Abstract
Causes of blindness differ across the globe; in higher-income countries, most blindness results from degeneration of specific classes of cells in the retina, including retinal pigment epithelium (RPE), photoreceptors, and retinal ganglion cells. Advances over the past decade in retinal regenerative medicine have allowed each of these cell types to be produced ex vivo from progenitor stem cells. Here, we review progress in applying these technologies to cell replacement — with the goal of vision restoration in degenerative disease. We discuss the landscape of human clinical trials for RPE transplantation and advanced preclinical studies for other cell types. We also review progress toward in situ repair of retinal degeneration using endogenous progenitor cells. Finally, we provide a high-level overview of progress toward prosthetic ocular vision restoration including advanced photovoltaic devices, opsin-based gene therapy, and small molecule photoswitches. Progress in each of these domains is at or near the human clinical trial stage, bringing the audacious goal of vision restoration within sight.
Introduction
Blinding eye disease remains a major global health challenge in the 21st century. An estimated 43 million people worldwide were blind in 2020, and another 295 million people had moderate to severe vision impairment1. The economic burden of vision loss in the US was estimated to be over $134B in 20212. The leading causes of irreversible blindness are neurodegenerative, including diseases caused by death of photoreceptors and associated retinal pigment epithelium (RPE) – including age related macular degeneration [AMD] and retinitis pigmentosa [RP], the most common form of inherited retinal disorder – and death of retinal ganglion cells (RGCs), the neurons of the optic nerve, in a condition known as glaucoma (Fig 1A). Although treatments that modestly slow neurodegeneration have been developed (such as vitamin therapy for AMD3), reversal of vision loss remains an elusive goal. To address this need, in 2013 the National Eye Institute (NEI) of the US National Institutes of Health organized the Audacious Goals Initiative (AGI) program which focused on developing cell-based therapies to replace lost photoreceptors and RGCs.
Figure 1:
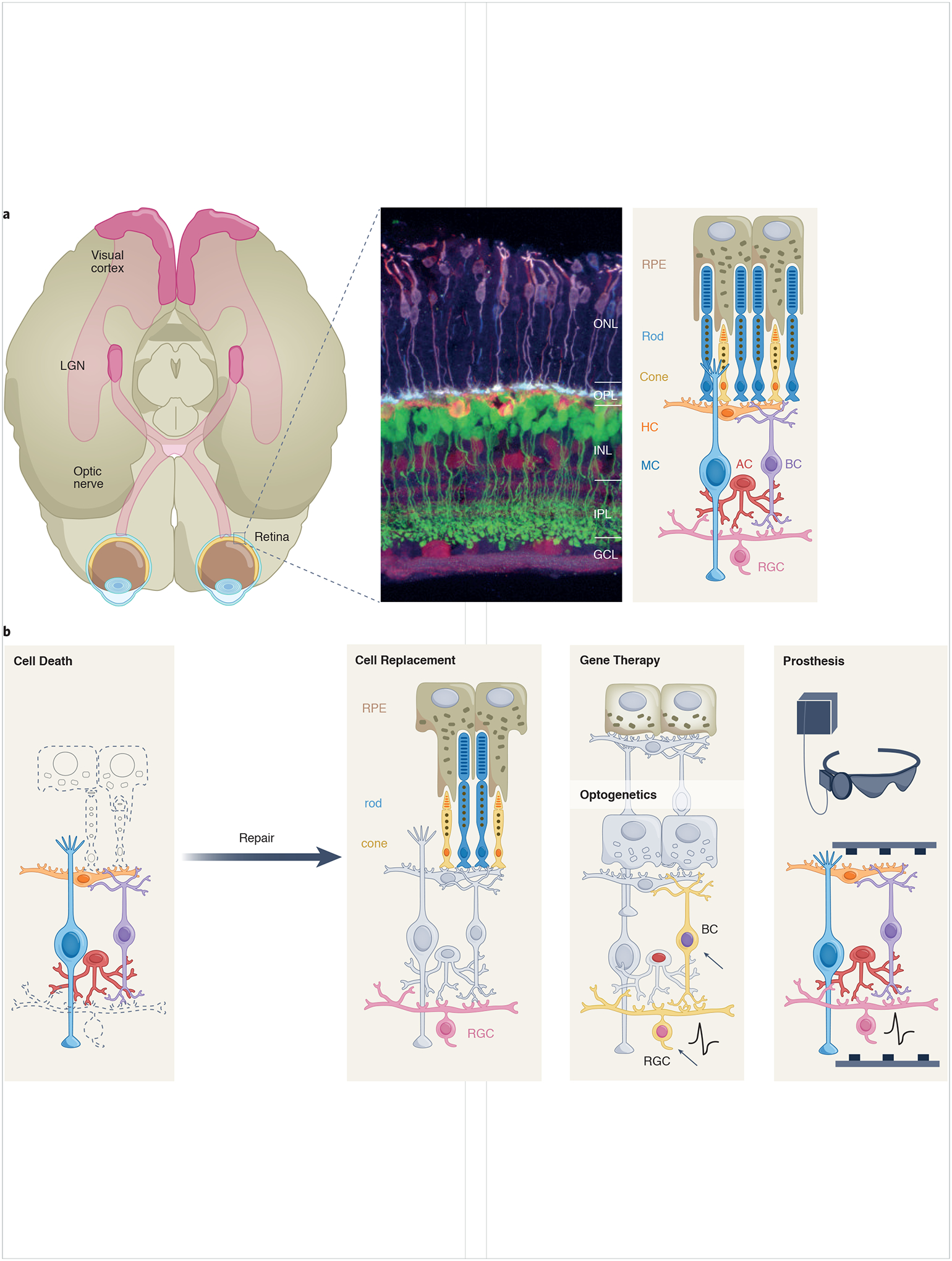
(A) Schematic of the primary visual pathway. Retinal output is conveyed along the optic nerve, comprising the axons of retinal ganglion cells, to a subcortical target, the lateral geniculate nucleus (LGN). Geniculate neurons project to the primary visual cortex. The retina is a layered structure comprising three cellular layers (outer and inner nuclear layers, ONL and INL, and the ganglion cell layer, GCL) and two synaptic layers (outer and inner plexiform layers, OPL and IPL). The middle panel shows a mouse retina with various cell types fluorescently labeled76. The schematic on the right illustrates the major cell types of the retina and the retinal pigment epithelium (RPE) including rod and cone photoreceptors; bipolar cells (BC); horizontal cells (HC); amacrine cells (AC); retinal ganglion cells (RGC); and Müller glial cells (MC). Cell types in the schematic are aligned with corresponding cells in the immunohistochemically-labeled retina.
(B) Vision loss arises largely due to the death or dysfunction of RPE cells, rod and cones and RGCs (leftmost panel). The panels to the right of this illustrate several major approaches to restore vision. Cell replacement therapy involves addition of new cells (RPE, rod, cone, RGC) to the retina, either by transdifferentiation of resident cells or introduction of transplanted tissue. Gene therapy involves restoring missing genetic function to a diseased cell. Optogenetics is mediated by introducing light-sensitive proteins (such as channelrhodopsins) to the genetic code of normally non-photoreceptive retinal neurons (arrows, yellow shading indicates cells expressing light-sensitive signaling proteins). Prosthetic approaches use artificially engineered materials (such as light-driven stimulating electrodes) to stimulate remaining signaling neurons in the retina.
The prospect of regenerating neural tissue to reverse blinding eye disease is indeed audacious; however, the retina is particularly well suited as a target tissue for such an endeavor. The retina has a highly organized and laminar anatomy, with well-understood physiology and biochemistry. It is readily accessible surgically, allowing for precise manipulation of tissue, as is required for implantation of newly differentiated neurons. Finally, the eye’s transparent optics make it possible to image the retina at the single-cell level in animals and humans, enabling monitoring of disease and treatment responses. As we approach the 10-year anniversary of this initiative, it is appropriate to review progress toward the overarching goal of restoring vision in degenerative blinding disease4. Approaches to achieving this goal are replacement of lost cells or restoration of cell function in damaged tissue, as well as others also showing promise including prosthetics and gene therapy, and optogenetics (Fig 1B). We discuss progress in each of these fields, as well as supportive imaging technologies, and outline the challenges and opportunities for translation into the clinic.
Cell replacement therapies
Retinal pigment epithelium
Human vision begins in the retina — with the absorption of a photon by the 11-cis-retinaldehyde in a molecule of rod or cone opsin. This isomerizes the retinaldehyde to the all-trans form, which in turn activates the G-protein transducin, ultimately resulting in altered synaptic signaling to bipolar interneurons. Signals from multiple bipolar interneurons are integrated via horizontal and amacrine interneurons to determine the firing of each of ~1.2 million retinal ganglion cells which project directly to the brain (Figure 1A). The all-trans-retinaldehyde must be re-isomerized to 11-cis, a function primarily carried out in the retinal pigment epithelium (RPE) adjacent to the outer segments of the rod and cone photoreceptors. Loss of RPE, as occurs in the atrophic or dry form of macular degeneration, results in photoreceptor dysfunction and ultimately degeneration, leading to blindness.
The retinal pigment epithelium is a hexagonally tiled cellular monolayer of about 4000 binucleated, pigmented cells cells/mm2. In 2004 it was discovered that RPE-like cells could be differentiated from primate pluripotent stem cells (PSCs)5, and subsequent work extended those results to human PSCs6 7. Preclinical studies using diverse animal models of retinal degeneration have shown that PSC-derived RPE can survive, function, and slow photoreceptor cell death following transplantation into an affected eye8,9;likely through a combination of anatomic, metabolic, and trophic support. Building on this, other sources of stem cells have been explored (Fig. 2), including embryonic stem cells (ESCs) and induced progenitor stem cells (iPSCs) — the latter being adult somatic cells which are reprogrammed into a pluripotent state. Translation of ESC- and iPSC-derived RPE cell transplantation to clinical use is the furthest advanced of any retinal cell transplantation technique.
Figure 2:

Cell sources for replacement therapies. A. Exogenous sources include cells harvested from 3D retinal organoids grown in vitro from progenitor stem cells, or differentiated retinal cell types (such as RPE, bottom) generated from embryonic or inducible progenitor stem cells. B. Endogenous sources for in situ repair are derived from Muller glial cells which may transdifferentiated into retinal progenitor cells and thence to mature photoreceptors, ganglion cells, and interneurons.
A number of human clinical trials are ongoing and several have reported results (Table 1). In particular, two prospective phase 1/2 studies examined outcomes of 18 patients with either AMD or a hereditary macular dystrophy who underwent RPE transplantation10. With median follow-up of 22 months, both macular and hereditary degeneration patients showed persistence of the transplanted cells and modest improvement of retinal function. Similar results were seen in a study of transplanted PSC-derived RPE in patients with Stargardt’s disease (a hereditary blinding disease featuring RPE cell death), which showed survival of grafted cells and some evidence for functional improvement in these patients11. In addition, differentiated RPE on biodegradable or synthetic scaffolds12–14 demonstrate significant integration and function in animal models of RPE injury. A Phase 1/2a clinical trial of 16 patients implanted with allogeneic RPE cells that were bioengineered on a perylene substrate demonstrated persistence of the cells following transplantation, with about 25% of patients showing some improvement in vision15. These successful early-stage clinical trials highlight the potential of vision restoration through regenerative medicine and provide a roadmap for replacing differentiated retinal cells.
Table 1:
Ongoing and completed clinical trials in vision restoration
| Type of treatment | Clinical trials identifier/references | Disease | Cell Source | Route |
|---|---|---|---|---|
| RPE replacement | NCT01469832 10,11 | Stargardt’s macular dystrophy | Human ESC-derived RPE | Subretinal transplantation |
| NCT02286089 | Non-exudative age related macular degeneration | Human ESC-derived RPE | Subretinal transplantation | |
| NCT03963154 | Retinitis pigmentosa | Human ESC-derived RPE | Subretinal transplantation | |
| NCT01674829 | Non-exudative age related macular degeneration | Human ESC-derived RPE | Subretinal transplantation | |
| NCT04339764 | Non-exudative age related macular degeneration | Human iPSC-derived RPE | Subretinal transplantation | |
| NCT02941991 | Stargardt’s macular dystrophy | Human ESC-derived RPE | Subretinal transplantation | |
| NCT01345006 74 | Stargardt’s macular dystrophy | Human ESC-derived RPE | Subretinal transplantation | |
| NCT02463344 | Non-exudative age related macular degeneration | Human ESC-derived RPE | Subretinal transplantation | |
| Retinal precursors transplantation | ||||
| NCT04284293 | Retinitis pigmentosa | Human fetal cortex-derived neural progenitor stem cells | Subretinal transplantation | |
| NCT02320812 | Retinitis pigmentosa | Human fetal retinal -derived progenitor stem cells | Intravitreal injection | |
| Optoelectronics | ||||
| NCT03333954 54 | Non-exudative age related macular degeneration | Optoelectronic prosthesis | Subretinal implant | |
| NCT04676854 | Non-exudative age related macular degeneration | Optoelectronic prosthesis | Subretinal implant | |
| NCT03406416 75 | Retinitis pigmentosa Choroideremia | Optoelectronic prosthesis | Suprachoroidal implant | |
| Optogenetics | ||||
| NCT03326336 62 | Retinitis pigmentosa | Recombinant adeno-associated viral vector (rAAV2.7m8) containing ChrimsonR-tdTomato gene under the control of the ubiquitous CAG promoter | Intravitreal injection of channelopsin gene therapy | |
| NCT02556736 | Retinitis pigmentosa | Recombinant adeno-associated viral vector containing channelrhodopsin 2 | Intravitreal injection of channelopsin gene therapy | |
| NCT04278131 | Retinitis pigmentosa | Recombinant adeno-associated viral vector containing ChronosFP | Intravitreal injection of channelopsin gene therapy | |
| NCT04945772 | Retinitis pigmentosa | Recombinant adeno-associated viral vector containing multi-characteristic opsin (vMCO-1) gene | Intravitreal injection of synthetic opsin gene therapy |
Retinal precursor transplantation
Retinal progenitor cells are PSC-derived cells that express some early pan-photoreceptor molecules such as CRX and recoverin, and which can further differentiate into retinal photoreceptors. These heterogeneous cell preparations have been tested by subretinal injection in non-human primate models where the cells clearly persist and appear to differentiate into cone photoreceptors at low density16. However, some non-human primate studies using retinal precursors have demonstrated significant anatomic disorganization on postmortem histology17,18. Human clinical trials are ongoing (see Table 1). Results are available from one Phase 1/2a trial in which 21 participants received an intravitreal injection of human retinal progenitor cells19); data suggested that the treatments were relatively safe and that vision improved in patients receiving relatively high doses of cells.
Photoreceptor replacement
While transplantation of RPE or neural progenitors may be utilized to support areas of retina with intact rod and cone photoreceptors, photoreceptor replacement will be required in the many cases where cell loss has occurred. Sheets of human fetal retinal tissue transplanted into immunodeficient rats with retinal degeneration show development of apparently mature photoreceptors and retinal interneurons, suggesting that the cell maturation can occur in the environment of the degenerated retina20. Whereas RPE can be readily differentiated from PSCs in 2-dimensional (2D) cultures, production of photoreceptors has been more challenging. Recent development and optimization of 3D cultures of retina (known as retinal ‘organoids’) from PSCs has provided researchers with a renewable source of retinal photoreceptors for transplantation21–23. Preclinical studies have demonstrated functional integration of human iPSC organoid-derived cones into rodent models of hereditary retinal degeneration, including reconstitution of retinal circuitry and functional visual outcomes24. Photoreceptor precursors have also been successfully transplanted into non-human primate models of thermally-induced retinal degeneration, where they appeared to migrate to the correct location, express opsin photopigments, and extend neurites25, all necessary prerequisites for functional restoration
While these preclinical studies are encouraging, there are several barriers that must be overcome for photoreceptor replacement to move into human clinical trials. One challenge is the integration of transplanted photoreceptors into existing neuronal circuits. Photoreceptor synapses are formed in a stepwise manner during development26. Following rod or cone specification at the apical surface of the retina, these cells differentiate and extend a single process toward the emerging outer plexiform layer. The first step in forming a functional photoreceptor synapse is invagination of a horizontal cell neurite, followed several days later by insertion of a bipolar neuron process to form a synaptic triad. It is unknown whether newly formed photoreceptor terminals can form a functional synaptic triad with horizontal and bipolar neurites at the outer plexiform layer in a retina that has had photoreceptor loss. Some evidence also suggests that aberrant wiring develops in the degenerated retina27,28 that may further complicate native visual restoration. Additionally, the human retina uses both rod and cone photoreceptors, which are specifically distributed during development, with cones predominant in fovea (the small centralregion of the retina where vision is sharpest). Novel methods for re-establishment of the native rod and cone mosaic may be necessary to optimize visual acuity. Additionally, there are functionally diverse subtypes of bipolar neurons29, and the rods and the newly formed rod and cone mosaic will need to appropriately align with existing retinal circuit architecture.
Another challenge to photoreceptor replacement is formation of photoreceptor outer segments that have functional RPE contacts necessary to support phototransduction. In most degenerative disease states, both photoreceptors and RPE will have degenerated, requiring transplantation of both cell types. Recent work shows that with some modifications to the differentiation protocols, 3D organoids can be generated that contain both RPE and neural retina and are responsive to light ex vivo30. This raises the exciting possibility of generating both cell types simultaneously for transplantation.
Retinal ganglion cell replacement
RGCs are the sole output neurons of the retina; in other words, they are the only neurons that send signals out of the retina to the brain. Neurons bearing the molecular signature and morphological hallmarks of RGCs have been produced successfully from PSCs31,32. Although RGCs do not survive long-term in PSC-derived organoid cultures, they can be harvested before undergoing cell death and enriched prior to transplantation. Relatively undifferentiated retinal precursor cells transplanted into the subretinal space of non-human primates elaborate axons which migrate primarily into the optic nerve and toward the brain18, suggesting regrowth of RGCs is feasible.
Despite these developments, the challenges for successful cell replacement therapy for retinal ganglion cells are more substantial than for RPE or photoreceptors. The mapping of photoreceptors to the areas they represent in vision is very straightforward: each photoreceptor responds directly to the image projected on it. For RGCs, the situation is more complex. First, these cells must reach specific locations within the retina and synapse with other neurons in the retina to form circuits. Unlike photoreceptors, which have few subtypes (i.e. three color cones, and one rod), there are over 30 specific subtypes of retinal ganglion cell; each with unique functions in vision conferred, at least in part, by the cell’s circuitry within the retina33. Second, after achieving integration and functional connectivity with afferent neurons in the retina, transplanted RGCs need to regenerate their axons and extend to the CNS targets necessary for visual perception, primarily the lateral geniculate nucleus. Primary RGCs purified from mice and transplanted into rats can integrate and generate axons reaching the appropriate CNS targets, and show evidence of electrophysiologic activity34, suggesting such an approach may be feasible. Similar results have been seen following transplant of PSC-derived RGCs in mouse models of retinal ganglion cell loss35; however, this has not yet been demonstrated in a primate model. Moreover, reconstruction of visual space depends on the spatially organized axonal projections of the ganglion cells to form a retinotopic map. The wiring precision of circuits throughout the visual pathway thus impose significant demands on how accurately axonal regeneration may need to be, in order to recapitulate circuitry damaged by disease or injury. This remains an active area of inquiry, but to date no human clinical trials of RGC replacement have commenced.
Activation of in vivo repair pathways
An ideal method for retinal regeneration would be to forgo replacement and instead activate latent repair pathways in the damaged tissue. In fish, the radial glia of the retina (Müller cells) can de-differentiate into retinal progenitor cells, undergo multiple rounds of cell division, and restore retinal neurons lost to injury36. In contrast, retinal injury in the mammalian retina leads to a damage response known as Müller cell reactive gliosis, without any restoration of lost neurons37. These reactive Müller glia undergo one or two rounds of cell division and then enter a quiescent state. To gain a deeper understanding of the molecular and cellular basis of Müller glial cell response to injury, comparative single cell transcriptomic and epigenomic analyses of purified Müller glia following injury identified species-specific programs in zebrafish and chick that promote proliferation and dedifferentiation of Müller glia into the retinal progenitor cell state38. Similar methods in mice identified unique Müller glia programs that prevent this process and favor quiescence associated with reactive gliosis38. When murine Müller glia are induced to dedifferentiate by blocking the reactive gliosis-quiescence program, they primarily produce amacrine and bipolar neurons rather than photoreceptors38, 39. Establishing the appropriate rod and cone mosaic may be particularly challenging because cones are produced early during development and rods at later stages,40 so fine-tuning of the progenitor stage of reprogrammed Müller glia may be required. There also are issues with generating a sufficient number of photoreceptors from Muller glia regeneration that would need to be addressed.
A better understanding of Müller glia transdifferentiation could enable regenerative approaches for human retinal and optic nerve, for example by using gene therapy to express factors that transdifferentiate Müller glia into retinal neuronal precursors. In mice with retina damage, expressing the transcription factor Ascl1 in Müller glia coincident with inhibition of histone deacetylase dedifferentiates these cells into retinal precursors in damaged retina41. The transcription factors Atoh7 and Ascl1 can also be co-expressed to reprogram Müller glia into neurogenic precursors in the absence of retinal injury in murine models42. While the extent of retinal damage that might be repaired using transdifferentiation has not yet been determined, this approach is an attractive alternative to stem-cell transplantation for retinal repair and holds promise for the future.
Advances in retinal cellular imaging
A necessary component of all forms of transplantation and cell-based therapies is the ability to visualize transplanted cells and monitor their integration, growth, and function. The optical clarity of the eye allows unparalleled imaging of the retina and anterior optic nerve. Two techniques are commonly used; these are optical coherence tomography (OCT) and adaptive optics (AO) scanning laser ophthalmoscopy. These techniques, especially when combined, allow imaging at single-cell resolution and have been used to characterize retinal organoids in vitro43, to track the fate of transplanted cells in clinical trials, and to image RGCs in nonhuman primates44 and humans45 in vivo. These techniques have also been used successfully to image transplanted photoreceptors in non-human primates25. More recently, methods that enable imaging of retinal neuronal function have been developed. Two-photon in vivo imaging permits functional analysis of retinoids in the retina and pigment epithelium, allowing demonstration of restoration of photocycle constituents in situ46. OCT and AO have been combined to yield an extremely high-resolution interferometric imaging method that can demonstrate microscopic (10 nm) cone deformations in response to light in humans47, indicating a reliable measure of photoreceptor function for potential clinical use.
Other vision restoration methods
In addition to the cell-based therapies discussed above, substantial progress has been made in technologies applied to restoring visual function to individuals with end-stage outer retinal degeneration. Three approaches – optoelectronics, photoreceptor gene therapy, and small molecule photo-switches -- have yielded significant advances over the past decade.
Optoelectronic prosthetics
Optoelectronic prosthetics are millimeter-sized electronic devices that translate visual information into stimulation of retinal cells using patterns of electric current. These devices are intended to restore function to patients who have photoreceptor function but have an intact optic nerve, as occurs in AMD and hereditary retinal degeneration. The initial devices of this category, the Argus and Argus II stimulators, were epiretinal electrode arrays of 16 and 60 electrodes, respectively, which transduced visual information provided by an external camera into local stimulation of RGCs48. The Argus II (which received humanitarian device exception approval from the US Food and Drug Administration in 2013 and is also approved in Europe) was able to generate useful percepts for some patients with end-stage inherited retinal disorder49. However, utility was limited by the relatively low electrode density, requirement for external power and wiring, and axonal stimulation of RGCs, which distorts retinotopic mapping50. Low acuity restoration was also noted in tests of the Alpha AMS subretinal implant, which has 1600 electrodes51. Newer generation devices offer significant improvements. To date, 11 devices have been tested in human clinical trials (reviewed in52). These devices have been placed both beneath (subretinal) and above (epiretinal) the retina. Newer devices are directly photovoltaic, eliminating necessity for a wired power supply53; have much finer pitch density; and may utilize infrared activation of the implant — thereby allowing simultaneous ‘natural’ and prosthetic function. These devices have shown promise in early clinical testing54, although they will likely be limited to stimulation of the immediate central visual field, as there are practical limits to the size of device that may be implanted beneath the retina.
Gene therapy
The first viral gene therapy approved for any neurologic disease was voretigene neparvovec, consisting of the RPE65 photoisomerase gene delivered to the retina via an adeno-associated virus (AAV)55. This biologic was approved by the US FDA in 2017. The virus is injected subretinally to treat the RPE65-deficient form of the congenital blinding disease Leber congenital amaurosis (LCA). In young patients with this condition, the therapy brought about restoration of useful vision in controlled clinical trials56 and established the feasibility of ocular gene therapy. However, this type of gene therapy is specific for one gene. Over 60 genes have been associated with retinitis pigmentosa, making replacement therapies challenging. There may be additional limitations to intraocular gene therapy – such as continued degeneration despite gene replacement57, or immune system activation in response to the viral vector58 – but this approach remains promising for a number of inherited retinal diseases. An alternate strategy involves the use of viral-based gene therapy to express trophic factors that provide growth and survival signals, and whose expression may slow the degenerative process. Such an approach has been demonstrated to be effective for a number of trophic factors in animal models, including ciliary neurotrophic factor (CNTF), pigment epithelial derived-factor, brain-derived neurotrophic factor, and rod-derived cone viability factor, although preservation of anatomy is typically greater than preservation of visual function (reviewed in59). One clinical device – an implantable, cell-based device secreting CNTF – was tested in clinical trials of inherited retinal disease, but did not significantly slow the degenerative process60; although results for slowing retinal degeneration due to a vascular condition, macular telangiectasia, appear promising61.
A different approach to gene therapy for vision restoration – known as ‘optogenetics’ – employs viral vectors to introduce phototransducing opsins (which convert light into electrical signals) into the bipolar or retinal ganglion cells that are preserved in outer retinal degeneration. DNA encoding microbial-derived channelopsins – seven transmembrane proteins that directly gate cation or anion channels following light exposure – are packaged in AAV under either general promoter or retinal ganglion cell-specific promoter. Following subretinal or intravitreal injection (depending on the vector) these channel opsins are expressed in inner retinal neurons and render them directly light responsive. At least three clinical trials using channel opsins delivered via gene therapy are presently underway (Table 1). Encouragingly, the first case report of partial visual function restoration in a blind patient treated with this approach was published in 202162, whereby the patient regained the ability to distinguish and count high contrast objects on a table. Channelopsins are not as sensitive to light as native human opsins, requiring optimal gene therapy vectors to achieve widespread expression. They also carry theoretical risks of immune responses to foreign proteins; nonetheless, this approach has substantial promise for vision restoration. A similar approach using human G-protein-coupled receptor rhodopsin or cone opsin has been shown to restore visual function in mice with outer retinal degeneration63. Mammalian opsins have higher sensitivity than channelopsins due to G-protein amplification and, as native proteins, would not elicit immune responses. This approach is in development for human clinical trials.
Vision restoration using azobenzene-based photoswitches has also shown efficacy for vision restoration in animal models of outer retinal degeneration. In this approach, voltage-gated potassium channel blocking drugs are rendered active in light though photoisomerization of a covalently coupled azobenzene moiety64,65. Following injection intravitreally, these compounds render retinal ganglion cells directly photosensitive66. This approach avoids the irreversibility of gene therapy but would require repeated treatments.
Challenges and opportunities
While great advances have been made in cell-replacement therapies for blinding eye disease over the past decade, real challenges remain. Cell sources for transplantation are based on allogeneic ESC or iPSC precursors; as a result, all clinical studies to date for RPE and neuronal precursor transplantation have required systemic immunosuppression similar to that used for other solid organ transplants, in order to avoid rejection of the transplanted tissue. The long-term consequences of systemic immunosuppression for patients is an important consideration for these treatments. In contrast, immunologic rejection is not an issue for patient-derived PSCs, but the practical considerations of generating bespoke ‘personal organoids’ may limit this approach. An alternative approach is the generation of human leukocyte antigen-edited iPSCs that may result in universal donor cells for transplantation67.
As noted previously, challenges also remain for photoreceptor transplants in terms of generating the correct circuitry (i.e. a functional mix of rods and cones, each transmitting through their appropriate retinal circuits), and particularly for RGCs, ensuring a retinotopic map of connectivity (a problem referred to as ‘the switchboard dilemma’68). PSC-derived retinal ganglion cells do appear to preferentially migrate toward explants of their natural brain targets in vitro (such as the lateral geniculate nucleus of the thalamus as opposed explants from olfactory bulb)69. Optimism that the the switchboard dilemma may be surmountable also comes from studies of the related problem of optic nerve axonal regeneration following crush injury. In murine genetic models of neuroprotection (such as BAX or PTEN knockouts), optic nerve axons regenerate after crush to innervate normal retinorecipient regions with partial recovery of visual function70,71. This suggests sufficient scaffold present in the injured optic nerve to facilitate axonal pathfinding.
A final challenge for all methods of vision restoration is the ‘encoding’ problem. The circuitry of the retina performs significant image processing, including encoding of orientation, motion detection, contrast, and color. At least 30 types of RGC encode this information in specific cell firing patterns, including ‘ON’, ‘OFF’, and ‘ON-OFF’ patterns. Vision restoration methods may replace native encoding with novel codes (for instance, optogenetics may convert ‘OFF’ cell firing patterns to ‘ON’). The retinal output from any of these methods will not be native. It remains to be determined the extent to which a novel retinal code can be correctly processed by the brain to yield useful images, although results with cochlear implants for hearing impairment suggest sufficient cortical plasticity exists to allow critical feature extraction from non-native encoding in users72. Recent results with subretinal prosthetic devices also suggest that form vision may be restored with subretinal implants stimulating bipolar cells.73
Conclusions
As recently as a decade ago, restoration of visual function in degenerative retinal disease seemed an audacious and perhaps unattainable goal. Progress in regenerative medicine, gene therapy, and micro-electronic prosthetic have brought us closer to this achievement and inspire confidence that it can be attained. PSC-derived cellular transplantation is in advanced clinical trials for RPE replacement and on the verge of human trials for photoreceptor replacement. While RGC transplants face greater hurdles, significant progress has been made in this domain as well. Prosthetic vision restoration through opto-electronic devices, optogenetic gene therapy, and small molecule photoswitches are all in or nearing human clinical trial work following successful animal model studies. Progress in the first decade since identifying this audacious goal has been substantial, and it is likely that multiple vision restoration technologies will reach clinical use in the next decade.
Acknowledgements
Special thanks to Dr. Paul Sieving for conceptualizing the Audacious Goals Initiative and pushing the field of regenerative medicine in the eye forward. Thanks also to Dr. Steven Becker for shepherding the vision of AGI and its progress.
References
- 1.Blindness GBD, Vision Impairment C & Vision Loss Expert Group of the Global Burden of Disease, S. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health 9, e130–e143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rein DB, et al. The economic burden of vision loss and blindness in the United States. Ophthalmology (2021). [DOI] [PubMed] [Google Scholar]
- 3.Age-Related Eye Disease Study Research, G., et al. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS Report No. 22. Arch Ophthalmol 125, 1225–1232 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Caras IW, Collins LR & Creasey AA A stem cell journey in ophthalmology: From the bench to the clinic. Stem cells translational medicine 10, 1581–1587 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haruta M, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest Ophthalmol Vis Sci 45, 1020–1025 (2004). [DOI] [PubMed] [Google Scholar]
- 6.Idelson M, et al. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell stem cell 5, 396–408 (2009). [DOI] [PubMed] [Google Scholar]
- 7.Osakada F, Ikeda H, Sasai Y & Takahashi M Stepwise differentiation of pluripotent stem cells into retinal cells. Nat Protoc 4, 811–824 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Luo J, et al. Human retinal progenitor cell transplantation preserves vision. J Biol Chem 289, 6362–6371 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carr AJ, et al. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PLoS One 4, e8152 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz SD, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet 385, 509–516 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Mehat MS, et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Pigment Epithelial Cells in Macular Degeneration. Ophthalmology 125, 1765–1775 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma R, et al. Clinical-grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Science translational medicine 11(2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chirco KR, et al. Preparation and evaluation of human choroid extracellular matrix scaffolds for the study of cell replacement strategies. Acta biomaterialia 57, 293–303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koss MJ, et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatan minipigs. Graefes Arch Clin Exp Ophthalmol 254, 1553–1565 (2016). [DOI] [PubMed] [Google Scholar]
- 15.Kashani AH, et al. One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration. Transl Vis Sci Technol 10, 13 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lingam S, et al. cGMP-grade human iPSC-derived retinal photoreceptor precursor cells rescue cone photoreceptor damage in non-human primates. Stem Cell Res Ther 12, 464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirai H, et al. Transplantation of human embryonic stem cell-derived retinal tissue in two primate models of retinal degeneration. Proc Natl Acad Sci U S A 113, E81–90 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chao JR, et al. Transplantation of Human Embryonic Stem Cell-Derived Retinal Cells into the Subretinal Space of a Non-Human Primate. Transl Vis Sci Technol 6, 4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kupperman BD, et al. ARVO annual meeting abstract: Safety and activity of a single, intravitreal injection of human retinal progenitor cells (JCell) for treatment of retinitis pigmentosa (RP). Invest Ophthal Vis Sci. 59(9):2987. [Google Scholar]
- 20.Lin B, McLelland BT, Mathur A, Aramant RB & Seiler MJ Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration. Exp Eye Res 174, 13–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano T, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Capowski EE, et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development 146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhong X, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun 5, 4047 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro J, et al. Restoration of visual function in advanced disease after transplantation of purified human pluripotent stem cell-derived cone photoreceptors. Cell Rep 35, 109022 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aboualizadeh E, et al. Imaging Transplanted Photoreceptors in Living Nonhuman Primates with Single-Cell Resolution. Stem cell reports 15, 482–497 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan J & Wong R Development of Cell Types and Synaptic Connections in the Retina. in Webvision: The Organization of the Retina and Visual System (eds. Kolb H, Fernandez E & Nelson R) (University of Utah Health Sciences, Salt Lake City (UT). (1995). [Google Scholar]
- 27.Marc RE, Jones BW, Watt CB & Strettoi E Neural remodeling in retinal degeneration. Prog Retin Eye Res 22, 607–655 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Sekirnjak C, et al. Changes in physiological properties of rat ganglion cells during retinal degeneration. J Neurophysiol 105, 2560–2571 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekhar K, et al. Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell 166, 1308–1323.e1330 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabriel E, et al. Human brain organoids assemble functionally integrated bilateral optic vesicles. Cell stem cell 28, 1740–1757 e1748 (2021). [DOI] [PubMed] [Google Scholar]
- 31.Miltner AM & La Torre A Retinal Ganglion Cell Replacement: Current Status and Challenges Ahead. Developmental dynamics : an official publication of the American Association of Anatomists 248, 118–128 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao D, et al. In vivo Regeneration of Ganglion Cells for Vision Restoration in Mammalian Retinas. Front Cell Dev Biol 9, 755544 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng YR, et al. Molecular Classification and Comparative Taxonomics of Foveal and Peripheral Cells in Primate Retina. Cell 176, 1222–1237 e1222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venugopalan P, et al. Transplanted neurons integrate into adult retinas and respond to light. Nat Commun 7, 10472 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu YR, et al. Transplanted Mouse Embryonic Stem Cell-Derived Retinal Ganglion Cells Integrate and Form Synapses in a Retinal Ganglion Cell-Depleted Mouse Model. Invest Ophthalmol Vis Sci 62, 26 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lahne M, Nagashima M, Hyde DR & Hitchcock PF Reprogramming Müller glia to regenerate retinal neurons. Annu Rev Vis Sci 6, 171–193 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyer MA & Cepko CL Control of Muller glial cell proliferation and activation following retinal injury. Nature neuroscience 3, 873–880 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Hoang T, et al. Gene regulatory networks controlling vertebrate retinal regeneration. Science 370(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorstad NL, et al. Stimulation of functional neuronal regeneration from Müller glia in adult mice. Nature 548, 103–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Livesey FJ & Cepko CL Vertebrate neural cell-fate determination: lessons from the retina. Nat Rev Neurosci 2, 109–118 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Jorstad NL, et al. Stimulation of functional neuronal regeneration from Muller glia in adult mice. Nature 548, 103–107 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Todd L, et al. Efficient stimulation of retinal regeneration from Muller glia in adult mice using combinations of proneural bHLH transcription factors. Cell Rep 37, 109857 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholler J, et al. Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci Appl 9, 140 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rossi EA, et al. Imaging individual neurons in the retinal ganglion cell layer of the living eye. Proc Natl Acad Sci U S A 114, 586–591 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Z, et al. Quantification of retinal ganglion cell morphology in human glaucomatous eyes. Invest Ophthalmol Vis Sci 62, 34 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Palczewska G, et al. Two-photon imaging of the mammalian retina with ultrafast pulsing laser. JCI Insight 3(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandiyan VP, et al. The optoretinogram reveals the primary steps of phototransduction in the living human eye. Sci Adv 6(2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahuja AK, et al. Blind subjects implanted with the Argus II retinal prosthesis are able to improve performance in a spatial-motor task. Br J Ophthalmol 95, 539–543 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dagnelie G, et al. Performance of real-world functional vision tasks by blind subjects improves after implantation with the Argus(R) II retinal prosthesis system. Clin Exp Ophthalmol 45, 152–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nanduri D, et al. Frequency and amplitude modulation have different effects on the percepts elicited by retinal stimulation. Invest Ophthalmol Vis Sci 53, 205–214 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stingl K, et al. Interim Results of a Multicenter Trial with the New Electronic Subretinal Implant Alpha AMS in 15 Patients Blind from Inherited Retinal Degenerations. Frontiers in neuroscience 11, 445 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ayton LN, et al. An update on retinal prostheses. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology 131, 1383–1398 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorach H, et al. Photovoltaic restoration of sight with high visual acuity. Nat Med 21, 476–482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palanker D, Le Mer Y, Mohand-Said S, Muqit M & Sahel JA Photovoltaic restoration of central vision in atrophic age-related macular degeneration. Ophthalmology 127, 1097–1104 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maguire AM, et al. Efficacy, Safety, and Durability of Voretigene Neparvovec-rzyl in RPE65 mutation-associated inherited retinal dystrophy: results of phase 1 and 3 trials. Ophthalmology 126, 1273–1285 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Russell S, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet 390, 849–860 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cideciyan AV, et al. Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement. Proc Natl Acad Sci U S A 110, E517–525 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bucher K, Rodriguez-Bocanegra E, Dauletbekov D & Fischer MD Immune responses to retinal gene therapy using adeno-associated viral vectors - Implications for treatment success and safety. Prog Retin Eye Res 83, 100915 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Van Gelder RN Gene therapy approaches to slow or reverse blindness from inherited retinal degeneration: growth factors and optogenetics. Int Ophthalmol Clin 61, 209–228 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kauper K, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Invest Ophthalmol Vis Sci 53, 7484–7491 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Chew EY, et al. Effect of ciliary neurotrophic factor on retinal neurodegeneration in patients with macular telangiectasia type 2: a randomized clinical trial. Ophthalmology 126, 540–549 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sahel JA, et al. Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 27, 1223–1229 (2021). [DOI] [PubMed] [Google Scholar]
- 63.Berry MH, et al. Restoration of high-sensitivity and adapting vision with a cone opsin. Nat Commun 10, 1221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polosukhina A, et al. Photochemical restoration of visual responses in blind mice. Neuron 75, 271–282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tochitsky I, et al. Restoring visual function to blind mice with a photoswitch that exploits electrophysiological remodeling of retinal ganglion cells. Neuron 81, 800–813 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tochitsky I, et al. How azobenzene photoswitches restore visual responses to the blind retina. Neuron 92, 100–113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koga K, Wang B & Kaneko S Current and furutre perspectives of HLA-edited induced pluripotent stem cells. Inflamm Regen 40, 223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y, & Lee RK Cell transplantation to replace retinal ganglion cells faces challenges – the Switchboard Dilemma. Neural Regen Res 16, 1138–1143 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fligor CM, et al. Extension of retinofugal projections in an assembled model of human pluripotent stem cell-derived organoids. Stem Cell Rep 16, 2228–2241 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yungher BJ, Ribeiro M & Park KK Regenerative responses and axon pathfinding of retinal ganglion cells in chronically injured mice. Invest Ophthalmol Vis Sci 58, 1743–1750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Lima S, Koriyama Y, Kurimoto T & Benowitz L Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci USA 109, 9149–9154 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Glennon E, Svirsky MA & Froemke RC Auditory cortical plasticity in cochlear implant users. Curr Opin Neurobiol 60, 108–114 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Palanker D, Le Mer Y, Mohand-Said S & Sahel JA Simultaneous perception of prosthetic and natural vision in AMD patients. Nat Commun 13, 513 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwartz SD, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet 379, 713–720 (2012). [DOI] [PubMed] [Google Scholar]
- 75.Petoe MA, et al. A Second-generation (44-channel) suprachoroidal retinal prosthesis: interim clinical trial results. Transl Vis Sci Technol 10, 12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan JL, Dhingra A, Vardi N & Wong ROL Axons and dendrites originate from neuroepithelial-like processes of retinal bipolar neurons. Nat Neurosci 9, 85–92 (2006). [DOI] [PubMed] [Google Scholar]


