Abstract
Mycobacterium indicus pranii (MIP), previously called Mw vaccine, is a one-of-a-kind immunomodulatory vaccine. It was indigenously developed in India for use in leprosy. MIP is heat-killed Mycobacterium w, which is a non-pathogenic atypical mycobacterium belonging to Class IV of Runyon classification. It shares epitopes with Mycobacterium leprae and Mycobacterium tuberculosis, which forms the rationale behind its use in leprosy and tuberculosis. MIP activates both innate and acquired immunity. It induces a Th1 and Th17 immune response along with downregulation of Th2 pathway and activates macrophages and dendritic cells. MIP vaccine is safe with adverse effects such as local site erythema, swelling, and rarely fever and other systemic reactions. Apart from leprosy, MIP has been used in dermatological diseases such as warts and psoriasis. Clinical trials have evaluated the efficacy of MIP in a plenitude of non-dermatological conditions such as category II tuberculosis, Gram-negative sepsis, non-small cell lung cancer, human immunodeficiency virus (HIV), muscle-invasive bladder cancer, and very recently, coronavirus 2019 (COVID-19). In vitro and animal studies have also demonstrated its utility in leishmaniasis, melanoma, and as a vaccine for the prevention of pregnancy. The PubMed database was searched using “Mycobacterium indicus pranii, MIP, Mycobacterium w” as the keyword in title. This comprehensive review provides useful information for healthcare professionals about immunotherapeutic potential of MIP vaccine, its composition, dosing schedule, administration, and side effects besides its efficacy in various indications other than leprosy.
Keywords: Immunotherapy, Leprosy, Mycobacterium indicus pranii
Introduction
Mycobacterium w (Mw) or Mycobacterium indicus pranii (MIP) is a coveted, first vaccine that has been indigenously developed in India for use in leprosy.[1] It is also particularly useful in advanced non-small cell lung cancer in combination with paclitaxel and cisplatin, and sepsis.[2,3] MIP vaccine stimulates innate as well as adaptive immune responses. It induces a Th1 and Th17 immune response along with downregulation of Th2 pathway and activates macrophages and dendritic cells.[4] MIP is a killed vaccine that contains heat-killed Mycobacterium w suspended in saline. Mycobacterium w is an atypical, cultivable, non-pathogenic, and rapidly growing mycobacterium classified under Runyon group IV of non-tubercular mycobacteria.[5] MIP vaccine is fairly safe with few adverse effects such as local site erythema, swelling, and rarely fever and other systemic reactions.[6,7,8]
Although originally developed for leprosy prevention and management, it has an additional plethora of uses [Figure 1], some approved and many still under evaluation including the prevention of tuberculosis and the most recent, COVID-19 infection. The PubMed database was searched using “Mycobacterium indicus pranii, MIP, Mycobacterium w” as the keyword in title. Abstracts were screened to include studies in the English language and those pertaining to the use of MIP. This comprehensive review provides useful information for healthcare professionals about immunotherapeutic potential of MIP vaccine, its composition, dosing schedule, administration, and side effects besides its efficacy in various indications other than leprosy.
Figure 1.
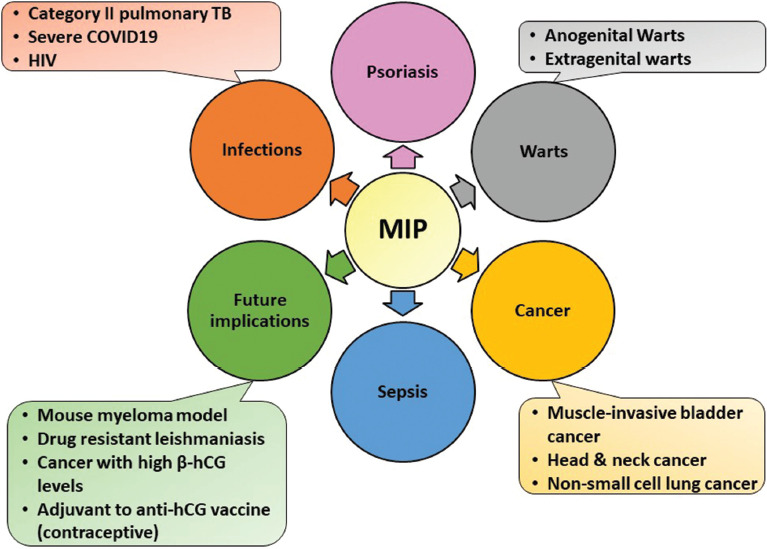
Dermatological and non-dermatological uses of MIP apart from leprosy
History
Vaccines are a cornerstone in the prevention of disease and eventually eliminating it. The basis for the development of a vaccine for leprosy was due to a specific defect that was observed in a subpopulation of T lymphocytes in certain individuals making them susceptible to the infection, especially lepromatous leprosy.[9] Use of killed or live attenuated M. leprae did not give the desired results in these individuals. Thus, in the quest for developing an effective leprosy vaccine, sixteen strains of atypical mycobacteria were tried of which five, including an unnamed strain, were found to be useful.[10] This strain was then called the Mycobacterium w. While initially developed for leprosy, the utility of the vaccine was found in multiple other diseases as well.
M. tuberculosis-W (Beijing genotype) is a distinct drug-resistant sub-strain that has different genetic makeup and molecular profile than Mycobacterium w. To avoid confusion, the name Mycobacterium Indicus Pranii (MIP) was suggested.[11] “Indicus” was derived from the place of origin, India, “Pran” from the family name of the discovering scientist (Gursaran Pran Talwar), and “NII” stood for National Institute of Immunology (New Delhi, India) where it was developed.
Immunomodulatory mechanism
MIP has a definite role in inducing both innate and adaptive immune responses [Figure 2]. Innate immunity is a potent inducer of pro-inflammatory responses in macrophages as well as in dendritic cells and mediates its effects through both TLR2 and NOD2 in a MyD88-dependent manner.[12] MIP-induced pro-inflammatory responses include induction of tumor necrosis factor- α, interleukin-12p40 (IL-12p40), IL-6, nitric oxide, enhanced reactive oxygen species, and IFN-induced chemokines such as CXCL10.[12,13,14] It causes significant upregulation of costimulatory molecules CD40, CD80, and CD86 in dendritic cells and also promotes classical as well as cross-presentation of antigens by them.[4]
Figure 2.
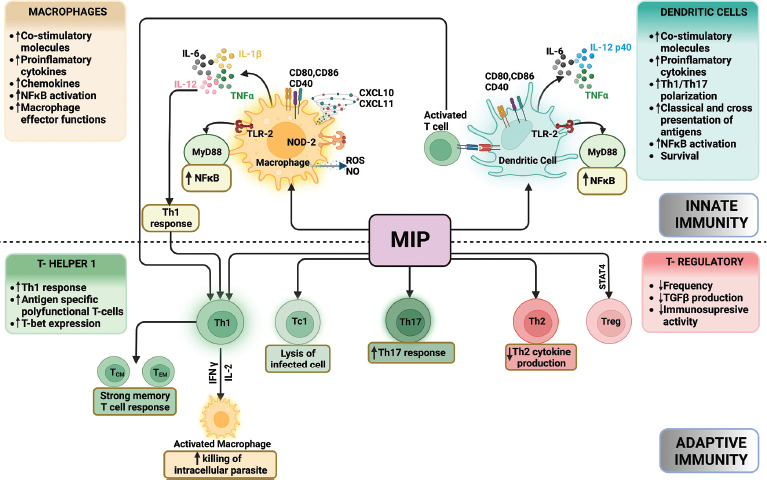
Immunomodulatory mechanism of MIP vaccine. “Created with BioRender.com”
MIP preferentially induces a strong host-protective Th1 immune response. It helps in the generation of antigen-specific polyfunctional T cells, that is, IFN-γ + TNF-α + IL-2+ T cells or IFN-γ + TNF-α + T cells.[15] A strong memory T-cell response including potent central memory (CD44+CD62L+cells) as well as a strong effector memory (CD44+ CD62L− cells) T-cell response in both CD4+ and CD8+ T-cell subsets has also been observed. It promotes Th17 polarization and abrogates Th2 and T-regulatory (T-reg) cell response.[16] MIP vaccine also has the ability to reduce the cytokine storm, which renders it good immunomodulatory capability in sepsis and COVID-19. The same mechanism underlies its utility in the reduction of type 2 lepra reactions and tubercular pericarditis.[8]
Composition and method of administration[1]
The vaccine is a colorless suspension of killed Mycobacterium w. It is available as a 0.6-mL vial, with each dose of 0.1 mL containing 0.5 × 109 cells of bacilli, 0.9% w/v of sodium chloride, and 0.01% w/v of thiomersal. It is also available as a 0.2-ml prefilled syringe, with each 0.1 ml composed of 0.5 × 109 cells of bacilli, 0.9% w/v of sodium chloride, and 1.2% w/v of benzyl alcohol. The vaccine is currently manufactured and marketed by M/s Cadila Pharmaceuticals Ltd and is available in India as Immunovac and Sepsivac. Sepsivac is the brand name for the 0.6-ml vial, while Immunovac is the 0.2-ml prefilled syringe.
MIP vaccine should be stored at +2 to +8 degrees Celsius, away from direct light. Freezing should be avoided, and if frozen by mistake, the vial should be discarded. The injection is administered intradermally, and the preferred sites for administration include insertion of the deltoid muscle in the middle of the upper arm and the upper and lateral aspects of the thigh. The upper arm is placed at 45 degrees to the body by placing the hand on the hip with the abduction of the arm. The area should be cleaned with spirit and allowed to dry following which the skin should be stretched between thumb and forefinger of one hand. A 26 G or smaller needle then should be inserted with the bevel upward at 5–15 degrees and the vaccine injected intradermally raising a tensed blanched bleb. Resistance should be felt while injecting the vaccine, and a bleb of about 6–10 mm should be raised. The second dose should be administered at least 1 inch away from the initial site of injection.
In case of warts (genital and extragenital), the site is cleaned and the vaccine is injected in the larger warts at the level of the dermis.
Precautions: If a bleb does not form after an intradermal injection or there is a diffuse swelling, then the injection has been wrongly administered subcutaneously. The needle should be withdrawn till it is more superficial in the dermal layer and then injected. The local site should not be infected, inflamed, or ulcerated, and cleaned with spirit or alcohol before giving the intradermal injection. The site should not be massaged after injection.
Contraindications: MIP vaccine is absolutely contraindicated in patients with history of allergic reaction to any of the components of the vaccine. It is not to be used in pregnant women due to lack of safety studies.
Use of MIP in different dermatological conditions and dosing schedule
MIP was first utilized in the early 1990s as an adjunct to WHO MDT-MBR (World Health Organisation Multidrug Therapy – Multibacillary regimen) in multibacillary (MB) leprosy patients.[5] It produced conversion of lepromin test, quicker clinical improvement, faster drop in BI, and rapid clearance of granulomas histologically. MIP was also investigated for the prevention of leprosy along with other vaccines as an immunoprophylactic agent in an general population in an endemic region, but it was of lower efficacy in comparison with the other vaccines.[17] MIP vaccine has been evaluated in household contacts of leprosy patients as an immunoprophylactic vaccination against the disease.[18]
The scope of the vaccine was eventually broadened beyond leprosy, and a number of studies have been carried out on its use in warts, anogenital as well as extragenital.[6,19,20,21,22,23,24,25,26] Few studies have also demonstrated the role of MIP vaccine in psoriasis.[27,28]
There is no standard dosing schedule described for the MIP vaccine. As elucidated below, the dosing schedules vary widely, even among the studies on the same disease. The only rule of thumb to be followed is that a maximum of 0.1 ml injection should be administered intradermally at each site in one visit.
Psoriasis: In the study by Rath et al., 16.6% of patients showed marked and 62.5% showed moderate improvement in Psoriasis Area and Severity Index (PASI) score at the end of 16 weeks. About 0.1 ml injection was given intradermally on the shoulder on the first visit and was repeated after three weeks.[27] In another study by Kumar et al., 0.1 ml injection was given each on both shoulders' deltoid region on the first visit followed by two more doses of 0.1 ml injections at three weekly intervals.[28] Both studies found that MIP can be used as an adjuvant in psoriasis.
Anogenital and extragenital warts: In about a third of the studies, sensitization dose of 0.1 ml in each shoulder was given intradermally. The sensitization response in the form of erythema or induration was examined two weeks later. In sensitized patients, 0.1 ml injection was given intralesionally, and divided and administered into 2–3 warts, preferably the largest ones. The injections were given for two weeks till there was complete resolution of the warts or a maximum of 10 doses, whichever was earlier.[19,20,21] In few other studies, the sensitization dose was given similarly but 0.1 ml injection in 3–5 largest warts was repeated weekly till clearance of the lesions or a maximum of 10 injections, whichever was earlier.[22,23,24] Lastly, in few studies the sensitization dose was not given and 0.1 ml of the vaccine was injected into the single largest wart every 2–4 weeks.[6,25,26] MIP was found to be useful in the treatment of anogenital warts in all the studies, and the efficacy was comparable to purified protein derivative (PPD), measles, mumps, and rubella vaccine (MMR vaccine), cryotherapy, and imiquimod.
The studies on various dermatological uses of the MIP vaccine are summarized in Table 1.
Table 1.
A brief summary of studies on various dermatological uses of the MIP vaccine besides leprosy
| Study | Year and design | Study population | Intervention | Outcome |
|---|---|---|---|---|
| Rath et al.[27] | 2003, pilot study | 36 patients with psoriasis | MIP/placebo | 16.6% of patients showed marked and 62.5% showed moderate improvement in PASI score at the end of 16 weeks. In the control group, all 12 patients showed less than a 25% reduction in PASI score at the end of one and four months. |
| Kumar et al.[5] | 2005, uncontrolled trial | 45 patients with mild-to-moderate psoriasis | MIP | Mean reduction in PASI score by 33% at 12 weeks; Mw vaccine can be an adjuvant but not the sole therapy in psoriasis |
| Gupta et al.[23] | 2008, pilot study | 10 patients with external anogenital warts | MIP | 88.9% of patients had complete clearance. No recurrence at mean follow-up of 5.1 months |
| Meena et al.[22] | 2013, uncontrolled open label | 40 patients with multiple cutaneous warts | MIP | Complete clearance in 83%, 50% clearance in one patient and 25–30% reduction in three patients; recurrence in three patients at 4.48 (1.32) months |
| Kumar et al.[20] | 2014, RCT | 89 patients with anogenital warts | MIP/imiquimod | 59% in the imiquimod group and 67% in the Mw group—complete resolution; significant decline in HPV-6 and HPV-11 viral load—Mw group, only in HPV-6 load in the imiquimod group |
| Gupta et al.[23] | 2014, retrospective study | 44 patients with extragenital warts | MIP | Complete clearance in 54.5%, >75% clearance in 84.1% of patients; response at distant sites in 86.3% of patients |
| Garg et al.[25] | 2014, prospective cohort | 30 patients with warts at difficult-to-treat places | MIP | Twenty-nine (93.33%) patients had complete resolution with meantime for clearance of 43.71 (32.82) days |
| Dhakar et al.[21] | 2015, RCT | 66 patients with refractory anogenital warts | MIP/cryotherapy | Both therapies are equally efficacious, Mw has an added advantage of clearance of distant warts |
| Khullar et al.[51] | 2017, case report | 1 patient with giant condyloma acuminata | MIP and acitretin | Improvement after three weekly doses, complete clearance after six months of acitretin |
| Chandra et al.[26] | 2019, RCT | 64 patients with multiple warts | MIP/PPD | Significant improvement in both groups |
| Kaur et al.[6] | 2021, RCT | 60 patients with cutaneous warts | MIP/MMR | MIP demonstrated a faster and a significantly comprehensive remission as compared to MMR. |
RCT=Randomized controlled trial, PPD=Purified protein derivative, MMR=Measles, mumps, and rubella vaccine
Use of MIP in different non-dermatological conditions and dosing schedule
MIP has been studied in a vast number of diseases. Some studies have found it useful in non-advanced small cell lung cancer and severe sepsis.[2,3,29,30] Few studies have also found it to be useful in HIV, muscle-invasive bladder cancer, category II tuberculosis, head and neck cancer, and most recently, COVID-19.[7,31,32,33,34,35,36,37]
HIV: About 0.1 ml of MIP injection is given on both shoulders followed by four doses of 0.1 ml injection every month.[31] The vaccine showed synergistic action with highly active antiretroviral therapy (HAART).
Non-small cell lung cancer: About 0.2 ml of the injection is given initially in two divided doses of 0.1 ml each in both arms, one week before initiation of chemotherapy. Subsequently, 0.1 ml of the vaccine is administered on days 8 and 15 of each chemotherapy cycle. After the completion of the four cycles of chemotherapy, MIP is continued monthly for 12 months from the initiation of therapy.[29] The study found significant improvement in quality of life and regression of tumor size and improvement in lung function.
Severe sepsis and COVID-19: About 0.3 ml of the vaccine is given every day for three days in the deltoid region divided into three aliquots of 0.1 ml each.[2,30,32,33] The vaccine should be initiated within 48 hours of first organ dysfunction. Statistically significant and clinically relevant faster recovery, decrease in length of ICU stay, and mortality were found in the MIP vaccine group.
Category II pulmonary TB: About 0.1 ml of the vaccine is given intradermally in both shoulders followed by 0.1 ml intradermal injections every two weeks for two months.[7] Sputum conversion rate and the cure rate in the MIP group were higher compared to the control.
The studies on the MIP vaccine's numerous non-dermatological applications are summarized in Table 2.
Table 2.
Studies on the utility of the MIP vaccine in non-dermatological conditions
| Study | Year and design | Study population | Intervention | Outcome |
|---|---|---|---|---|
| Kharkar[31] single author so no et al. | 2002, RCT | 50 patients with HIV | MIP/MIP + 2 antiretroviral agents/MIP + HAART | 108.96% increase in CD4 counts in patients receiving MIP+HAART suggesting a synergistic action; 80.22% increase with MIP alone |
| Chaudhary et al.[34] | 2003 | 5 patients with muscle-invasive bladder cancer | MIP with external beam radiotherapy | Complete remission for more than two years |
| Sur et al.[3] | 2003, RCT | Patients with non-small cell lung cancer | Cisplatin, etoposide, radiotherapy with or without Mw vaccine | Significant improvement in quality of life and regression of tumor size and improvement in lung function |
| Patel et al.[35] | 2003, RCT | Patients with category II pulmonary TB | Short-course chemotherapy with or without MIP | Sputum conversion rate in the MIP group was 75.51% compared to 51.85% in control, and the cure rate was 97.96% in Mw compared to 77.77% in control |
| Pant et al.[36] | 2005, RCT | 91 patients with head and neck cancer | Chemo-radiotherapy with or without MIP | Better tumor response, significantly lesser therapy-related side effects, and higher improvement in quality of life in the MIP group. |
| Belani et al.[29] | 2011, open-label phase II clinical trial | 221 patients with stage IIIB and IV non-small cell lung carcinoma | Paclitaxel and cisplatin with or without 0.1 ml MIP | Significant improvement in progression-free survival and overall survival with MIP |
| Mayosi et al.[8] | 2014, 2×2 RCT | 1400 patients with definite or probable tuberculous pericarditis | Prednisolone or placebo MIP or placebo |
No significant difference in the composite of death, cardiac tamponade requiring pericardiocentesis, or constrictive pericarditis |
| Sehgal et al.[2] | 2014, RCT | 50 patients with sepsis | MIP/placebo | Statistically significant and clinically relevant faster recovery, length of ICU stay |
| Sharma et al.[7] | 2017, RCT | 890 patients with category II TB | MIP/placebo | Significant conversion of sputum culture at four weeks in MIP arm compared to placebo |
| Sehgal et al.[32] | 2021, RCT | 42 patients with severe COVID-19 | MIP/placebo | Clinical status distribution was significantly better in the MIP group on days 14 and 21 but there was no difference in SOFA score or mortality |
| Sehgal et al.[30] | 2021, RCT | 202 patients with presumed Gram-negative sepsis | MIP/placebo | Significant decrease in mortality after adjusting for culture-positive sepsis, baseline SOFA score, age, and sex |
| Dixit et al.[33] | 2022, retrospective study | 448 critically ill COVID-19 patients | MIP | Early initiation of Mw (<3 days) resulted in significant decrease in mortality, intubation requirement, and lesser duration of stay in the ICU |
ICU=Intensive care unit, HIV=Human immunodeficiency virus, HAART=Highly active antiretroviral therapy, SOFA=Sequential organ failure assessment, TB=Tuberculosis
Adverse effects
Common side effects
Mild-to-moderate injection site erythema, induration, nodules, and ulceration are the most common adverse effects observed with the MIP vaccine [Figure 3a-d].[6,7,8] Such reactions are most often conservatively managed with analgesics and observation. Dressing is usually avoided except if oozing occurs; then, a dry dressing can be applied. Low-grade fever in the first three days after injection has also been reported commonly.[19] It does not warrant treatment usually. Pain and itching are common complaints at the site of intralesional injection of the vaccine, which resolve on their own without any specific treatment. Local site edema and swelling can also be noted, especially in cases of warts.[20]
Figure 3.
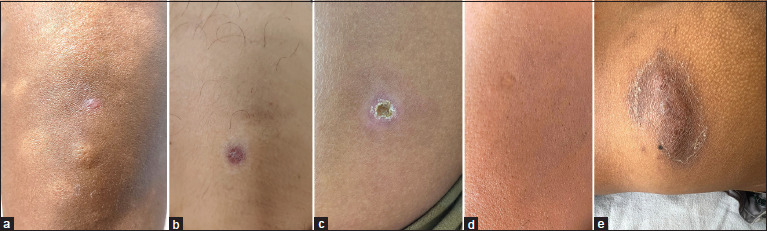
(a) Bleb formation at the time of injection, (b) erythema and induration after one week, (c) self-healing ulcer formed after three weeks, (d) scar at the injection site, and (e) abscess formation due to erroneous intramuscular administration of the vaccine
Uncommon side effects
Keloid formation at the site of vaccine administration can largely be attributed to injection of the vaccine higher than the insertion of the deltoid in the mid-arm, especially at the tip of the shoulder. Improper injection technique leading to a deeper delivery of the vaccine into the subcutaneous or intramuscular plane can lead to ulceration and abscess formation [Figure 3e]. In the event of a severe local site reaction in the form of a large ulcer or abscess, the subsequent dose should be deferred till at least eight weeks after the previous dose. In one study, the ulcers were treated with chemotherapeutic regimen for atypical mycobacteria including doxycycline, azithromycin, and ofloxacin till the resolution of the ulcer.[26] The studies on the use of MIP in psoriasis patients demonstrated occurrence of a psoriatic plaque at the site of injection in some patients.[27,28] This is probably a result of koebnerization phenomenon. Paresthesias have been reported adjacent to the site of intralesional injection of MIP vaccine in warts. It resolves without treatment in 7–10 days.[19] Submandibular lymph nodes draining the facial warts were found to be swollen and tender in two patients in one study. They were treated with oral amoxicillin or clavulanic acid and had complete resolution.[22] Another study also noted regional lymphadenopathy after the first intralesional injection into the warts.[21] Cellulitis was observed in two of 30 patients treated with intralesional heat-killed Mw vaccine for warts.[21]
Rare side effects
The pilot study on the use of MIP vaccine in anogenital warts reported the occurrence of granulomatous balanitis in a seropositive patient with genital warts. The diagnosis was confirmed histopathologically, and the patient was treated with topical corticosteroids for three weeks.[23] Herpes zoster was also noted in an immunosuppressed patient with anogenital warts after Mw vaccination.[23] Three patients have been reported to have developed erythematous and tender nodules at all the injection sites about a week after the first injection. They were suffering from severe COVID-19 and had received three injections every day for three days. One of the patients had abscess followed by ulcer formation at those sites. All the lesions were managed conservatively with analgesics with or without topical steroids.[38]
A persistent nodular swelling at the injection site of MIP for the treatment of warts should raise the suspicion of exaggerated granulomatous hypersensitivity reaction.[39,40] Skin biopsy for histopathology shows evidence of granulomas, and the cultures for mycobacteria are sterile. The patients respond well to oral minocycline.
Animal and in vitro studies
Mouse models have shown MIP to have an effective anti-tumor action, especially on myeloma cells.[41] In vitro studies have also demonstrated the cytotoxic action of heat-killed MIP fraction against multiple human cancer cell lines, which can pave the way for its future application in cancers other than non-small cell lung cancer and bladder cancer.[42]
Leishmaniasis is a tropical infection caused by various species of Leishmania. While cutaneous leishmaniasis is self-resolving, visceral leishmaniasis and post-kala-azar dermal leishmaniasis are progressively becoming resistant to conventional treatment such as antimonial and amphotericin B. In vitro studies have shown promising results of treatment of leishmaniasis with the use of MIP vaccine.[43]
During the development of an anti-human chorionic gonadotropin (hCG) vaccine for prevention of pregnancy, MIP has been found to be a potent adjuvant.[44] This can prove to be highly useful in prevention of pregnancy as well as treatment of carcinomas with high beta-hCG levels.
In a novel study, aerosol immunization by alginate-coated mycobacterium (BCG/MIP) particles provided enhanced immune response and protective efficacy than aerosol of plain mycobacterium against MTB H37Rv infection in mice. This can pave the way for a more safe, effective, and economical vaccine for tuberculosis and leprosy.[45]
Currently ongoing trials
Few of the clinical trials underway for application of MIP vaccine in conditions other than leprosy include prevention of COVID-19 and tuberculosis (TB) in contacts, effect on the course of COVID-19, and advanced non-small cell lung cancer [Table 3].[46,47,48,49,50]
Table 3.
Currently ongoing clinical trials on MIP for conditions other than leprosy
| Study | Primary objective | Number of participants |
|---|---|---|
| ICMR TB Consortium study et al.[46] | To compare the percentage of confirmed TB cases among healthy household contacts of newly diagnosed sputum-positive pulmonary TB patients in the vaccinated and placebo groups from two months after the first dose of vaccine till 38-month follow-up period. | 12000 |
| Patel et al.[47] | Number of participants (healthy subjects with recent history of close contact with COVID-19 patients) acquiring COVID-19 till eight weeks after the first dose | 4000 |
| Sudan et al.[48] | To compare the COVID-19 symptoms relief over the time, to compare the difference in proportion of patients with improved clinical outcome, and to compare the duration for conversion of COVID-19-positive status to negative in patients receiving MIP vs placebo | 50 |
| Prabhash et al.[49] | To compare safety, assess tumor response rate, compare PFS, and compare EORTC QOL | 834 |
TB=Tuberculosis, PFS=Progression-free survival, EORTC QOL=European Organization for Research and Treatment of Cancer Quality of Life
Conclusions
Several studies have demonstrated that the MIP vaccine exhibits strong immunomodulatory properties and has been effective in both infectious and non-infectious diseases. In the context of sepsis, TB, and warts, there is promising evidence suggesting its efficacy in mitigating the severity of these conditions. Additionally, 'MIP's potential extends beyond infectious diseases, offering favorable outcomes in malignancies as well. By potentially reducing mortality, morbidity, and healthcare costs, the MIP vaccine holds promise as a valuable intervention for improving overall patient outcomes across various medical conditions. This emphasizes the scope of this multipurpose vaccine and the vast arena of other uses that still need to be looked into closely. The safety profile and affordable cost are two conducive factors for further research and a widespread availability and application of this unique vaccine.
Financial support and sponsorship
Financial support by Indian Council of Medical Research (ICMR), Government of India - (5/8/3/7/2020/ECD-1).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to thank the Indian Council of Medical Research (ICMR), Government of India, for the financial support (5/8/3/7/2020/ECD-1) and for providing Senior Research Fellowship to Ayush Sharma. The authors acknowledge BioRender.com for providing cartoon components in the design of illustrations.
References
- 1.Talwar GP, Gupta JC, Mustafa AS, Kar HK, Katoch K, Parida SK, et al. Development of a potent invigorator of immune responses endowed with both preventive and therapeutic properties. Biologics. 2017;11:55–63. doi: 10.2147/BTT.S128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sehgal IS, Agarwal R, Aggarwal AN, Jindal SK. A randomized trial of Mycobacterium w in severe sepsis. J Crit Care. 2015;30:85–9. doi: 10.1016/j.jcrc.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Sur PK, Dastidar AG. Role of mycobacterium w as adjuvant treatment of lung cancer (non-small cell lung cancer) J Indian Med Assoc. 2003;101:118–20. [PubMed] [Google Scholar]
- 4.Kumar P, John V, Marathe S, Das G, Bhaskar S. Mycobacterium indicus pranii induces dendritic cell activation, survival, and Th1/Th17 polarization potential in a TLR-dependent manner. J Leukoc Biol. 2015;97:511–20. doi: 10.1189/jlb.1A0714-361R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talwar GP, Zaheer SA, Mukherjee R, Walia R, Misra RS, Sharma AK, et al. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine. 1990;8:121–9. doi: 10.1016/0264-410x(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaur A, Brar BK, Kumar S, Brar SK, Boparai AS, Puri N. A randomized comparative study of MIP and MMR vaccine for the treatment of cutaneous warts. Indian J Dermatol. 2021;66:151–8. doi: 10.4103/ijd.IJD_700_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma SK, Katoch K, Sarin R, Balambal R, Kumar Jain N, Patel N, et al. Efficacy and safety of Mycobacterium indicus pranii as an adjunct therapy in Category II pulmonary tuberculosis in a randomized trial. Sci Rep. 2017;7:3354. doi: 10.1038/s41598-017-03514-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayosi BM, Ntsekhe M, Bosch J, Pandie S, Jung H, Gumedze F, et al. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014;371:1121–30. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nath I, Curtis J, Bhutani LK, Talwar GP. Reduction of a subpopulation of T lymphocytes in lepromatous leprosy. Clin Exp Immunol. 1974;18:81–7. [Google Scholar]
- 10.Mustafa AS, Talwar GP. Five cultivable mycobacterial strains giving blast transformation and leukocyte migration inhibition of leukocytes analogous to mycobacterium leprae. Lepr India. 1978;50:498–508. [PubMed] [Google Scholar]
- 11.Talwar GP, Ahmed N, Saini V. The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): A suggestion. Infect Genet Evol. 2008;8:100–1. doi: 10.1016/j.meegid.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Kumar P, Das G, Bhaskar S. Mycobacterium indicus pranii therapy induces tumor regression in MyD88- and TLR2-dependent manner. BMC Res Notes. 2019;12:648. doi: 10.1186/s13104-019-4679-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar P, Tyagi R, Das G, Bhaskar S. Mycobacterium indicus pranii and Mycobacterium bovis BCG lead to differential macrophage activation in Toll-like receptor-dependent manner. Immunology. 2014;143:258–68. doi: 10.1111/imm.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rawat KD, Chahar M, Srivastava N, Gupta UD, Natrajan M, Katoch VM, et al. Expression profile of CXCL12 chemokine during M. tuberculosis infection with different therapeutic interventions in guinea pig. Indian J Tuberc. 2018;65:152–8. doi: 10.1016/j.ijtb.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Saqib M, Khatri R, Singh B, Gupta A, Bhaskar S. Cell wall fraction of Mycobacterium indicus pranii shows potential Th1 adjuvant activity. Int Immunopharmacol. 2019;70:408–16. doi: 10.1016/j.intimp.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Saqib M, Singh B, Pal L, Nishikanta A, Bhaskar S. Mycobacterium indicus pranii Induced Memory T-Cells in Lung Airways Are Sentinels for Improved Protection Against M.tb Infection. Front Immunol. 2019;10:2359. doi: 10.3389/fimmu.2019.02359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupte MD, Vallishayee RS, Anantharaman DS, Nagaraju B, Sreevatsa, Balasubramanyam S, et al. Comparative leprosy vaccine trial in south India. Indian J Lepr. 1998;70:369–88. [PubMed] [Google Scholar]
- 18.Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, Parida SK, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: Clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127–43. [PubMed] [Google Scholar]
- 19.Singh S, Chouhan K, Gupta S. Intralesional immunotherapy with killed Mycobacterium indicus pranii vaccine for the treatment of extensive cutaneous warts. Indian J Dermatol Venereol Leprol. 2014;80:509–14. doi: 10.4103/0378-6323.144145. [DOI] [PubMed] [Google Scholar]
- 20.Kumar P, Dar L, Saldiwal S, Varma S, Datt Upadhyay A, Talwar D, et al. Intralesional injection of Mycobacterium w vaccine vs imiquimod, 5%, cream in patients with anogenital warts: A randomized clinical trial. JAMA Dermatol. 2014;150:1072–8. doi: 10.1001/jamadermatol.2014.794. [DOI] [PubMed] [Google Scholar]
- 21.Dhakar AK, Dogra S, Vinay K, Sarangal R, Kanwar AJ, Singh MP. Intralesional Mycobacterium w Vaccine Versus Cryotherapy in Treatment of Refractory Extragenital Warts: A Randomized, Open-Label, Comparative Study. J Cutan Med Surg. 2016;20:123–9. doi: 10.1177/1203475415616962. [DOI] [PubMed] [Google Scholar]
- 22.Meena JK, Malhotra AK, Mathur DK, Mathur DC. Intralesional immunotherapy with Mycobacterium w vaccine in patients with multiple cutaneous warts: Uncontrolled open study. JAMA Dermatol. 2013;149:237–9. doi: 10.1001/jamadermatol.2013.866. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Malhotra AK, Verma KK, Sharma VK. Intralesional immunotherapy with killed Mycobacterium w vaccine for the treatment of ano-genital warts: An open label pilot study. J Eur Acad Dermatol Venereol. 2008;22:1089–93. doi: 10.1111/j.1468-3083.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 24.Khullar G, Narang T, De D, Nahar Saikia U, Dogra S, Handa S. Recalcitrant giant condyloma acuminatum treated successfully with a novel combination of Mycobacterium indicus pranii immunotherapy and acitretin. Int J STD AIDS. 2017;28:1155–7. doi: 10.1177/0956462417694805. [DOI] [PubMed] [Google Scholar]
- 25.Garg S, Baveja S. Intralesional immunotherapy for difficult to treat warts with Mycobacterium w vaccine. J Cutan Aesthet Surg. 2014;7:203–8. doi: 10.4103/0974-2077.150740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandra S, Sil A, Datta A, Pal S, Das NK. A double-blind, randomized controlled trial to compare the effectiveness and safety of purified protein derivative of tuberculin antigen with Mycobacterium w vaccine in the treatment of multiple viral warts. Indian J Dermatol Venereol Leprol. 2019;85:355–66. doi: 10.4103/ijdvl.IJDVL_549_18. [DOI] [PubMed] [Google Scholar]
- 27.Rath N, Kar HK. Efficacy of intradermal heat-killed Mycobacterium w in psoriasis: A pilot study. Int J Dermatol. 2003;42:756–7. doi: 10.1046/j.1365-4362.2003.01962.x. [DOI] [PubMed] [Google Scholar]
- 28.Kumar B, Sandhu K, Kaur I. Role of Mycobacterium w vaccine in the management of psoriasis. Br J Dermatol. 2005;152:380–2. doi: 10.1111/j.1365-2133.2005.06343.x. [DOI] [PubMed] [Google Scholar]
- 29.Belani CP, Desai D, Khamar BM, Cadi-05 Investigators Study Group Open-label, randomized multicenter phase II clinical trial of a toll-like receptor-2 (TLR2) agonist mycobacterium w (Cadi-05) in combination with paclitaxel plus cisplatin versus paclitaxel plus cisplatin in advanced non-small cell lung cancer (NSCLC) J Clin Oncol. 2011;29(15_suppl):7501. [Google Scholar]
- 30.Sehgal IS, Basumatary NM, Dhooria S, Prasad KT, Muthu V, Aggarwal AN, et al. A randomized trial of Mycobacterium w in severe presumed gram-negative sepsis. Chest. 2021;160:1282–91. doi: 10.1016/j.chest.2021.03.062. [DOI] [PubMed] [Google Scholar]
- 31.Kharkar R. Immune recovery in HIV with Mycobacterium W. J Indian Med Assoc. 2002;100:578–9. [PubMed] [Google Scholar]
- 32.Sehgal IS, Guleria R, Singh S, Siddiqui MS, Agarwal R. A sernamed trial of Mycobacterium w in critically ill patients with COVID-19: ARMY-1. ERJ Open Res. 2021;7:00059–2021. doi: 10.1183/23120541.00059-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixit S, Zirpe K, Suryawanshi P, Borawake K, Prasad S, Ambapkar S, et al. Retrospective cohort observational study to compare the effect of Mycobacterium w along with Standard of Care vs Standard of Care alone in critically ill COVID-19 patients. J Assoc Physicians India. 2022;70:11–2. doi: 10.5005/japi-11001-0044. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhuri P, Mukhopadhyay S. Bladder preserving approach for muscle invasive bladder cancer—role of Mycobacterium w. J Indian Med Assoc. 2003;101:559–60. [PubMed] [Google Scholar]
- 35.Patel N, Trapathi SB. Improved cure rates in pulmonary tuberculosis category II (retreatment) with Mycobacterium w. J Indian Med Assoc. 2003;101:680–682. [PubMed] [Google Scholar]
- 36.Pant MC, Hadi R, Prasad R, Dalela D, Pant R, Parmar D, et al. Role of 10 serna-therapy as a adjuvant treatment in advance head and neck cancer, patient receiving chemo radiotherapy. J Clin Oncol. 2005;23(16_suppl):2598. [Google Scholar]
- 37.Sehgal IS, Bhalla A, Puri GD, Yaddanapudi LN, Singh M, Malhotra P, et al. Safety of an immunomodulator Mycobacterium w in COVID-19. Lung India. 2020;37:279–81. doi: 10.4103/lungindia.lungindia_242_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chawla RK, Chawla AK, Chaudhary G, Chopra K, Chawla MK. Mycobacterium W. — An unusual side effect. Indian J Tuberc. 2022;69:250–2. doi: 10.1016/j.ijtb.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinay K, Narang T, Saikia UN, Kumaran MS, Dogra S. Minocycline successfully treats exaggerated granulomatous hypersensitivity reaction to Mw immunotherapy. Dermatol Ther. 2017;30:e12452. doi: 10.1111/dth.12452. [DOI] [PubMed] [Google Scholar]
- 40.Vinay K, Narang T, Dogra S, Saikia UN. Grotesque face secondary to immunotherapy: Cure circumvallating to curse. Skinmed. 2017;15:157–9. [PubMed] [Google Scholar]
- 41.Ahmad F, Mani J, Kumar P, Haridas S, Upadhyay P, Bhaskar S. Activation of anti-tumor immune response and reduction of regulatory T cells with Mycobacterium indicus pranii (MIP) therapy in tumor bearing mice. PloS One. 2011;6:e25424. doi: 10.1371/journal.pone.0025424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Subramaniam M, In LL, Kumar A, Ahmed N, Nagoor NH. Cytotoxic and apoptotic effects of heat killed Mycobacterium indicus pranii (MIP) on various human cancer cell lines. Sci Rep. 2016;6:19833. doi: 10.1038/srep19833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dey S, Mukherjee D, Sultana SS, Mallick S, Dutta A, Ghosh J, et al. Combination of Mycobacterium indicus pranii and heat-induced promastigotes cures drug-resistant Leishmania infection: Critical role of interleukin-6-producing classical dendritic cells. Infect Immun. 2020;88:e00222–19. doi: 10.1128/IAI.00222-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purswani S, Talwar GP. Development of a highly immunogenic recombinant candidate vaccine against human chorionic gonadotropin. Vaccine. 2011;29:2341–8. doi: 10.1016/j.vaccine.2010.11.069. [DOI] [PubMed] [Google Scholar]
- 45.Nagpal PS, Kesarwani A, Sahu P, Upadhyay P. Aerosol immunization by alginate coated Mycobacterium (BCG/MIP) particles provide enhanced immune response and protective efficacy than aerosol of plain Mycobacterium against M.tb. H37Rv infection in mice. BMC Infect Dis. 2019;19:568. doi: 10.1186/s12879-019-4157-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clinical Trials Registry India. New Delhi: database publisher (India) 2019 Jan 10. Identifier CTRI/2019/01/017026, A Phase III, Randomized, Double-blind, three arm Placebo controlled Trial to Evaluate the Efficacy and Safety of two vaccines VPM1002 and Immuvac in Preventing Tuberculosis (TB) in Healthy Household Contacts of Newly Diagnosed Sputum Positive Pulmonary TB Patients. [Last accessed on 2022 Oct 06];2021 Jan 28; Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=27411&EncHid=&userName= [Google Scholar]
- 47.Clinical Trials Registry India. New Delhi: database publisher (India) 2020 May 21. Identifier CTRI/2020/05/025277, A Randomized, Double-blind, Two arm, Placebo Controlled Clinical Trial to Evaluate the Efficacy and Safety of Mycobacterium w in preventing COVID-19 in subjects at risk of getting infected with COVID-19. [Last accessed on 2022 Oct 06];2021 Feb 03; Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=43176& EncHid=&userName= [Google Scholar]
- 48.Clinical Trials Registry India. New Delhi: database publisher (India) 2020 Sep 10. Identifier CTRI/2020/09/027741, A Randomized Double Blind Controlled study to assess the dose related effect of Mycobacterium w on clinical course of Corona Virus Disease 2019 (COVID-19) [Last accessed on 2022 Oct 06];2020 Sep 29; Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=47197&EncHid=&userName= [Google Scholar]
- 49.Clinical Trials Registry India. New Delhi: database publisher (India) 2020 Sep 17. Identifier CTRI/2020/09/027853, An, open label, randomized, clinical trial of Mycobacterium w (Mw) as an Adjuvant to chemotherapy in Advanced Non-small Cell Lung Cancer. [Last accessed on 2022 Oct 06];2020 Sep 15; Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=46579&EncHid=&userName= [Google Scholar]
- 50.Clinical Trials Registry India. New Delhi: database publisher (India) 2021 Aug 04. Identifier CTRI/2021/08/035408, Study of intravenous Inj Sepsivac (heat killed Mycobacterium w) in gram negative sepsis. [Last accessed on 2022 Oct 06];2021 Aug 03; Available from: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=58471&EncHid=&userName= [Google Scholar]
- 51.Khullar G, Narang T, Nahar Saikia U, Dogra S. Generalized granulomatous dermatitis following Mycobacterium w (Mw) immunotherapy in lepromatous leprosy. Dermatol Ther. 2017;30:e12441. doi: 10.1111/dth.12441. [DOI] [PubMed] [Google Scholar]


