activity of RpoH1 may then suffice to keep the DnaK concentration at an elevated level.
ACKNOWLEDGMENTS
We thank Hans-Martin Fischer for his continuous interest in our work and for many stimulating discussions. We are grateful to Bernd Bukau and Alan Easton for the generous gift of antisera, and we thank Wolfgang Weiglhofer for constructing the plasmid pRJ5086.
This study was supported by a grant from the Swiss National Foundation for Scientific Research.
REFERENCES
- 1.Allen S P, Polazzi J O, Gierse J K, Easton A M. Two novel heat shock genes encoding proteins produced in response to heterologous protein expression in Escherichia coli. J Bacteriol. 1992;174:6938–6947. doi: 10.1128/jb.174.21.6938-6947.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babst M, Hennecke H, Fischer H M. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol Microbiol. 1996;19:827–839. doi: 10.1046/j.1365-2958.1996.438968.x. [DOI] [PubMed] [Google Scholar]
- 3.Beck C, Marty R, Kläusli S, Hennecke H, Göttfert M. Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J Bacteriol. 1997;179:364–369. doi: 10.1128/jb.179.2.364-369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benvenisti L, Koby S, Rutman A, Giladi H, Yura T, Oppenheim A B. Cloning and primary sequence of the rpoH gene from Pseudomonas aeruginosa. Gene. 1992;155:73–76. doi: 10.1016/0378-1119(94)00829-h. [DOI] [PubMed] [Google Scholar]
- 5.Cowing D W, Bardwell J C A, Craig E A, Woolford C, Hendrix R W, Gross C A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci USA. 1985;82:2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fischer H M, Babst M, Kaspar T, Acuña G, Arigoni F, Hennecke H. One member of a groESL-like chaperonin multigene family in Bradyrhizobium japonicum is co-regulated with symbiotic nitrogen fixation genes. EMBO J. 1993;12:2901–2912. doi: 10.1002/j.1460-2075.1993.tb05952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamer J, Bujard H, Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, and GrpE and the bacterial heat shock transcription factor ς32. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 8.Gross C A, Lonetto M, Losick R. Bacterial sigma factors. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Vol. 1. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 129–176. [Google Scholar]
- 9.Gross C A, Chan C L, Lonetto M A. A structure function analysis of Escherichia coli RNA polymerase. Philos Trans R Soc London B Ser. 1996;351:475–482. doi: 10.1098/rstb.1996.0045. [DOI] [PubMed] [Google Scholar]
- 10.Helman J D, Chamberlin M J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- 11.Ishihama A. Protein-protein communication within the transcription apparatus. J Bacteriol. 1993;175:2483–2489. doi: 10.1128/jb.175.9.2483-2489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins A J, March J B, Oliver I R, Masters M. A DNA fragment containing the groE genes can suppress mutations in the Escherichia coli dnaA gene. Mol Gen Genet. 1986;202:446–454. doi: 10.1007/BF00333275. [DOI] [PubMed] [Google Scholar]
- 13.Kündig C, Beck C, Hennecke H, Göttfert M. A single rRNA gene region in Bradyrhizobium japonicum. J Bacteriol. 1995;177:5151–5154. doi: 10.1128/jb.177.17.5151-5154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusukawa N, Yura T. Heat shock protein GroE of Escherichia coli: key protective roles against thermal stress. Genes Dev. 1988;2:874–882. doi: 10.1101/gad.2.7.874. [DOI] [PubMed] [Google Scholar]
- 15.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 16.Minder A C, Narberhaus F, Babst M, Hennecke H, Fischer H M. The dnaKJ operon belongs to the ς32 dependent class of heat shock genes in Bradyrhizobium japonicum. Mol Gen Genet. 1997;254:195–206. doi: 10.1007/s004380050408. [DOI] [PubMed] [Google Scholar]
- 17.Missiakas D, Raina S, Georgopoulos C. Heat shock regulation. In: Lin E C C, Lynch A S, editors. Regulation of gene expression in Escherichia coli. R. G. Georgetown, Tex: Landes Co.; 1996. pp. 481–501. [Google Scholar]
- 18.Narberhaus F, Weiglhofer W, Fischer H M, Hennecke H. The Bradyrhizobium japonicum rpoH1 gene encoding a ς32-like protein is part of a unique heat shock gene cluster together with groESL1 and three small heat shock genes. J Bacteriol. 1996;178:5337–5346. doi: 10.1128/jb.178.18.5337-5346.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narberhaus F, Krummenacher P, Fischer H M, Hennecke H. Three disparately regulated genes for ς32-like transcription factors in Bradyrhizobium japonicum. Mol Microbiol. 1997;24:93–104. doi: 10.1046/j.1365-2958.1997.3141685.x. [DOI] [PubMed] [Google Scholar]
- 20.Narberhaus F, Käser R, Nocker A, Hennecke H. A novel DNA element that controls bacterial heat shock gene expression. Mol Microbiol. 1998;28:315–323. doi: 10.1046/j.1365-2958.1998.00794.x. [DOI] [PubMed] [Google Scholar]
- 21.Newlands J T, Gaal T, Mescas J, Gourse R L. Transcription of the Escherichia coli rrnB P1 promoter by the heat shock RNA polymerase (Eς32) in vitro. J Bacteriol. 1993;175:661–668. doi: 10.1128/jb.175.3.661-668.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regensburger B, Hennecke H. RNA polymerase from Rhizobium japonicum. Arch Microbiol. 1983;135:103–109. doi: 10.1007/BF00408017. [DOI] [PubMed] [Google Scholar]
- 23.Sambrook J E, Fritsch F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 24.Segal G, Ron E R. The dnaKJ operon of Agrobacterium tumefaciens: transcriptional analysis and evidence for a new heat shock promoter. J Bacteriol. 1995;177:5952–5958. doi: 10.1128/jb.177.20.5952-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straus D B, Walter W A, Gross C A. The heat shock response of E. coli is regulated by changes in the concentration of ς32. Nature. 1987;329:348–351. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 26.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 27.Tilly K, Spence J, Georgopoulos C. Modulation of stability of the Escherichia coli heat shock regulatory factor ς32. J Bacteriol. 1989;171:1585–1589. doi: 10.1128/jb.171.3.1585-1589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu J G, Newton A. Isolation, identification, and transcriptional specificity of the heat shock sigma factor ς32 from Caulobacter crescentus. J Bacteriol. 1996;178:2094–2101. doi: 10.1128/jb.178.7.2094-2101.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J G, Newton A. The Caulobacter heat shock sigma factor gene rpoH is positively autoregulated from a ς32-dependent promoter. J Bacteriol. 1997;179:514–521. doi: 10.1128/jb.179.2.514-521.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou Y N, Kusukawa N, Erickson J W, Gross C A, Yura T. Isolation and characterization of Escherichia coli mutants that lack the heat shock sigma factor ς32. J Bacteriol. 1988;170:3640–3649. doi: 10.1128/jb.170.8.3640-3649.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]