Abstract
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death worldwide, and its global health burden is increasing. COPD is characterized by emphysema, mucus hypersecretion, and persistent lung inflammation, and clinically by chronic airflow obstruction and symptoms of dyspnea, cough, and fatigue in patients. A cluster of pathologies including chronic bronchitis, emphysema, asthma, and cardiovascular disease in the form of hypertension and atherosclerosis variably coexist in COPD patients. Underlying causes for COPD include primarily tobacco use but may also be driven by exposure to air pollutants, biomass burning, and workplace related fumes and chemicals. While no single animal model might mimic all features of human COPD, a wide variety of published models have collectively helped to improve our understanding of disease processes involved in the genesis and persistence of COPD. In this review, the pathogenesis and associated risk factors of COPD are examined in different mammalian models of the disease. Each animal model included in this review is exclusively created by tobacco smoke (TS) exposure. As animal models continue to aid in defining the pathobiological mechanisms of and possible novel therapeutic interventions for COPD, the advantages and disadvantages of each animal model are discussed.
Keywords: chronic obstructive pulmonary disease (COPD), animal models, emphysema, chronic bronchitis, tobacco smoke
Introduction
Chronic obstructive pulmonary disease (COPD) encompasses a cluster of pathological respiratory conditions that affect approximately 300 million individuals globally. As the third leading cause of death worldwide (WHO 2014), COPD kills approximately three million individuals annually. In the United States (US) alone, an individual dies from COPD every 4 min (CDC 2020a). The global burden of this chronic lung disease is substantial and rising. In 2010, the national annual medical costs for COPD were $32 billion in the US, with a projected increase to $49 billion by 2020 (CDC 2020b).
Those suffering from COPD with chronic airflow obstruction that disrupts normal breathing results in increased hospitalization and mortality rates. The prevalence of COPD is rising due to elevated ambient air pollution exposure, biomass burning, dust and fume exposures in the workplace, extracellular vesicles, continued cigarette and e-cigarette smoking, and contribution of individual genetic/epigenetic factors (Benedikter et al. 2018; Duffy and Criner 2019; Hikichi et al. 2019; GOLD 2020; Easter et al. 2020; Silverman 2020; Jin et al. 2021; O’Farrell et al. 2021; Kamal et al. 2022; Effah et al. 2022; Snoderly et al 2023). Thus, it is imperative to better understand the risk factors that result in COPD among those exposed to environmental contaminants and tobacco smoke (TS), and improve therapeutic strategies.
Through use of animal models and clinical studies, significant insights have been gained into the biological mechanisms underlying COPD development including varied manifestations of bronchitis and emphysema, as well as associated overlapping asthma, pulmonary hypertension, and lung infections. The goal of this review was to identify the ideal applications of different animal models for developing treatment strategies. Tobacco smoking is a major risk factor for developing COPD and most commonly used to induce COPD in in vivo studies (Fricker, Deane, and Hansbro 2014; Ghorani et al. 2017). To better define smoke-induced COPD-like features and pathogenesis, the review includes only models developed using TS exposures. COPD animal models developed through other targeted interventions, with exception of biomass burning, are not included. The review focuses on 1) critical advances regarding the mechanisms underlying COPD-induced pathological alterations and associated disease risk factors; 2) wide variety of animal models of TS exposure that mimic specific pathologies of human COPD (Tables 1) advantages and disadvantages of each TS-exposed animal model (Table 2).
Table 1.
A Catalog of the Animal Models of COPD, and Summary of their Exposure Conditions and Observational Changes.
| Species | Exposure condition | Observation | Possible Mechanism | Ref. |
|---|---|---|---|---|
| Mice (Neutrophil elastase++ wild type littermates, C57Bl/6 Neutrophil Elastase KO) | 2 cig/day, 6 day/wek for 6 months (no CO toxicity). | Airspace enlargement determined by chord length was less by 59% in NE KO mice in comparison to mice with NE++. | NE plays a role in development of emphysema. | (Shapiro et al. 2003) |
| Mice (C57bl/129 TNF-αR KO, C57Bl/129) | 4 cig/day, 5 day/week for 6 months. 2R1 reference cig University of Kentucky. |
11% increase in Lm in TNFRKO mice and 38% in WT mice. Increased matrix metalloproteinase in WT mice. | TNF-α mediates airspace enlargement, neutrophil influx and matrix breakdown. | (Churg et al. 2004) |
| Mice (WT Balb/c mice and immunodeficient Scid mice) | 5 cig 4 times a d with 30 ms smoke free interval for 5 week & 24 week. 2R4F reference cig University of Kentucky. |
Increase in neutrophils, macrophages and dendritic cells in WT and Scid mice after 5 weeks. Increase in neutrophils, macrophages, dendritic cells, CD4+ and CD8+ cells in WT mice after 24 weeks. Pulmonary emphysema demonstrated by increased Lm and associated with increased MMP-12 in lungs and TNF-α in BALF in WT mice and Scid mice after 24 weeks of cig smoke exposure. |
Pulmonary Emphysema can occur in the absence of adaptive immunity after chronic smoke exposure. | (D’Hulst A et al. 2005) |
| Mice (C57Bl/6 (C57) and ICR) | 2 hr/d 5 day/week for 6 months. 2s, 35-ml puff, 60s interval between puffs. 75, 250 or 600 µg/l of total PM concentrations. 2R4F reference cig University of Kentucky. |
Mild emphysema demonstrated by increased Lm in ICR mice after 6 m of exposure to 600 µg/l total particulate matter. Macrophages and inflammatory cells infiltration around alveolar ducts. | There is a role of chronic inflammation in the pathogenesis of emphysema but the inflammation depends on the concentration and duration of exposure. | (Hodge-Bell et al. 2007) |
| Mice (C57Bl/6 and IL-18Rα−/−) | 2 cig twice a day, 5 day/weeks for 6 months. 2R4 reference cig University of Kentucky. |
Emphysema in WT mice demonstrated by increased Lm and alveolar destruction. | IL-18Rα plays a role in cig smoke induced pulmonary emphysema. | (Kang et al. 2007) |
| Mice (C57Bl/6 WT; C57Bl/6 CD8+ KO) | 2 cig/day, 6 day/week for 6 months. Research cig from University of Kentucky. |
WT showed macrophage, neutrophil and lymphocyte recruitment to the lung. Emphysema demonstrated by increased Lm in WT. No changes seen in C57Bl/6 CD8+ KO. | CD8+ plays a role in inflammation and alveolar destruction in cig smoke induced pulmonary emphysema. | (Maeno et al. 2007) |
| Mice (C57Bl/6 WT; C57Bl/6 IL-1RKO; TNFR KO) | Acute exposure: 4 2R1 cig/day for a single day. Chronic exposure: 3 2R1 cig/day, 5 day/week for 6 month. |
Acute exposure: No influx of inflammatory cells and matrix breakdown in IL-1RKO mice. Increased IL-1β, neutrophils, macrophages, desmosin and hydroxyproline in C57Bl/6. Chronic exposure: Increased IL-18, neutrophils, macrophages, desmosin and hydroxyproline in C57Bl/6. Increased neutrophil and macrophages in IL-1RKO. IL-1RKO and TNFRKO were 65% and 83% protected against emphysema respectively. Emphysema was demonstrated by analysis of Lm and matrix breakdown. |
There is a role of IL-1 and TNF-α in the development of smoke induced emphysema and small airway remodeling in mice. | (Churg et al. 2009) |
| Mice (C57Bl/6 clarithromycin p.o.) | 2 unfiltered cig/d, 6 day/w for 6 months. Research cig University of Kentucky. |
Decreased airspace enlargement, decreased alveolar wall destruction and decreased macrophages in BAL in mice treated with clarithromycin for 6 months. | Clarithromycin treatment prevents smoke induced emphysema and airway inflammation in mice. | (Nakanishi et al. 2009) |
| Mice (C57Bl/6J curcumin p.o.) | Commercially available filtered cig (Marlboro, 12 mg tar/1 mg nicotine; Philip Morris, Richmond, VA). Short term exposure: 5% CS (12puffs/min) for 60 ms/day for 10 consecutive days. Long term exposure: 12 puffs/min, 60 ms/day, 5 day/week for 12 weeks. Curcumin was added daily 1 hr before exposure. |
Decreased neutrophil and macrophages after short term exposure and decreased airspace enlargement after long term exposure in animals administered with curcumin. | Administration of oral curcumin decreases smoke induced emphysema and pulmonary inflammation. | (Suzuki et al. 2009) |
| Mice (WT mice; adiponectin deficient mice) | 2 cig/day, 5 day/week for 6 months. 2R4F reference cig University of Kentucky (2.45 mg nicotine/cig). |
Airspace enlargement (increased Lm), decreased tissue elastance and lung inflammation (increased total BAL cells, neutrophils and macrophages) in wild type mice. | There is a proinflammatory role of adiponectin in development of tobacco smoke induced emphysema. | (Miller et al. 2010) |
| Mice (C57Bl/6; C57Bl/6 IL-17a KO; C57Bl/6 Cc10-IL17a) | 4 cig/day (4–5min/cig) (1 hr), 5 day/week for 4 month Commercial cig (Marlboro 100s). 5 s of puffing of active smoke followed by 25 s of forced air to prevent CO2 asphyxiation. |
Increased lung inflammatory cells in Cc10-IL17a compared to C57Bl/6 and IL-17a KO mice. Exaggeration of tobacco smoke induced emphysema in Cc10-IL17a mice demonstrated by alveolar destruction under microscope and quantitative microcomputed tomography. | IL-17a plays a role in pathogenesis of tobacco smoke induced emphysema. | (Shan et al. 2012) |
| Mice (C57Bl/6J; DBA/2; ICR) | 3 cig/d, 5 day/w for 7 months. Commercial Virginia cig (12 mg of tar and 0.9 mg of nicotine). |
Decreased lung elastin in C57Bl/6 and DBA/2 but not in ICR. Focal areas of emphysema in lung parenchyma in C57Bl/6 and DBA/2 determined by morphometric measurement (Lm, ISA, DI). | In mice, development of tobacco smoke induced emphysema is strain dependent. | (Cavarra et al. 2001) |
| Mice (ICR Nrf2 KO) | 2 cig/day, 7 hr, 7 day/week for 6 month; each cig puffed for 2 sec, 1 puff/min for total of 8 puffs. 2R4F reference cig University of Kentucky (2.45 mg nicotine/cig). |
Emphysema demonstrated by histopathological analysis, Lm and alveolar destruction more extensive in comparison to WT mice. Increased endothelial and type II epithelial cells. Increased alveolar expression of 8-oxo-7,8-dihydro-2′-deoxyguanosine. | There is a role of Nrf2 pathway in upregulation of oxidant defense which can prevent tobacco smoke induced lung inflammation and emphysema. | (Rangasamy et al. 2004) |
| Mice (C57Bl/6J; DBA/2) | 3 cig/day, 5 day/week for 1,3,6 and 10 months. Commercial Virginia filter cig (12 mg of tar and 0.9 mg of nicotine). |
Patchy emphysema analyzed by inter-alveolar distance and ISA in both strains. DBA/2: uniform parenchymal dilatation with apoptotic bodies. Fibrotic areas in parenchyma. C57Bl/6J: mucous (goblet) cell metaplasia. | Development of emphysema and fibrosis is strain dependent. | (Bartalesi et al. 2005) |
| Mice (C57Bl/CBA; A/J) | C57Bl/CBA: 6 hr/day, 5 days/week for 1 year A/J: 6hr/day, 5day/week for 6 months. PM in the chamber: 250 mg/m3. |
17% increase in Lm in C57Bl/CBA and 32% increase in Lm in A/J. Increased neutrophils and lung lavage elastase like activity in CS exposed animals. No changes in lung compliance observed. | There is no correlation between structural emphysema and lung compliance. | (Foronjy et al. 2005) |
| Mice (C57Bl/6 CCR6 KO) | 5 cig, 4 times/day with 30 ms smoke free interval 5 day/week for 4 weeks or 24 weeks. 2R4F without filter University of Kentucky. |
Increased dendritic cells, neutrophils and T lymphocytes in WT in comparison to CCR6 KO and control animals. Statistically significant higher Lm and DI in WT mice exposed to TS in comparison to CCR6 KO mice. |
There is a role of CCR6 in the development of TS induced pulmonary inflammation and emphysema. | (Bracke et al. 2006) |
| Mice (C57Bl/6J mice rFGF-2 intranasally administered) | 20 filtered commercial cig/day for 1, or 4 days or 6 months (5 days/week). Eighty-Eight Lights, KT&G, Daejeon, Korea (8.5 mg tar and 0.9 mg nicotine/cig). |
Decreased FGF-2 production in lung tissues after short-term smoke exposure (1 and 4 days). After 4-day smoke exposure, recruitment of inflammatory cells was increased, macrophages were increased in the BALF, increased MLI, lung resident cell apoptosis and protease activity developed in the destroyed alveoli. |
Intranasal use of rFGF-2 reduced macrophage-dominant inflammation and alveolar destruction in the lungs. | (Kim et al. 2018) |
| Mice (C57Bl/6J mice) | 75 min exposure of smoke generated from ~25 cig/session, 2 sessions/day (morning and afternoon, separated by a recovery period), 5 day/week for 12 weeks. Commercial cig Marlboro, Philips Morris, USA (1 mg nicotine and 11 mg tar/cig). |
Lung function decline and airflow limitation. Profound peribronchial and/or perivascular inflammatory infiltration, collagen deposition, and mucus secretion. Enlargement of alveolar and profound airway inflammation with significantly increased number of total cell count, neutrophils, macrophages, and lymphocytes. |
CXCL5 levels increased and correlated to granulocyte-colony stimulating factor levels. Circulating levels of CXCL5 correlated to lung functions decline. |
(Chen et al. 2019) |
| Mice (BALB/c) | Whole-body exposure with 5 cig, 4 times/day with 30 min smoke-free intervals, 5 day/week for 24 weeks. Nanning Jiatianxia unfiltered cig (12 mg of tar and 0.9 mg of nicotine). Total PM concentrations 140–160 mg/m3. |
Elevated IL-27 was accompanied by an exaggerated IFN-γ+CD8+Tc1 response. | Lung dendritic cells were one of the main sources of IL-27. IL-27 negatively regulated the differentiation of IFN-γ +CD8+Tc1 cells in a STAT1- and STAT3-independent manner. |
(Qiu et al. 2022) |
| Mice (C57BL/6, CCL17−/−, and CCL17+/+ littermate) | Whole body exposure with 5 commercial non-filtered cig (Peace®; Japan Tobacco Inc., Japan) for 30 min, twice/daily for 8 weks. | Increases CCL17 production in bronchial epithelial cells and accumulation of alveolar macrophages. Alveolar macrophage accumulation significantly reduced in CCL17-deficient mice. |
CCL17 involved in CS-induced accumulation of alveolar macrophages possibly through CCL17-induced production of CCL2 by macrophages | (Machida et al. 2022) |
| Mice (Balb/cByJ) | Whole body exposure to unfiltered cig for 72 days, except for days 42, 52, and 62. 3R4F reference cig University of Kentucky, Lexington, Kentucky (carbon monoxide level 200–400 ppm). |
Increased alveolar enlargement and numbers of macrophages and neutrophils in BALF. Lower body weight accompanied by lower serum leptin levels, more time spent in the inner zone of the open field, and decreased claudin-5 and occludin protein expression levels in brain microvessels. | Neuroinflammation is present in the brain area involved in cognitive functioning and that blood-brain barrier integrity is compromised. | (Pelgrim et al. 2022) |
| Rat (SH with s.c. administration of sEH inhibitor) | 35 ml puff, 2 sec duration, 1 puff/m 6 hr/ day for 3 days. 2R4F cig University of Kentucky. |
Increase in total cells in BAL in animals exposed to tobacco smoke. Decreased total cells in BAL, neutrophils, alveolar macrophages and lymphocytes in tobacco smoke exposed rats treated with sEH inhibitors. | sHE helps in attenuation of inflammation induced by tobacco smoke. | (Smith et al. 2005) |
| Rat (SH with simvastatin administered i.p.) | 35 ml puff, 2 sec duration once each min, 80–90 mg/m3 total suspended PM 6 hr/day for 3 days. 3R4F cig University of Kentucky. |
Decreased influx of leukocytes, macrophages and neutrophils in rats pretreated with simvastatin prior to and throughout the exposure. | Simvastatin attenuates pulmonary inflammation induced by acute TS exposure. | (Davis et al. 2013) |
| Rat (SH) | 35 ml puff, 2 sec duration once each min, 80–90 mg/m3 total suspended PM 6 hr/day, 3 days/week for 3 days, 4 weeks or 12 weeks. 3R4F cig University of Kentucky. |
Correlation between neutrophil accumulation and expression of adhesion molecules and chemokines in bronchial blood vessels and leucocyte accumulation recovered from lungs. There was no correlation between leucocytes recovered from BAL and other vascular beds. | In SH rat model of COPD, leucocytes are recruited through bronchial circulation. | (Davis et al. 2012) |
| Rat (Sprague-Dawley) | 5 cig every 9 ms, 6 hr/day with 2 hr resting period for 2–4 months. Total PM concentrations in the chamber were 100–120 µg/m3. 1R3F reference cig University of Kentucky. |
Increased IL-18 in BAL. Decreased vascular endothelial growth factor (VEGF) in the lung tissue. Progressive alveolar airspace enlargement, cell death, pulmonary vessel loss, vessel muscularization, collagen deposition, and right ventricular hypertrophy. | There is a role of IL-18 in endothelial cell death that can lead to pulmonary vasculature destruction and emphysema. | (Kratzer et al. 2013) |
| Rat (SH, male; SH, female; WKY, male) | 6 hr/day, 3 day/week for 4 or 12 weeks. Total suspended PM in chamber was 90 mg/m3. 2R4F research cig University of Kentucky. |
Increased total leucocytes, macrophages, TNF-α and lactate dehydrogenase in BAL after 4 weeks of exposure and enlarged alveolar air space after 12 weeks of exposure in SH male rats in comparison to SH female, control and WKY. | Cellular, inflammatory and structural changes similar to COPD changes in SH male rats exposed to tobacco smoke. | (Shen et al. 2016) |
| Rat (SH, male) | 6 hr/day, 3 day/week for 3 day or 4 weeks or 12 weeks. Total suspended PM in chamber was 90 mg/m3. 2R4F reference cig University of Kentucky. |
Epithelial cell injury in the proximal airways, loss of ciliated epithelium and sloughing of airway epithelium after 3 d of TS exposure. Stratified squamous metaplasia in proximal airways and hypertrophic epithelial cells in mid airway level after 4 and 12 weeks TS exposure. Few TGF-β positive epithelial cells in proximal airways after 4 weeks of exposure. In mid and distal airway, epithelial cells were strongly positive for TGF-β after both 4 and 12 weeks of exposure. Increase in TGF-β2 after 4 and 12 weeks of exposure whereas decrease in TGF-β1 at 12 weeks of exposure demonstrated by ELISA of whole lung homogenates. | Airway generation and exposure duration determine changes in airway structure after TS exposure. During airway remodeling due to TS exposure, there is increase in TGF-β in epithelial and inflammatory cells. |
(Hoang et al. 2016) |
| Guinea pig | 10 commercial non filter cig/day, 5 day/week for 1,3,6 and 12 months. | Progressive destruction of lungs parenchyma. Alteration of pulmonary function test. | Development of morphological and physiological changes of emphysema in guinea pigs after chronic exposure to TS. | (Wright and Churg 1990) |
| Guinea pig | 10 cig/day, 5 day/week for 4–8 months. | Increased alveolar air space size, decreased alveolar surface area to volume ratio. Changes in pulmonary vasculature. Pulmonary arteriolar muscularization. | Long term exposure of TS to guinea pigs induce emphysematous changes in lung. | (Wright and Sun 1994) |
| Guinea pig (Hartley with p.o. administration of NE inhibitor) | Acute exposure: 20 ml cig smoke/1.5 m for 24 hr. Chronic exposure: 20 ml cig smoke/ 1.5 m 5 day/week for 6 m. 2R1 research cig University of Kentucky. |
Increased neutrophils, desmosine, hydroxyproline returned to normal and airspace enlargement reduced by 45% after administration of NE inhibitor. | NE inhibitor attenuates TS induced airway inflammation and destruction. | (Wright, Farmer, and Churg 2002) |
| Guinea pig (Hartley with administration of simvastatin) | 5 cig/day, 5 day/week for 6 months. 2R1/2R4F research cig University of Kentucky. |
TS increased pulmonary arterial systolic pressure. Reversal of pulmonary hypertension and emphysema (demonstrated by morphological and physiological measurement) after administration of simvastatin but no improvement in small airway remodeling. | Simvastatin prevents TS induced emphysema and reverses pulmonary hypertension but there was no effect on small airway remodeling. | (Wright et al. 2011) |
| Guinea pig | 20 commercial non filter cig/d, 5 day/week for 1,3,4,6 and 8 weeks. | Interstitial and peribronchiolar inflammation and emphysematous changes (demonstrated by morphological measurement) at 6 and 8 weeks. Increased collagenolytic activity by 6 and 8 weeks. | There is a role of collagen degradation in the development of TS induced emphysema. | (Selman et al. 1996) |
| Guinea pig (Hartley) | Two 70 ml puffs/min, 4 hr/day 5 day/week for 1,2,4,8 or 12 weeks. Total PM concentration of 250 mg/m3. 2RF research cig University of Kentucky. |
Inflammatory changes after 4 weeks and emphysematous changes after 12 weeks of exposure. Decreased elastin and collagen in the alveolar wall. Increased cathepsin K activity. Increased airspace enlargement. | There is a role of cathepsin K in destruction of an extracellular matrix in TS induced emphysema. | (Golovatch et al. 2009) |
| Dogs (beagle dogs) | Concentration and puffs of smoke increased gradually. Within 10 w of exposure, the exposure was 16 cig/day, 5 day/week for 12 months. Dogs used mouth pipe to inhale diluted smoke (1:4) from the cig. 1R1 research cig University of Kentucky. |
Tracheal epithelial basal cell hyperplasia. Peribronchiolar hypercellularity. Increase in number of mucous (goblet) cells in large airways. No significant differences observed in bronchiolar size distribution, volume proportion of parenchymal structure and alveolar surface area. | Prolonged period of smoking is necessary for the development of irreversible damage after TS. | (Park et al. 1977) |
| Dogs (adult greyhound) | 30–45 ms of smoke, twice/daily, 5 day/week for more than a year (mean 14.74 m). | Inflammation of bronchioles and small bronchi. Alveolar inflammation and disruption. | Concentrated TS can cause parenchymal disruption resembling human emphysema. | (Hernandez et al. 1966) |
| Dogs (beagle dogs) | 2–7 cig/day, 7 day/week for 2–4 months. 1 cig at a time, 35 ml puff volume in 2 sec, puff interval every 20 sec via tracheostomy tube. Code 26 research cig National Cancer Institute (nonfiltered, 27 mg of tar and 3.25 mg of nicotine). |
Enlargement of alveolar ducts and alveolar spaces. Destruction of alveolar walls. Interstitial fibrosis of the interalveolar septa. | Short term TS exposure to dogs can cause early emphysematous and fibrotic changes in the lungs. | (Frasca et al. 1983) |
| Monkeys (cynomolgus macaque) | 6 hr/day, 5 day/week for 12 weeks (250 mg/m3 Total suspended PM. | Airway inflammation. Mucus metaplasia. Submucosal gland hypertrophy and hyperplasia. Peribronchial fibrosis. | No emphysematous changes in a given exposure setting. | (Polverino et al. 2015) |
| Ferrets | 60 ms twice daily for 6 months. 3R4F research cig (200µg/l total particulate matter). |
Airway obstruction. Mucous (goblet) cells metaplasia/hyperplasia. Increased mucus expression in small airways. Early morning spontaneous cough. | Ferrets after TS exposure can develop clinical and pathological features of chronic bronchitis. | (Raju, Kim, et al. 2016) |
Abbreviations: cig – cigarette(s); d – day(s); hr – hour(s); ms – minute(s); sec – second(s);; WT – wild type; NE – neutrophil elastase; MMP – matrix metalloproteinase; TNFR – TNF-α receptor; ISA – internal surface area of the lungs; DI – destructive index; Lm – mean linear intercept; KO – knock out; p.o. – peroral; TS – tobacco smoke; BAL – bronchoalveolar lavage; i.p. – intraperitoneal; sEH – soluble epoxide hydrolase; s.c. – subcutaneous; rFGF – recombinant fibroblast growth factor; CXCL5 – C-X-C motif chemokine 5; IFN – interferon; CS – cigarette smoke; TS – tobacco smoke.
Significant different from control at p <0.05.
Table 2.
Advantages and Disadvantages of Animal Models.
| Animal Models | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Mice | Low cost Easy handling Ease of genetic manipulation Rapid breeding rate Availability of antibodies and molecular probe Numerous strains |
Takes longer time to develop features like human COPD No bronchial glands Strain dependent Inconsistent reproducibility of studies Difficult to perform pulmonary function test and telemetry |
(Groneberg and Chung 2004) (Wright, Cosio, and Churg 2008) (Wright and Churg 2008a) (Churg, Cosio, and Wright 2008) (Kodavanti, Costa, and Bromberg 1998) |
| Rats | Mimics human chronic bronchitis and airway hyperreactivity Develops similar drug resistance as humans Useful in studying comorbidities of COPD |
Few knockout strains Lacks submucosal glands |
(Groneberg and Chung 2004) (Wright, Cosio, and Churg 2008) (Wright and Churg 2008a) (Churg, Cosio, and Wright 2008) (Cavailles et al. 2013) (Kodavanti et al. 2000) (Bolton et al. 2009) |
| Guinea Pigs | Useful in studying comorbidities of COPD Develops emphysema, marked airway remodeling and mucous (goblet) cell metaplasia following smoke exposure. Cough reflex present. |
Limited availability and costlier than mice and rats Insufficient availability of immunologic reagents Less genetic information Sparse distribution or absence of bronchial glands |
(Groneberg and Chung 2004) (Padilla-Carlin, McMurray, and Hickey 2008) |
| Dogs | Short term and long-term cigarette exposures produce changes similar to human chronic bronchitis and emphysema respectively Easy to measure pulmonary mechanics and ventilation, bronchoscopy Cough reflex can be measured Relatively large size and easy handling |
Costly Lack of genetic manipulation |
(Chapman 2008) |
| Ferrets | Similar to humans with respect to pulmonary physiology and submucosal gland distribution Smoke exposure causes impaired mucus transport |
Less studies done in comparison to other animal model | (Raju, Kim, et al. 2016) |
| Monkey | Pulmonary anatomy and immune system similar to humans Symptoms that develop after exposure are reproducible and comparable to human patients Similar molecular reagents can be used in human and primates Easy to perform pulmonary measurements and bronchoscopy |
Expensive Ethical issues Difficulty in purchasing, housing and exposing Takes longer time to develop emphysema (>12 weeks) |
(Plopper and Hyde 2008) (Polverino et al. 2015) |
Methods Approach to Literature Review
A systematic literature review was performed according to PRISMA (preferred reporting items for systematic review and meta-analysis) guidelines. PubMed and Web of Science databases were systematically searched, and references of retrieved articles were examined. The search was not limited to a year of publication/study or geographical region, and not done in collaboration with a librarian or methodologist. The search occurred 03/2023 for articles published from 1960 – 12/2022.
Keywords included the following: animal models, COPD, chronic obstructive pulmonary disease, emphysema, chronic bronchitis, cigarette smoke, cigarette exposure, tobacco exposure. A flow chart describing the literature review process is provided in supplemental Figure E1.
In total, 865 articles were identified, out of which 626 articles were excluded based upon criteria such as publication language, duplicates, and relevance determined through title and abstract reviews. To be included in the review, articles needed to meet the following criteria: (1) present distinct animal models of COPD; (2) provide a clear and detailed description of the method used to expose animals to TS, which leads to development of COPD; and (3) evaluate parameters that indicate the successful induction of a COPD animal model. Abstracts or unpublished articles, human studies and non-English language articles were excluded. Finally, 177 articles were found to be eligible and were included in the systematic review.
Results and Discussion
COPD Pathogenesis
Chronic obstructive pulmonary disease (COPD) patients suffer from chronic airflow obstruction attributed to mucous hypersecretion and persistent inflammation which manifests as chronic bronchitis with or without emphysema (alveolar septal wall destruction). These pathologies lead to wheezing, cough, and dyspnea with acute exacerbations of disease which, if severe, may result in hospitalization and death. COPD also overlaps with asthma in some patients (Zeki et al. 2011; Postma and Rabe 2015), and is associated with pulmonary hypertension and cardiovascular diseases such as systemic hypertension and atherosclerosis (Chaouat, Naeije, and Weitzenblum 2008). Current smokers and those suffering from chronic bronchitis attributed to other environmental exposures exhibit increased mucous (goblet) cell numbers as well as cellular hyperplasia in small and large airways (Mullen et al. 1987; Wright, Ngai, and Churg 1992). In comparison to non-smokers, smokers display an elevated risk of developing chronic bronchitis and COPD-associated lung ailments, and the extent of symptom exacerbation appears to be associated with host susceptibility (Liu et al. 2010; Raju et al. 2014).
Patients diagnosed with emphysema exhibit mucous cell hyperplasia, resembling manifestations of chronic bronchitis. These individuals exhibit destruction of lung parenchymal tissues and alveolar septal walls, which result in airspace enlargement. Destruction of alveolar walls includes loss of the alveolar capillary bed and alveolar surface area, which impairs gas exchange. One potential mechanism underlying alveolar loss involves proteases released by the sustained recruitment, retention, and activation of inflammatory cells, or by alveolar and bronchial epithelial cells following exposure to toxic irritants, allergens, or TS (Churg, Zhou, and Wright 2012; Fischer, Voynow, and Ghio 2015). Loss of alveolar septa reduces lung function, increases compliance (reduces elastance), and induces breathlessness and hyperventilation (Suki et al. 2013; Boutou et al. 2015). In smokers, the loss contributes to persistent airway inflammation (Gamble et al. 2007; Barnes 2016b) and airflow obstruction. Host genetic predisposition also induces COPD pathogenesis and symptoms (Alam et al. 2014; Sorroche et al. 2015; Silverman 2020).
Inflammatory Cells in COPD
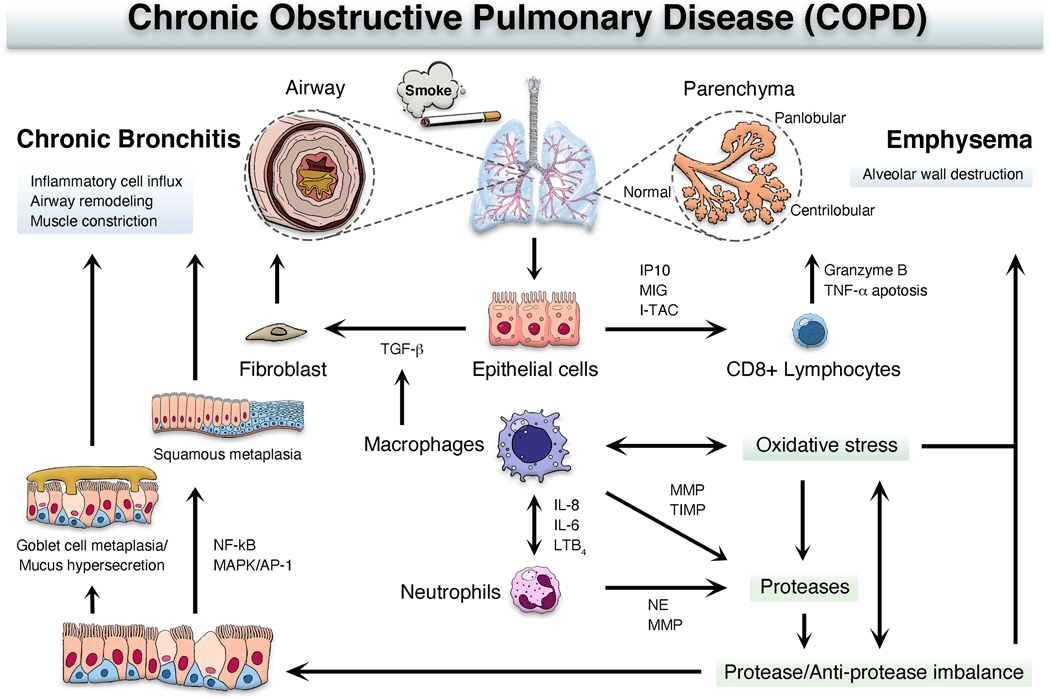
Long-term TS exposure and systemic inflammation that results from chronic airway inflammation also play significant roles in lung tissue damage, adverse remodeling, and vascular dysfunction when mediators are released by inflammatory cells, epithelial cells, and fibroblasts (Figure 1). Inhaled TS in the lungs stimulates activation of macrophages and airway epithelial cells to release chemotactic factors, including interferon-γ-induced protein 10 (IP10; CXCL10), monokine induced by interferon-γ (MIG; CXCL9), interferon-induced T cell α-chemoattractant (I-TAC; CXCL11), interleukin (IL)-6, IL-8, and leukotriene B4, which results in accumulation of neutrophils and CD8+ T cells within the airways (Mabley, Gordon, and Pacher 2011; Barnes et al. 2015; Tanner and Single 2020). The protease, Granzyme B, excreted by CD8+ T cells, was implicated in degradation of extracellular matrix (ECM) leading to tissue remodeling associated with emphysema (Tanner and Single 2020). Proteases released by neutrophils and alveolar macrophages break down connective tissue and elastin in the alveoli contributing to localized (centrilobular) and generalized (panlobular) emphysema (Barnes et al. 2015; Tanner and Single 2020). TS-induced protease/antiprotease imbalance and oxidative stress are key inflammatory processes that promote COPD pathogenesis (Fricker, Deane, and Hansbro 2014; Barnes et al. 2015; Fischer, Voynow, and Ghio 2015). Neutrophil elastase, matrix metalloproteinases (MMPs), and MMP inhibitors play critical roles in development and progression of emphysema and COPD (Vlaykova and Dimov 2014; Gharib, Manicone, and Parks 2018; Ghosh et al. 2019; Zhou et al. 2020). Accumulation and hyperactivation of neutrophils produces mucous (goblet) cell metaplasia and mucus hypersecretion (Shaykhiev 2019). Macrophages and epithelial cells also secrete transforming growth factor (TGF)-β to stimulate proliferation of the epithelium, smooth muscle, and fibroblasts, resulting in fibrosis, airway remodeling, and chronic bronchitis (Herfs et al. 2012; Barnes et al. 2015; Tanner and Single 2020).
Figure 1. Potential pathways for the development of chronic obstructive pulmonary disease (COPD).
IP10, interferon-γ-induced protein 10; MIG, monokine induced by interferon-γ; I-TAC, interferon-inducible T cell α-chemoattractant; IL, interleukin; LTB4, leukotriene B4; NE, neutrophil elastase; MMPs, matrix metalloproteinases; TIMPs, tissue inhibitors of metalloproteinases; TGF-β, transforming growth factor beta; NF-κB, nuclear factor-kappa B; MAPK/AP-1, mitogen-activated protein kinase/activator protein-1.
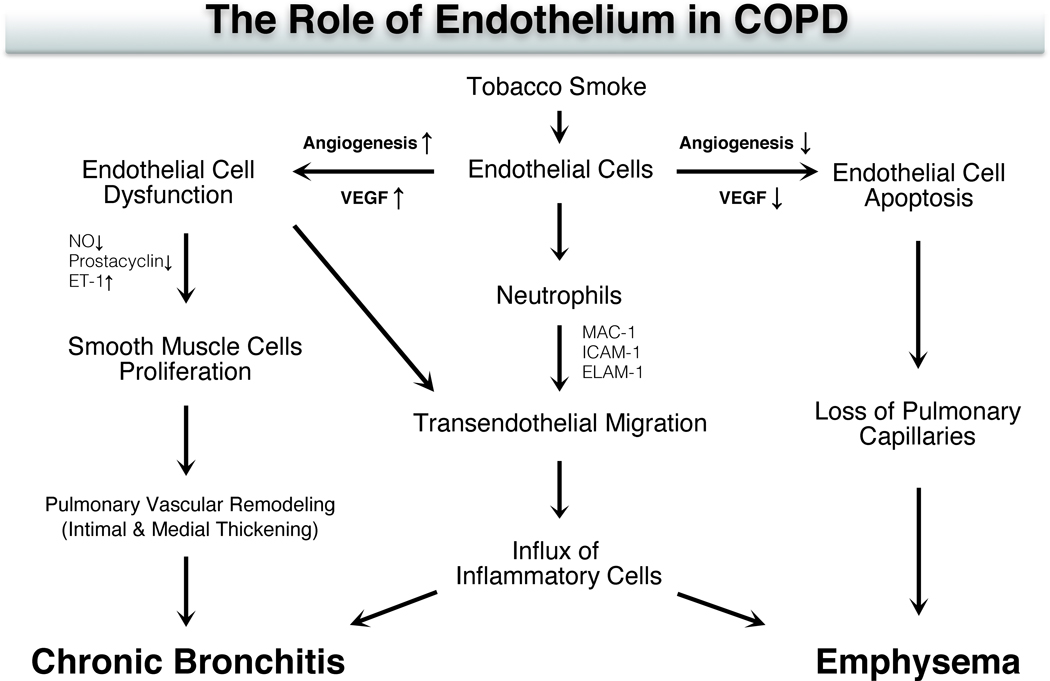
Inflammatory, epithelial, fibroblast, and smooth muscle cells also alter the function of vascular endothelial cells [Figure 2; (Maiellaro and Taylor 2007; Wright and Churg 2008a; Barberà and Blanco 2009)]. TS-induced endothelial injury results in endothelial cell apoptosis, which was shown in animal studies to induce emphysema by blocking vascular endothelial growth factor [VEGF; (Kasahara et al. 2000; Polverino, Celli, and Owen 2018)]. Elevated VEGF concentrations enhance bronchial vascularity and leakage of plasma proteins resulting in airway narrowing in chronic bronchitis (Green and Turner 2017).
Figure 2. Potential mechanisms of endothelial cell injury in the development of chronic obstructive pulmonary disease (COPD).
In the inflammatory response of COPD induced by tobacco smoke, neutrophils migrate through the endothelium by trans-endothelial migration (TEM). Macrophage (MAC)-1 antigen, intracellular adhesion molecule (ICAM)-1 and endothelial-leucocyte adhesion molecule (ELAM)-1 on the surface of endothelial cells involved in TEM are upregulated. The expression of cell adhesion molecules also appears to be induced by endothelial dysfunction in COPD. Vascular endothelial growth factor (VEGF) is a regulator that increases endothelial permeability and leads to endothelial cell growth. By blockade of VEGF, the disappearance of lung tissue in emphysema appears to involve the progressive loss of capillary endothelial and alveolar epithelial cells through the process of apoptosis. On the other hand, VEGF concentration in induced sputum is significantly elevated in chronic bronchitis, which increases bronchial vascularity and leakage of plasma proteins resulting in airway narrowing in chronic bronchitis. In COPD, the synthesis and release of NO (nitric oxide) and prostacyclin are reduced, and the endothelin (ET)-1 is increased in pulmonary arteries. These changes of vasoactive mediators result in endothelial dysfunction with the subsequent proliferation of smooth muscle cell, which may contribute to intimal hyperplasia with the ensuing reduction of arterial lumen and increase pulmonary vascular resistance.
Macrophages
Macrophages are key players in the genesis and persistence of COPD. These cells may either mount an inflammatory response directly, or participate in modulating the immune response by promoting healing and repair of damaged airways and parenchyma. Macrophages might acquire different functions as M1- or M2-polarized phenotypes. M1, known as the “classical macrophage”, releases cytokines that induce the expression of pro-inflammatory genes following exposure to inhaled irritants. In contrast, the M2-polarized macrophage participates in repair and healing mechanisms, and immune-regulation. COPD patients may exhibit phenotypic alterations of M1 and M2 populations (Kaku et al. 2014). Such alterations might persist even after smoking cessation and contribute to chronic airway inflammation seen in former smokers (Harada and Basrur 1998; Hodge et al. 2011). In addition, macrophage phagocytic function which is necessary for the resolution of infections and inflammation is impaired in COPD, a process known as “efferocytosis” (Ito et al. 2020; Liu et al. 2020). Efferocytosis refers to the key role macrophages play in the elimination of apoptotic neutrophils and epithelial cells, which if not cleared might lead to secondary inflammatory cascades which contributes to chronic airway inflammation in COPD. Further, Machida et al (2022) recently demonstrated that lung epithelial cells produce CC motif chemokine ligand 17 (CCL17), a key mediator of cell accumulation in type 2 inflammation that binds to CC chemokine receptor (CCR) 4, upon exposure to cigarette smoke. This CCL17 production is associated with accumulation of alveolar macrophages and development of elastase-induced pulmonary emphysema, possibly through CCL17-induced production of CCL2, a chemoattractant, present in macrophages. These findings may shed new light on the pathogenesis of COPD, and further research is warranted to gain a better understanding of the underlying mechanisms and explore potential therapeutic strategies.
Neutrophils
Granulocytic airway inflammation, typically associated with acute inflammatory episodes, is prominent in COPD. Neutrophils, which contain proteases and other cationic peptides, migrate into lung tissue utilizing a variety of chemoattractants including biological molecules and chemokines secreted by damaged resident macrophages, epithelial cells, and endothelial cells. In the process of killing bacteria and other foreign substances, neutrophils also destroy structural elements of alveolar units and promote emphysema onset. In COPD, neutrophils might produce nearly 25-fold greater quantity of proteases found in healthy individuals (Overbeek et al. 2013). For this reason, neutrophilic inflammation likely contributes to development of emphysema in COPD during acute inflammatory events.
Lymphocytes
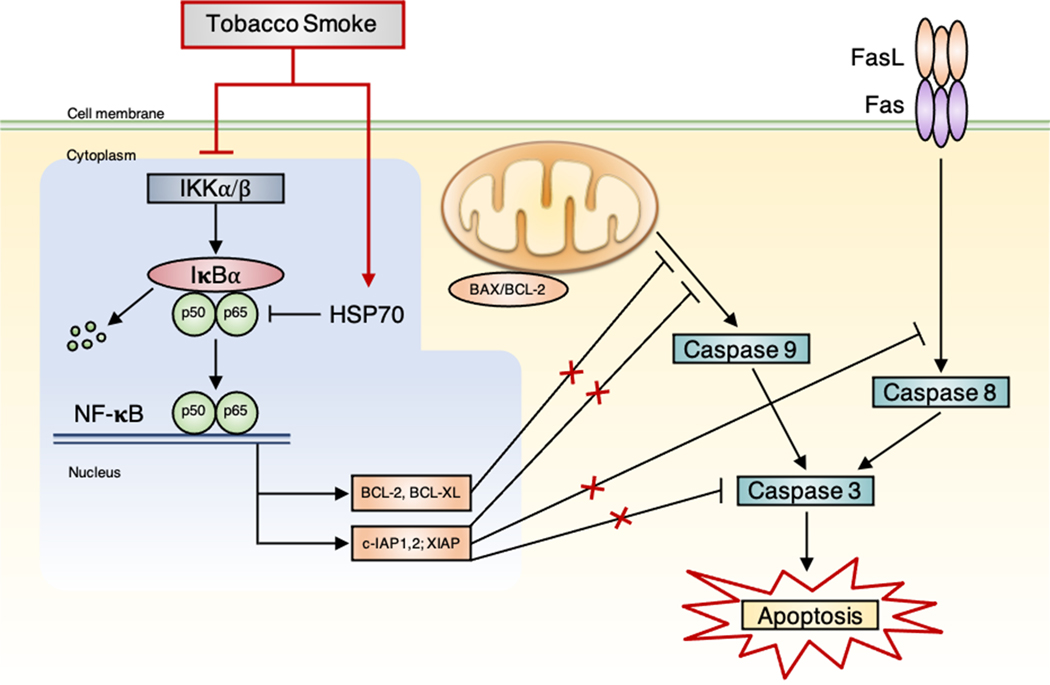
CD8+ and CD4+ T lymphocytes play complex, but interrelated roles in COPD pathogenesis (Caramori et al. 2016). CD8+ cytotoxic T cells are thought to contribute to development of emphysema by releasing perforins and granzymes that initiate tissue destruction and apoptosis (Figure 1). CD8+ cytotoxic T cells also produce inflammatory cytokines such as IFN-γ and tumor necrosis factor-alpha (TNF-α) that provoke emphysema (Maeno et al. 2007; Fairclough et al. 2008; Freeman et al. 2013). T helper 1 (Th1) lymphocytes, a lineage of CD4+ cells, produce cytokines like IFN-γ, which contributes to COPD pathogenesis by promoting autoimmune responses and inflammation (Barnes and Cosio 2004). These and other T cells collectively, through increased enzymatic lytic activity, Fas-ligand mediated-apoptosis (Figure 3), and pro-inflammatory cytokine release, contribute to development of emphysema and other inflammatory responses in COPD (Green and Ferguson 2001; Fairclough et al. 2008; Hotchkiss et al. 2009). The contribution of T cells to TS-induced COPD is evident from the tissue destruction that ensues in healthy mice injected with T cells obtained from TS-exposed mice (Eppert et al. 2013).
Figure 3. Proposed mechanisms of NF-κB pathway in tobacco smoke (TS)-induced apoptosis in the lungs of rats.
Fas-ligand (FasL) mediated-apoptosis involves binding of ligands to the death receptor (Fas), which leads to activation of caspase 8. Both mitochondrial (caspase 9) and death receptor (caspase 8) pathways lead to activation of caspase 3, which ultimately result in cell death by apoptosis. Nuclear factor-kappa B (NF-κB) is the major anti-apoptosis transcription factor, which regulates the expression of anti-apoptotic genes, such as cellular inhibitors of apoptosis (c-IAP) and BCL-2 family members (e.g., BCL-2 and BCL-XL). It exists as a complex consisting of p50 and p65 (RelA) subunits. NF-κB is maintained in the cytoplasm by the inhibitory protein IκB, mainly IκBα, which is rapidly phosphorylated by IκB kinases (IKK) upon stimulation. Through suppression of IKK and induction of heat shock proteins (HSP70), TS inhibits NF-κB activity and downregulates NF-κB-dependent anti-apoptotic proteins, including members of BCL-2 and c-IAPs, leading to caspase activation, eventually inducing apoptosis. Reproduced from Zhong CY, Zhou YM, Pinkerton KE. NF-κB inhibition is involved in TS-induced apoptosis in the lungs of rats. Toxicology and Applied Pharmacology 2008; 230: 150–158.
Epigenetic Regulation of Gene Expression in COPD Pathogenesis
As epigenetic modulation of gene expression is increasingly recognized as an important regulator of pathobiological processes, COPD pathogenesis and TS exposure are postulated to be linked to changes in circulating micro-ribonucleic acid (miRNA; non-coding RNA which plays a role in regulating gene expression) profiles in ex-vivo, epidemiological, and in vivo mouse studies (Takahashi et al. 2013; Gross et al. 2014; Leuenberger et al. 2016; Yu et al. 2019). Although the causal roles of specific miRNA molecules have not been confirmed in COPD pathogenesis, a number of miRNAs associated with inflammatory processes may be elevated in COPD patients. In vitro and human population studies demonstrated pathological indices of COPD including (1) inflammation, tissue remodeling, and emphysema, (2) involve a distinct set of miRNA changes that correspond to alterations in expression of genes associated with each of the listed pathologies (Bracke and Mestdagh 2017). While current therapeutic strategies are not designed to specifically alter the genetic susceptibility or complex pathology associated with COPD, miRNAs were targeted for potential therapeutic intervention (Liao et al. 2017; Bracke and Mestdagh 2017). Although a number of animal investigations involving TS-induced pathology examined the role of specific miRNAs in cell signaling (Halappanavar et al. 2013; Xi et al. 2013; Yu et al. 2019), it needs to be emphasized that the relevance of miRNA changes in smokers and COPD patients as compared to animal models of TS-induced inflammation has not been established.
In addition to RNA interference, other epigenetic modifications might be mediated by different mechanisms, including DNA methylation and histone modification, which might also play essential roles in COPD development. DNA methylation is a chemical modification that involves addition of a methyl group to cytosine residues in CpG dinucleotides, resulting in formation of 5-methylcytosine. DNA methylation might occur in promoter regions of genes, leading to gene silencing or reduced gene expression. In COPD, alterations in DNA methylation patterns were noted in genes involved in inflammation, oxidative stress, and tissue remodeling, which are critical processes in COPD pathogenesis (Alfahad et al. 2021). Previously Zeng et al ( 2020) suggested that cigarette-induced oxidative stress plays a role in mediating pulmonary apoptosis and hypermethylation of the B-cell lymphoma/leukemia-2 (Bcl-2) promoter, an apoptosis regulator, in COPD through DNA methyltransferase enzyme 1 (DNMT1), a key DNA methyltransferase enzyme. Similarly, aberrant DNA methylation was reported to be a widespread occurrence in small airways of COPD patients and was associated with altered expression of genes and pathways related to COPD, such as NF-E2-related factor 2 oxidative response pathway (Vucic et al. 2014).
Histone modifications, on the other hand, involve chemical modifications of the proteins called histones that package DNA into a compact structure termed chromatin. These modifications might either activate or repress gene expression by altering accessibility of DNA to transcriptional machinery. Histone modifications including acetylation, methylation, phosphorylation, and ubiquitination, might influence expression of genes involved in inflammation, immune responses, and other processes relevant to COPD (Zhang et al. 2020). It is of interest that Wu et al (2018) found altered histone deacetylase (HDAC) activity, which leads to dysregulated histone acetylation in COPD patients and was suggested to be implicated in regulation of inflammation in COPD.
Taken together investigations involving miRNA, DNA methylation, and histone modifications in COPD revealed epigenetic changes that contribute to this disease pathogenesis. These modifications might alter gene expression, affecting crucial biological processes involved in COPD development and progression. Understanding these epigenetic mechanisms may lead to potential therapeutic targets and personalized treatment strategies for COPD patients. However, further research is needed to fully comprehend the complex interplay between epigenetic modifications and COPD pathogenesis.
COPD Risk Factors
Environmental Determinants of COPD
Although various environmental stimuli including air pollutants including second-hand smoke, nitrogen dioxide, biomass smoke and allergens as well as infectious diseases attributed to viruses and bacteria may be associated with or possibly exacerbate COPD (Eisner 2007; Bhalla et al. 2009; Salvi and Barnes 2010; Eisner et al. 2010; Andersen et al. 2011; Harting et al. 2012; Schikowski et al. 2014; Sana et al. 2018; Cochard, Ledoux, and Landkocz 2020), the major contributor to the pathogenesis of COPD is TS exposure (Hylkema et al. 2007; Laniado-Laborin 2009; Tamimi, Serdarevic, and Hanania 2012; Lugade et al. 2014). TS contains over 6,000 constituents including nicotine, aldehydes, polycyclic aromatic hydrocarbons, glycoproteins, and metals, which are known to be both antigenic and carcinogenic (Oldham, Coggins, and McKinney 2013; Hou et al. 2019). In humans, exposure to first- and possibly second-hand TS might lead to COPD and cancer (Hou et al. 2019; Song, Chen, and Liu 2021), and potentially lead to development of asthma and cardiovascular diseases (Saha and Brightling 2006; Onor et al. 2017). Even non-smokers with long-term exposure to second-hand TS or smoke from biomass burning might develop bronchitis leading to COPD (Whittemore, Perlin, and DiCiccio 1995; Coultas et al. 2001; Goldklang, Marks, and D’Armiento 2013; Ramirez-Venegas et al. 2019).
Physiological Determinants of COPD: Comorbidities and Sex
The severity of the development of COPD is partly dependent upon individual risk factors, such as a history of cardiovascular, metabolic, or autoimmune diseases, which increases susceptibility (Campo et al. 2015; Hizawa 2016). It is known that patients with α1-antitrypsin deficiency, as well as polymorphisms and mutations in genes associated with cancer and cardiovascular diseases, exhibit an elevated risk of developing COPD (Liu et al. 2010; Kaur-Knudsen et al. 2011; Castaldi et al. 2014).
Although gender may also influence COPD development, this continues to be an active area of research. While previous investigators noted that COPD prevalence was higher among men than women (Lindberg et al. 2005a, 2005b; de Torres et al. 2006). Evidence suggests it may now be greater in women (Pinkerton et al. 2015; Barnes 2016a; Ramirez-Venegas et al. 2019). These findings might be attributed to changing gender patterns of smoking (GOLD 2020), but several investigators suggested women are at greater risk to the adverse effects of cigarettes leading to COPD and carcinogenesis (Viegi et al. 2001; Langhammer et al. 2003; Stabile and Siegfried 2003; Han et al. 2007).
In addition to respiratory decrements, COPD patients often suffer from cardiovascular pathologies including atherosclerosis, hypertension, and heart failure, as well as metabolic diseases such as diabetes and osteoporosis (Agusti et al. 2010). Significant clinical evidence demonstrates a positive association between COPD and enhanced risk of developing other diseases including coronary artery, cerebrovascular, and peripheral arterial diseases, independent of TS exposure (Sin and Man 2005; Finkelstein, Cha, and Scharf 2009).
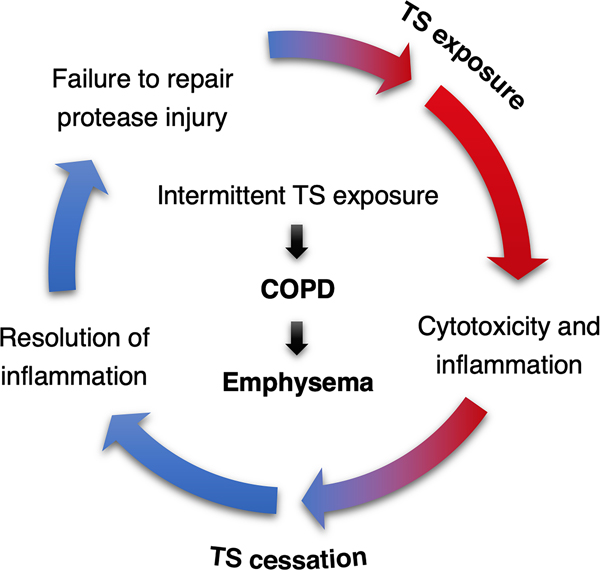
At this time, smoking cessation is the most effective means of reducing the progression of cellular and tissue-based injury, and the exacerbation of COPD symptoms produced by years of smoking (Groneberg and Chung 2004; Tashkin 2015). It is well-established that in those who quit smoking, the rate of lung function decline lessens compared to active smokers but remains higher than healthy non-smokers over time (Wu and Sin 2011; Jimenez-Ruiz et al. 2015). Although smoking cessation might improve quality of life and attenuate some features of COPD, the effects of prolonged TS exposure are often irreversible and difficult to treat with pharmacological intervention alone (Hersh 2010; Chang and Rivera 2013; Hawkins, Virani, and Ceconi 2013). Recently, Wu et al (2020) demonstrated the inflammatory and morphological changes induced by long-term TS exposure in lungs of rats became irreversible even after prolonged smoking cessation. Further, intermittent exposure to TS may contribute to persistent chronic inflammation, increased severity of emphysema, and a higher incidence of tumorigenesis in lungs compared with continuous TS exposure as illustrated in Figure 4 (Pinkerton and Poindexter 2018; Kameyama et al. 2018).
Figure 4. Possible mechanisms for the genesis of tobacco smoke (TS)-induced lung disease.
During smoking exposure inflammation, injury and remodeling are highly activated, while smoking cessation leads to a resolution in inflammation. Repeated TS exposure and cessation creates a renewed insult to the lungs, resulting in renewed inflammation that repeats and amplifies the injury process. Release of proteolytic enzymes during this injury further damages and remodels the lungs through a loss of elastic fibers, thus causing airspace enlargement of the alveoli. Intermittent exposure to TS promulgates these effects, leading to enhanced emphysema, which is one of the most common conditions that contributes to chronic obstructive pulmonary disease (COPD). Reproduced from Pinkerton KE, Poindexter ME. Harmful interruptions: Impact of smoking patterns on tumorigenesis and emphysema. Am J Respir Cell Mol Biol 2018;59:133–134.
TS Exposure in COPD Animal Models Mimicking Real-Life Human Exposure
The generation and exposure conditions of tobacco smoke (TS) are crucial factors in interpreting animal studies investigating the development of chronic obstructive pulmonary disease (COPD). The variability in TS exposure conditions, such as yield measurement methods, smoking regimes, smoke characteristics, and dosage, significantly impact the consistency and reproducibility of study results.
An excessively high dose of TS in animals may be employed to achieve the desired effects of COPD development, whereas this approach may be criticized for its relevance to real-life human exposure. On the other hand, some investigators attempted to model COPD using lower doses of TS that more closely resemble human exposure levels. However, lower doses of TS in animal models may fail to induce definitive phenotypes of COPD, as seen in the study by Hodge-Bell et al. (2007), where only mild emphysema was observed in a minimal number of mice exposed to mainstream cigarette smoke (MS) with varying total particulate matter (PM) concentrations, suggesting that MS exposure in this study may not have been sufficient to induce a definitive COPD phenotype. This highlights the importance of carefully selecting appropriate dosages of TS in animal models for both better mimicking real-life exposure in humans and understanding the pathogenesis of the disease.
To better mimic human exposure, dosages of TS in animal models might include measurements of PM concentration, nicotine levels, smoke volume, and other relevant factors recalculated per airway square footage or lung volume/respiratory volume to provide a more accurate assessment of the dose-response relationship. In addition, standardized methods for measuring the yield of tar, nicotine, and carbon monoxide (CO) from cigarettes (Chae, Walters, and Holman 2017), such as the Federal Trade Commission (FTC) or International Organization of Standardization (ISO) methods, require consideration of animal studies. Further, smoking regimes, including number of cigarettes smoked per day, duration of smoking, and depth of inhalation, as well as type of cigarette (filtered, non-filtered or e-cigarette) and characteristics of the smoke such as particle size distribution or concentration of toxic constituents also needs to be considered with caution and reported in animal studies.
Further research and discussion on appropriate dosages and TS characteristics in animal models are needed to improve the translatability of findings to humans and enhance our understanding of the pathogenesis of COPD. Careful consideration and standardization of TS generation and exposure conditions in animal studies are critical to ensure the robustness and relevance of research observations in advancing our understanding of COPD and developing effective interventions for this complex disease.
Measured Parameters to Define COPD in Animal Models
Lung Function Measurement
Pulmonary function testing used to define COPD in animals involves measuring various parameters related to lung function, including forced expiratory volume (FEV1), forced vital capacity (FVC), peak expiratory flow (PEF), lung compliance, resistance, functional residual capacity (FRC), and total lung capacity (TLC) (Bailey 2012). These parameters are measured using a variety of techniques, including plethysmography, spirometry, and forced oscillation techniques. By measuring these parameters, investigators are able to assess the extent of airway obstruction, inflammation, and other features associated with COPD in animals.
Cell Counting in Bronchoalveolar Lavage Fluid (BALF)
The BALF is collected from the lungs through lavage, followed by analyzing the cell population within the fluid (Lofdahl et al. 2005). Cell counting is performed using a hemocytometer or an automated cell counter to quantify total number of cells present in the BALF. The cell population is typically divided into different types of cells, such as macrophages, neutrophils, lymphocytes, and eosinophils. The relative proportions of these different cell types provide important information regarding the inflammatory response in lungs and severity of COPD.
Histological Examination
Pathological changes in animal models are analyzed using lung tissue samples under a microscope, involving collecting lung tissue samples and processing by standard histological techniques (Shu et al. 2017). The most frequently used stains for histological examination of lung tissue are hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) stains for identifying cell types and structural components of the lung, and mucus substances in the airways, respectively. Histological examination provides information regarding the morphological alterations that occur in lungs following TS exposure. The morphological alterations detected include inflammation, fibrosis, destruction of alveolar walls, and mucus accumulation in the airways.
Biomedical assays
Biomedical assays are used to examine biological fluids and tissues for various biomarkers of inflammation, oxidative stress, and lung function which provide a sensitive approach for measuring progression of COPD in animal models, and are often employed in combination with other techniques such as pulmonary function testing and histological examination (Churg et al. 2009; Barnes et al. 2015). Some frequently utilized biomedical assays include (1) enzyme-linked immunosorbent assay (ELISA) to measure levels of specific proteins, such as cytokines and chemokines, in biological fluids in blood, BALF, and lung tissue homogenates; (2) western blotting, for detecting changes in protein expression levels; (3) immunohistochemistry, for determining specific proteins in lung tissue sections using specific antibodies and visualizing the spatial distribution of specific proteins within lung tissue; and (4) oxidative stress assays, used to measure levels of reactive oxygen species (ROS), which might result in oxidative damage in cells and tissues, and postulated to play a key role in development of COPD.
In addition to the above commonly employed methods, several other techniques may also be utilized to define COPD in animal models including (1) gene expression analysis, measurement of alterations in gene expression levels, which are performed using microarray or RNA sequencing technologies, and provide insights into the molecular mechanisms underlying COPD development; (2) proteomics, a large-scale analysis of proteins in biological samples to identify proteins that are differentially expressed in response to TS exposure; (3) metabolomics, an analysis of metabolites in biological samples to provide information on the metabolic pathways that are dysregulated in COPD; and (4) imaging techniques, such as computed tomography (CT) and magnetic resonance imaging (MRI), which are used to assess lung structure and function in animal models of COPD (Al Faraj et al. 2014; Kim et al. 2022). By using a combination of different methods, investigators might gain a more comprehensive understanding of the mechanisms underlying COPD pathogenesis.
Animal Models of COPD
While no animal model might truly recapitulate the decades-long TS exposures that humans endure, numerous animal models were developed to examine the cellular and molecular mechanisms underlying COPD pathogenesis (Table 1). Regardless of the animal model, the fundamental features include airspace enlargement, mucus hypersecretion, chronic inflammation, and some form of airway remodeling. Each of these conditions exist to varying degrees of severity in COPD patients. In fact, disease heterogeneity may explain the lack of a universally reliable therapeutic approach for COPD, and require a regime of personalized medicine (Franssen et al. 2019). Unfortunately, the best available animal models display only limited aspects of COPD pathologies, thus reducing their ability to fully recapitulate the entire spectrum of clinical COPD. It is of interest most animal models of emphysema do not truly mimic the human disease, but rather reflect airspace enlargement due to either loss of collagen/elastin or TS-induced insults and incomplete alveolarization of the lungs during developmental windows of morphogenesis (Wright, Cosio, and Churg 2008). Ideally, an animal model of COPD needs to recapitulate the aforementioned morphological and biological changes in a manner similar to human disease. Therefore, it is critical to acknowledge the limits of animal models during any experimental process to reliably interpret and clinically contextualize data relevant to human disease.
Several animal models clearly replicate one or more key pathologic features of COPD such as emphysema and bronchitis. However, when selecting a COPD model, several factors need to be considered. These include but are not limited to inter-species (animal-to-human) differences in genetics, lung development, and susceptibility to a given pulmonary insult.
Since humans and rodents possess a number of genetic homologies, mice and rats were widely used to study human respiratory diseases. For measurement of TS-related lung responses, mice and rats are generally exposed via a whole-body or nose-only exposure systems (Perez-Rial, Giron-Martinez, and Peces-Barba 2015). Depending upon the objective of the investigation, the exposure regimen might range from days to months. In general, to induce emphysema-like changes and airway remodeling, animals are exposed to TS for at least 4–6 months. However, exposure duration might vary with model species, exposure methods, and exposure concentrations/internalized doses (Wright and Churg 2002).
There are a number of structural and immunological differences to consider when extrapolating findings from animal models to humans. Although humans and rodents mount similar host responses to exogenous stimuli, there are inter-species differences in the anatomy of the tracheobronchial tree. These include (1) dichotomous symmetrical versus asymmetrical airway branching in humans versus rodents, respectively; (2) absence of respiratory bronchioles in mice and rats compared to humans; and (3) lack of a bronchial circulation in mice but not rats or humans (Mitzner et al. 2000; Kotoulas et al. 2014). Indeed, while humans, dogs, and ferrets exhibit several generations of membranous and respiratory bronchioles (Wright and Churg 2008a; Churg, Cosio, and Wright 2008), there exists in rats and mice an abrupt transition from the conducting airways to the alveolar duct. Despite inter-species structural variability in the respiratory system, animal models provide approximations of human disease pathology that continue to serve a critical role in the study underlying COPD pathogenesis.
Mice
Mice are commonly used to model lung disease. Advantages include low cost, easy handling, rapid reproductive turnover with large litters, and a potential for genetic manipulation that fosters their use over other animal species for lab research. This is because the mouse genome is similar to humans, diverse inbred strains are well-characterized, and generation of transgenic mice provides a rapid and relatively inexpensive approach to determine the exact function of particular genes (Vandamme 2014; Gurumurthy and Lloyd 2019). The wide availability of mouse antibodies and molecular probes are additional advantages. Mouse models of COPD include emphysema-like phenotypes induced by administration of elastase (Mano et al. 2022; Jo et al. 2022) or an extract solution of cigarette smoke (Yang et al. 2022), bronchitis and emphysema phenotypes induced by whole body or nose-only TS exposure (Shapiro et al. 2003; Pelgrim et al. 2022), and other COPD phenotypes initiated chemically by nitrogen dioxide or sulfur dioxide (Groneberg and Chung 2004).
Disadvantages of COPD mouse models include the time it takes to develop morphological changes consistent with human COPD, and the reversibility of these alterations, the latter being highly inconsistent with progressive and irreversible nature of human COPD. Mice are also poor models of chronic bronchitis due to their lack of bronchial submucosal glands, and minimal development of mucous (goblet) cell metaplasia in the smaller airways (Churg, Cosio, and Wright 2008). Further, development of emphysema in mice is strain-dependent and cannot always be consistently reproduced (Guerassimov et al. 2004; Nadziejko et al. 2007). Finally, the lengthy process required to produce disease in the mouse further limits the utility of telemetry and pulmonary function testing. These tests are often used in studies of cardiovascular disease, a common comorbidity of COPD (Kodavanti, Costa, and Bromberg 1998; Wright, Cosio, and Churg 2008).
Rats
Rats are frequently employed to study respiratory, cardiovascular, metabolic, and autoimmune diseases, all of which were reported to be associated with TS-induced COPD (Cavailles et al. 2013). One of the advantages to using rats is the ability to produce emphysema-like injury within 2 to 6 months of TS exposure. Rats also mimic human chronic bronchitis and airway hyperreactivity (Shore et al. 1995). With prolonged exposure to TS, rats demonstrate mucous metaplasia in the larger and smaller airways (Wright, Cosio, and Churg 2008), a common feature of human COPD. Similar to humans, rats display drug resistance, a critical factor when considering therapeutic glucocorticoid and antibiotic treatments in human COPD (Cowan et al. 1986; Maslarova et al. 2011; Kabir and Morshed 2015; Fedosenko et al. 2015).
Limitations to using rats compared to mice in COPD investigations include greater expense and fewer knockout strains available. Rats also lack submucosal glands; although, mucous (goblet) cells might increase and proliferate from a small baseline population (Kodavanti, Costa, and Bromberg 1998).
Given the comorbidities of diabetes, atherosclerosis, and hypertension commonly reported in COPD patients, rats were used to study such comorbidities following TS exposure. TS-exposed rats develop elevated pulmonary arterial pressure, suggesting development of pulmonary hypertension (Wright, Cosio, and Churg 2008; Lee et al. 2005). Spontaneously hypertensive rats (SHRs), established during the 1960s by selective breeding of Wistar-Kyoto (WKY) rats for high blood pressure traits, were found to be useful in studies of respiratory and cardiovascular diseases.
Spontaneously Hypertensive Rats (SHR) Model of Human COPD
Spontaneously hypertensive rats (SHR) demonstrate COPD-like phenotypes similar to humans following inhalation of PM (Kodavanti et al. 2000) and TS (Yu et al. 2008; Bolton et al. 2009; Shen et al. 2016), as well as intratracheal instillation of lipopolysaccharide (LPS), also known as endotoxin present in the outer cell membrane of gram negative bacteria (Wang et al. 2013). Since SHRs naturally develop elevated blood pressure, these animals have been widely utilized as genetic models of essential hypertension, arise in blood pressure without any secondary cause (Doggrell and Brown 1998; Zhou and Frohlich 2007). In SHRs, systemic blood pressure begins to rise by 5 to 6 weeks of age, and plateaus at values ranging from 180–200 mm mercury (Hg) by adulthood (12–13 weeks). In contrast, normotensive WKY rat strains display a systemic blood pressure ranging from 110–150 mm Hg (Buttner et al. 1984). Given the variety of more than 22 different SHR strains ranging from stroke-prone to Dahl/Rapp salt-sensitive rats and also exhibiting an array of different cardiovascular and metabolic pathologies, the SHR model may be ideal for study of COPD (Bolton et al. 2009; Chen et al. 2015; Shen et al. 2016; Wu et al. 2020; Yu et al. 2008).
SHRs afford us the opportunity to investigate mechanisms underlying COPD pathogenesis and enable testing of novel drug therapies (Davis et al. 2013). Previously investigators s demonstrated that SHRs manifest neutrophilic airway inflammation and nuclear factor-kappa B (NF-κB) and mitogen activated protein kinase-/activator protein-1 (MAPK/AP-1) activation which likely play a role in TS-induced pathogenesis (Zhong et al. 2005; Zhong, Zhou, and Pinkerton 2008). NF-κB and AP-1 regulate the expression of the inflammatory genes, IL-17, IL-8, and TNF-α, which are produced in COPD (Schuliga 2015). These TS-induced pro-inflammatory cytokines upregulate airway epithelial hyperplasia and squamous metaplasia through NF-κB and MAPK/AP-1 signaling [Figure 1; (Zhong et al. 2005)]. In addition, TS inhibits NF-κB activity and downregulates NF-κB-dependent anti-apoptotic proteins (BCL-2 and c-IAPs), leading to activation of caspases 3, 8, and 9, to induce programmed cell death by apoptosis as illustrated in Figure 3 (Zhong, Zhou, and Pinkerton 2008)]. Similarly, TS produces pulmonary toxicity via MAPK signaling and AP-1 transactivation (Mossman, Lounsbury, and Reddy 2006). SHRs also demonstrate increased expression of TGF-β in epithelial and inflammatory cells, and leukocyte recruitment in response to TS seems to occur mainly through bronchial circulation (Davis et al. 2012; Hoang et al. 2016). Overall, these studies demonstrate that SHRs provide new and unique insights into the molecular mechanisms underlying TS-associated lung diseases that cannot be attained with other COPD models.
Limited data are available on the combined effects of enhanced susceptibility to cardiopulmonary complications and long-term TS exposure on pulmonary structural and cellular alterations frequently detected in COPD patients. SHRs exhibit risk factors similar to those found in COPD patients making this rat strain a promising human-relevant animal model of lung disease induced by inhaled irritants (Kodavanti et al. 2006; Bolton et al. 2009; Yu et al. 2008). Recently Pham et al. (2020) used SHR and WKY rats to examine the effects of long-term TS exposure on lung injury and airway inflammation, and elucidate the inherent risk factors and pathophysiology of COPD. The degree and severity of TS-induced pathological changes noted in SHRs were significantly different from WKY rats with long-term progressive exposure to TS up to 12 weeks. Data suggest a genetic contribution, which plays an important role for predicting TS-induced COPD onset in humans. Previous investigations with prolonged TS exposure and subsequent cessation demonstrated that SHRs responded similarly to humans. Such findings encourage further exploration of the SHR model in future studies of smoking cessation and COPD treatments (Wu et al. 2020; Pham et al. 2020).
It is also important to note that while genetically modified animal models may provide useful insights into the biological mechanisms underlying COPD, there may be limitations in extrapolating these findings to humans. The use of these models may be criticized as not necessarily representative of normal genetic diversity as found in humans. Therefore, it is important to consider the advantages and limitations of different animal models and their relevance to human disease when interpreting findings from such studies.
Guinea Pigs
Guinea pigs are also widely used to model COPD. TS inhalation studies in guinea pigs ranged from 1 to 12 months to produce progressive pulmonary function abnormalities and emphysema-like lesions (Groneberg and Chung 2004) that are easily recognizable and more prominent than those observed in mice (Wright and Churg 2008b).
In guinea pigs, long-term TS exposure produced a rise of number of polymorphonuclear leukocytes (PMNs) and accumulation of macrophages and CD4+ T-cells (Meshi et al. 2002). Pulmonary inflammation and emphysematous destruction induced by chronic TS exposure were amplified by the presence of latent adenoviral infection (Meshi et al. 2002). Long-term TS exposure also induced selective endothelial dysfunction in pulmonary arteries of guinea pigs confirming that smoking exerts deleterious effects on pulmonary vessels and may contribute to development of pulmonary hypertension in COPD (Ferrer et al. 2009). Advantages of using guinea pigs include the ability to model common COPD comorbidities including pulmonary hypertension, autoimmune disease, and viral infection (Meshi et al. 2002; Groneberg and Chung 2004; Ferrer et al. 2009; Gu et al. 2012; Dominguez-Fandos et al. 2015).
Shortcomings associated with the guinea pig COPD model include irregularly distributed or absent bronchial glands, which make it a poor model for chronic bronchitis (Goco, Kress, and Brantigan 1963), and increased cost relative to mice and rats. Substantial molecular studies using guinea pigs, and commercially available antibodies and probes that cross react with guinea pig proteins are also lacking (Padilla-Carlin, McMurray, and Hickey 2008; Perez-Rial, Giron-Martinez, and Peces-Barba 2015).
Dogs
In general, COPD-related studies in dogs have been beset by inconsistent findings, confounding factors, incomplete reporting, and/or lack of controls. This may be attributed to the timeframe in which experiments were conducted and published. Some of the earliest studies examining the effects of cigarette smoking were in dogs. Repeated chamber exposures over the course of approximately one year yielded epithelial hyperplasia (Reece and Ball 1972), peribronchiolar hypercellularity with basal and mucous (goblet) cell hyperplasia (Park et al. 1977), and inflammatory cell influx in peribronchiolar regions (Hernandez et al. 1966; Park et al. 1977). When exercise stress was combined with TS exposure for 11 months, signs of hypoxemia manifested (Reece and Ball 1972).
In some early investigations, expected changes in pulmonary function were mostly absent despite indications that TS inhalation might lead to chronic bronchitis and emphysema. For example Park et al. (1977) and Wanner et al. (1973), demonstrated that TS inhalation using mask or “mouthpipe” exposures for 6 or 12 months yielded decreased tracheal mucous velocity, while alterations in pulmonary function parameters, including pulmonary resistance and dynamic compliance, lung diffusing capacity of carbon monoxide, arterial blood gases, or lung volumes were not apparent. Similar experiments using tracheostomy exposures for 10 months yielded mucus hypersecretion without a change in mucous velocity (King et al. 1989). No changes in ciliary beat frequency were noted (Park et al. 1977) suggesting TS-induced alterations in mucus viscosity played a role in protecting airway epithelium and parenchyma (King et al. 1989; Park et al. 1977). However, at least one study suggested that TS-induced mucus hypersecretion protected against nonspecific airway reactivity to inhaled methacholine (Desanctis et al. 1987).
Emphysema induced by TS was reported by several investigators (Frasca et al. 1983; Hernandez et al. 1966; Auerbach et al. 1967; Zwicker et al. 1978). However, these studies were criticized for confounding factors, such as pulmonary infections, and lack of sham controls (Thomas and Vigerstad 1989).
Interestingly, most investigations utilized young adult dogs even though COPD was proposed to be a disease of accelerated lung aging (Ito and Barnes 2009). Given that dog lungs undergo restructuring, “senile emphysema”, as in humans later in life (Hyde et al. 1977; Mauderly 2000), there may still be an opportunity to better define this aspect of COPD with this model.
Ferrets
One of the significant components of COPD pathogenesis is bronchitis with mucus hypersecretion and airway inflammation. Bronchitis pathogenesis is associated with chronic impairment in epithelial sodium channels and hindrance of mucociliary clearance through inhibition of the cystic fibrosis transmembrane conductance regulator (CFTR) protein (Rab et al. 2013; Raju et al. 2016a; Mall 2016; Cantin 2016). Ferret and human CFTRs function in similar ways to produce changes in epithelial permeability (Fisher et al. 2012). Distribution of submucosal mucus glands and airway physiology are similar in ferrets and humans enabling the bronchitis component of TS-induced human COPD to be mimicked (Raju et al. 2016b). Long-term TS exposure in ferrets induces clinical features of human bronchitis such as cough, airway mucous (goblet) cell hyperplasia, and mucus hypersecretion with associated bronchiolitis (Raju et al. 2016b). Considering the molecular and pathological similarities between human and ferret TS-induced bronchitis, this animal model has the potential to enhance our understanding of the pathogenesis of human bronchitis, and enable subsequent testing of novel therapeutics (Solomon et al. 2016).
Nonhuman Primates (Monkeys)
As nonhuman primates (NHPs) exhibit anatomical, physiological, and immunological similarities to human lungs, this species might serve as reliable models for COPD (Miller et al. 2017). However, there are several limitations to their use in research. First is the cost of purchase, per diem, and exposure conditions required to begin to simulate a COPD-like condition. Exposures require up to several years to recapitulate human COPD, although shorter term exposure may elicit inflammation (bronchitis), mucous metaplasia, and airspace enlargement when exposure begins in infancy. NHP models also present challenges in genetic manipulation techniques, while specialized facilities required to house monkeys demand high associated costs. Thus, only a few studies have attempted to model COPD through exposure of monkeys to TS.
Over 35 years ago, baboons were trained to smoke and assessed after 6 pack-years of smoking (over the course of three years). Interestingly, bronchial reactivity was blunted, residual lung volume was preserved following methacholine challenge, and no other changes in lung volumes or flow-volume measures were observed in smoking monkeys (Roehrs, Rogers, and Johanson 1981). While inhalation of nicotine was found to acutely blunt the methacholine response without affecting baseline lung function, the response to histamine was unaffected (Wallis, Rogers, and Johanson 1982). Notwithstanding, nicotine also produces bronchoconstriction by decreasing guanylate cyclase activity in male Sprague Dawley rats (Vesely and Levey 1978). Aspects of lung barrier function, such as mucus secretion and mucociliary function were not assessed in these studies.
In a more recent examination of heavy smoking approximating 4 packs per day, female macaques (average age = 11 years) were exposed in chambers over the course of 4 or 12 weeks to determine TS-induced pathological processes in the model (Polverino et al. 2015). Inflammation, mucus metaplasia, glandular hypertrophy/hyperplasia, and peribronchial fibrosis were evident. A “pre-emphysematous” profile was indicated by elevated MMP, apoptosis of alveolar septal cells, and oxidative stress in lung tissue. After 12 weeks, pulmonary function changes were minimal relative to sham exposure. These included a trend toward reduced forced expiratory volume in one second, and an approximately 20% increase in quasistatic compliance versus a 7% decrease in sham-exposed controls. Further studies in this model showed that a combination of TS and influenza infection enhanced connective tissue growth factor expression in bronchial epithelial cells which might accelerate cell senescence (Jang et al. 2017).
COPD Animal Models in Clinical Studies
Animal models were instrumental in advancing our understanding of COPD and its potential treatments. Studies utilizing animal models provided insights into mechanisms underlying COPD pathophysiology, determined novel interventions, explored regenerative potential, and identified potential biomarkers. Qiu et al. (2022) observed that elevated levels of IL-27, a member of IL-12 family cytokines, was accompanied by an exaggerated IFN-γ+CD8+Tc1 response in a smoking mouse model of emphysema. These observations suggested that IL-27 negatively regulated IFN-γ+CD8+Tc1 response in the late stage of chronic cigarette smoke exposure, which may provide a new strategy for the anti-inflammatory treatment of smoking-related COPD/emphysema. Kim et al. (2018) demonstrated that fibroblast growth factor-2 (FGF2) was decreased in lungs of cigarette smoke-exposed mice and intranasal recombinant FGF-2 (rFGF-2) significantly reduced macrophage-dominant inflammation and alveolar destruction in lungs, suggesting that inhaling rFGF-2 may be a new therapeutic option for patients with COPD. Chen et al. (2019) established a mouse model of COPD utilizing cigarette smoke exposure and found that C-X-C motif chemokine 5 levels correlated with granulocyte-colony stimulating factor levels in both plasma/serum and BALF obtained from patients and the mouse model. Chen et al. (2019) suggested that the elevated circulating C-X-C motif chemokine 5 are associated with lung function decline and might serve as a blood-based biomarker for preliminary screening and diagnosis of COPD. In a study using an animal model of hypoxia-induced pulmonary hypertension (PH), Jiang et al. (2018) showed that circulating levels of miR-190a-5p correlated with the severity of PH secondary to COPD and suggested that miR-190a-5p may serve as a biomarker for diagnosis and prognosis of COPD-PH. Turino et al. (2018) noted that hyaluronan aerosol reduced the severity of elastase-induced emphysema in animal models and indicating promising results in preliminary human studies by decreasing levels of desmosine and isodesmosine (DI), which might serve as biomarkers of elastin degradation.
Previously Baker, Donnelly, and Barnes (2020) reported that senolytic therapies induce apoptosis and clearance of senescent cells, as well as diminish senescence-associated secretory phenotype response in animal models of aging and other age-related diseases. Data suggested that the combination of senolytics and senostatics, which are drugs that inhibit cellular senescence, may hold promise as a novel approach for COPD treatment, and need to be evaluated in animal models of COPD. Further, Ng-Blichfeldt et al.( 2019) found that numerous preclinical studies showed induction of regeneration in animal models of COPD/emphysema, proposing an important role for COPD animal studies to be considered in clinical trials. Overall, these studies highlight the invaluable role of animal models in developing potential therapeutic strategies for COPD treatment in clinical studies.
Conclusions
The current global burden of COPD is substantial, and prevalence of this devastating disease is rising. Multiple rational models for human COPD were developed (Table 1), and these are essential to the study of TS-induced lung dysfunction, diseases, and pathologies related to COPD (Table 2). Significant progress was made in our understanding of the biological mechanisms underlying COPD, specifically the role of macrophage phenotypes, lymphocyte subpopulations, and protease/antiprotease defense mechanisms. However, key factors contributing to the progression of inflammation and exacerbation of COPD remain unclear.
While there are considerable limitations to the use of rodent models and questions as to whether these accurately reflect human COPD-like features, SHRs seem to best represent the biological mechanisms and features of human COPD including progressive airway inflammation, mucus hypersecretion, and emphysema compared to other lab animal models (Nadadur, Pinkerton, and Kodavanti 2002; Zhong et al. 2005; Kodavanti et al. 2006; Yu et al. 2008; Zhong, Zhou, and Pinkerton 2008; Bolton et al. 2009; Davis et al. 2013; Chen et al. 2015; Shen et al. 2016; Hoang et al. 2016; Pham et al. 2020). SHRs also exhibit genetic risk factors towards hypertension that are found in COPD patients suggesting that SHR may be a more clinically relevant model of TS-induced disease. The application of new animal models might also be necessary to test novel therapeutic interventions and elucidate the risk factors that lead to disease onset, progression, and disease exacerbations.
Supplementary Material
Acknowledgments
We thank the editorial assistance of Dr. Rona M. Silva.
Disclosure statement
The research described in this article has been reviewed by the National Health and Environmental Effects Research Laboratory, U.S. Environmental Protection Agency and approved for publication. Approval does not signify that the contents necessarily reflect the view and the policies of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Funding
The preparation of this article has been facilitated through the following: National Institute for Occupational Safety and Health (NIOSH) grant U54 OH07550, National Institute of Environmental Health Sciences (NIEHS) grants U01 ES027288, P30 ES023513, and P51 OD011107.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- Agusti A, Calverley PM, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, Tal-Singer R, Wouters E, Yates JC, Vestbo J, and COPD longitudinally to identify predictive surrogate endpoints investigators evaluation of 2010. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res 11:122.20831787 [Google Scholar]
- Al Faraj A, Sultana Shaik A, Pureza MA, Alnafea M, and Halwani R. 2014. Preferential macrophage recruitment and polarization in LPS-induced animal model for COPD: Noninvasive tracking using MRI. PLoS One 9: e90829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S, Li Z, Atkinson C, Jonigk D, Janciauskiene S, and Mahadeva R. 2014. Z alpha1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease. Am J Respir Crit Care Med 189: 909–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfahad AJ, Alzaydi MM, Aldossary AM, Alshehri AA, Almughem FA, Zaidan NM, and Tawfik EA 2021. Current views in chronic obstructive pulmonary disease pathogenesis and management. Saudi Pharm J 29: 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen ZJ, Hvidberg M, Jensen SS, Ketzel M, Loft S, Sorensen M, Tjonneland A, Overvad K, and Raaschou-Nielsen O. 2011. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: A cohort study. Am J Respir Crit Care Med 183: 455–461. [DOI] [PubMed] [Google Scholar]
- Auerbach O, Hammond EC, Kirman D, and Garfinkel L. 1967. Emphysema produced in dogs by cigarette smoking.J Am Med Assoc 199: 241–246. [PubMed] [Google Scholar]
- Bailey KL 2012. The importance of the assessment of pulmonary function in COPD Med Clin North Am 96: 745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JR, Donnelly LE, and Barnes PJ 2020. Senotherapy: A new horizon for COPD therapy.Chest 158: 562–570. [DOI] [PubMed] [Google Scholar]
- Barberà JA, and Blanco I. 2009. Pulmonary hypertension in patients with chronic obstructive pulmonary disease. Drugs 69: 1153–1171. [DOI] [PubMed] [Google Scholar]
- Barnes PJ 2016a. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease.J Allergy Clin Immunol 138: 16–27. [DOI] [PubMed] [Google Scholar]
- Barnes PJ 2016b. Sex differences in chronic obstructive pulmonary disease mechanisms.Am J Respir Crit Care Med 193: 813–814. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Burney PG, Silverman EK, Celli BR, Vestbo J, Wedzicha JA, and Wouters EF 2015. Chronic obstructive pulmonary disease. Nat Rev Dis Primers 1:15076. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, and Cosio MG 2004. Characterization of T lymphocytes in chronic obstructive pulmonary disease. PLOS Med 1: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartalesi B, Cavarra E, Fineschi S, Lucattelli M, Lunghi B, Martorana PA, and Lungarella G. 2005. Different lung responses to cigarette smoke in two strains of mice sensitive to oxidants. Eur Respir J 25: 15–22. [DOI] [PubMed] [Google Scholar]
- Benedikter BJ, Wouters EFM, Savelkoul PHM, Rohde GGU, and Stassen FRM 2018. Extracellular vesicles released in response to respiratory exposures: Implications for chronic disease. J Toxicol Environ Health B 21: 142–160. [DOI] [PubMed] [Google Scholar]
- Bhalla DK, Hirata F, Rishi AK, and Gairola CG 2009. Cigarette smoke, inflammation, and lung injury: A mechanistic perspective. J Toxicol Environ Health B 12: 45–64. [DOI] [PubMed] [Google Scholar]
- Bolton SJ, Pinnion K, Oreffo V, Foster M, and Pinkerton KE 2009. Characterisation of the proximal airway squamous metaplasia induced by chronic tobacco smoke exposure in spontaneously hypertensive rats. Respir Res 10:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutou AK, Zoumot Z, Nair A, Davey C, Hansell DM, Jamurtas A, Polkey MI, and Hopkinson NS 2015. The impact of homogeneous versus heterogeneous emphysema on dynamic hyperinflation in patients with severe COPD assessed for lung volume reduction. COPD 12: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracke KR, D’hulst AI, Maes T, Moerloose KB, Demedts IK, Lebecque S, Joos GF, and Brusselle GG 2006. Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice. J Immunol 177: 4350–4359. [DOI] [PubMed] [Google Scholar]
- Bracke KR, and Mestdagh P. 2017. MicroRNAs as future therapeutic targets in COPD? Eur Respir J 49: 1700431. [DOI] [PubMed] [Google Scholar]
- Buttner D, Hackbarth H, Wollnik F, and Borggreve H. 1984. Blood pressure in rats: A comparison of a multifactorial experimental design to measurements in an outbred stock. Lab Animal 18 :110–114. [DOI] [PubMed] [Google Scholar]
- Campo G, Pavasini R, Biscaglia S, Contoli M, and Ceconi C. 2015. Overview of the pharmacological challenges facing physicians in the management of patients with concomitant cardiovascular disease and chronic obstructive pulmonary disease. Eur Heart J Cardiovasc Pharmacother 1: 205–211. [DOI] [PubMed] [Google Scholar]
- Cantin AM 2016. Cystic fibrosis transmembrane conductance regulator. Implications in cystic fibrosis and chronic obstructive pulmonary disease. Ann Am Thorac Soc 13 (Suppl_2): S150–S155. [DOI] [PubMed] [Google Scholar]
- Caramori G, Casolari P, Barczyk A, Durham AL, Di Stefano A, and Adcock I. 2016. COPD immunopathology. Semin Immunopathol 38: 497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldi PJ, Cho MH, San Jose Estepar R, McDonald ML, Laird N, Beaty TH, Washko G, Crapo JD, Silverman EK, and OPDGene Investigators C. 2014. Genome-wide association identifies regulatory Loci associated with distinct local histogram emphysema patterns. Am J Respir Crit Care Med 190: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavailles A, Brinchault-Rabin G, Dixmier A, Goupil F, Gut-Gobert C, Marchand-Adam S, Meurice JC, Morel H, Person-Tacnet C, Leroyer C, and Diot P. 2013. Comorbidities of COPD. Eur Respir Rev 22 : 454–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavarra E, Bartalesi B, Lucattelli M, Fineschi S, Lunghi B, Gambelli F, Ortiz LA, Martorana PA, and Lungarella G. 2001. Effects of cigarette smoke in mice with different levels of α1-proteinase inhibitor and sensitivity to oxidants. Am J Respir Crit Care Med 164: 886–890. [DOI] [PubMed] [Google Scholar]
- CDC. 2020a. Disease or condition of the week. COPD. Centers for Disease Control and Prevention, Nov 5, 2019 2020a [cited Nov 10 2020]. Available from https://www.cdc.gov/dotw/copd/index.html. [Google Scholar]
- CDC. 2020b. Chronic obstructive pulmonary disease (COPD). COPD costs. National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health, Feb 21, 2018 2020b [cited Nov 10 2020]. Available from https://www.cdc.gov/copd/infographics/copd-costs.html. [Google Scholar]
- Chae C, Walters MJ, and Holman MR 2017. International organization of standardization (ISO) and cambridge filter test (CFT) smoking regimen data comparisons in tobacco product marketing applications. Tob Regul Sci 3: 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang LH, and Rivera MP 2013. Respiratory diseases: Meeting the challenges of screening, prevention, and treatment. N C Med J 74: 385–392. [PubMed] [Google Scholar]
- Chaouat A, Naeije R, and Weitzenblum E. 2008. Pulmonary hypertension in COPD. Eur Respir J 32: 1371–1385. [DOI] [PubMed] [Google Scholar]
- Chapman RW 2008. Canine models of asthma and COPD. Pulm Pharmacol Ther 21:731–742. [DOI] [PubMed] [Google Scholar]
- Chen J, Dai L, Wang T, He J, Wang Y, and Wen F. 2019. The elevated CXCL5 levels in circulation are associated with lung function decline in COPD patients and cigarette smoking-induced mouse model of COPD. Ann Med 51: 314–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu C, Pan M, Li T, Li W, Cai S, and Meng Y. 2015. Comparison of matrix metalloproteinase-9 between Wistar rat and spontaneously hypertensive rat in pulmonary injury model. Zhonghua Yi Xue Za Zhi 95 : 1415–1420. [PubMed] [Google Scholar]
- Churg A, Cosio M, and Wright JL 2008. Mechanisms of cigarette smoke-induced COPD: Insights from animal models. Am J Physiol Lung Cell Mol Physiol 294: L612–L631. [DOI] [PubMed] [Google Scholar]
- Churg A, Wang RD, Tai H, Wang X, Xie C, and Wright JL 2004. Tumor necrosis factor-alpha drives 70% of cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 170: 492–498. [DOI] [PubMed] [Google Scholar]
- Churg A, Zhou S, Wang X, Wang R, and Wright JL 2009. The role of interleukin-1β in murine cigarette smoke-induced emphysema and small airway remodeling. Am J Respir Cell Mol Biol 40: 482–490. [DOI] [PubMed] [Google Scholar]
- Churg A, Zhou S, and Wright JL 2012. Series “matrix metalloproteinases in lung health and disease”: Matrix metalloproteinases in COPD. Eur Respir J 39: 197–209. [DOI] [PubMed] [Google Scholar]
- Cochard M, Ledoux F, and Landkocz Y. 2020. Atmospheric fine particulate matter and epithelial mesenchymal transition in pulmonary cells: State of the art and critical review of the in vitro studies. J Toxicol Environ Health B 23: 293–318. [DOI] [PubMed] [Google Scholar]
- Coultas DB, Mapel D, Gagnon R, and Lydick E. 2001. The health impact of undiagnosed airflow obstruction in a national sample of United States adults. Am J Respir Crit Care Med 164: 372–377. [DOI] [PubMed] [Google Scholar]
- Cowan KH, Batist G, Tulpule A, Sinha BK, and Myers CE 1986. Similar biochemical changes associated with multidrug resistance in human breast cancer cells and carcinogen-induced resistance to xenobiotics in rats. Proc Natl Acad Sci U S A 83: 9328–9332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hulst A I, Maes T, Bracke KR, Demedts IK, Tournoy KG, Joos GF, and Brusselle GG 2005. Cigarette smoke-induced pulmonary emphysema in scid-mice. Is the acquired immune system required? Respir Res 6 :147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BB, Shen YH, Tancredi DJ, Flores V, Davis RP, and Pinkerton KE 2012. Leukocytes are recruited through the bronchial circulation to the lung in a spontaneously hypertensive rat model of COPD. PLoS One 7: e33304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis BB, Zeki AA, Bratt JM, Wang L, Filosto S, Walby WF, Kenyon NJ, Goldkorn T, Schelegle ES, and Pinkerton KE 2013. Simvastatin inhibits smoke-induced airway epithelial injury: Implications for COPD therapy. Eur Respir J 42: 350–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres JP, Campo A, Casanova C, Aguirre-Jaime A, and Zulueta J. 2006. Gender and chronic obstructive pulmonary disease in high-risk smokers. Respiration 73: 306–310. [DOI] [PubMed] [Google Scholar]
- Desanctis GT, Kelly SM, Saetta MP, Shiner RJ, Stril JL, Hakim TS, Cosio MG, and King M. 1987. Hyporesponsiveness to aerosolized but not to infused methacholine in cigarette-smoking dogs. Am Rev Respir Dis 135: 338–344. [DOI] [PubMed] [Google Scholar]
- Doggrell SA, and Brown L. 1998. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovasc Res 39: 89–105. [DOI] [PubMed] [Google Scholar]
- Dominguez-Fandos D, Valdes C, Ferrer E, Puig-Pey R, Blanco I, Tura-Ceide O, Paul T, Peinado VI, and Barbera JA 2015. Sildenafil in a cigarette smoke-induced model of COPD in the guinea-pig. Eur Respir J 46: 346–354. [DOI] [PubMed] [Google Scholar]
- Duffy SP, and Criner GJ 2019. Chronic obstructive pulmonary disease: Evaluation and management. Med Clin North Am 103: 453–461. [DOI] [PubMed] [Google Scholar]
- Easter M, Bollenbecker S, Barnes JW, and Krick S. 2020. Targeting aging pathways in chronic obstructive pulmonary disease. Int J Mol Sci 21: 6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effah F, Taiwo B, Baines D, Bailey A, and Marczylo T. 2022. Pulmonary effects of e-liquid flavors: A systematic review. J Toxicol Environ Health B 25: 343–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner MD 2007. Indoor air, passive smoking, and COPD. Am J Respir Crit Care Med 176: 426–427. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, and Balmes JR 2010. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 182: 693–718. [DOI] [PubMed] [Google Scholar]
- Eppert BL, Wortham BW, Flury JL, and Borchers MT 2013. Functional characterization of T cell populations in a mouse model of chronic obstructive pulmonary disease. J Immunol 190: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairclough L, Urbanowicz RA, Corne J, and Lamb JR 2008. Killer cells in chronic obstructive pulmonary disease. Clin Sci (Lond) 114: 533–541. [DOI] [PubMed] [Google Scholar]
- Fedosenko SV, Ogorodova LM, Il’ina EN, Senina ME, Lisitsina ES, Karnaushkina MA, Kostryukova ES, Govorun VM, Deev IA, Kulikov ES, and Kirillova NA 2015. Genetic determinants of antibiotic resistance in oropharyngeal streptococci in patients with chronic obstructive pulmonary disease and in those with asthma. Ter Arkh 87: 51–57. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Peinado VI, Diez M, Carrasco JL, Musri MM, Martinez A, Rodriguez-Roisin R, and Barbera JA 2009. Effects of cigarette smoke on endothelial function of pulmonary arteries in the guinea pig. Respir Res 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein J, Cha E, and Scharf SM 2009. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int J Chron Obstruct Pulmon Dis 4 :337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BM, Voynow JA, and Ghio AJ 2015. COPD: Balancing oxidants and antioxidants. Int J Chron Obstruct Pulmon Dis 10: 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JT, Liu X, Yan Z, Luo M, Zhang Y, Zhou W, Lee BJ, Song Y, Guo C, Wang Y, Lukacs GL, and Engelhardt JF 2012. Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator. J Biol Chem 287: 21673–21685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D’Armiento J, and Okada Y. 2005. Structural emphysema does not correlate with lung compliance: Lessons from the mouse smoking model. Exp Lung Res 31: 547–562. [DOI] [PubMed] [Google Scholar]
- Franssen FM, Alter P, Bar N, Benedikter BJ, Iurato S, Maier D, Maxheim M, Roessler FK, Spruit MA, Vogelmeier CF, Wouters EF, and Schmeck B. 2019. Personalized medicine for patients with COPD: Where are we? Int J Chron Obstruct Pulmon Dis 14: 1465–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca JM, Auerbach O, Carter HW, and Parks VR 1983. Morphologic alterations induced by short-term cigarette smoking. Am J Pathol 111: 11–20. [PMC free article] [PubMed] [Google Scholar]
- Freeman CM, Martinez FJ, Han MK, Washko GR Jr., McCubbrey AL, Chensue SW, Arenberg DA, Meldrum CA, McCloskey L, and Curtis JL 2013. Lung CD8+ T cells in COPD have increased expression of bacterial TLRs. Respir Res 14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker M, Deane A, and Hansbro PM 2014. Animal models of chronic obstructive pulmonary disease. Expert Opin Drug Discov 9: 629–645. [DOI] [PubMed] [Google Scholar]
- Gamble E, Grootendorst DC, Hattotuwa K, O’Shaughnessy T, Ram FS, Qiu Y, Zhu J, Vignola AM, Kroegel C, Morell F, Pavord ID, Rabe KF, Jeffery PK, and Barnes NC 2007. Airway mucosal inflammation in COPD is similar in smokers and ex-smokers: a pooled analysis. Eur Respir J 30: 467–471. [DOI] [PubMed] [Google Scholar]
- Gharib SA, Manicone AM, and Parks WC 2018. Matrix metalloproteinases in emphysema. Matrix Biol 73: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorani V, Boskabady MH, Khazdair MR, and Kianmeher M. 2017. Experimental animal models for COPD: A methodological review. Tob Induc Dis 15:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Coakley RD, Ghio AJ, Muhlebach MS, Esther CR Jr., Alexis NE, and Tarran R. 2019. Chronic E-cigarette use increases neutrophil elastase and matrix metalloprotease levels in the lung. Am J Respir Crit Care Med 200: 1392–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goco RV, Kress MB, and Brantigan OC 1963. Comparison of mucus glands in the tracheobronchial tree of man and animals. Ann N Y Acad Sci 106 :555–571. [DOI] [PubMed] [Google Scholar]
- GOLD. 2020. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report).Global Initiative for Chronic Obstructive Lung Disease. [Google Scholar]
- Goldklang MP, Marks SM, and D’Armiento JM 2013. Second hand smoke and COPD: Lessons from animal studies. Front Physiol 4: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovatch P, Mercer BA, Lemaitre V, Wallace A, Foronjy RF, and D’Armiento J. 2009. Role for cathepsin K in emphysema in smoke-exposed guinea pigs. Exp Lung Res 35: 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, and Turner AM 2017. The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respir Res 18: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, and Ferguson TA 2001. The role of Fas ligand in immune privilege. Nat Rev Mol Cell Biol 2: 917–924. [DOI] [PubMed] [Google Scholar]
- Groneberg DA, and Chung KF 2004. Models of chronic obstructive pulmonary disease. Respir Res 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TJ, Powers LS, Boudreau RL, Brink B, Reisetter A, Goel K, Gerke AK, Hassan IH, and Monick MM 2014. A microRNA processing defect in smokers’ macrophages is linked to SUMOylation of the endonuclease DICER. J Biol Chem 289: 12823–12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Chen ZW, Siegel A, Koshy R, Ramirez C, Raabe TD, Devries GH, and Ilyas AA 2012. Analysis of humoral immune responses to LM1 ganglioside in guinea pigs. J Neuroimmunol 246: 58–64. [DOI] [PubMed] [Google Scholar]
- Guerassimov A, Hoshino Y, Takubo Y, Turcotte A, Yamamoto M, Ghezzo H, Triantafillopoulos A, Whittaker K, Hoidal JR, and Cosio MG 2004. The development of emphysema in cigarette smoke-exposed mice is strain dependent. Am J Respir Crit Care Med 170: 974–980. [DOI] [PubMed] [Google Scholar]
- Gurumurthy CB, and Lloyd KCK 2019. Generating mouse models for biomedical research: Technological advances. Dis Model Mech 12: dmm029462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halappanavar S, Nikota J, Wu D, Williams A, Yauk CL, and Stampfli M. 2013. IL-1 receptor regulates microRNA-135b expression in a negative feedback mechanism during cigarette smoke-induced inflammation. J Immunol 190: 3679–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Postma D, Mannino DM, Giardino ND, Buist S, Curtis JL, and Martinez FJ 2007. Gender and chronic obstructive pulmonary disease: Why it matters. Am J Respir Crit Care Med 176: 1179–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada T, and Basrur PK 1998. Pulmonary macrophage mobilization in hamsters after cessation of smoking. Exp Animal 47: 43–47. [DOI] [PubMed] [Google Scholar]
- Harting JR, Gleason A, Romberger DJ, Von Essen SG, Qiu F, Alexis N, and Poole JA 2012. Chronic obstructive pulmonary disease patients have greater systemic responsiveness to ex vivo stimulation with swine dust extract and its components versus healthy volunteers. J Toxicol Environ Health A 75: 1456–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins NM, Virani S, and Ceconi C. 2013. Heart failure and chronic obstructive pulmonary disease: the challenges facing physicians and health services. Eur Heart J 34: 2795–803. [DOI] [PubMed] [Google Scholar]
- Herfs M, Hubert P, Poirrier AL, Vandevenne P, Renoux V, Habraken Y, Cataldo D, Boniver J, and Delvenne P. 2012. Proinflammatory cytokines induce bronchial hyperplasia and squamous metaplasia in smokers: Implications for chronic obstructive pulmonary disease therapy. Am J Respir Cell Mol Biol 47: 67–79. [DOI] [PubMed] [Google Scholar]
- Hernandez JA, Anderson AE Jr., Holmes WL, and Foraker AG 1966. Pulmonary parenchymal defects in dogs following prolonged cigarette smoke exposure. Am Rev Respir Dis 93: 78–83. [DOI] [PubMed] [Google Scholar]
- Hersh CP 2010. Pharmacogenetics of chronic obstructive pulmonary disease: Challenges and opportunities. Pharmacogenomics 11: 237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikichi M, Mizumura K, Maruoka S, and Gon Y. 2019. Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis 11 (Suppl 17): S2129-S2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hizawa N. 2016. Clinical approaches towards asthma and chronic obstructive pulmonary disease based on the heterogeneity of disease pathogenesis. Clin Exp Allergy 46:678–687. [DOI] [PubMed] [Google Scholar]
- Hoang LL, Nguyen YP, Aspee R, Bolton SJ, Shen YH, Wang L, Kenyon NJ, Smiley-Jewell S, and Pinkerton KE 2016. Temporal and spatial expression of transforming growth factor-beta after airway remodeling to tobacco smoke in rats. Am J Respir Cell Mol Biol 54: 872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge S, Matthews G, Mukaro V, Ahern J, Shivam A, Hodge G, Holmes M, Jersmann H, and Reynolds PN 2011. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. Am J Respir Cell Mol Biol 44: 673–681. [DOI] [PubMed] [Google Scholar]
- Hodge-Bell KC, Lee KM, Renne RA, Gideon KM, Harbo SJ, and McKinney WJ 2007. Pulmonary inflammation in mice exposed to mainstream cigarette smoke. Inhal Toxicol 19: :361–376. [DOI] [PubMed] [Google Scholar]
- Hotchkiss RS, Strasser A, McDunn JE, and Swanson PE 2009. Cell death. N Engl J Med 361: 1570–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou W, Hu S, Li C, Ma H, Wang Q, Meng G, Guo T, and Zhang J. 2019. Cigarette smoke induced lung barrier dysfunction, EMT, and tissue remodeling: A possible link between COPD and lung cancer. Biomed Res Int 2019: 2025636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde DM, Robinson NE, Gillespie JR, and Tyler WS 1977. Morphometry of the distal air spaces in lungs of aging dogs. J Appl Physiol 43: 86–91. [DOI] [PubMed] [Google Scholar]
- Hylkema MN, Sterk PJ, de Boer WI, and Postma DS 2007. Tobacco use in relation to COPD and asthma. Eur Respir J 29: 438–445. [DOI] [PubMed] [Google Scholar]
- Ito H, Yamashita Y, Tanaka T, Takaki M, Minh N. Le L-M Yoshida, and Morimoto K. 2020. Cigarette smoke induces endoplasmic reticulum stress and suppresses efferocytosis through the activation of RhoA. Sci Rep 10: 12620–12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, and Barnes PJ 2009. COPD as a disease of accelerated lung aging. Chest 135: 173–180. [DOI] [PubMed] [Google Scholar]
- Jang JH, Chand HS, Bruse S, Doyle-Eisele M, Royer C, McDonald J, Qualls C, Klingelhutz AJ, Lin Y, Mallampalli R, Tesfaigzi Y, and Nyunoya T. 2017. Connective tissue growth factor promotes pulmonary epithelial cell senescence and is associated with COPD severity. COPD 14: 228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Xia Y, Liang Y, Yang M, Zeng W, and Zeng X. 2018. miR-190a-5p participates in the regulation of hypoxia-induced pulmonary hypertension by targeting KLF15 and can serve as a biomarker of diagnosis and prognosis in chronic obstructive pulmonary disease complicated with pulmonary hypertension. Int J Chron Obstruct Pulmon Dis 13: 3777–3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Ruiz CA, Andreas S, Lewis KE, Tonnesen P, van Schayck CP, Hajek P, Tonstad S, Dautzenberg B, Fletcher M, Masefield S, Powell P, Hering T, Nardini S, Tonia T, and Gratziou C. 2015. Statement on smoking cessation in COPD and other pulmonary diseases and in smokers with comorbidities who find it difficult to quit. Eur Respir J 46: 61–79. [DOI] [PubMed] [Google Scholar]
- Jin SW, Im JS, Park JH, Kim HG, Lee GH, Kim SJ, Kwack SJ, Kim KB, Chung KH, Lee BM, Kacew S, Jeong HG, and Kim HS 2021. Effects of tobacco compound 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) on the expression of epigenetically regulated genes in lung carcinogenesis. J Toxicol Environ Health A 84: 1004–1019. [DOI] [PubMed] [Google Scholar]
- Jo YS, Rhee CK, Yoon HK, Park CK, Lim JU, An TJ, and Hur J. 2022. Evaluation of asthma-chronic obstructive pulmonary disease overlap using a mouse model of pulmonary disease. J Inflamm (Lond) 19 : 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir ER, and Morshed N. 2015. Different approaches in the treatment of obstructive pulmonary diseases. Eur J Pharmacol 764: 306–317. [DOI] [PubMed] [Google Scholar]
- Kaku Y, Imaoka H, Morimatsu Y, Komohara Y, Ohnishi K, Oda H, Takenaka S, Matsuoka M, Kawayama T, Takeya M, and Hoshino T. 2014. Overexpression of CD163, CD204 and CD206 on alveolar macrophages in the lungs of patients with severe chronic obstructive pulmonary disease. PLoS One 9: e87400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal R, Srivastava AK, Kesavachandran CN, Bihari V, and Singh A. 2022. Chronic obstructive pulmonary disease (COPD) in women due to indoor biomass burning: A meta analysis. Int J Environ Health Res 32: 1403–1417. [DOI] [PubMed] [Google Scholar]
- Kameyama N, Chubachi S, Hegab AE, Yasuda H, Kagawa S, Tsutsumi A, Fukunaga K, Shimoda M, Kanai Y, Soejima K, and Betsuyaku T. 2018. Intermittent exposure to cigarette smoke increases lung tumors and the severity of emphysema more than continuous exposure. Am J Respir Cell Mol Biol 59: 179–188. [DOI] [PubMed] [Google Scholar]
- Kang M-J, Homer RJ, Gallo A, Lee CG, Crothers KA, Cho SJ, Rochester C, Cain H, Chupp G, Yoon HJ, and Elias JA 2007. IL-18 Is induced and IL-18 receptor α plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol 178: 1948–1959. [DOI] [PubMed] [Google Scholar]
- Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, and Voelkel NF 2000. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur-Knudsen D, Bojesen SE, Tybjaerg-Hansen A, and Nordestgaard BG 2011. Nicotinic acetylcholine receptor polymorphism, smoking behavior, and tobacco-related cancer and lung and cardiovascular diseases: A cohort study. J Clin Oncol 29: 2875–2882. [DOI] [PubMed] [Google Scholar]
- Kim H-Y, Lee H-S, Kim I-H, Kim YK, Ji M, Oh S, Kim D-Y, Lee W, Kim S-H, and Paik M-J 2022. Comprehensive targeted metabolomic study in the lung, plasma, and urine of PPE/LPS-induced COPD mice model. Int J Mol Sci 23: 2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Hong G, Kim DH, Kim YM, Kim YK, Oh YM, and Jee YK 2018. The role of FGF-2 in smoke-induced emphysema and the therapeutic potential of recombinant FGF-2 in patients with COPD. Exp Mol Med 50: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M, Wight A, DeSanctis GT, el-Azab J, Phillips DM, Angus GE, Cosio MG, and De Sanctis GT 1989. Mucus hypersecretion and viscoelasticity changes in cigarette-smoking dogs. Exp Lung Res 15: 375–389. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Costa DL, and Bromberg PA 1998. Rodent models of cardiopulmonary disease: Their potential applicability in studies of air pollutant susceptibility. Environ Health Perspect 106 Suppl 1: 111–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, Ortuno RV, Suffia M, Evansky P, Richards JH, Jaskot RH, Thomas R, Karoly E, Huang YC, Costa DL, Gilmour PS, and Pinkerton KE 2006. The spontaneously hypertensive rat: An experimental model of sulfur dioxide-induced airways disease. Toxicol Sci 94: 193–205. [DOI] [PubMed] [Google Scholar]
- Kodavanti UP, Schladweiler MC, Ledbetter AD, Watkinson WP, Campen MJ, Winsett DW, Richards JR, Crissman KM, Hatch GE, and Costa DL 2000. The spontaneously hypertensive rat as a model of human cardiovascular disease: Evidence of exacerbated cardiopulmonary injury and oxidative stress from inhaled emission particulate matter. Toxicol Appl Pharmacol 164: 250–263. [DOI] [PubMed] [Google Scholar]
- Kotoulas C, Panagiotou I, Tsipas P, Melachrinou M, Alexopoulos D, and Dougenis D. 2014. Experimental studies in the bronchial circulation. Which is the ideal animal model? J Thorac Dis 6: 1506–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzer A, Salys J, Nold-Petry C, Cool C, Zamora M, Bowler R, Koczulla AR, Janciauskiene S, Edwards MG, Dinarello CA, and Taraseviciene-Stewart L. 2013. Role of IL-18 in second-hand smoke-induced emphysema. Am J Respir Cell Mol Biol 48: 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhammer A, Johnsen R, Gulsvik A, Holmen TL, and Bjermer L. 2003. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J 21: 1017–1023. [DOI] [PubMed] [Google Scholar]
- Laniado-Laborin R. 2009. Smoking and chronic obstructive pulmonary disease (COPD). Parallel epidemics of the 21 century. Int J Environ Res Public Health 6: 209–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, Kim SE, Lee YS, and Lee SD 2005. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med 172: 987–993. [DOI] [PubMed] [Google Scholar]
- Leuenberger C, Schuoler C, Bye H, Mignan C, Rechsteiner T, Hillinger S, Opitz I, Marsland B, Faiz A, Hiemstra PS, Timens W, Camici GG, Kohler M, Huber LC, and Brock M. 2016. MicroRNA-223 controls the expression of histone deacetylase 2: A novel axis in COPD. J Mol Med 94: 725–734. [DOI] [PubMed] [Google Scholar]
- Liao W, Dong J, Peh HY, Tan LH, Lim KS, Li L, and Wong W-SF 2017. Oligonucleotide therapy for obstructive and restrictive respiratory diseases. Molecules 22: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, and Lundback B. 2005a. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor’s diagnosis, symptoms, age, gender, and smoking habits. Respiration 72 : 471–479. [DOI] [PubMed] [Google Scholar]
- Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, and Lundback B. 2005b. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest 127: 1544–1552. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liang WB, Gao LB, Pan XM, Chen TY, Wang YY, Xue H, Zhang LS, and Zhang L. 2010. CTLA4 and CD86 gene polymorphisms and susceptibility to chronic obstructive pulmonary disease. Human Immunol 71: 1141–1146. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Li C, Ma C, and Ge W. 2020. Proteome profiling of lung tissues in chronic obstructive pulmonary disease (COPD): Platelet and macrophage dysfunction contribute to the pathogenesis of COPD. Int J Chron Obstruct Pulmon Dis 15: 973–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofdahl JM, Cederlund K, Nathell L, Eklund A, and Skold CM 2005. Bronchoalveolar lavage in COPD: Fluid recovery correlates with the degree of emphysema. Eur Respir J 25: 275–281. [DOI] [PubMed] [Google Scholar]
- Lugade AA, Bogner PN, Thatcher TH, Sime PJ, Phipps RP, and Thanavala Y. 2014. Cigarette smoke exposure exacerbates lung inflammation and compromises immunity to bacterial infection. J Immunol 192: 5226–5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabley J, Gordon S, and Pacher P. 2011. Nicotine exerts an anti-inflammatory effect in a murine model of acute lung injury. Inflammation 34: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H, Inoue S, Igarashi A, Saitoh S, Yamauchi K, Nishiwaki M, Nemoto T, Otaki Y, Sato M, Sato K, Nakano H, Yang S, Furuyama K, Murano H, Ishibashi Y, Ota T, Nakayama T, Shibata Y, and Watanabe M. 2022. Role of CC chemokine ligand 17 in mouse models of chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol 66 : 428–438. [DOI] [PubMed] [Google Scholar]
- Maeno T, Houghton AM, Quintero PA, Grumelli S, Owen CA, and Shapiro SD 2007. CD8+ T cells rre required for inflammation and destruction in cigarette smoke-induced emphysema in mice. J Immunol 178: 8090. [DOI] [PubMed] [Google Scholar]
- Maiellaro K, and Taylor WR 2007. The role of the adventitia in vascular inflammation. Cardiovasc Res 75: 640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall MA 2016. Unplugging mucus in cystic fibrosis and chronic obstructive pulmonary disease. Ann Am Thorac Soc 13 (Suppl 2): S177–S185. [DOI] [PubMed] [Google Scholar]
- Mano Y, Tsukamoto M, Wang KY, Nabeshima T, Kosugi K, Tajima T, Yamanaka Y, Suzuki H, Kawasaki M, Nakamura E, Zhou Q, Azuma K, Nakashima T, Tamura Y, Kozaki K, Nakazato K, Li YS, Kawai K, Yatera K, and Sakai A. 2022. Oxidative stress causes muscle structural alterations via p38 MAPK signaling in COPD mouse model. J Bone Miner Metab 40: 927–939. [DOI] [PubMed] [Google Scholar]
- Maslarova A, Alam M, Reiffurth C, Lapilover E, Gorji A, and Dreier JP 2011. Chronically epileptic human and rat neocortex display a similar resistance against spreading depolarization in vitro. Stroke 42: 2917–2922. [DOI] [PubMed] [Google Scholar]
- Mauderly JL 2000. Animal models for the effect of age on susceptibility to inhaled particulate matter. Inhal Toxicol 12: 863–900. [DOI] [PubMed] [Google Scholar]
- Meshi B, Vitalis TZ, Ionescu D, Elliott WM, Liu CY, Wang X-D, Hayashi S, and Hogg JC 2002. Emphysematous lung destruction by cigarette smoke. Am J Respir Cell Mol Biol 26: 52–57. [DOI] [PubMed] [Google Scholar]
- Miller LA, Royer CM, Pinkerton KE, and Schelegle ES 2017. Nonhuman primate models of respiratory disease: Past, present, and future. ILSR J 58: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Pham A, Cho JY, Rosenthal P, and Broide DH 2010. Adiponectin-deficient mice are protected against tobacco-induced inflammation and increased emphysema. Am J Physiol Lung Cell Mol Physiol 299: L834–L842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitzner W, Lee W, Georgakopoulos D, and Wagner E. 2000. Angiogenesis in the mouse lung. Am J Pathol 157: 93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossman BT, Lounsbury KM, and Reddy SP 2006. Oxidants and signaling by mitogen-activated protein kinases in lung epithelium. Am J Respir Cell Mol Biol 34: 666–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JB, Wright JL, Wiggs BR, Pare PD, and Hogg JC 1987. Structure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: Relationship to lung function. Thorax 42: 843–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadadur SS, Pinkerton KE, and Kodavanti UP 2002. Pulmonary gene expression profiles of spontaneously hypertensive rats exposed to environmental tobacco smoke. Chest 121 (3 Suppl): 83s–84s. [PubMed] [Google Scholar]
- Nadziejko C, Fang K, Bravo A, and Gordon T. 2007. Susceptibility to pulmonary hypertension in inbred strains of mice exposed to cigarette smoke. J Appl Physiol 102: 1780–1785. [DOI] [PubMed] [Google Scholar]
- Nakanishi Y, Kobayashi D, Asano Y, Sakurai T, Kashimura M, Okuyama S, Yoneda Y, Shapiro SD, and Takayama K. 2009. Clarithromycin prevents smoke-induced emphysema in mice. Am J Respir Crit Care Med 179: 271–278. [DOI] [PubMed] [Google Scholar]
- Ng-Blichfeldt JP, Gosens R, Dean C, Griffiths M, and Hind M. 2019. Regenerative pharmacology for COPD: Breathing new life into old lungs. Thorax 74: 890–897. [DOI] [PubMed] [Google Scholar]
- O’Farrell HE, Brown R, Brown Z, Milijevic B, Ristovski ZD, Bowman RV, Fong KM, Vaughan A, and Yang IA 2021. E-cigarettes induce toxicity comparable to tobacco cigarettes in airway epithelium from patients with COPD. Toxicol In Vitro 75:105204. [DOI] [PubMed] [Google Scholar]
- Oldham MJ, Coggins CR, and McKinney WJ Jr. 2013. A comprehensive evaluation of selected components and processes used in the manufacture of cigarettes: Approach and overview. Inhal Toxicol 25 Suppl 2: 1–5. [DOI] [PubMed] [Google Scholar]
- Onor IO, Stirling DL, Williams SR, Bediako D, Borghol A, Harris MB, Darensburg TB, Clay SD, Okpechi SC, and Sarpong DF 2017. Clinical effects of cigarette smoking: Epidemiologic impact and review of pharmacotherapy options. Int J Environ Res Public Health 14: 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeek SA, Braber S, Koelink PJ, Henricks PA, Mortaz E, LoTam Loi AT, Jackson PL, Garssen J, Wagenaar GT, Timens W, Koenderman L, Blalock JE, Kraneveld AD, and Folkerts G. 2013. Cigarette smoke-induced collagen destruction; Key to chronic neutrophilic airway inflammation? PLoS One 8: e55612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Carlin DJ, McMurray DN, and Hickey AJ 2008. The guinea pig as a model of infectious diseases. Comp Med 58: 324–340. [PMC free article] [PubMed] [Google Scholar]
- Park SS, Kikkawa Y, Goldring IP, Daly MM, Zelefsky M, Shim C, Spierer M, and Morita T. 1977. An animal model of cigarette smoking in beagle dogs: Correlative evaluation of effects on pulmonary function, defense, and morphology. Am Rev Respir Dis 115: 971–979. [DOI] [PubMed] [Google Scholar]
- Pelgrim CE, Wang L, Peralta Marzal LN, Korver S, van Ark I, Leusink-Muis T, Braber S, Folkerts G, Garssen J, van Helvoort A, and Kraneveld AD 2022. Increased exploration and hyperlocomotion in a cigarette smoke and LPS-induced murine model of COPD: Linking pulmonary and systemic inflammation with the brain. Am J Physiol Lung Cell Mol Physiol 323: L251–L265. [DOI] [PubMed] [Google Scholar]
- Perez-Rial S, Giron-Martinez A, and Peces-Barba G. 2015. Animal models of chronic obstructive pulmonary disease. Arch Bronconeumol 51: 121–127. [DOI] [PubMed] [Google Scholar]
- Pham AK, Wu CW, Qiu X, Xu J, Smiley-Jewell S, Uyeminami D, Upadhyay P, Zhao D, and Pinkerton KE 2020. Differential lung inflammation and injury with tobacco smoke exposure in Wistar Kyoto and spontaneously hypertensive rats. Inhal Toxicol 32:328–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton KE, Harbaugh M, Han MK, Jourdan Le Saux C, Van Winkle LS, Martin WJ 2nd, Kosgei RJ, Carter EJ, Sitkin N, Smiley-Jewell SM, and George M. 2015. Women and lung disease. Sex differences and global health disparities. Am J Respir Crit Care Med 192: 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkerton KE, and Poindexter ME 2018. Harmful interruptions: Impact of smoking patterns on tumorigenesis and emphysema. Am J Respir Cell Mol Biol 59: 133–134. [DOI] [PubMed] [Google Scholar]
- Plopper CG, and Hyde DM 2008. The non-human primate as a model for studying COPD and asthma. Pulm Pharmacol Ther 21: 755–766. [DOI] [PubMed] [Google Scholar]
- Polverino F, Celli BR, and Owen CA 2018. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm Circ 8: 2045894018758528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polverino F, Doyle-Eisele M, McDonald J, Wilder JA, Royer C, Laucho-Contreras M, Kelly EM, Divo M, Pinto-Plata V, Mauderly J, Celli BR, Tesfaigzi Y, and Owen CA 2015. A novel nonhuman primate model of cigarette smoke-induced airway disease. Am J Pathol 185: 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma DS, and Rabe KF 2015. The asthma-COPD overlap syndrome. N Engl J Med 373: 1241–1249. [DOI] [PubMed] [Google Scholar]
- Qiu SL, Sun QX, Zhou JP, Tang HJ, Chen YQ, Chen FS, Feng T, He ZQ, Qin HJ, and Duan MC 2022. IL-27 mediates anti-inflammatory effect in cigarette smoke induced emphysema by negatively regulating IFN-gamma producing cytotoxic CD8(+) T cells in mice. Eur J Immunol 52: 222–236. [DOI] [PubMed] [Google Scholar]
- Rab A, Rowe SM, Raju SV, Bebok Z, Matalon S, and Collawn JF 2013. Cigarette smoke and CFTR: Implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol 305: L530–L541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju SV, Tate JH, Peacock SK, Fang P, Oster RA, Dransfield MT, and Rowe SM 2014. Impact of heterozygote CFTR mutations in COPD patients with chronic bronchitis. Respir Res 15: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju SV, Lin VY, Liu L, McNicholas CM, Karki S, Sloane PA, Tang L, Jackson PL, Wang W, Wilson L, Macon KJ, Mazur M, Kappes JC, DeLucas LJ, Barnes S, Kirk K, Tearney GJ, and Rowe SM 2016a. The cystic fibrosis transmembrane conductance regulator potentiator ivacaftor augments mucociliary clearance abrogating cystic fibrosis transmembrane conductance regulator inhibition by cigarette smoke. Am J Respir Cell Mol Biol 56: 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju SV, Kim H, Byzek SA, Tang LP, Trombley JE, Jackson P, Rasmussen L, Wells JM, Libby EF, Dohm E, Winter L, Samuel SL, Zinn KR, Blalock JE, Schoeb TR, Dransfield MT, and Rowe SM 2016b. A ferret model of COPD-related chronic bronchitis. JCI Insight 1 : e87536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Venegas A, Torres-Duque CA, Guzman-Bouilloud NE, Gonzalez-Garcia M, and Sansores RH 2019. Small airway disease in COPD associated to biomass exposure. Rev Invest Clin 71: 70–78. [DOI] [PubMed] [Google Scholar]
- Rangasamy T, Cho CY, Thimmulappa RK, Zhen L, Srisuma SS, Kensler TW, Yamamoto M, Petrache I, Tuder RM, and Biswal S. 2004. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J Clin Invest 114: 1248–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece WO, and Ball RA 1972. Inhaled cigarette smoke and treadmill-exercised dogs. Arch Environ Health 24: 262–270. [PubMed] [Google Scholar]
- Roehrs JD, Rogers WR, and Johanson WG Jr. 1981. Bronchial reactivity to inhaled methacholine in cigarette-smoking baboons. J Appl Physiol Respir Environ Exerc Physiol 50: 754–760. [DOI] [PubMed] [Google Scholar]
- Saha S, and Brightling CE 2006. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis 1: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S, and Barnes PJ 2010. Is exposure to biomass smoke the biggest risk factor for COPD globally? Chest 138: 3–6. [DOI] [PubMed] [Google Scholar]
- Sana A, Somda SMA, Meda N, and Bouland C. 2018. Chronic obstructive pulmonary disease associated with biomass fuel use in women: A systematic review and meta-analysis. BMJ Open Respir Res 5: e000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikowski T, Mills IC, Anderson HR, Cohen A, Hansell A, Kauffmann F, Krämer U, Marcon A, Perez L, Sunyer J, Probst-Hensch N, and Künzli N. 2014. Ambient air pollution: A cause of COPD? Eur Respir J 43: 250–263. [DOI] [PubMed] [Google Scholar]
- Schuliga M. 2015. NF-kappaB dsignaling in chronic inflammatory airway disease. Biomolecules 5: 1266–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selman M, Montano M, Ramos C, Vanda B, Becerril C, Delgado J, Sansores R, Barrios R, and Pardo A. 1996. Tobacco smoke-induced lung emphysema in guinea pigs is associated with increased interstitial collagenase. Am J Physiol 271: L734–L743. [DOI] [PubMed] [Google Scholar]
- Shan M, Yuan X, Song L-Z, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang S-H, Dong C, Corry DB, and Kheradmand F. 2012. Cigarette smoke induction of osteopontin (SPP1) mediates T(H)17 inflammation in human and experimental emphysema. Sci Transl Med 4: 117ra9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, and Belaaouaj A. 2003. Neutrophil elastase contributes to cigarette smoke-induced emphysema in mice. Am J Pathol 163: 2329–2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaykhiev R. 2019. Emerging biology of persistent mucous cell hyperplasia in COPD. Thorax 74: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YH, Pham AK, Davis B, Smiley-Jewell S, Wang L, Kodavanti UP, Takeuchi M, Tancredi DJ, and Pinkerton KE 2016. Sex and strain-based inflammatory response to repeated tobacco smoke exposure in spontaneously hypertensive and Wistar Kyoto rats. Inhal Toxicol 28: 677–685. [DOI] [PubMed] [Google Scholar]
- Shore S, Kobzik L, Long NC, Skornik W, Van Staden CJ, Boulet L, Rodger IW, and Pon DJ 1995. Increased airway responsiveness to inhaled methacholine in a rat model of chronic bronchitis. Am J Respir Crit Care Med 151: 1931–1938. [DOI] [PubMed] [Google Scholar]
- Shu J, Li D, Ouyang H, Huang J, Long Z, Liang Z, Chen Y, Chen Y, Zheng Q, Kuang M, Tang H, Wang J, and Lu W. 2017. Comparison and evaluation of two different methods to establish the cigarette smoke exposure mouse model of COPD. Sci Rep 7: 15454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman EK 2020. Genetics of COPD. Annu Rev Physiol 82: 413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin DD, and Man SF 2005. Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Am Thorac Soc 2: 8–11. [DOI] [PubMed] [Google Scholar]
- Smith KR, Pinkerton KE, Watanabe T, Pedersen TL, Ma SJ, and Hammock BD 2005. Attenuation of tobacco smoke-induced lung inflammation by treatment with a soluble epoxide hydrolase inhibitor. Proc Natl Acad Sci U S A 102: 2186–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GM, Raju SV, Dransfield MT, and Rowe SM 2016. Therapeutic approaches to acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis. Ann Am Thorac Soc 13(Suppl 2): S169–S176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Chen P, and Liu XM 2021. The role of cigarette smoke-induced pulmonary vascular endothelial cell apoptosis in COPD. Respir Res 22 : 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorroche PB, Fernandez Acquier M, Lopez Jove O, Giugno E, Pace S, Livellara B, Legal S, Oyhamburu J, and Saez MS 2015. Alpha-1 antitrypsin deficiency in COPD patients: A cross-sectional study. Arch Bronconeumol 51: 539–543. [DOI] [PubMed] [Google Scholar]
- Snoderly HT, Alkhadrawi H, Panchal DM, Weaver KL, Vito JN, Freshwater KA, Santiago SP, Olfert IM, Nurkiewicz TR and Bennewitz MF 2023. Short-term exposure of female BALB/cJ miceto e-cigarette aerosol promotes neutrophil recruitment and enhances neutrophil-platelet aggregartion in pulmonary microvasculature. J Toxicol Environ Health A 86: 246–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile LP, and Siegfried JM 2003. Sex and gender differences in lung cancer. J Gend Specif Med 6: 37–48. [PubMed] [Google Scholar]
- Suki B, Sato S, Parameswaran H, Szabari MV, Takahashi A, and Bartolak-Suki E. 2013. Emphysema and mechanical stress-induced lung remodeling. Physiology (Bethesda) 28: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Betsuyaku T, Ito Y, Nagai K, Odajima N, Moriyama C, Nasuhara Y, and Nishimura M. 2009. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am J Physiol Lung Cell Mol Physiol 296: L614–L623. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, and Nakajima M. 2013. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol 272: 154–160. [DOI] [PubMed] [Google Scholar]
- Tamimi A, Serdarevic D, and Hanania NA 2012. The effects of cigarette smoke on airway inflammation in asthma and COPD: Therapeutic implications. Respir Med 106: 319–328. [DOI] [PubMed] [Google Scholar]
- Tanner L, and Single AB 2020. Animal models reflecting chronic obstructive pulmonary disease and related respiratory disorders: Translating pre-clinical data into clinical relevance. J Innate Immun 12: 203–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin DP 2015. Smoking cessation in chronic obstructive pulmonary disease. Semin Respir Crit Care Med 36: 491–507. [DOI] [PubMed] [Google Scholar]
- Thomas RD, and Vigerstad TJ 1989. Use of laboratory animal models in investigating emphysema and cigarette smoking in humans. Regul Toxicol Pharmacol 10: 264–271. [DOI] [PubMed] [Google Scholar]
- Turino GM, Ma S, Lin YY, and Cantor JO 2018. The therapeutic potential of hyaluronan in COPD. Chest 153: 792–798. [DOI] [PubMed] [Google Scholar]
- Vandamme TF 2014. Use of rodents as models of human diseases. J Pharm Bioallied Sci 6: 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely DL, and Levey GS 1978. Nicotine decreases guanylate cyclase activity. Bull Environ Contam Toxicol 20: 31–34. [DOI] [PubMed] [Google Scholar]
- Viegi G, Scognamiglio A, Baldacci S, Pistelli F, and Carrozzi L. 2001. Epidemiology of chronic obstructive pulmonary disease (COPD). Respiration 68: 4–19. [DOI] [PubMed] [Google Scholar]
- Vlaykova T, and Dimov D. 2014. Polymorphisms of matrix metalloproteinases (MMP) in COPD. Biotechnol Biotechnol Equip 26 (Supp1): 111–119. [Google Scholar]
- Vucic EA, Chari R, Thu KL, Wilson IM, Cotton AM, Kennett JY, Zhang M, Lonergan KM, Steiling K, Brown CJ, McWilliams A, Ohtani K, Lenburg ME, Sin DD, Spira A, Macaulay CE, Lam S, and Lam WL 2014. DNA methylation is globally disrupted and associated with expression changes in chronic obstructive pulmonary disease small airways. Am J Respir Cell Mol Biol 50: 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis TW, Rogers WR, and Johanson WG Jr. 1982. Effects of acute and chronic exposure to nicotine aerosol on bronchial reactivity to inhaled methacholine. J Appl Physiol Respir Environ Exerc Physiol 52: 1071–1076. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu SJ, Ma J, Qu GB, Wang XY, Yu SJ, He JY, Liu JF, Xia T, and Jiang GB 2013. Silver nanoparticles induced RNA polymerase-silver binding and RNA transcription inhibition in erythroid progenitor cells. ACS Nano 7: 4171–4186. [DOI] [PubMed] [Google Scholar]
- Wanner A, Hirsch JA, Greeneltch DE, Swenson EW, and Fore T. 1973. Tracheal mucous velocity in beagles after chronic exposure to cigarette smoke. Arch Environ Health 27: 370–371. [DOI] [PubMed] [Google Scholar]
- Whittemore AS, Perlin SA, and DiCiccio Y. 1995. Chronic obstructive pulmonary disease in lifelong nonsmokers: Results from NHANES. Am J Public Health 85: 702–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. 2014. Global status report on noncommunicable diseases. World Health Organization. [Google Scholar]
- Wright JL, and Churg A. 1990. Cigarette smoke causes physiologic and morphologic changes of emphysema in the guinea pig. Am Rev Respir Dis 142: 1422–1428. [DOI] [PubMed] [Google Scholar]
- Wright JL, and Churg A. 2002. Animal models of cigarette smoke-induced COPD. Chest 122 (6 Suppl): 301s–306s. [DOI] [PubMed] [Google Scholar]
- Wright JL, and Churg A. 2008a. Animal models of COPD: Barriers, successes, and challenges. Pulm Pharmacol Ther 21: 696–698. [DOI] [PubMed] [Google Scholar]
- Wright JL, and Churg A. 2008b. Short-term exposure to cigarette smoke induces endothelial dysfunction in small intrapulmonary arteries: Analysis using guinea pig precision cut lung slices. J Appl Physiol 104: 1462–1469. [DOI] [PubMed] [Google Scholar]
- Wright JL, Cosio M, and Churg A. 2008. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol 295: L1–L15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JL, Farmer SG, and Churg A. 2002. Synthetic serine elastase inhibitor reduces cigarette smoke-induced emphysema in guinea pigs. Am J Respir Crit Care Med 166: 954–960. [DOI] [PubMed] [Google Scholar]
- Wright JL, Ngai T, and Churg A. 1992. Effect of long-term exposure to cigarette smoke on the small airways of the guinea pig. Exp Lung Res 18: 105–114. [DOI] [PubMed] [Google Scholar]
- Wright JL, and Sun JP 1994. Effect of smoking cessation on pulmonary and cardiovascular function and structure: Analysis of guinea pig model. J Appl Physiol 76: 2163–2168. [DOI] [PubMed] [Google Scholar]
- Wright JL, Zhou S, Preobrazhenska O, Marshall C, Sin DD, Laher I, Golbidi S, and Churg AM 2011. Statin reverses smoke-induced pulmonary hypertension and prevents emphysema but not airway remodeling. Am J Respir Crit Care Med 183: 50–58. [DOI] [PubMed] [Google Scholar]
- Wu C-W, Yau T, Fulgar CC, Mack SM, Revilla AM, Kenyon NJ, and Pinkerton KE 2020. Long-term sequelae of smoking and cessation in spontaneously hypertensive rats. Toxicol Pathol 48: 422–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu DD, Song J, Bartel S, Krauss-Etschmann S, Rots MG, and Hylkema MN 2018. The potential for targeted rewriting of epigenetic marks in COPD as a new therapeutic approach. Pharmacol Ther 182: 1–14. [DOI] [PubMed] [Google Scholar]
- Wu J, and Sin DD 2011. Improved patient outcome with smoking cessation: When is it too late? Int J Chron Obstruct Pulmon Dis 6: 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi S, Xu H, Shan J, Tao Y, Hong JA, Inchauste S, Zhang M, Kunst TF, Mercedes L, and Schrump DS 2013. Cigarette smoke mediates epigenetic repression of miR-487b during pulmonary carcinogenesis. J Clin Invest 123: 1241–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HJ, Tsou WH, Shen MC, Liu CY, Saunders HM, Wang KY, and Douglas FL 2022. The effects of hydrogen treatment in a cigarette smoke solution-induced chronic obstructive pulmonary disease-like changes in an animal model. J Thorac Dis 14: 4246–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Kodavanti UP, Takeuchi M, Witschi H, and Pinkerton KE 2008. Acute tobacco smoke-induced airways inflammation in spontaneously hypertensive rats. Inhal Toxicol 20: 623–633. [DOI] [PubMed] [Google Scholar]
- Yu XF, Wang J, UYang N O, Guo S, Sun H, Tong J, Chen T, and Li J. 2019. The role of miR-130a-3p and SPOCK1 in tobacco exposed bronchial epithelial BEAS-2B transformed cells: Comparison to A549 and H1299 lung cancer cell lines. J Toxicol Environ Health A 82: 862–869. [DOI] [PubMed] [Google Scholar]
- Zeki AA, Schivo M, Chan A, Albertson TE, and Louie S. 2011. The asthma-COPD overlap syndrome: A common clinical problem in the elderly. J Allergy (Cairo) 2011: 861926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Li T, He X, Cai S, Luo H, Chen P, and Chen Y. 2020. Oxidative stress mediates the apoptosis and epigenetic modification of the Bcl-2 promoter via DNMT1 in a cigarette smoke-induced emphysema model. Respir Res 21: 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Valizadeh H, Alipourfard I, Bidares R, Aghebati-Maleki L, and Ahmadi M. 2020. Epigenetic modifications and therapy in chronic obstructive pulmonary disease (COPD): An update review. COPD 17: 333–342. [DOI] [PubMed] [Google Scholar]
- Zhong CY, Zhou YM, Douglas GC, Witschi H, and Pinkerton KE 2005. MAPK/AP-1 signal pathway in tobacco smoke-induced cell proliferation and squamous metaplasia in the lungs of rats. Carcinogenesis 26: 2187–2195. [DOI] [PubMed] [Google Scholar]
- Zhong CY, Zhou YM, and Pinkerton KE 2008. NF-kappaB inhibition is involved in tobacco smoke-induced apoptosis in the lungs of rats. Toxicol Appl Pharmacol 230: 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JS, Li ZY, Xu XC, Zhao Y, Wang Y, Chen HP, Zhang M, Wu YF, Lai TW, Di CH, Dong LL, Liu J, Xuan NX, Zhu C, Wu YP, Huang HQ, Yan FG, Hua W, Wang Y, Xiong WN, Qiu H, Chen T, Weng D, Li HP, Zhou X, Wang L, Liu F, Lin X, Ying SM, Li W, Imamura M, Choi ME, Stampfli MR, Choi AMK, Chen ZH, and Shen HH 2020. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice. Eur Respir J 56 : 2000404. [DOI] [PubMed] [Google Scholar]
- Zhou X, and Frohlich ED 2007. Analogy of cardiac and renal complications in essential hypertension and aged SHR or L-NAME/SHR. Med Chem 3: 61–65. [DOI] [PubMed] [Google Scholar]
- Zwicker GM, Filipy RE, Park JF, Loscutoff SM, Ragan HA, and Stevens DL 1978. Clinical and pathological effects of cigarette smoke exposure in beagle dogs. Arch Pathol Lab Med 102: 623–628. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.