Abstract
The chimeric antigen receptor (CAR) design, first invented by Zelig Eshhar, paved the way for the use of genetically modified T-cells in targeted therapy against cancer cells. Since then, it has gone through many generations, especially with the integration of co-stimulation in the second and third-generation CARs. However, it also mounts a hyperactive immune response named as cytokine release syndrome with the release of several cytokines eventually resulting in multiple end-organ toxicities. The severity of cytokine release syndrome depends upon certain factors such as the tumor burden, choice of co-stimulation, and degree of lymphodepletion, and can manifest as pulmonary edema, vascular leak, renal dysfunction, cardiac problems, hepatic failure, and coagulopathy. Many grading criteria have been used to define these clinical manifestations but they lack harmonization. Neurotoxicity has also been significantly associated with CAR T-cell therapy but it has not been studied much in previous literature. This review aims to provide a comprehensive account of the clinical manifestations, diagnosis, management, and treatment of CAR T-cell associated neurotoxicity.
Keywords: CAR T-cell therapy, chimeric antigen receptor, cytokine release syndrome (CRS), neurological toxicity, neurology, pharmacology
Introduction
Highlights
Hyperactive immune response in chimeric antigen receptor-based therapy can lead to neurotoxicity.
This has also been significantly underreported in previous literature.
This review aims to provide a comprehensive account of the treatment of chimeric antigen receptor T-cell associated neurotoxicity.
The usage of targeted adoptive cell therapy is aimed at using the body’s immune system against tumor cells. The use of chimeric antigen receptor (CAR) in genetically modified T-cells was first invented by Zelig Eshhar; however, there are some ambiguities about this fact. It paved the way for allogeneic or autologous T-cells to be genetically modified using CAR, providing therapy for cancer patients. The first successful cancer treatment using CAR T-cell therapy was performed in 2010 for an advanced follicular lymphoma patient. Since then, it has gone through five generations and undergone multiple upgrades to provide a more sophisticated treatment1. Since then, it has been used in B-cell acute lymphoblastic leukemia (ALL), B-cell non-Hodgkin lymphoma, including diffuse large B-cell lymphoma (DLBCL), follicular lymphoma with (DLBCL), high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and mantle cell lymphoma, and more recently in targeted therapy of multiple myeloma. However, it also develops an overwhelming immune response in some patients in the form of cytokine release syndrome (CRS). Clinically, this is seen as fever, low blood pressure, respiratory distress, and multiple organ failure amongst other manifestations. As a result of on/off-tumor toxicity, antigens targeted by CAR T-cells can destroy normal B-cells apart from cancerous ones. Therefore, B-cell aplasia can result2, which is also an indicator of the CAR T-cell activity. Neurotoxicity is another common pathology associated with CAR T-cell therapy in the clinical setting. Neurologic toxicity is considered separate from CRS even if certain common cytokines are involved in its development. It is still early days for targeted CAR T-cell therapies but studies have shown promising results with cancer remissions right after treatment and a cancer-free period of more than 12 months reported in the literature. Future and ongoing research indicates that there can be more insights of role of CAR T-cell therapy in treating solid organ tumors like breast cancer, lung cancer, and possibly brain cancer. Other researchers are extensively finding ways to reduce therapy side effects and investigating the ways to extend the length of time that CAR T-cells might contain cancer.
In this review, we present a detailed discussion of the pathophysiology, clinical manifestations, diagnosis, and management of the neurological risk profile in patients receiving CAR T-cell therapy.
Methods
An extensive literature was carried out using PUBMED/MEDLINE, Scopus, Web of Science (WoS), and Google Scholar from its inception to June 2020. The following search string was employed: (‘CAR T-cell therapy’) AND (‘neurotoxicity’) AND (‘pathophysiology’ OR ‘clinical manifestations’ OR ‘diagnosis’ OR ‘management’ OR ‘treatment’). All articles in a language other than the English were excluded from our review. We tried to formulate this narrative review by including relevant text and knowledge from prior literature.
Overview of CAR T-cell therapy
CARs commonly contain three modules3. They are all in series, namely: an antigen recognition domain, a transmembrane element, and a signaling endodomain. The first generation of CARs contained a single-chain variable fragment, coexpressing elements from a monoclonal antibody such as the antigen-binding proteins, with the CD3ζ endodomain of the TCR/CD3 complex. However, they failed to show the required T-cell expansion and persistence4. The invention of second-generation and third-generation CARs came with the integration of one or two co-stimulatory domains, respectively. CD28 or 4-1BB signaling elements are known to be the best known and widely tested co-stimulatory domains. Co-stimulation prevents the unresponsive state seen in primary TCR stimulation, known as ‘anergy’. CAR T-cell therapy determines the target specificity and affinity, similar to the light chain region of an antibody. It does so without the need for histocompatibility complex activation, which imparts more flexibility to it. This is particularly useful in targeting tumor cells with down-regulated HLA expression and proteasomal antigen processing5. CAR T-cell therapy is important for T-cell expansion and persistence. Another important advantage is its ability to bind to protein as well as glycolipid and carbohydrate structures. It can be active in both CD4+ and CD8+ cells and there is only a minimal risk of autoimmunity and graft-versus-host disease. Other than that, role of different chemokines (GM-CSF, CXCL8, etc.) have a more comprehensive pathophysiology that contributes to ICANS.
Clinical manifestations and pathophysiology of CRS
Clinical manifestations
CRS is an acute systemic inflammatory response syndrome caused by the release of inflammatory cytokines such as Interleukin-2 (IL-2), IL-2 receptor a, IL-6, IL-8, IL-10, interferon-γ, and tumor necrosis factor. It varies from being self-limiting to being treated in an ICU6. The first symptom observed in CRS is fever7–9. According to the clinical trials7,10, the onset and the duration of the fever varied with the grading of CRS. Patients with a grade greater than 4, experienced fever within 25 h whereas patients with a grade less than 3, experienced fever after 12 days of the CAR T-cell infusion. In addition to fever, the patients also experience tachycardia, hypotension, hypoxia, and some neurological changes such as decreased attention span, language disturbance, and impaired handwriting. Some of the severe neurological manifestations include obtundation, seizures, and cerebral edema11,12.
The severity of CRS is determined by the elevation of IL-6, IFN-Y, and soluble IL2Ra serum markers, which show a marked increase in severe CRS as compared to the CRS without severity13. Severe CRS manifests as pulmonary edema, vascular leak, renal dysfunction, cardiac problems, hepatic failure, and coagulopathy9. According to a phase 1 trial at Memorial Sloan Kettering Cancer Center (MSKCC)14, the severity of CRS is associated with a higher disease burden as compared to a lower disease burden. 41% of the patients with high disease burden were observed to have severe CRS, whereas only 5% of patients with lower disease burden experienced it.
Multiple end-organ toxicities caused by the CAR T-cell infusion are mostly reversible. The constitutional symptoms include fever, malaise, fatigue, and headache. CRS has an impact on the human heart leading to cardiac problems like QT-prolongation6, troponinemia15, arrhythmias including sinus tachycardia15–17, and decreased left ventricular ejection fraction6,15,17. It also causes hepatic impairment by increasing the hepatic enzymes and bilirubin as observed in the clinical trials conducted in 201218 and 201619. Similarly, there is an increase in the serum creatinine level, which suggests renal insufficiency6,18 which further leads to hypokalemia, hyponatremia, and hypophosphatemia. Tumor lysis syndrome20 and muscle damage6,19 has also been reported as an effect of CRS. Furthermore, there are respiratory problems following the CAR T-cell infusion and they include dyspnea, increased respiratory rate, and pleural effusions. Hematologic toxicities have also been observed in some reports6,15 which show that there is a development of anemia, neutropenia, and thrombocytopenia where conditioning chemotherapy regimens have been used. An increased prothrombin time, partial thromboplastin time, and decreased fibrinogen levels have also been seen in a clinical trial21. In some cases8, disseminated intravascular coagulation may be the consequence of the hematological toxicity.
Pathophysiology
CAR T-cells target antigens, proliferate, and become activated to secrete large amounts of cytokines such as IL-1, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, MCP-1, and MIP-1α. Immune cells stimulated include lymphocytes such as B-cells, T-cells and natural killer cells, and/or myeloid cells including macrophages, dendritic cells, and monocytes. Uninterrupted stimulation of the immune system, particularly macrophages, can explain the development of hemophagocytic lymphohistiocytosis/macrophage activation syndrome. Again, some cytokines are involved13 and a genetic predisposition also exists in these patients. Moreover, IL-6 is an important cytokine of CRS, highly associated with macrophages22, which initiates a proinflammatory IL-6 mediated signaling cascade9. The severity of CRS depends upon the tumor burden. The choice of co-stimulatory ligand and the level of lymphodepletion, both of which are associated with enhanced T-cell proliferation, are also known to affect the severity of CRS. Endothelial activation is also involved. High serum concentrations of VWF, Ang2, and endothelium-activating cytokines, such as IL-6 and interferon-γ, can explain the capillary leak and coagulopathy associated with severe CRS7.
Grading of CRS
The Common Terminology Criteria for Adverse Events (CTCAE) 4.3 (Table 1)24 was the first grading scheme used, which was modified by later clinical trials (Table 2). Lee et al. and others redefined the clinical presentations associated with CRS grading in CTCAE 4.3. Guidelines were altered with regards to hypoxia requiring oxygen support, hypotension, and responsiveness to vasopressors and other end-organ toxicities, particularly in grades 2 and 3. The CARTOX consensus group defined hypotension in their criteria as systolic blood pressure less than 90 mmHg in adults25. Interestingly, other symptoms of CRS were not included in the grading criteria because they were always associated with hypotension and/or hypoxia. It is evident from the grading criteria in Tables 1 and 2 that disparities exist in the guidelines. Hypoxia and hypotension have not been consistently defined as well. Therefore, ASTCT, the consensus grading system was formulated in 2018, which called for the harmonization of CRS grading and definitions23.
Table 1.
Comparison of cytokine release syndrome grading using CTCAE versions 4.03 and 5.023.
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|
| Version 4.0324 | Mild reaction; infusion interruption not indicated; intervention not indicated | Therapy or infusion interruption indicated, but responds promptly to symptomatic treatment (antihistamines, NSAIDS, narcotics, IV fluids); prophylactic medications indicated for ≤24 h | Prolonged (e.g. not rapidly responsive to symptomatic medication and/or brief interruption of infusion); recurrence of symptoms following initial improvement; hospitalization indicated for clinical sequel (such as renal impairment, pulmonary infiltrate) | Life-threatening consequences; pressor or ventilator support indicated | Death |
| Version 5.025 | Fever, with or without constitutional symptoms | Hypotension responding to fluids. Hypoxia responding to <40% FiO2 | Hypotension is managed with one pressor. Hypoxia requiring ≥40% FiO2 | Life-threatening consequences; urgent intervention needed | Death |
Lee et al. 201923. Table is adapted from above publication.
Table 2.
Comparison of grading criteria utilized in different clinical trials23.
| Lee et al. criteria9 | Porter et al. criteria26 | MSKCC criteria14 | CARTOX criteria12 | |
|---|---|---|---|---|
| Grade 1 | Symptoms are not life-threatening and require symptomatic treatment only, e.g. fever, nausea, fatigue, headache, myalgia, malaise | Mild reaction: treated with supportive care such as antipyretics, antiemetic | Mild symptoms, requiring observation or symptomatic management only (e.g. antipyretics, antiemetic, pain medications, etc.) | Temperature >38°C (fever Grade 1 organ toxicity |
| Grade 2 | Symptoms require and respond to moderate intervention. Oxygen requirement <40% or hypotension responsive to fluids or low-dose pressor or Grade 2 organ toxicity | Moderate reaction: some signs of organ dysfunction (e.g. Grade 2 creatinine or Grade 3 LFTs) related to CRS and not attributable to any other condition. Hospitalization for management of CRS-related symptoms, including fevers with associated neutropenia, need for IV therapies (not including fluid resuscitation for hypotension) | Hypotension requiring any vasopressors <24 h, or Hypoxia or dyspnea requiring supplemental oxygen <40% (up to 6L NC) | Hypotension responding to IV fluids or low-dose vasopressors, hypoxia requiring FiO2 <40%, Grade 2 organ toxicity |
| Grade 3 | Symptoms require and respond to aggressive intervention. Oxygen requirement ≥40% or hypotension requiring high-dose or multiple pressor or Grade 3 organ toxicity or Grade 4 transaminitis | More severe reaction: hospitalization required for management of symptoms related to organ dysfunction, including Grade 4 LFTs or Grade 3 creatinine related to CRS and not attributable to any other conditions; this excludes management of fever or myalgia; includes hypotension treated with intravenous fluids (defined as multiple fluid boluses for blood pressure support) or low-dose vasopressors, coagulopathy requiring fresh frozen plasma or cryoprecipitate, or fibrinogen concentrate, and hypoxia requiring supplemental oxygen (nasal cannula oxygen, high-flow oxygen, CPAP, or BiPAP). Patients admitted for management of suspected infection due to fevers and/or neutropenia may have Grade 2 CRS |
Hypotension requiring any vasopressors ≥24 h, or Hypoxia or dyspnea requiring supplemental oxygen ≥40% | Hypotension needing high-dose or multiple vasopressors, hypoxia requiring FiO2 ≥40%, Grade 3 or Grade 4 transaminitis |
| Grade 4 | Life-threatening symptoms. Requirements for ventilator support or grade 4 oxygen toxicity (excluding transaminitis) | Life-threatening complications such as hypotension requiring high-dose vasopressors, hypoxia requiring mechanical ventilation | Life-threatening symptoms Hypotension refractory to high-dose vasopressors *Hypoxia or dyspnea requiring mechanical ventilation |
Life-threatening hypotension, Needing ventilator support, Grade 4 organ toxicity except for Grade 4 transaminitis |
Lee et al. 201923. Table is adapted from above publication.
Davila et al. also defined the severity of CRS based on cytokine levels and clinical features16. Under their criteria, severe CRS was characterized by fever greater than or less than 38°C for at least 3 consecutive days, two serum cytokines elevated at 75-fold over baseline, or one serum cytokine elevated 250-fold over baseline, and one clinical sign of severe toxicity. Severe toxicity could be in the form of hypotension requiring at least one intravenous vasoactive pressor or hypoxia (PO2 <90%) or neurologic disorders including mental status changes, obtundation, and seizures.
Pathophysiology of CAR T-cell associated neurotoxicity
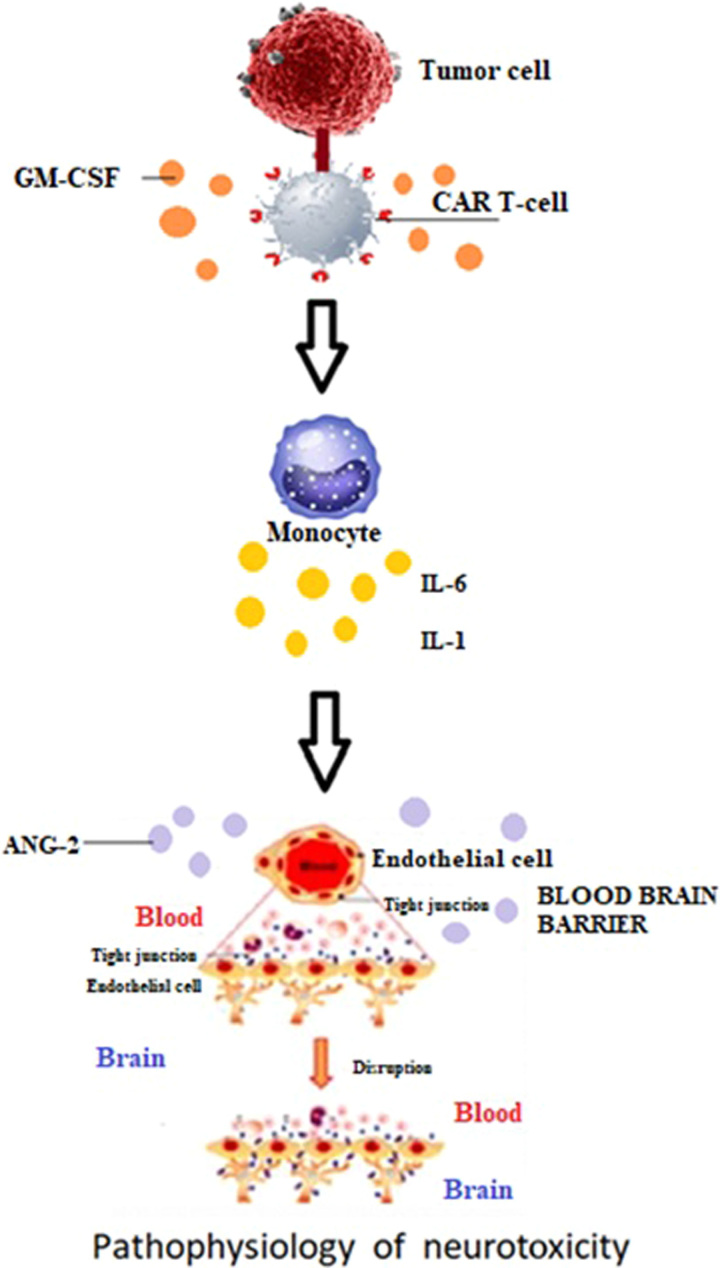
The pathophysiology of neurotoxicity and that of CRS has not been completely understood as of yet; however, certain mechanisms have been worked upon. The pathogenesis underlying neurological risk profile in CAR T-cell patients is demonstrated in Figure 1. In about 90% of patients, the onset of neurotoxicity occurs with CRS or after its resolution, the neurotoxicity that occurs without manifestation of CRS is mild or of grade 1. The mechanism of CRS leads to the activation of immune cells, that is monocytes and macrophages. The activated macrophages secrete large amounts cytokines including IL-6, IL-1, IL-10 inducible nitric oxide synthase (iNOS), and other mediators for inflammation. In a study of leukemic mice, monocytes were the main source of IL-1 and IL-6 during CRS, and inhibition of the IL-6 receptor (IL6R) with tocilizumab prevented CRS but did not affect neurotoxicity. Blocking IL1 with the IL1 receptor (IL1R) antagonist known as anakinra, prevented both CRS and neurotoxicity27.
Figure 1.
Pathophysiology underlying neurotoxicity in CAR T-cell therapy.
Initially, neurotoxicity was linked to direct parenchymal CAR T-cell toxicity, however, new studies suggest that the dysfunction of the blood-brain barrier (BBB) is the main culprit28. Autopsy studies in patients who developed severe CRS along with high-grade neurotoxicity that progressed to fatal cerebral edema support dysfunctional BBB as well29. Another case report of a patient who developed fatal cerebral edema, showed evidence of BBB disruption30. BBB dysfunction has been associated with high levels of TNF-α, IL-6, and IL-131,32. IL-6 has been proven to disrupt the endothelium of the BBB in vitro due to the low expression of intracellular tight junction molecules33. Furthermore, severe neurotoxicity has been linked with elevated levels of IL-15 and granulocyte-macrophage colony-stimulating factor (GM-CSF)34. It was suggested that the interaction of CAR T-cells with the tumor causes CAR T-cells to produce GM-CSF35, which acts as a bridge between the specific immune activity of the CAR T-cells and the off-target inflammatory cascade initiated by immune cells, which leads to myeloid cells to expand synthesis of other inflammatory chemokine and cytokines, including monocyte chemokine protein-1 (MCP-1), IL-1, and IL-6, and others.
In addition to this, a relatively higher level of angiopoietin 2 (ANG2) has been linked with severe neurotoxicity36,37. Angiopoietin 2 (ANG2) is secreted upon activation of endothelial cells by inflammatory cytokines and binds to the TIE2 receptor, which is present on the endothelial cell causing increased vascular permeability38. Angiopoietin 1 is a protein that is produced by perivascular cells, which surrounds the BBB, can be produced by platelets as well, and is usually found bound to the TIE2 receptor. Patients with high-grade neurotoxicity exhibited an increased ratio of ANG2 to angiopoietin 1 (ANG1). The earlier rise in ANG2 levels in the first 24 h following CAR T-cell therapy was associated with a higher risk of developing high-grade neurotoxicity, suggesting that endothelial activation precedes the development of clinical toxicity. Severe neurotoxicity was also linked with higher levels of von Willebrand factor (vWF), a blood glycoprotein involved in hemostasis, and IL-8 also known as a neutrophil chemotactic factor, both of which are stored in the same weibel-palade bodies, which are small storage granules located in endothelial cells as ANG228. Endothelial activation by cytokines and inflammation following the CAR T-cell therapy causes the release of ANG2 and high molecular weight vWF, which results in increased vascular permeability and coagulopathy28. High-grade neurotoxicity is also linked with a higher concentration of biomarkers of diffuse intravascular coagulation with decreased levels of fibrinogen before the manifestation of neurologic signs or symptoms28.
Clinical manifestations of CAR T-cell associated neurotoxicity
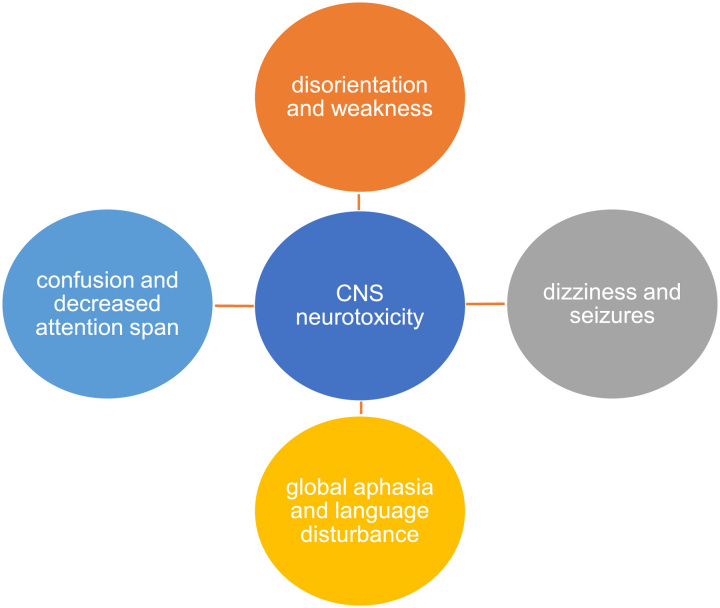
The most frequent and severe toxicity of the CAR T-cell therapy is neurotoxicity, also called as CAR T-cell–related encephalopathy syndrome and immune effector cell-associated neurologic toxicity syndrome (ICANS)39. It is clinically presented with delirium, seizures, dizziness, decreased attention span, disorientation, ataxia, weakness, and sometimes headache as illustrated in Figure 2. It may gradually progress to confusion, difficulty in speaking, and global aphasia after expressive aphasia in severe cases16,40,41. Neurotoxicity greater than grade 2 is severe and is presented with motor weakness, incontinence, mental obtundation, and increased intracranial pressure, which causes papilledema and cerebral edema12. In addition to that, electroencephalography (EEG) detects the encephalopathy in patients presenting with neurotoxicity28,42. Severe neurotoxicity shows abnormal findings on MRI, which includes micro-hemorrhages, white matter changes, and leptomeningeal enhancement28,43. Severe ICANS often develop in patients with severe CRS, with a higher pretreatment tumor burden, younger age, and in patients with pre-existing neurological conditions28. Other than ICANS, CAR T neurotoxicity also include movement disorders, personality and cognitive changes as well as low incidence of peripheral neuropathies.
Figure 2.
Clinical manifestations of CAR T-cell associated neurotoxicity.
Relative frequencies of CRS occurrence is 3–71% as opposed to ICANS (0–56%). Generally, there is a lower rate of neurotoxicity observed in 4-1BB compared to CD28 co-stimulatory CD19 CAR T-cells. According to a study conducted in 2017 with CAR T-cells containing a 4-1BB co-stimulatory domain28, the median time for the presentation of neurotoxicity was reported to be 4 days after the CAR T-cell infusion. Around 40% of the patients had 1 and greater than 1 neurological toxicity whereas only 5% developed neurotoxicity greater than or equal to 4 whereas in the phase 2 ZUMA 1 trial with the CD28 co-stimulatory domain, the median duration of onset was observed to be 5 days and lasted 2–4 days while around 28% had neurotoxicity greater than or equal to grade 344.
Diagnosis of CAR T-cell-associated neurotoxicity
One of the most important findings for the detection of neurotoxicity is the change of the cerebrospinal fluid (CSF) composition. Neurotoxicity shows an increase in the serum protein levels along with an increase in the white blood cell count28,45. However, these markers are not specific as they can also be used to detect other dysfunctions28,45,46. Other serum biomarkers include C-reactive protein (CRP) and ferritin, which upon the administration of CAR T-cells rise above their normal levels. The peak concentration of ferritin varies with the low-grade and high-grade neurotoxicity whereas the peak CRP concentration does not depend on the severity10. Other laboratory findings include lactate dehydrogenase, coagulation assays, metabolites, and electrolyte levels47.
The physical examination includes neurological assessment, monitoring oxygen saturation, blood pressure, and temperature47. The neuroimaging includes brain MRI, which shows patchy T2 hyperintensities in the white matter, and symmetric T2 hyperintensities in the thalami in case of neurotoxicity45,48. The neurological injury can also be detected by the identification of global cerebral edema on imaging. In addition to MRI, a head computed tomography (CT) scan is performed, and according to a clinical trial10 only grade 4 neurotoxicity patients developed subdural hematoma almost 2 weeks after the onset of neurological symptoms, the rest showed normal CT imaging. Furthermore, electroencephalography which is more critical than MRI and CT scans detects the high-grade neurotoxicity characterized by the periodic or rhythmic EEG patterns on the ictal-interictal continuum10. The seizures associated with neurotoxicity are also detected by using EEG49. Along with that, to rule out papilledema, EEG and fundoscopic examination is performed in all cases of neurotoxicity12.
Management and treatment of CAR T-cell-associated neurotoxicity
The management of neurotoxicity varies between different institutions and guidelines while the treatment of neurotoxicity depends on the severity which is determined by the grading criteria (Table 3) and is initiated by providing supportive care42,50. For patients with grade 1 ICANS, the platelet count and sodium levels are frequently monitored in addition to frequent neurological assessment, and corticosteroids are not administered unlike in patients presenting with symptoms showing grade 2 ICANS. The most common first-line corticosteroid used is dexamethasone as it is reported to penetrate the central nervous system well. Along with that, according to a report in 201812, methylprednisolone is given in case of severe ICANS such as grade 4 ICANS depending on the neuroinflammatory disorders. In grade 3 ICANS, patients are admitted in the intensive care unit and electroencephalography, CT, and MRI are performed from time to time in case of increased intracranial pressure.
Table 3.
Grading of immune effector cell-associated neurologic toxicity syndrome (ICANS)23.
| Signs/Symptoms | GRADE 1 | GRADE 2 | GRADE 3 | GRADE 4 |
|---|---|---|---|---|
| ICE scores | 7–9 | 3–6 | 0–2 | 0 |
| Impairment | Mild | Moderate | Severe | Patient in critical condition |
| Seizures | No | No | Any clinical seizure focal or generalized that resolves rapidly; or nonconvulsive seizures on EEG that resolve with Intervention. |
Life-threatening prolonged seizure (>5 min); or Repetitive clinical or electrical seizures without return to baseline in between. |
| Motor weakness | No | No | No | Hemiparesis and paraparesis. |
| Raised intracranial pressure | No | No | Focal/local edema on Neuroimaging. Stage 1–2 papilledema |
Diffuse cerebral edema on neuroimaging; decerebrate or decorticate posturing; or cranial nerve VI palsy or stage 3–5 papilledema or Cushing’s triad. |
| Level of consciousness | Awakens spontaneously | Awakens to voice | Tactile stimulus is needed to awaken | Repetitive tactile stimulus is needed to awaken/ coma |
Lee et al. 201923. Table is adapted from above publication with a formal consent taken from the corresponding author.
Grade 4 ICANS is detected when there are repetitive seizures and increased intracranial pressure and is treated with high doses of the two corticosteroids51. Furthermore, according to a 2016 study52, siltuximab, a chimeric monoclonal antibody can be used to manage CRS and neurotoxicity both by directly binding interleukin-6, preventing it from binding with the IL-6 receptors. If the neurotoxicity is associated with CRS, tocilizumab 8 mg/kg IV can also be used instead of siltuximab12.
Grade 1 ICANS
Patients are managed by supportive care, which includes minimizing the aspiration risks and giving intravenous fluids for hydration. For patients who have a disability in swallowing food or medications are also fed intravenously. Along with that, for grade 1 ICANS, MRI is performed in patients with focal peripheral neurological deficits, lumbar puncture for diagnostic purposes, CT scan is performed where an MRI is not feasible and EEG is carried out for 30 min every day until the symptoms resolve. Medications that can lead to central nervous system depression are avoided and levetiracetam 750 mg is given every 12 h as antiseizure prophylaxis10,49. They also include phenobarbital, which is used for seizures due to neurotoxicity and is preferred after levetiracetam. Low doses of lorazepam are likely to be administered every 8 h for patients who appear disconcerted12.
Grade 2 ICANS
The patients are provided with supportive care and 10 mg IV dexamethasone is given every 6 h or 1 mg/kg IV methylprednisolone is given every 12 h when ICANS is not associated with CRS12.
Grade 3 ICANS
Along with the repetitive neuroimaging every 2–3 days, the patients are given corticosteroids mainly dexamethasone 10–20 mg every 6 h in addition to supportive care42. If stage 1–2 papilledema is detected due to increased intracranial pressure in grade 3 ICANS, 1000 comparison of CRS grading using CTCAE versions 4.03 and 5.0 mg acetazolamide is administered intravenously, which is then followed by 250–1000 mg IV every 12 h12.
Grade 4 ICANS
High-dose corticosteroids are administered until the neurotoxicity reaches grade 1 ICANS and then it is tapered. Repetitive neurological consultation and neuroimaging are also performed in addition to providing supportive care. Patients with grade 4 ICANS are often monitored in the ICU and mechanical ventilation is also provided to protect the airway as it is a severe condition12,42. Stages 3, 4, and 5 papilledema may be detected in patients with grade 4 ICANS. For treatment, high-dose corticosteroids are administered along with the hyperventilation and a 30° elevation of the head of the patient’s bed. In addition to that, hyperosmolar therapy with either mannitol (20 g/dl solution) or hypertonic saline (3% or 23.4%) can be administered12.
Some patients may develop nonconvulsive status epilepticus or convulsive status epilepticus regardless of the grade. For the management of the nonconvulsive status epilepticus, benzodiazepine42, which includes lorazepam, and antiepileptic, which includes levetiracetam are administered and maintenance doses for both are given even after the resolution of seizures. If the seizures persist, the patient is to be monitored in the intensive care unit along with the intravenous administration of phenobarbital 60 mg. Similar treatment is followed in the case of the convulsive status epilepticus except that the dosage of lorazepam is increased and there is a constant electroencephalogram monitoring12.
Conclusion
CRS and neurotoxicity have a close association due to elevation in certain cytokines common in both types of toxicities. However, neurotoxicity is known to be caused by a compromised BBB and endothelial activation. The authors would like to emphasize that both have distinct pathophysiology that led to immune pathways that may be common. The CNS pathway coincides with the immune dysregulation of CRS. The severity of CRS is determined by the elevation of IL-6, IFN-Y, and soluble IL2Ra serum markers, which formulates the common pathway for CNS neurotoxicity. Management depends on the severity of the clinical symptoms determined by the grading criteria.
Ethical approval
Ethical approval was waived by the institutional review board because of the study protocol as narrative review, which does not involve human subjects.
Consent
Informed consent was not required for this review.
Sources of funding
None to declare.
Author contribution
F.Y.: conception of the study, major drafting of the work, final approval, and agreeing to the accuracy of the work; S.M.I.S.: conception of the study, major drafting of the work, final approval, and agreeing to the accuracy of the work; A.R.: conception of the study, major drafting of the work, final approval, and agreeing to the accuracy of the work; S.K.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; I.S.S.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; J.U.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; F.K.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; C.A.H.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; N.R.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; M.S.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; S.K.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; F.N.U.S.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; D.M.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work. D.K.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work; M.S.A.: help in design of the study, drafting of the work, final approval, and agreeing to the accuracy of the work.
Conflicts of interest disclosures
The authors declare that they have no financial conflict of interest with regard to the content of this report.
Research registration unique identifying number (UIN)
Name of the registry: not applicable.
Unique identifying number or registration ID: not applicable.
Hyperlink to your specific registration (must be publicly accessible and will be checked): not applicable.
Guarantor
Muhammad Sohaib Asghar.
Data availability statement
Used existing datasets publically available. No new datasets generated.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Acknowledgements
None to declare.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 20 October 2023
Contributor Information
Muhammad Sohaib Asghar, Email: farahyasmin972@yahoo.com.
Syed M. Ismail Shah, Email: ismailshah6551@gmail.com.
Anooja Rani, Email: anoojarani99@gmail.com.
Sana Kazmi, Email: sanakazmi999@gmail.com.
Ilma S. Savul, Email: ilma.savul@gmail.com.
Janta Ukrani, Email: jukrani@northwell.edu.
Farmanullah Khan, Email: farman018@gmail.com.
Chaudhary A. Hasan, Email: ahmedhasan95@hotmail.com.
Navin Rathore, Email: naveenrathore97@gmail.com.
Maria Syed, Email: mariasyed0@gmail.com.
Shiwani Keswani, Email: shiwani.keswani@gmail.com.
FNU Surkasha, Email: surakshautmani04@gmail.com.
Doongro Mal, Email: doongarmalchettan@gmail.com.
Dileep Kumar, Email: dileepbajeer@gmail.com.
References
- 1.Nasiri F, Kazemi M, Mirarefin SMJ, et al. CAR-T cell therapy in triple-negative breast cancer: hunting the invisible devil. Front Immunol 2022;13:1018786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu S, Yi M, Qin S, et al. Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer 2019;18:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guedan S, Posey AD, Jr, Shaw C, et al. Enhancing CAR T cell persistence through ICOS and 4-1BB costimulation. JCI Insight 2018;3:e96976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov 2013;3:388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hay KA, Hanafi LA, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017;130:2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126:2123–2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karschnia P, Jordan JT, Forst DA, et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood 2019;133:2212–2221. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty R, Sidana S, Shah GL, et al. Patient-reported outcomes with chimeric antigen receptor T Cell therapy: challenges and opportunities. Biol Blood Marrow Transplant 2019;25:e155–e162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 2018;15:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teachey DT, Lacey SF, Shaw PA, et al. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov 2016;6:664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JH, Rivière I, Gonen M, et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018;378:449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali SA, Shi V, Maric I, et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 2016;128:1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brudno JN, Maric I, Hartman SD, et al. T cells genetically modified to express an Anti-B-cell maturation antigen chimeric antigen receptor cause remissions of poor-prognosis relapsed multiple myeloma. J Clin Oncol 2018;36:2267–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kochenderfer JN, Dudley ME, Feldman SA, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119:2709–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brudno JN, Somerville RP, Shi V, et al. Allogeneic T cells that express an Anti-CD19 chimeric antigen receptor induce remissions of b-cell malignancies that progress after allogeneic hematopoietic stem-cell transplantation without causing graft-versus-host disease. J Clin Oncol 2016;34:1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giavridis T, van der Stegen SJC, Eyquem J, et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med 2018;24:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant 2019;25:625–638. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE). Version 4.0: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf
- 25.National Cancer Institute. . Common Terminology Criteria for Adverse Events (CTCAE). Version 5.0: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- 26.Porter D, Frey N, Wood PA, et al. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J Hematol Oncol 2018;11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norelli M, Camisa B, Barbiera G, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 2018;24:739–748. [DOI] [PubMed] [Google Scholar]
- 28.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 2017;7:1404–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hay KA. Cytokine release syndrome and neurotoxicity after CD19 chimeric antigen receptor-modified (CAR-) T cell therapy. Br J Haematol 2018;183:364–374. [DOI] [PubMed] [Google Scholar]
- 30.Torre M, Solomon IH, Sutherland CL, et al. Neuropathology of a Case With Fatal CAR T-cell-associated cerebral edema. J Neuropathol Exp Neurol 2018;77:877–882. [DOI] [PubMed] [Google Scholar]
- 31.Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006;7:41–53. [DOI] [PubMed] [Google Scholar]
- 32.de Vries HE, Blom-Roosemalen MC, van Oosten M, et al. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol 1996;64:37–43. [DOI] [PubMed] [Google Scholar]
- 33.Blecharz-Lang KG, Wagner J, Fries A, et al. Interleukin 6-mediated endothelial barrier disturbances can be attenuated by blockade of the il6 receptor expressed in brain microvascular endothelial cells. Transl Stroke Res 2018;9:631–642. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed O. CAR-T-cell neurotoxicity: hope is on the horizon. Blood 2019;133:2114–2116. [DOI] [PubMed] [Google Scholar]
- 35.Barrett DM, Singh N, Hofmann TJ, et al. Interleukin 6 is not made by chimeric antigen receptor T cells and does not impact their function. Blood 2016;128:654. [Google Scholar]
- 36.Zhang ZG, Zhang L, Croll SD, et al. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience 2002;113:683–687. [DOI] [PubMed] [Google Scholar]
- 37.Nag S, Papneja T, Venugopalan R, et al. Increased angiopoietin2 expression is associated with endothelial apoptosis and blood-brain barrier breakdown. Lab Invest 2005;85:1189–1198. [DOI] [PubMed] [Google Scholar]
- 38.Page AV, Liles WC. Biomarkers of endothelial activation/dysfunction in infectious diseases. Virulence 2013;4:507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santomasso B, Bachier C, Westin J, et al. The other side of CAR T-cell therapy: cytokine release syndrome, neurologic toxicity, and financial burden. Am Soc Clin Oncol Educ Book 2019;39:433–444. [DOI] [PubMed] [Google Scholar]
- 40.Baer B, Dudley CV, Simons RM. Management principles associated with cytokine release syndrome. Semin Oncol Nurs 2019;35:150931. [DOI] [PubMed] [Google Scholar]
- 41.Anderson K, Latchford T. Associated toxicities: assessment and management related to CAR T-cell therapy. Clin J Oncol Nurs 2019;23:13–19. [DOI] [PubMed] [Google Scholar]
- 42.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol 2019;37(Suppl 1):48–52. [DOI] [PubMed] [Google Scholar]
- 43.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst 2019;111:646–654. [DOI] [PubMed] [Google Scholar]
- 44.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-Cell therapy in refractory large B-Cell lymphoma. N Engl J Med 2017;377:2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santomasso B, Park JH, Riviere I, et al. Biomarkers associated with neurotoxicity in adult patients with relapsed or refractory B-ALL (R/R B-ALL) treated with CD19 CAR T cells. J Clin Oncol 2017;35:3019. [Google Scholar]
- 46.Rheingold SR, Chen LN, Maude SL, et al. Efficient trafficking of chimeric antigen receptor (CAR)-modified T cells to CSF and induction of durable CNS remissions in children with CNS/combined relapsed/refractory ALL. Blood 2015;126:3769. [Google Scholar]
- 47.Rivera AM, May S, Lei M, et al. CAR T-cell-associated neurotoxicity: current management and emerging treatment strategies. Crit Care Nurs Q 2020;43:191–204. [DOI] [PubMed] [Google Scholar]
- 48.Gust J, Finney O, Gardner R. Brain inflammation in CD19 CAR T cell treatment-related neurotoxicity 2017. Accessed 27 April 2018. https://virtual.keystonesymposia.org/ks/articles/767/view. [Google Scholar]
- 49.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity associated with CD19-Targeted CAR-T cell therapies. CNS Drugs 2018;32:1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahadeo KM, Khazal SJ, Abdel-Azim H, et al. Management guidelines for paediatric patients receiving chimeric antigen receptor T cell therapy. Nat Rev Clin Oncol 2019;16:45–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou CK, Turtle CJ. Assessment and management of cytokine release syndrome and neurotoxicity following CD19 CAR-T cell therapy. Expert Opin Biol Ther 2020;20:653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah B, Huynh V, Sender LS, et al. High rates of minimal residual disease-negative (MRD−) complete responses (CR) in adult and pediatric and patients with relapsed/refractory acute lymphoblastic leukemia (R/R ALL) treated with KTE-C19 (anti-CD19 chimeric antigen receptor [CAR] T cells): preliminary results of the ZUMA-3 and ZUMA-4 trials. Blood 2016;128:2803. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Used existing datasets publically available. No new datasets generated.