Abstract
BACKGROUND
Pegozafermin is a long-acting glycopegylated (pegylated with the use of site-specific glycosyltransferases) fibroblast growth factor 21 (FGF21) analogue in development for the treatment of nonalcoholic steatohepatitis (NASH) and severe hypertriglyceridemia. The efficacy and safety of pegozafermin in patients with biopsy-proven noncirrhotic NASH are not well established.
METHODS
In this phase 2b, multicenter, double-blind, 24-week, randomized, placebo-controlled trial, we randomly assigned patients with biopsy-confirmed NASH and stage F2 or F3 (moderate or severe) fibrosis to receive subcutaneous pegozafermin at a dose of 15 mg or 30 mg weekly or 44 mg once every 2 weeks or placebo weekly or every 2 weeks. The two primary end points were an improvement in fibrosis (defined as reduction by ≥1 stage, on a scale from 0 to 4, with higher stages indicating greater severity), with no worsening of NASH, at 24 weeks and NASH resolution without worsening of fibrosis at 24 weeks. Safety was also assessed.
RESULTS
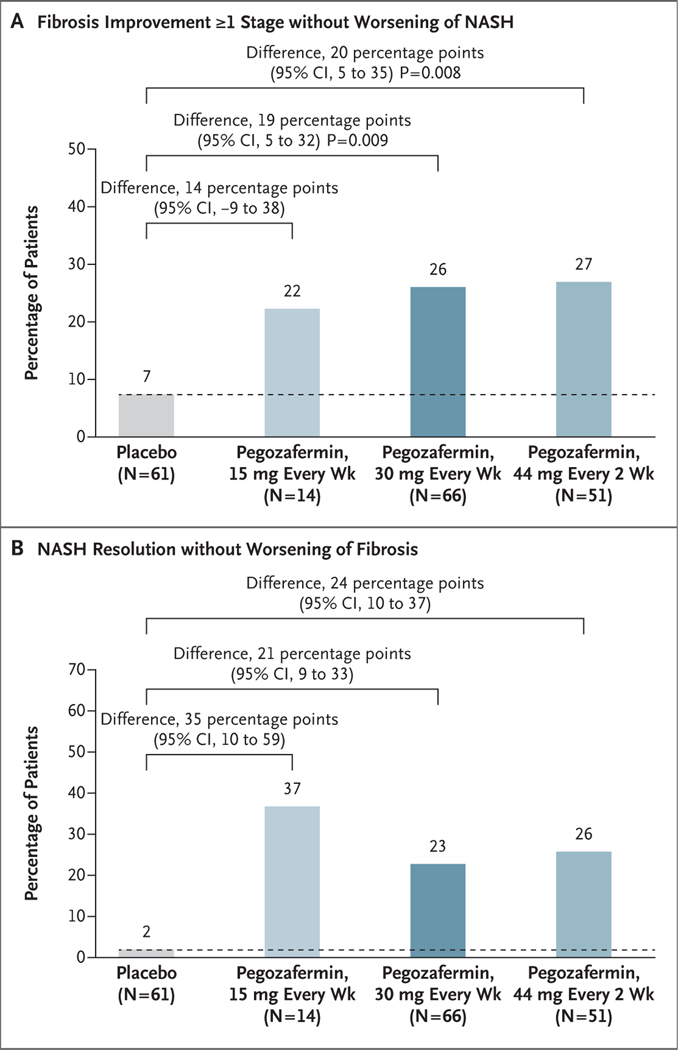
Among the 222 patients who underwent randomization, 219 received pegozafermin or placebo. The percentage of patients who met the criteria for fibrosis improvement was 7% in the pooled placebo group, 22% in the 15-mg pegozafermin group (difference vs. placebo, 14 percentage points; 95% confidence interval [CI], −9 to 38), 26% in the 30-mg pegozafermin group (difference, 19 percentage points; 95% CI, 5 to 32; P = 0.009), and 27% in the 44-mg pegozafermin group (difference, 20 percentage points; 95% CI, 5 to 35; P = 0.008). The percentage of patients who met the criteria for NASH resolution was 2% in the placebo group, 37% in the 15-mg pegozafermin group (difference vs. placebo, 35 percentage points; 95% CI, 10 to 59), 23% in the 30-mg pegozafermin group (difference, 21 percentage points; 95% CI, 9 to 33), and 26% in the 44-mg pegozafermin group (difference, 24 percentage points; 95% CI, 10 to 37). The most common adverse events associated with pegozafermin therapy were nausea and diarrhea.
CONCLUSIONS
In this phase 2b trial, treatment with pegozafermin led to improvements in fibrosis. These results support the advancement of pegozafermin into phase 3 development. (Funded by 89bio; ENLIVEN ClinicalTrials.gov number, NCT04929483.)
NONALCOHOLIC STEATOHEPATITIS (NASH) is characterized by excess fat accumulation, hepatic inflammation, and cellular injury, with or without fibrosis.1,2 NASH is associated with the metabolic syndrome and an increased risk of cardiovascular disease.3–5 The development of clinically significant fibrosis in NASH is associated with worse liver-related outcomes (e.g., progression to cirrhosis and its complications and hepatocellular carcinoma), cardiovascular events, and death.2,6 The prevalence of NASH among adults has been reported to be 5.3% worldwide and 14% among middle-age adults in the United States7,8 and is increasing,9,10 but no pharmacologic treatment has been approved.11
Fibroblast growth factor 21 (FGF21) regulates lipid and glucose metabolism and energy expenditure.12 Pegozafermin, a long-acting glycopegylated (pegylated with the use of site-specific glycosyltransferases) recombinant FGF21 analogue, is being developed for the treatment of NASH and severe hypertriglyceridemia.13–16 A phase 1b–2a study involving patients with NASH did not show safety concerns and suggested that pegozafermin therapy may improve hepatic steatosis, markers of inflammation and fibrosis, circulating lipid levels, and glycemic control.15 Benefits with regard to liver histologic features were observed in an open-label cohort of patients with biopsy-confirmed NASH.17 The objective of the ENLIVEN trial was to evaluate the efficacy and safety of pegozafermin in patients with noncirrhotic NASH.
METHODS
TRIAL DESIGN AND OVERSIGHT
We conducted this phase 2b, randomized, double-blind, placebo-controlled trial at 61 sites in the United States to evaluate the efficacy and safety of pegozafermin over a treatment period of 24 weeks. The trial included a 12-week screening period and a 24-week treatment period. The trial was conducted in accordance with the principles of the Declaration of Helsinki, the International Ethical Guidelines of the Council for International Organizations of Medical Sciences, the Good Clinical Practice guidelines of the International Council for Harmonisation, and applicable laws and regulations. The trial protocol (available with the full text of this article at NEJM.org) and amendments were approved by the institutional review board or independent ethics committee for each site. A placebo-controlled, single-blind, 24-week extension study is ongoing under the same protocol. All the patients provided written informed consent.
The sponsor (89bio) designed the trial with the academic steering committee and performed site monitoring, data collection, and data analysis. All the authors had access to the data and participated in data interpretation. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. The steering committee and first author made the decision to submit the manuscript for publication. The first author wrote the first draft of the manuscript, which was further developed with the assistance of a medical writer (funded by the sponsor) under the guidance of the authors.
PATIENTS
Eligible patients were 21 to 75 years of age and had NASH (defined as a Clinical Research Network fibrosis stage of F2 or F3 and a nonalcoholic fatty liver disease [NAFLD] activity score of ≥4, with ≥1 point for steatosis, ballooning, and lobular inflammation), as confirmed on a biopsy that was performed at screening or no more than 6 months before screening. A fibrosis stage of F2 indicates moderate (perisinusoidal and portal or periportal) fibrosis, and F3 severe (bridging) fibrosis. The NAFLD activity score is assessed on a scale of 0 to 8, with higher scores indicating more severe disease; the components of this measure are steatosis (assessed on a scale of 0 to 3), lobular inflammation (assessed on a scale of 0 to 3), and hepatocellular ballooning (assessed on a scale of 0 to 2). There was no criterion for a minimum liver fat content.
Key exclusion criteria were liver disease other than NASH, cirrhosis, uncontrolled or newly diagnosed type 2 diabetes, or any illness that, in the opinion of the investigator, might affect the results of the trial or pose an additional risk to the participant. Clinically relevant abnormalities in laboratory measurements, electrocardiograms, or vital signs were also exclusionary. The full inclusion and exclusion criteria are listed in the Supplementary Appendix (available at NEJM.org).
PROCEDURES
Patients were randomly assigned by means of a central interactive Web-response system to receive placebo once weekly or once every 2 weeks or pegozafermin at a dose of 15 mg once weekly, 30 mg once weekly, or 44 mg every 2 weeks; randomization was initially conducted in a 2:1:3:3:3 ratio. A maximum effect model that was based on magnetic resonance imaging proton density fat fraction (MRI-PDFF) data from the phase 1b–2a study guided dose selection. (MRI-PDFF is a noninvasive, quantitative, imaging-based biomarker of liver fat content.) After the second protocol amendment, the randomization ratio was updated to 16:8:6:24:15, which resulted in limited randomization to the 15-mg pegozafermin group, owing to concern about potentially lower efficacy (as assessed histologically) with this dose (see the Supplementary Appendix).
Randomization was stratified according to type 2 diabetes status and fibrosis stage (F2 vs. F3). Patients, investigators, and site personnel were unaware of the trial-group assignments but were aware of the dose frequency. Details of the administration of pegozafermin or placebo and lifestyle counseling18,19 are provided in the Supplementary Appendix.
Follow-up biopsy was performed at week 24. Initially, biopsies were assessed by one central pathologist. In response to advances in methodologic approaches to consensus reading in clinical trials involving patients with NASH,20,21 a consensus scoring method that involved a central panel of three pathologists replaced the original biopsy-reading approach. Assessments of biopsies, which were blinded to patient, treatment, and sequence, were performed by three expert liver pathologists according to the NASH Clinical Research Network NAFLD activity score grading and fibrosis staging system.22 A consensus score was derived from the individual reader scores according to an algorithm designed to minimize interaction among the readers (Fig. S1 in the Supplementary Appendix). Baseline biopsies that were initially assessed by one pathologist were reread by the panel. Protocol-specified reasons for trial discontinuation, withdrawal, or interruption are provided in the Supplementary Appendix.
END POINTS
The two primary end points, evaluated at week 24 as compared with baseline, were an improvement (reduction) in fibrosis of at least one stage, without worsening of NASH (defined as an increase in ballooning, inflammation, or steatosis), and NASH resolution (defined as the total absence of ballooning and absent or mild inflammation) without worsening of fibrosis (increase of ≥1 stage). Key secondary end points included an improvement of at least 2 points in the NAFLD activity score and no worsening of fibrosis. Other secondary end points included changes from baseline to week 24 in liver variables (MRI-PDFF, liver chemistry tests, and N-terminal type III collagen propeptide [Pro-C3]) and metabolic variables (adiponectin, serum triglycerides, high-density lipoprotein [HDL] cholesterol, non-HDL cholesterol, low-density lipoprotein [LDL] cholesterol, and glycated hemoglobin). Exploratory end points included changes from baseline to week 24 in iron-corrected T1 (which assesses fibroinflammation), the Enhanced Liver Fibrosis test score, liver stiffness (as assessed by vibration-controlled transient elastography [FibroScan, Echosens]), the FibroScan–aspartate aminotransferase (FAST) score, the Fibrosis-4 index score, and liver and spleen volumes.
Safety end points included the frequency and severity of adverse events, which were classified according to the Medical Dictionary for Regulatory Activities, version 24.0. Additional safety assessments included safety laboratory variables, vital signs, electrocardiograms, and dual-energy x-ray absorptiometry (DXA) scans. A list of all the primary, secondary, and safety end points is provided in Table S1.
STATISTICAL ANALYSIS
We calculated that a sample size of approximately 184 would provide the trial with 83 to 94% power to detect between-group differences of 30 percentage points in the two primary end points, on the basis of assumptions regarding response to placebo and an assumption that 15% of the patients would withdraw (see the Supplementary Appendix). To match the intended target population as defined by regulatory authorities, the prespecified primary efficacy analyses included all the patients with F2 or F3 fibrosis and a NAFLD activity score of at least 4 at baseline who received at least one dose of pegozafermin or placebo (full analysis population). For the primary and key secondary end points, results were also analyzed in the full analysis population plus any patients with F2 or F3 fibrosis and a NAFLD activity score of at least 4 who had undergone randomization but had not received pegozafermin or placebo and in the population of all the patients who had undergone randomization. Safety analyses included all the patients who received at least one dose of pegozafermin or placebo. The two placebo groups (administration once weekly and once every 2 weeks) were pooled for all the analyses.
A multiple imputation strategy and a stratified Cochran–Mantel–Haenszel method were used for analysis of the primary and key secondary end points (see the Supplementary Appendix). Sensitivity analyses (analysis involving only patients with biopsy data at both baseline and week 24 [completer analysis] and analysis with imputation of missing biopsy data as nonresponse) were conducted to assess the robustness of the results of the primary analysis. Continuous efficacy end points were analyzed with the use of a mixed-model repeated-measures analysis. There was no prespecified plan to adjust for multiple comparisons. Comparisons with placebo of the 30-mg and 44-mg dose groups for the first primary end point (fibrosis improvement) are reported with P values at a two-sided significance level of 0.05. All the other results are reported as point estimates with 95% confidence intervals. The widths of 95% confidence intervals have not been adjusted for multiplicity and should not be used to infer definitive treatment effects. Statistical analyses were conducted with the use of SAS software, version 9.4 (SAS Institute).
RESULTS
PATIENTS
Patients were enrolled between September 28, 2021, and August 15, 2022. A total of 222 patients underwent randomization, of whom 219 received pegozafermin or placebo and were included in the safety analysis population (Fig. S2). The demographic and disease characteristics of the patients are shown in Table 1. Most of the patients were White; Black patients were underrepresented in this trial (Table S2).
Table 1.
Baseline Characteristics of the Patients Who Underwent Randomization.*
| Characteristic | Placebo (N = 71) | Pegozafermin, 15 mg Weekly (N = 21) | Pegozafermin, 30 mg Weekly (N = 73) | Pegozafermin, 44 mg Every 2 Wk (N = 57) | Total (N = 222) |
|---|---|---|---|---|---|
| Age ― yr | 56.3±9.0 | 55.0±10.5 | 55.3±11.2 | 55.2±11.2 | 55.6±10.4 |
| Male sex ― no. (%) | 32 (45) | 12 (57) | 23 (32) | 20 (35) | 87 (39) |
| White race ― no. (%)† | 67 (94) | 18 (86) | 69 (95) | 54 (95) | 208 (94) |
| Hispanic or Latino ethnic group ― no. (%)† | 24 (34) | 9 (43) | 32 (44) | 20 (35) | 85 (38) |
| Body weight ― kg | 108.7±20.1 | 108.0±21.0 | 95.7±20.2 | 100.2±20.4 | 102.2±20.9 |
| Body-mass index‡ | 38.1±5.6 | 37.8±4.8 | 35.1±6.4 | 36.1±5.5 | 36.6±5.9 |
| Type 2 diabetes ― no. (%) | 49 (69) | 18 (86) | 45 (62) | 35 (61) | 147 (66) |
| Glycated hemoglobin ― % | 6.6±1.0 | 7.0±1.2 | 6.6±1.2 | 6.7±1.3 | 6.7±1.2 |
| Alanine aminotransferase ― U/liter | 49.6±25.7 | 61.1±34.8 | 60.0±32.1 | 56.3±32.0 | 55.8±30.6 |
| Aspartate aminotransferase ― U/liter | 40.6±20.2 | 47.7±27.9 | 46.7±25.3 | 41.7±23.3 | 43.6±23.5 |
| NASH CRN fibrosis stage ― no. (%)§ | |||||
| F1 | 2 (3) | 3 (14) | 2 (3) | 0 | 7 (3) |
| F2 | 20 (28) | 6 (29) | 21 (29) | 21 (37) | 68 (31) |
| F3 | 47 (66) | 9 (43) | 47 (64) | 30 (53) | 133 (60) |
| F4 | 2 (3) | 3 (14) | 3 (4) | 6 (11) | 14 (6) |
| NAFLD activity score¶ | 5.0±1.2 | 4.8±1.2 | 5.3±1.1 | 5.2±1.0 | 5.1±1.1 |
| Liver fat content ― %‖ | 16.7±7.1 | 15.8±6.4 | 16.7±7.0 | 15.8±7.8 | 16.4±7.2 |
| Liver stiffness ― kPa** | 14.1±7.7 | 11.2±2.9 | 12.5±4.2 | 13.2±10.3 | 13.0±7.3 |
| Pro-C3 ― ng/ml†† | 49.8±17.5 | 61.6±30.7 | 53.6±22.3 | 52.3±18.8 | 52.8±21.1 |
| Triglycerides ― mg/ml | 170.3±84.6 | 186.2±118.7 | 175.0±83.1 | 164.7±77.7 | 171.9±85.8 |
Plus–minus values are means ±SD. Data from the placebo groups were pooled. Percentages may not total 100 because of rounding. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Race and ethnic group were reported by the patient. Patients could be recorded as both White and Hispanic or Latino.
The body-mass index is the weight in kilograms divided by the square of the height in meters.
The fibrosis stages according to the Clinical Research Network (CRN) in patients with nonalcoholic steatohepatitis (NASH) are as follows: F1 indicates mild (perisinusoidal or periportal) fibrosis, F2 moderate (perisinusoidal and portal or periportal) fibrosis, F3 severe (bridging) fibrosis, and F4 cirrhosis. A total of 21 patients were initially assessed as having F2 or F3 fibrosis but were later assessed as having F1 or F4 fibrosis by the consensus-panel read and thus as not meeting the histologic inclusion criteria of the trial.
The nonalcoholic fatty liver disease (NAFLD) activity score is assessed on a scale of 0 to 8, with higher scores indicating more severe disease; the components of this measure are steatosis (assessed on a scale of 0 to 3), lobular inflammation (assessed on a scale of 0 to 3), and hepatocellular ballooning (assessed on a scale of 0 to 2). A total of six patients were initially assessed as having F2 or F3 fibrosis with an NAFLD activity score of 4 or higher but were later assessed as having F2 or F3 fibrosis with an NAFLD activity score of less than 4 by the consensus-panel read and thus as not meeting the histologic inclusion criteria of the trial.
Liver fat content was assessed by means of the magnetic resonance imaging proton density fat fraction (MRI-PDFF). Baseline data were missing for two patients (3%) in the pooled placebo group and for one (1%) in the 30-mg pegozafermin group.
Liver stiffness was assessed by means of vibration-controlled transient elastography (FibroScan). Higher liver-stiffness scores as assessed with the use of vibration-controlled transient elastography indicate a higher risk of advanced fibrosis. A score of 20 kPa or higher is associated with cirrhosis, but for ruling out cirrhosis, the cutoff point is less than 8 kPa.1
Baseline data on the level of N-terminal propeptide of type III collagen (Pro-C3) were missing for two patients (3%) in the pooled placebo group, for one (5%) in the 15-mg pegozafermin group, and for two (3%) in the 30-mg pegozafermin group. Pro-C3 is a measure of collagen cleavage during active fibrogenesis and was analyzed on a high-precision Cobas platform (Roche).
The mean baseline body-mass index and liver-stiffness results as assessed on vibration-controlled transient elastography were somewhat higher in the placebo group than in the pegozafermin groups. Of the 222 patients who had undergone randomization, 27 who were initially assessed as having F2 or F3 fibrosis and a NAFLD activity score of at least 4 by a single reader were later assessed by the consensus-panel read as not meeting the histologic inclusion criteria of the trial. These 27 patients were excluded from the full analysis population, as were 3 patients who underwent randomization but did not receive any pegozafermin or placebo. Therefore, 192 patients were included in the full analysis population. Full agreement or mode (agreement between two pathologists) determined 91 to 99% of the final biopsy scores (Table S3).
EFFICACY
Biopsy results were available at baseline and at week 24 for 164 patients; end points were imputed for the remaining 28 patients in the full analysis population. At 24 weeks, the percentage of patients with an improvement in fibrosis of at least one stage without worsening of NASH was significantly higher with pegozafermin than with placebo at both the weekly 30-mg dose (26% vs. 7%; difference, 19 percentage points, 95% confidence interval [CI], 5 to 32; P = 0.009) and the every-2-week 44-mg dose (27% vs. 7%; difference, 20 percentage points; 95% CI, 5 to 35; P = 0.008) (Fig. 1A). In the group that received 15 mg of pegozafermin weekly, 22% of the patients had an improvement in fibrosis (difference vs. placebo, 14 percentage points; 95% CI, −9 to 38).
Figure 1. Primary End Points at Week 24 (Full Analysis Population).
The two primary end points were an improvement in fibrosis (defined as reduction by ≥1 stage, on a scale from 0 to 4, with higher stages indicating greater severity), with no worsening of nonalcoholic steatohepatitis (NASH) at 24 weeks, and NASH resolution (defined as the total absence of ballooning and absent or mild inflammation) without worsening of fibrosis (increase of ≥1 stage) at 24 weeks. Patients were assigned to receive placebo or pegozafermin at a dose of 15 mg every week, 30 mg every week, or 44 mg every 2 weeks. Data from the placebo groups were pooled; the dashed line indicates the results in the pooled placebo group for comparison across the pegozafermin groups. Data were analyzed with the use of a Cochran–Mantel–Haenszel method with adjustment for baseline stratification factors (type 2 diabetes status and fibrosis stage). Missing end-point data (for 28 patients) were imputed with the use of multiple imputation by means of logistic regression with all collected outcomes. The full analysis population included all the enrolled patients who had undergone randomization and received at least one dose of pegozafermin or placebo and who had confirmed fibrosis stage F2 or F3 (indicating moderate or severe fibrosis) and a nonalcoholic fatty liver disease activity score of at least 4 at baseline, as assessed on independent review by a three-pathologist panel. The widths of the confidence intervals have not been adjusted for multiplicity, so the confidence intervals should not be used to reject or not reject treatment effects.
The percentage of patients with NASH resolution without worsening of fibrosis also favored pegozafermin over placebo in both the 30-mg pegozafermin group (23% vs. 2%; difference, 21 percentage points; 95% CI, 9 to 33) and the 44-mg pegozafermin group (26% vs. 2%; difference, 24 percentage points; 95% CI, 10 to 37) (Fig. 1B). In the 15-mg pegozafermin group, 37% of the patients had NASH resolution without worsening of fibrosis (difference vs. placebo, 35 percentage points; 95% CI, 10 to 59) (Table S4).
Results were consistent in prespecified sensitivity analyses (completer analysis and imputation of missing biopsy data as nonresponse) and in analyses in the full analysis population plus the three patients with F2 or F3 fibrosis and a NAFLD activity score of at least 4 who did not receive pegozafermin or placebo and in the population of all the patients who had undergone randomization (Tables S5 and S6 and Figs. S3 and S4). Post hoc analysis showed that 89% of the patients treated with pegozafermin who met the criteria for the fibrosis primary end point had an improvement of at least 2 points in the NAFLD activity score. Primary end-point results for the two placebo groups are shown in Figure S5. Subgroup analyses are shown in Figures S6 and S7. In a post hoc analysis, positive results regarding fibrosis regression in patients with F4 fibrosis (cirrhosis) were observed (Fig. S8).
Analyses of key secondary end points were generally supportive of the primary end-point findings (Figs. S9, S10, and S11). The percentage of patients with a reduction in the NAFLD activity score of at least 2 points and no worsening of fibrosis was 37% in the 15-mg pegozafermin group, 65% in the 30-mg pegozafermin group, 62% in the 44-mg pegozafermin group, and 24% in the placebo group.
At week 24, the least-squares mean percentage change from baseline in liver fat content (as assessed by MRI-PDFF) was −27.1% in the 15-mg pegozafermin group, −48.2% in the 30-mg pegozafermin group, and −41.9% in the 44-mg pegozafermin group, as compared with −5.0% in the placebo group (Table 2 and Table S7). A reduction in liver fat of at least 50% from baseline occurred in 63% of the patients in the 30-mg pegozafermin group and in 58% of those in the 44-mg pegozafermin group, as compared with 12% of the patients in the placebo group. Post hoc analyses involving patients who had more than 10% liver fat at baseline are shown in Figure S12.
Table 2.
Changes from Baseline to Week 24 in Selected Liver and Metabolic End Points (Full Analysis Population).*
| End Point | Placebo (N = 61) | Pegozafermin, 15 mg Weekly (N = 14) | Pegozafermin, 30 mg Weekly (N = 66) | Pegozafermin, 44 mg Every 2 Wk (N = 51) |
|---|---|---|---|---|
| Alanine aminotransferase | ||||
| Absolute change ― U/liter | −8.8±2.5 | −24.3±5.1 | −26.3±2.4 | −23.5±2.7 |
| Percentage change | −4.6±5.0 | −37.7±10.1 | −41.6±4.8 | −31.8±5.4 |
| Liver fat content† | ||||
| Absolute change ― percentage points | −1.5±0.7 | −4.6±1.4 | −8.1±0.7 | −8.2±0.8 |
| Percentage change | −5.0±5.2 | −27.1±10.3 | −48.2±5.1 | −41.9±5.6 |
| Enhanced Liver Fibrosis test score‡ | 0.2±0.1 | −0.3±0.1 | −0.3±0.1 | −0.3±0.1 |
| Liver stiffness ― kPa§ | 0.8±0.8 | −1.4±1.5 | −3.1±0.8 | −2.4±0.9 |
| Pro-C3 | ||||
| Absolute change ― ng/ml | −1.2±1.8 | −9.9±3.7 | −13.8±1.8 | −11.4±2.0 |
| Percentage change | 6.4±4.1 | −5.4±8.3 | −18.1±4.0 | −17.3±4.4 |
| Iron-corrected T1 ― msec† | −6.1±11.7 | −46.7±21.3 | −92.4±10.7 | −69.8±12.3 |
| Liver volume | ||||
| Absolute change ― ml | −62±38 | −127±78 | −304±37 | −241±42 |
| Percentage change | −2.5±1.5 | −5.9±3.1 | −12.5±1.5 | −9.5±1.7 |
| Spleen volume | ||||
| Absolute change ― ml | 3±6 | −23±12 | −37±6 | −19±7 |
| Percentage change | 1.3±1.7 | −6.1±3.5 | −9.8±1.7 | −5.2±1.9 |
| Adiponectin | ||||
| Absolute change ― μg/ml | −0.6±0.4 | 1.1±0.7 | 1.1±0.3 | 1.2±0.4 |
| Percentage change | −7.2±5.9 | 20.6±11.9 | 30.0±5.6 | 27.7±6.1 |
| Median triglyceride level (IQR)¶ | ||||
| Absolute change ― mg/dl | −7.5 (−29.0 to 19.0) | −8.3 (−26.8 to 10.3) | −40.5 (−73.0 to −7.0) | −14.0 (−45.5 to 7.0) |
| Percentage change | −6.4 (−17.8 to 13.6) | −5.9 (−22.9 to 6.4) | −26.6 (−39.7 to −5.8) | −10.1 (−28.6 to 3.5) |
| Glycated hemoglobin ― percentage points | −0.0±0.1 | −0.1±0.2 | −0.3±0.1 | −0.2±0.1 |
Plus–minus values are least-squares means ±SE. The end points shown in this table were selected on the basis of interest for this type of trial involving patients with noncirrhotic NASH. Absolute changes are shown unless otherwise specified. The full analysis population included all the patients with F2 or F3 fibrosis and a NAFLD activity score of at least 4 at baseline who received at least one dose of pegozafermin or placebo. Data were analyzed with the use of a mixed-model with trial group, week, and interaction between trial group and week as main effects and with baseline measurements and stratifications (type 2 diabetes status and fibrosis stage) as covariates. Observed data were used. Data from the placebo groups were pooled. The Enhanced Liver Fibrosis test score, liver stiffness, iron-corrected T1 (which assesses fibroinflammation), liver volume, and spleen volume were exploratory end points.
Liver fat content was assessed by means of MRI-PDFF. Iron-corrected T1 was also assessed in the MRI-PDFF analysis population, which included all the patients in the full analysis population who had a baseline and at least one follow-up MRI-PDFF assessment. Data were available for 57 patients in the pooled placebo group, for 14 in the 15-mg pegozafermin group, for 61 in the 30-mg pegozafermin group, and for 49 in the 44-mg pegozafermin group.
The Enhanced Liver Fibrosis test score is derived from an algorithm that combines for hyaluronic acid, type III procollagen peptide, and tissue inhibitor of matrix metalloproteinase 1. A score of less than 7.7 indicates no or mild fibrosis, and a score of 11.3 or higher indicates cirrhosis.
Liver stiffness was assessed with the use of vibration-controlled transient elastography. Data were analyzed with the use of analysis of covariance with trial group, baseline measurements, and stratifications (type 2 diabetes status and fibrosis stage) as covariates.
Data are shown as medians with interquartile ranges (IQRs) for nonnormal distribution. Data were analyzed by means of the van Elteren method; patients with missing values at week 24 were excluded from the nonparametric analysis. Data on triglyceride levels were missing for four patients (7%) in the placebo group, for two (14%) in the 15-mg pegozafermin group, for nine (14%) in the 30-mg pegozafermin group, and for six (12%) in the 44-mg pegozafermin group.
Pegozafermin treatment for 24 weeks was associated with reductions in liver chemistry variables (Table 2). In a post hoc analysis, the alanine aminotransferase level was normalized (defined as an end-of-trial level of ≤30 U per liter in patients with a baseline level of >30 U per liter) in 59% of the patients in the 30-mg pegoza- fermin group and in 65% of those in the 44-mg pegozafermin group, as compared with 24% of those in the placebo group (Fig. S13). The results suggested reductions in the iron-corrected T1 (which assesses fibroinflammation) and in markers of fibrosis such as the Enhanced Liver Fibrosis test score, liver stiffness (as assessed by vibration-controlled transient elastography), the FAST score, the Pro-C3 level, and the Fibrosis-4 index score, as well as a decrease in liver and spleen volumes (Table 2 and Fig. S14).
The results also suggested that pegozafermin treatment was associated with a greater decrease in the level of serum triglycerides and a greater increase in the HDL cholesterol level with the 30-mg dose of pegozafermin than with placebo and with increases in the adiponectin level in all pegozafermin dose groups as compared with a decrease in the placebo group (Table 2). Results for glycated hemoglobin and LDL and HDL cholesterol levels are shown in Table 2 and Table S8. No apparent effect on body weight was observed.
SAFETY
Adverse events were reported in 95% of the patients in the group that received 15 mg of pegozafermin weekly, in 85% of those in the group that received 30 mg pegozafermin weekly, and in 67% of those in the group that received 44 mg of pegozafermin every 2 weeks, as compared with 68% of those in the placebo group (Table 3). The most frequent adverse events were nausea, diarrhea, and injection-site erythema. Grade 3 adverse events were reported in 10% of the patients in the 15-mg pegozafermin group, in 4% of those in the 30-mg pegozafermin group, and in 9% of those in the 44-mg pegozafermin group, as compared with 9% of those in the placebo group. No adverse events with a severity above grade 3 or deaths were reported.
Table 3.
Adverse Events (Safety Analysis Population).*
| Event | Placebo (N = 69) | Pegozafermin, 15 mg Weekly (N = 21) | Pegozafermin, 30 mg Weekly (N = 72) | Pegozafermin, 44 mg Every 2 Wk (N = 57) |
|---|---|---|---|---|
| number (percent) | ||||
| Any adverse event | 47 (68) | 20 (95) | 61 (85) | 38 (67) |
| Serious adverse event | 3 (4) | 1 (5) | 3 (4) | 6 (11) |
| Adverse event related to pegozafermin or placebo | 20 (29) | 10 (48) | 39 (54) | 24 (42) |
| Adverse event leading to the discontinuation of pegozafermin or placebo | 1 (1) | 1 (5) | 6 (8) | 1 (2) |
| Adverse event related to pegozafermin or placebo leading to its discontinuation | 0 | 1 (5) | 4 (6) | 1 (2) |
| Adverse event from any system organ class, according to preferred term† | ||||
| Nausea | 6 (9) | 4 (19) | 23 (32) | 11 (19) |
| Diarrhea | 4 (6) | 5 (24) | 14 (19) | 8 (14) |
| Injection-site erythema | 3 (4) | 3 (14) | 11 (15) | 3 (5) |
| Increased appetite | 1 (1) | 2 (10) | 10 (14) | 4 (7) |
| Vomiting‡ | 2 (3) | 1 (5) | 10 (14) | 2 (4) |
| Coronavirus disease 2019‡ | 6 (9) | 1 (5) | 9 (12) | 5 (9) |
| Urinary tract infection | 5 (7) | 0 | 8 (11) | 2 (4) |
| Injection-site rash | 1 (1) | 0 | 7 (10) | 2 (4) |
| Muscle spasms | 1 (1) | 1 (5) | 7 (10) | 0 |
| Headache | 5 (7) | 2 (10) | 6 (8) | 6 (11) |
| Sinusitis | 3 (4) | 0 | 2 (3) | 7 (12) |
| Lower abdominal pain | 0 | 3 (14) | 1 (1) | 0 |
| Procedural pain | 1 (1) | 3 (14) | 0 | 1 (2) |
The safety analysis population included all the patients who received at least one dose of pegozafermin or placebo. Data from the placebo groups were pooled. The relatedness of adverse events to pegozafermin or placebo was determined by the investigator.
Shown are adverse events that had an incidence of at least 10% and that were reported in at least three patients in any trial group.
The data-cutoff date for the main trial was February 14, 2023. As of April 24, 2023, one additional case of coronavirus disease 2019 was reported in the placebo group and one additional case of vomiting in the 30-mg pegozafermin group.
Serious adverse events were reported in 5% of the patients in the 15-mg pegozafermin group, in 4% of those in the 30-mg pegozafermin group, and in 11% of those in the 44-mg pegozafermin group, as compared with 4% of those in the placebo group (Table S9). One serious adverse event was considered to be related to pegozafermin therapy by the investigator: acute pancreatitis in a patient who received a single 44-mg dose of pegozafermin and had gallbladder sludge on imaging. The clinical course was typical for uncomplicated acute pancreatitis. Adverse events that were considered by the investigator to be related to pegozafermin or placebo and that led to its discontinuation were reported in 5% of patients receiving the 15-mg dose of pegozafermin (due to diarrhea in one patient), in 6% of those receiving the 30-mg dose (due to diarrhea in two, nausea in one, and injection-site erythema in one), in 2% of those receiving the 44-mg dose (due to pancreatitis in one), and in no patients receiving placebo.
No consistent patterns were observed in safety-related laboratory variables (Table S10), and there were no clinically relevant findings in vital signs or electrocardiograms. No clinically relevant changes were observed in insulin-like growth factor 1, thyrotropin, or bone biomarkers. No adverse changes were observed in DXA scans after 24 weeks (Table S11). Traumatic fracture was reported in 1 of 150 patients across the pegozafermin dose groups (in the group that received 44 mg every 2 weeks) and in 3 of 69 patients in the placebo group. No occurrences of liver injury or tremor were reported in any trial patient.
DISCUSSION
In this trial, treatment with the FGF21 analogue pegozafermin at doses of 30 mg once weekly and 44 mg every 2 weeks for 24 weeks led to significant improvements, as compared with placebo, in fibrosis without worsening of NASH. The results also supported a benefit with regard to NASH resolution without worsening of fibrosis. Fibrosis progression is a key predictor of clinical outcomes in NASH, including liver-related death and death from any cause.6 Improvement in both steatohepatitis and fibrosis indicates that pegozafermin may affect key aspects of the pathophysiology of NASH.
Marked variability in biopsy reading may contribute to the highly variable frequency of response with placebo that has been reported in clinical trials involving patients with NASH.23 In this trial, we used an objective consensus biopsyreading method, in which a consensus score, which was based on the individual scores submitted by three expert pathologists, was determined by a prespecified algorithm rather than by consensus discussion or the use of an adjudicator. It is possible that the low percentage of patients meeting the criteria for the primary end points in the placebo group in our trial reflected greater accuracy of the biopsy-reading method, with better estimation of the actual occurrence of spontaneous regression in NASH (i.e., “placebo response”). Use of this method may have reduced the percentages of patients with a response in the pegozafermin groups as well.
Corroboration of biopsy findings by noninvasive tests increases confidence in the histologic findings. No formal hypothesis testing was conducted for the secondary and exploratory end points; however, the results suggest that pegozafermin was associated with reductions in liver fat and in noninvasive markers of liver injury, inflammation, and fibrosis, including iron-corrected T1 (which assesses fibroinflammation) and markers of fibrosis such as the Enhanced Liver Fibrosis score, liver stiffness (as assessed by vibration-controlled transient elastography), the FAST score, Pro-C3 level, and Fibrosis-4 index score.
NASH is highly associated with the metabolic syndrome. Given that metabolic derangements contribute to progression of NASH and increase the risk of atherosclerotic cardiovascular disease, a common coexisting condition and cause of death in this population,3,4 NASH drugs would ideally improve metabolic coexisting conditions, but this has not been the case for some classes of therapeutic agents in development for NASH.24–29 In line with findings from previous studies, the results of this trial suggest that pegozafermin may have positive effects on adiponectin, serum triglyceride, and HDL cholesterol levels.
Nausea and diarrhea were the most common adverse events with pegozafermin. A single serious adverse event of acute pancreatitis was considered by the investigator to be related to pegozafermin treatment. Effects on bone turnover have been reported in nonclinical studies with FGF21 analogues and in early clinical studies with two FGF21 analogues.30–32 There was no signal for reduced bone-mass density or fractures in this trial, but longer studies are needed to fully assess this potential risk. No hepatotoxic effects were observed.
One limitation of this trial is its short duration. The single-blind extension study for 24 additional weeks may provide data on longer-term safety and noninvasive biomarker assessments. Another limitation is the lack of racial diversity, given that most of the patients were White, which potentially limits the generalizability of the data.
In this phase 2b trial, pegozafermin treatment for 24 weeks led to improvements in fibrosis with both weekly and every-2-week administration in patients with biopsy-confirmed NASH. A potential for administration once every 2 weeks may increase patient convenience and adherence to treatment. Results of this trial may be informative for guiding dose selection for larger and longer phase 3 trials involving patients with NASH.
Supplementary Material
Acknowledgments
Supported by 89bio. Dr. Loomba is also supported by a grant (P30DK120515) from the National Institute of Diabetes and Digestive and Kidney Diseases.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
We thank all the patients who participated in this trial and Sarah Graham, Ph.D., of Oxford PharmaGenesis, for medical writing assistance with an earlier version of the manuscript.
Footnotes
A Quick Take is available at NEJM.org
REFERENCES
- 1.Rinella ME, Neuschwander-Tetri BA, Siddiqui MS, et al. AASLD Practice Guidance on the clinical assessment and management of nonalcoholic fatty liver disease. Hepatology 2023; 77: 1797–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021; 184:2537–64. [DOI] [PubMed] [Google Scholar]
- 3.Pouwels S, Sakran N, Graham Y, et al. Non-alcoholic fatty liver disease (NAFLD): a review of pathophysiology, clinical management and effects of weight loss. BMC Endocr Disord 2022; 22: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shroff H, VanWagner LB. Cardiovascular disease in nonalcoholic steatohepatitis: screening and management. Curr Hepatol Rep 2020;19:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016; 64:73–84. [DOI] [PubMed] [Google Scholar]
- 6.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017; 65: 1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology 2023; 77:1335–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrison SA, Gawrieh S, Roberts K, et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J Hepatol 2021; 75: 284–91. [DOI] [PubMed] [Google Scholar]
- 9.Riazi K, Azhari H, Charette JH, et al. The prevalence and incidence of NAFLD worldwide: a systematic review and metaanalysis. Lancet Gastroenterol Hepatol 2022;7 : 851–61. [DOI] [PubMed] [Google Scholar]
- 10.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018; 67: 123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loomba R, Ratziu V, Harrison SA. Expert panel review to compare FDA and EMA guidance on drug development and endpoints in nonalcoholic steatohepatitis. Gastroenterology 2022; 162: 680–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee Y, Lim S, Hong E-S, et al. Serum FGF21 concentration is associated with hypertriglyceridaemia, hyperinsulinaemia and pericardial fat accumulation, independently of obesity, but not with current coronary artery status. Clin Endocrinol (Oxf) 2014; 80: 57–64. [DOI] [PubMed] [Google Scholar]
- 13.Rosenstock M, Ayalon M, Liu Y, Mansbach H, Margalit M. BIO89–100, a novel PEG-FGF21 analogue, is efficacious following weekly and every 2-week subcutaneous dosing in spontaneous diabetic cynomolgus monkeys. J Hepatol 2019; 70: e155. abstract. [Google Scholar]
- 14.Loomba R, Mansbach H, Tseng L, et al. BIO-89–100, a glycoPEGylated FGF21 analogue, demonstrates robust reduction in serum lipids and long half-life in a phase 1 randomized, controlled single ascending dose trial in healthy subjects. Hepatology 2019; 70: 1266A. abstract. [Google Scholar]
- 15.Loomba R, Lawitz EJ, Frias JP, et al. Safety, pharmacokinetics, and pharmacodynamics of pegozafermin in patients with non-alcoholic steatohepatitis: a randomised, double-blind, placebo-controlled, phase 1b/2a multiple-ascending-dose study. Lancet Gastroenterol Hepatol 2023;8 : 120–32. [DOI] [PubMed] [Google Scholar]
- 16.Bhatt DL, Bays HE, Miller M, et al. The FGF21 analog pegozafermin in severe hypertriglyceridemia: a randomized phase 2 trial. Nat Med (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomba R, Alkhouri N, Lazas D, et al. Pegozafermin improved liver histology, liver-related non-invasive tests (NITs) and metabolic profiles in an open-label cohort of a phase 1b/2a study in subjects with non-alcoholic steatohepatitis (NASH). J Hepatol 2022;77: S730. abstract. [Google Scholar]
- 18.Glass O, Filozof C, Noureddin M, et FGF21 Analogue Pegozafermin in NASH al. Standardisation of diet and exercise in clinical trials of NAFLD-NASH: recommendations from the Liver Forum. J Hepatol 2020; 73: 680–93. [DOI] [PubMed] [Google Scholar]
- 19.Pais R, Cariou B, Noureddin M, et al. A proposal from the Liver Forum for the management of comorbidities in nonalcoholic steatohepatitis therapeutic trials. J Hepatol 2023. March 29 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 20.Sanyal AJ, Loomba R, Anstee QM, et al. Minimizing variability and increasing concordance for NASH histological scoring in NASH clinical trials. Hepatology 2021;74: 968A. abstract. [Google Scholar]
- 21.Sanyal AJ, Loomba R, Anstee QM, et al. Topline results from a new analysis of the REGENERATE trial of obeticholic acid for the treatment of nonalcoholic steatohepatitis. AASLD The Liver Meeting 2022: 5008. (https://www.aasld.org/sites/default/files/2022-11/2022%20Late%20Breaking%20Abstracts%20rev2.pdf).abstract. [Google Scholar]
- 22.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41:1313–21. [DOI] [PubMed] [Google Scholar]
- 23.Ng CH, Xiao J, Lim WH, et al. Placebo effect on progression and regression in NASH: evidence from a meta-analysis. Hepatology 2022; 75:1647–61. [DOI] [PubMed] [Google Scholar]
- 24.Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology 2021; 160:219–231.e1. [DOI] [PubMed] [Google Scholar]
- 25.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for noncirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015; 385: 956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pockros PJ, Fuchs M, Freilich B, et al. CONTROL: a randomized phase 2 study of obeticholic acid and atorvastatin on lipoproteins in nonalcoholic steatohepatitis patients. Liver Int 2019; 39: 2082–93. [DOI] [PubMed] [Google Scholar]
- 27.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394: 2184–96. [DOI] [PubMed] [Google Scholar]
- 28.Kim C-W, Addy C, Kusunoki J, et al. Acetyl CoA carboxylase inhibition reduces hepatic steatosis but elevates plasma triglycerides in mice and humans: a bedside to bench investigation. Cell Metab 2017; 26: 394–406.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Francque SM, Bedossa P, Ratziu V, et al. A randomized, controlled trial of the pan-PPAR agonist lanifibranor in NASH. N Engl J Med 2021; 385: 1547–58. [DOI] [PubMed] [Google Scholar]
- 30.Rader DJ, Maratos-Flier E, Nguyen A, et al. LLF580, an FGF21 analog, reduces triglycerides and hepatic fat in obese adults with modest hypertriglyceridemia. J Clin Endocrinol Metab 2022; 107(1):e57–e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Talukdar S, Zhou Y, Li D, et al. A long-acting FGF21 molecule, PF-05231023, decreases body weight and improves lipid profile in non-human primates and type 2 diabetic subjects. Cell Metab 2016; 23:427–40. [DOI] [PubMed] [Google Scholar]
- 32.Kim AM, Somayaji VR, Dong JQ, et al. Once-weekly administration of a long-acting fibroblast growth factor 21 analogue modulates lipids, bone turnover markers, blood pressure and body weight differently in obese people with hypertriglyceridaemia and in non-human primates. Diabetes Obes Metab 2017;19: 1762–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.