Abstract
Background:
A few recent studies have shown fungal elements within the hair follicle epithelium, which may act as a reservoir and responsible for recurrent dermatophytosis.
Objectives:
To assess the clinical patterns, mycological profile, and histopathology of recurrent dermatophytosis and to determine the prevalence of fungal hyphae in the hair follicle epithelium and other appendages.
Materials and Methods:
One hundred and fifty clinically diagnosed cases of recurrent dermatophytic infection were included. Skin samples were taken for direct microscopy, fungal culture, and histopathological analysis. Haematoxylin and eosin and special staining with periodic acid Schiff (PAS) and Gomori's methenamine silver (GMS) were performed to detect the fungal hyphae in the skin and hair follicle epithelium.
Results:
The most common clinical pattern observed was tinea corporis et cruris in 64 patients (42.66%). On direct microscopy and fungal culture, positive results were obtained in 116 cases (77.33%) and 78 (52%) cases, respectively. Presence of fungal hyphae in the stratum corneum, hair follicle, and acrosyringium was seen in 107 patients (71.33%), 47 patients (31.33%), and five patients (3.33%), respectively. Out of the 52 cases with hair follicle and eccrine gland involvement, history of fixed drug combinations (FDC) cream use was present in 42 cases (80.76%) and absent in ten cases (19.24%) (P = 0.000062).
Limitations:
Skin samples were taken only from a single skin lesion. Higher incidence of follicular invasion may have been detected if multiple biopsy samples were taken.
Conclusion:
Hair follicle/eccrine sweat gland involvement was observed in nearly one-third of the patients, which may act as a reservoir and may be responsible for recurrence and chronicity. Histopathology should be considered as an important adjuvant tool in recurrent dermatophytosis to establish the extent of the infection, which guides the further management.
Keywords: Fungal hyphae, hair follicle epithelium, histopathology, recurrent dermatophytosis
Introduction
Recurrent dermatophytosis is defined as the re-occurrence of dermatophyte infection within a few weeks (<6 weeks), after completion of treatment.[1] The prevalence of recurrent dermatophytosis varies in different regions, having a range of 0.39%–75.67%.[2] Recent studies have shown an increasing prevalence of recurrent and clinically unresponsive dermatophytosis in India.[3] Injudicious use of irrational fixed drug combinations (FDC) cream containing steroid and antifungal drugs, poor compliance to treatment, various host and environmental factors, and a growing resistance to antifungal drugs may be responsible for the recurrence and chronicity.[4,5]
Study of histopathology is becoming increasingly important in the assessment of recurrent dermatophytosis in view of the findings of a few recent studies that have shown the presence of fungal elements within the hair follicle unit or epithelium.[6,7] These fungal elements act as a reservoir, leading to recurrence and recalcitrance of dermatophytosis. Dermatophytic infection of vellus hair in sites other than the scalp, may also give rise to recurrence.[8] In the current milieu of increasing recurrent dermatophytosis, there is paucity of studies focusing on the histopathological aspects. Hence, the present study was undertaken to assess the clinical patterns, mycological profile, and histopathology of recurrent dermatophytosis and to determine the prevalence of fungal hyphae in hair follicle epithelium and other appendageal structures.
Materials and Methods
The study was a prospective, randomized, hospital-based, investigative study in which 150 patients diagnosed with recurrent dermatophytosis attending the dermatology outpatient department at a suburban medical college hospital were enrolled. The study was done over 22 months from January 2020 to October 2021, after being approved by the institutional ethics committee. The sample size was calculated by the difference of means formula, and to achieve a power of study of 80% and a precision alpha of 0.05 with a confidence interval of 95%, the estimated sample size was determined to be 150. Clinically diagnosed cases of recurrent dermatophytic infection with atleast one episode of relapse within six weeks of completing the full duration of antifungal therapy at the recommended dosage with good compliance, between the ages of 18 and 70 years, were included in the study. Immunocompromised patients including uncontrolled diabetes and HIV infection along with pregnant and lactating women were excluded.
In all enrolled subjects, a detailed clinical history including demographic data, socioeconomic status, and occupation was taken. History of similar complaints in the family, history of recurrences, history of previous treatment including use of FDC creams, type of lesions, and duration of disease were recorded. General physical examination and systemic examination were done. After a detailed dermatologic examination, the patients were classified into different clinical types based on the sites of involvement. Routine investigations including complete blood count and serum biochemistry profile were performed.
Skin scrapings were collected by scraping the active edge of the affected skin and subjected to direct microscopic examination using 10% potassium hydroxide (KOH). A positive KOH mount showed the presence of refractile, thready, septate hyphae. The collected specimens of skin scrapings were also sent for fungal culture. Specimens were inoculated on Sabouraud dextrose agar (SDA) slopes with cycloheximide (0.05 g/L) and chloramphenicol (0.005 g/L) for primary isolation. Test tubes were incubated for four weeks at 28°C before labelling it as negative. Dermatophyte test media (DTM) was used as a selective media and on incubation at 25ºC, the colour of DTM changes to red due to change in colour of phenol red indicator because of increased pH through increased metabolic activity. Species identification was done by colony morphology and microscopy on lactophenol cotton blue mount.
For histopathological analysis, a punch biopsy from the active edge of the skin lesion, including hair follicles, was taken under aseptic conditions and transported to the lab. Specimen was formalin fixed and embedded in paraffin and then subjected to vertical serial sectioning. Hematoxylin and eosin (H and E) as well as special staining with periodic acid Schiff (PAS) and Gomori's methenamine silver (GMS) were performed to detect and highlight the fungal hyphae. Six sections in H and E stained specimens and three sections each in PAS and GMS stained specimens were examined in all cases. In the H and E stained specimens, histopathological analysis was done to demonstrate the changes in the epidermis and dermis, to detect ‘sandwich sign’, and to look for involvement of the follicular unit or other appendageal structures with the presence of fungal spores and hyphae. In the GMS and PAS stained specimens, the presence of fungal hyphae in all layers of the epidermis, hair follicle as well as other appendages including eccrine sweat gland apparatus was studied.
Data collected from all patients were tabulated and analyzed. Statistical analysis was performed using the statistical package for social sciences (SPSS) trial version 25. All the data were presented in the form of percentages with the help of tables, bar diagrams, and pie diagrams. To test the statistical difference between groups, Chi-square test was used and P-value < 0.05 was considered as statistically significant.
Results
Among the 150 patients included in the study, 90 (60%) were males and 60 (40%) were females with a male to female ratio of 1.5:1. Most of the patients were in the age group of 18–-30 years (n = 62, 41.33%), followed by 31–40 years (n = 38, 25.33%). Duration of disease was less than three months in 36 (24%) patients and between three and six months in 46 (30.66%) patients. Chronic disease of more than six months was present in 53 patients (35.33%) and for more than one year in 15 patients (10%). The mean duration of illness was 4.73 months (standard deviation = 2.41). A history of similar recurrent lesions in the past was seen in 64 patients accounting for 42.66% of the cases whereas positive family history was recorded in 57 patients (38%). Eighty-nine patients (59.33%.) had used FDC creams before consulting a physician. Laboratory investigations were normal in all patients except in five patients in whom diabetes mellitus was detected.
The most common clinical patterns observed were tinea corporis et cruris in 64 patients (42.66%) followed by extensive tinea corporis (involving more than one region of the body) in 53 patients (35.33%) and tinea corporis et faciei in 13 patients (8.66%) [Table 1]. On direct microscopy, the hyphae were seen as a highly refractile, branched, septate threads suggestive of dermatophyte infection in 116 cases (77.33% KOH positivity). On fungal culture, microbiological confirmation of dermatophytic infection was obtained in 78 (52%) patients. Overall, both KOH and culture positivity was seen in 68 cases (45.33%). Of the 78 culture positive cases, Trichophyton mentagrophytes species (spp.) complex was isolated in 52 cases, making it the most common isolate (66.66%). It was followed by Trichophyton schoenleinii, seen in 17 patients (21.79%) [Table 2 and Figures 1-3].
Table 1.
Clinical profile of dermatophytosis
| Distribution of dermatophytosis over body sites | No. of cases (n=150) | Percentage |
|---|---|---|
| Tinea corporis | 53 | 35.33% |
| Tinea corporis et cruris | 64 | 42.66% |
| Tinea capitis et faciei | 13 | 8.66% |
| Tinea corporis et manuum | 3 | 2% |
| Tinea corporis et cruris et faciei | 9 | 6% |
| Tinea cruris | 8 | 5.33% |
Table 2.
Dermatophyte species identified on fungal culture (total 78 cases)
| Species identified on culture | No. of cases (n=78) | Percentage |
|---|---|---|
| T. mentagrophytes | 52 | 66.66% |
| T. schoenleinii | 17 | 21.79% |
| T. violaceum | 4 | 5.12% |
| T. rubrum | 2 | 2.56% |
| M. audouinii | 3 | 3.84% |
T=Trichophyton, M=Microsporum
Figure 1.

White flat powdery growth of fungal colonies suggestive of Trichophyton mentagrophytes species complex on (a) SDA medium and (b) dermatophyte test medium
Figure 2.
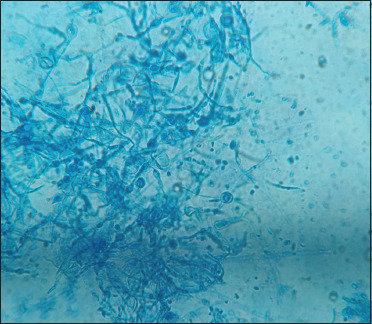
Septate hyphae with numerous microconidia arranged in clusters and spiral hyphae seen on lactophenol cotton blue mount suggestive of Trichophyton mentagrophytes species complex, 40×
Figure 3.

Smooth, waxy, and brownish growth of fungal colonies suggestive of Trichophyton schoenleinii on SDA medium
In most of the cases, histopathological changes seen in the epidermis were compact orthokeratosis, focal parakeratosis, and mild acanthosis. Superficial dermis showed dilated blood vessels and mild perivascular inflammatory infiltrate composed of lymphocytes and a few eosinophils, while deep dermis was unremarkable. Fungal hyphae were seen in the surface epithelium (stratum corneum) on H and E staining in 57 patients (38%), on PAS staining in 103 patients (68.88%), and on GMS staining in 107 patients (71.33%) [Figures 4 and 5]. Presence of ‘sandwich sign’ was observed in 57 cases (38%). Presence of fungal hyphae in the hair follicle was seen in 47 patients (31.33%), whereas in five cases (3.33%), fungal elements were detected in the acrosyringium [Table 3 and Figures 6-9]. Out of these 52 cases with either follicular or acrosyringeal involvement, fungal culture positivity was obtained in 48 cases. Follicular involvement with Trichophyton mentagrophytes spp. complex was seen in 32 cases and in 16 cases with all other isolated fungal species combined (P = 0.001091) [Table 4].
Figure 4.
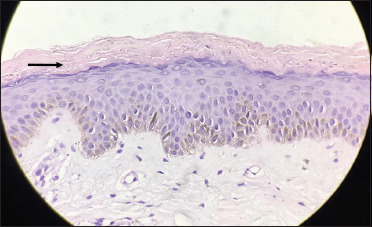
Numerous refractile (clear spaces), long fungal hyphae seen in the stratum corneum (black arrow) (H and E, 40×)
Figure 5.
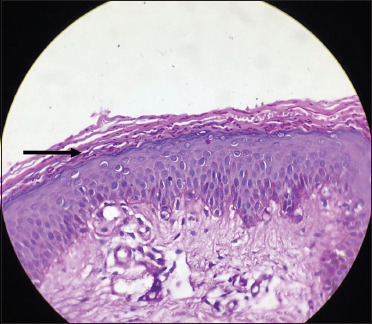
Numerous dark pink to purplish coloured fungal hyphae seen in the stratum corneum (black arrow) (periodic acid Schiff, 40×)
Table 3.
Demonstration of fungal elements on histopathological examination
| Presence of fungal hyphae and spores |
||
|---|---|---|
| No. of cases (n=150) | Percentage | |
| H and E staining | 57 | 38 |
| PAS staining | 103 | 68.66 |
| GMS staining | 107 | 71.33 |
| Within hair follicle or acrosyringium | 52 | 34.66 |
Figure 6.
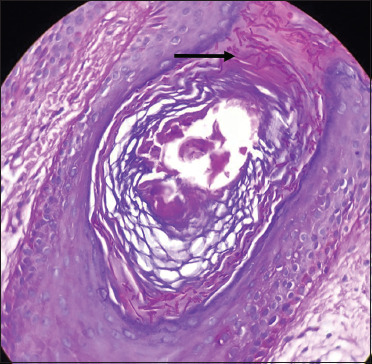
Numerous dark pinkcoloured fungal hyphae seen within the hair follicle (black arrow) (periodic acid Schiff, 40×)
Figure 7.
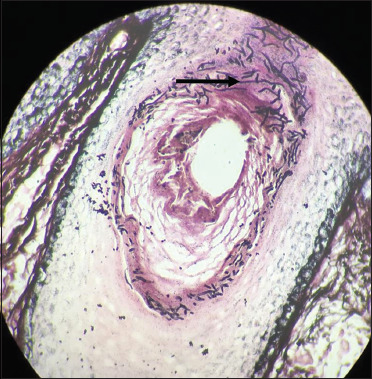
Numerous blackcoloured fungal hyphae are noted within the hair follicle (black arrow) (Gomori methenamine silver, 40×)
Figure 8.
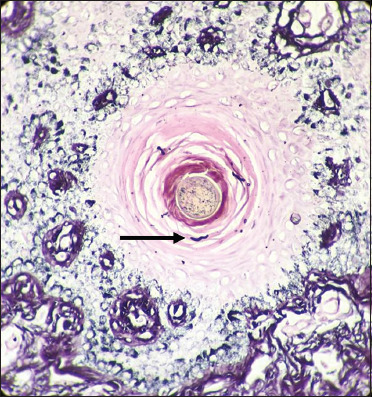
Few black coloured fungal hyphae are seen in the hair follicle (black arrow) (Gomori methenamine silver, 40×)
Figure 9.
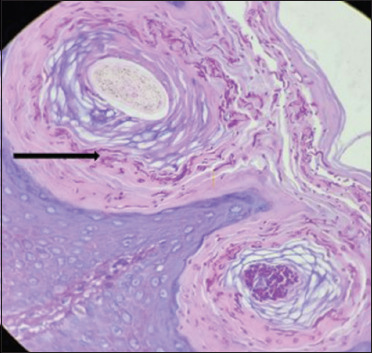
Numerous dark pink coloured hyphae are seen in the stratum corneum and hair follicle (black arrow) (periodic acid Schiff, 40×)
Table 4.
Comparison of hair follicle involvement with T. mentagrophytes and all other isolated fungal species combined
| Causative dermatophyte species | Presence of fungal elements in the hair follicle/eccrine sweat glands |
|
|---|---|---|
| No. of cases (n=48) | Percentage (%) | |
| Trichophyton mentagrophytes | 32 | 66.66% |
| All other isolated species combined | 16 | 33.33% |
Chi-square=10.667 and P<0.05 (P=0.001091)
In majority of cases, fungal elements were detected in the upper part of hair follicle affecting the infundibulum, isthmus, and keratinized portion of inner root sheath. In seven cases, fungal hyphae were demonstrated in the follicular plugging; one case showed presence of fungal hyphae in the acrosyringeal opening [Figures 10 and 11]. On correlating histopathological findings with FDC usage, out of the 52 cases with hair follicle/acrosyringeal involvement, 42 (80.76%) cases had a history of FDC cream use whereas only ten (19.23%) cases without history of FDC cream application showed hair follicle involvement. There was a statistically significant relation between previous FDC cream use and invasion of hair follicle and eccrine sweat gland (P = 0.000062) [Table 5].
Figure 10.
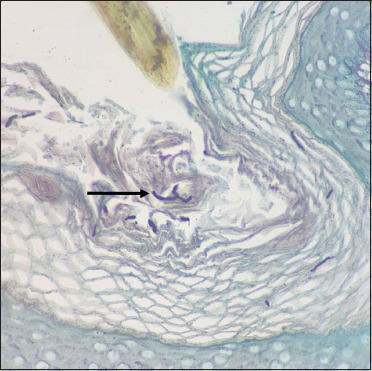
Few fungal hyphae are seen in the follicular plug (black arrow) (Gomori methenamine silver, 40×)
Figure 11.
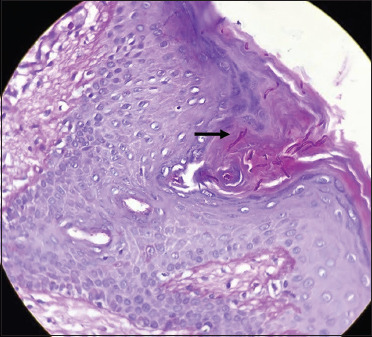
Few pinkish fungal hyphae are noted in the acrosyringeal opening (black arrow) (periodic acid Schiff, 40×)
Table 5.
Correlation between presence of fungal elements in the skin appendages and previous FDC cream use
| Recurrent dermatophytosis | Presence of fungal elements in the hair follicle/eccrine sweat glands |
|
|---|---|---|
| No. of cases (n=52) | Percentage (%) | |
| With history of FDC cream use | 42 (37 cases – hair follicle, 5 cases – eccrine gland) | 80.76% |
| Without history of FDC cream use | 10 (10 cases–hair follicle) | 19.23% |
Chi-square=16.03 and P<0.05 (P=0.000062)
Discussion
In recent years, dermatophytosis has attained unprecedented dimensions in India. Its clinical presentation is now multifarious, showing atypical morphology and an unusually extensive disease affecting all age groups and having a significant negative impact on social health, occupational health, and quality of life.[9] The significant increase in the number of chronic recalcitrant cases could be due to the unchecked availability of FDC creams containing topical corticosteroids and development of in-vitro resistance to systemic antifungals.[10] A few recent studies have postulated that presence of fungal hyphae in hair follicle could serve as a reservoir and enable clinical resistance, recurrence, and chronicity resulting in chronic and recurrent dermatophytosis.[6,7]
The extent of spread of the infection to the hair follicle or other appendageal structures can be determined by histopathology along with special stains, making it an essential diagnostic tool for the assessment and management in chronic and recalcitrant dermatophytosis.[11,12] In view of the above alarming and rapidly changing trends, the present study was undertaken to determine the clinical patterns, mycological profile, and histopathological changes including invasion of hair follicle in cases of recurrent dermatophytosis.
In this present study, patients were clinically diagnosed as having recurrent dermatophytosis as per the definition given by the expert panel during the first Delphi round in the article published by Rajagopalan et al.[1] All the patients had completed the recommended full course of anti-fungals and the lesions had cleared completely but then reappeared within a few days to less than six weeks of stopping the treatment. In the present study, the duration of the disease ranged from seven days to two years with a mean duration of 4.73 months. Highest number of patients had a recurrent disease duration of more than six months (n = 53,35.33%) followed by 3–6 months (n = 46, 30.66%). A similar high prevalence of dermatophytic infection lasting for 1–6 months (40.4%) and upto two years (35.8%) had been reported previously by Mahajan et al. and Pathania et al., respectively.[13,14] In the current study, 89 (59.33%) patients had a past history of using FDC medications before using the recommended systemic antifungal drugs which is similar to the high topical steroid usage observed in studies by Singh et al. and Nenoff et al.[5,15]
In the present study, out of the 78 culture positive cases, Trichophyton mentagrophytes spp. complex was isolated in 66.66% of cases and Trichophyton schoenleinii in 21.79% cases. Although T. schoenleinii is usually associated with favus and is an uncommon cause of tinea corporis, sporadic cases of tinea corporis caused by T. schoenleinii have been reported in the previous publications.[7,16,17] However, isolation of T. schoenleinii from tinea corporis has not been reported in our geographic region. Differences in severity and extent of dermatophytosis were not seen in the cases caused by the above two species.
Histological evidence of hair follicle involvement was also significantly more common with Trichophyton mentagrophytes when compared to all other isolated fungal species combined. Review of current literature shows that Trichophyton indotineae, which is identical to genotype VIII within the Trichophyton mentagrophytes/T. interdigitale species complex, has been found in a near-epidemic form all over the country and has spread to several countries worldwide. T. indotineae causes inflammatory and itchy, often widespread, chronic dermatophytosis and has largely displaced other previously prevalent dermatophytes on the Indian subcontinent.[18]
In this study, histopathological changes in the epidermis seen were compact orthokeratosis, focal parakeratosis, and mild acanthosis. Superficial dermis showed dilated blood vessels and mild perivascular inflammatory infiltrate composed of lymphocytes and few eosinophils. These findings are in accordance with the findings of Patil et al. and Vineetha et al.[6,7] The ‘sandwich sign’ was seen on histopathological examination and indicates the presence of hyphae between two zones of stratum corneum with parakeratotic lamellae below and orthokeratotic lamellae above and formation of a fissure in between in which fungal elements are seen. This sign was first described by Gottlieb and Ackermann and is specific to dermatophytosis.[19] In our study, this sign was observed in 57 cases (38%) which was higher than the observations made by Patil et al. (11%) and Al-Amiri et al. (12%).[6,20]
In our study, fungal hyphae were seen in the surface epithelium with H and E staining, PAS staining, and GMS staining in 57 patients (38%), 103 patients (68.88%), and 107 patients (71.33%), respectively, whereas higher rates of surface fungal detection was reported by Patil et al. in 91% patients with GMS stain.[6] Our study highlights the immense usefulness of special stains (PAS and GMS) in demonstrating the fungal elements in dermatophytoses when compared to the poor visibility of hyphae in H and E stained sections. Though many previous studies have suggested that GMS stain is superior to PAS in demonstrating fungal elements, based on our experience, the efficacy of PAS and GMS in detecting fungal elements was nearly equal.[6,21,22]
Presence of fungal hyphae within the hair follicle and acrosyringium were seen in 47 cases (31.33%) and five cases (3.33%), respectively. Among the above cases, fungal hyphae in the follicular plugs and acrosyringeal opening were seen in seven and one case, respectively. In our cases, fungal hyphae and arthrospores were detected in the upper part of hair follicle affecting the infundibulum, isthmus, and keratinized portion of inner root sheath. In recurrent and chronic dermatophytosis, the upper hair follicle may act as a reservoir and could be responsible for chronicity and recalcitrance. In all such cases, histopathological examination is a useful adjuvant tool which helps to ascertain hair follicle involvement. Like the duration of treatment in tinea capitis, all cases with histological evidence of involvement of hair follicle or eccrine glands should be treated with systemic antifungal medication at an appropriate dose based on the current guidelines for a sufficiently long duration of 6–8 weeks to achieve adequate penetration and concentration and to attain complete fungal clearance.[6,7,9,23]
Out of the 52 cases with hair follicle and eccrine gland involvement, history of FDC cream use was present in 80.76% cases and absent in 19.24% cases, which was statistically significant (P = 0.000062). Topical steroid abuse has a profound effect on the local host immune responses. Steroids inhibit the Th1-type of cell mediated immunity, which mediates the overall elimination of a fungal infection, by decreasing Th1 cytokines, interleukin -1, interferon-γ, and neutrophil chemotaxis. Th2 type of response, which predisposes to infection, is enhanced by the production of antibodies and cytokines such as IL-4, IL-5, and IL-13, which impair fungal clearance from the skin, leading to chronicity and recurrence.[24,25] Therefore, profound suppression of the local cell mediated immunity and long standing dermatophytosis caused by topical steroid application could be the possible reasons for the spread of fungus from the stratum corneum to the adjoining structures including hair follicles and eccrine sweat glands which in turn may act as a reservoir. This scenario has been brought about by the unchecked availability of inexpensive and irrational over the counter FDC medications and prescriptions by general physicians or self-medication by the patients.[26]
Limitations
In all the patients, skin samples were taken only from a single skin lesion. Higher incidence of follicular invasion may have been detected if multiple biopsy samples were taken. A repeat biopsy after completion of treatment to demonstrate the clearance of fungus could not be done. A control population without recurrent infection was not included in the present study. Future case control studies may be carried out to demonstrate that the histological changes are significantly higher in recurrent cases compared to control cases.
Conclusion
Hair follicle or eccrine sweat gland involvement was observed in nearly one-third of the patients, which may act as a reservoir and could be responsible for clinical resistance, recurrence, and chronicity. Histopathology should be considered as an important adjuvant tool in chronic and recurrent dermatophytosis to establish the extent of infection, which guides further management. A significant association was found between previous use of steroid creams and follicular unit invasion. This reinforces the urgent need to raise awareness levels regarding the abuse of topical steroids. Strict control over production and sale of FDC creams is warranted.
Financial support and sponsorship
The first author received IADVL THESIS GRANT in 2019 for the conduct of this research work as post graduate dissertation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors are grateful to Dr. Supriya Panda, professor and Head of the Department and Dr. R. Sarath Babu, M.D, Professor, Department of Microbiology, Maharajah's Institute of Medical Sciences, Nellimarla, Vizianagaram for their help in performing fungal cultures.
References
- 1.Rajagopalan M, Inamadar A, Mittal A, Miskeen AK, Srinivas CR, Sardana K, et al. Expert consensus on the management of dermatophytosis in India (ECTODERM India) BMC Dermatol. 2018;18:6. doi: 10.1186/s12895-018-0073-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shenoy MM, Rengasamy M, Dogra S, Kaur T, Asokan N, Sarveswari KN, et al. A multicentric clinical and epidemiological study of chronic and recurrent dermatophytosis in India. Mycoses. 2022;65:13–23. doi: 10.1111/myc.13360. [DOI] [PubMed] [Google Scholar]
- 3.Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: I. Epidemiology, risk factors and clinical features. Indian J Dermatol Venereol Leprol. 2021;87:154–75. doi: 10.25259/IJDVL_301_20. [DOI] [PubMed] [Google Scholar]
- 4.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227–36. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh S, Verma P, Chandra U, Tiwary NK. Risk factors for chronic and chronic-relapsing tinea corporis, tinea cruris and tinea faciei: Results of a case-control study. Indian J Dermatol Venereol Leprol. 2019;85:197–200. doi: 10.4103/ijdvl.IJDVL_807_17. [DOI] [PubMed] [Google Scholar]
- 6.Patil PD, Pande S, Mahore S, Borkar M. Histopathology of hair follicle epithelium in patients of recurrent and recalcitrant dermatophytosis: A diagnostic cross-sectional study. Int J Trichology. 2019;11:159–66. doi: 10.4103/ijt.ijt_7_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vineetha M, Sheeja S, Celine MI, Sadeep MS, Palackal S, Shanimole PE, et al. profile of dermatophytosis in a tertiary care center. Indian J Dermatol. 2018;63:490–5. doi: 10.4103/ijd.IJD_177_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sentamilselvi G, Janaki C, Murugusundram S. Trichomycoses. Int J Trichology. 2009;1:100–7. doi: 10.4103/0974-7753.58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verma SB, Panda S, Nenoff P, Singal A, Rudramurthy SM, Uhrlass S, et al. The unprecedented epidemic-like scenario of dermatophytosis in India: III. Antifungal resistance and treatment options. Indian J Dermatol Venereol Leprol. 2021;87:468–82. doi: 10.25259/IJDVL_303_20. [DOI] [PubMed] [Google Scholar]
- 10.Sardana K, Gupta A, Mathachan SR. Immunopathogenesis of dermatophytoses and factors leading to recalcitrant infections. Indian Dermatol Online J. 2021;12:389–99. doi: 10.4103/idoj.IDOJ_503_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gocev Đ, Damevska K. The role of histopathology in the diagnosis of dermatophytoses. Serbian J Dermatol Venerol. 2010;2:45–53. [Google Scholar]
- 12.Kaliyamoorthy S, Srinivasan S. Histopathological study of cutaneous and soft tissue fungal infections. Int J Res Med Sci. 2016;4:1933–7. [Google Scholar]
- 13.Mahajan S, Tilak R, Kaushal SK, Mishra RN, Pandey SS. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care centre. Indian J Dermatol Venereol Leprol. 2017;83:436–40. doi: 10.4103/ijdvl.IJDVL_519_16. [DOI] [PubMed] [Google Scholar]
- 14.Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol Venereol Leprol. 2018;84:678–84. doi: 10.4103/ijdvl.IJDVL_645_17. [DOI] [PubMed] [Google Scholar]
- 15.Nenoff P, Verma SB, Vasani R, Burmester A, Hipler U-C, Wittig F, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes - A molecular study. Mycoses. 2019;62:336–56. doi: 10.1111/myc.12878. [DOI] [PubMed] [Google Scholar]
- 16.Surendran K, Bhat RM, Boloor R, Nandakishore B, Sukumar D. A clinical and mycological study of dermatophytic infections. Indian J Dermatol. 2014;59:262–7. doi: 10.4103/0019-5154.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansouri P, Farshi S, Khosravi AR, Naraghi ZS, Chalangari R. Trichophyton schoenleinii-induced widespread tinea corporis mimicking parapsoriasis. J Mycol Med. 2012;22:201–5. doi: 10.1016/j.mycmed.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Uhrlaß S, Verma SB, Gräser Y, Rezaei-Matehkolaei A, Hatami M, Schaller M, et al. Trichophyton indotineae-An emerging pathogen causing recalcitrant dermatophytoses in India and worldwide-A multidimensional perspective. J Fungi (Basel) 2022;8:757. doi: 10.3390/jof8070757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gottlieb GJ, Ackerman AB. The “sandwich sign” of dermatophytosis. Am J Dermatopathol. 1986;8:347–50. doi: 10.1097/00000372-198608000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Al-Amiri A, Chatrath V, Bhawan J, Stefanato CM. The periodic acid-schiff stain in diagnosing tinea: Should it be used routinely in inflammatory skin diseases? J Cutan Pathol. 2003;30:611–5. doi: 10.1034/j.1600-0560.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 21.D'Hue Z, Perkins SM, Billings SD. GMS is superior to PAS for diagnosis of onychomycosis. J Cutan Pathol. 2008;35:745–7. doi: 10.1111/j.1600-0560.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 22.Weedon D. Weedon's skin pathology. 3rd. Edinburgh: Churchill Livingstone Elsevier; 2010. Mycoses and algal infections; pp. 581–606.e24. [Google Scholar]
- 23.Gómez-Moyano E, Crespo-Erchiga V. Tinea of vellus hair: An indication for systemic antifungal therapy. Br J Dermatol. 2010;163:603–6. doi: 10.1111/j.1365-2133.2010.09811.x. [DOI] [PubMed] [Google Scholar]
- 24.Waldman A, Segal R, Berdicevsky I, Gilhar A. CD4+ and CD8+T cells mediated direct cytotoxic effect against Trichophyton rubrum and Trichophyton mentagrophytes. Int J Dermatol. 2010;49:149–57. doi: 10.1111/j.1365-4632.2009.04222.x. [DOI] [PubMed] [Google Scholar]
- 25.Bressani VO, Santi TN, Domingues-Ferreira M, Almeida A, Duarte AJS, Moraes-Vasconcelos D. Characterization of the cellular immunity in patients presenting extensive dermatophytoses due to Trichophyton rubrum. Mycoses. 2013;56:281–8. doi: 10.1111/myc.12018. [DOI] [PubMed] [Google Scholar]
- 26.Tigga RA, Das S, Bhattacharya SN, Saha R, Pandhi D, Datt S, et al. Burden of chronic dermatophytosis in a tertiary care hospital: Interaction of fungal virulence and host immunity. Mycopathologia. 2018;183:951–9. doi: 10.1007/s11046-018-0303-4. [DOI] [PubMed] [Google Scholar]


