Abstract
Introduction:
From its historical identification to modern times with advancements in management modalities globally, the mortality of necrotizing fasciitis (NF) is high ranging from 19 to 30% for all affected sites. Although many diagnostic adjuncts have been developed to assist with the prompt and accurate diagnosis of NF, the primary diagnosis is still based on high clinical suspicion. The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed as a tool for distinguishing NF from other soft tissue infections. The main objective of this study is to evaluate LRINEC as a tool for early diagnosis of NF and differentiating it from other soft tissue infections like cellulitis.
Methods:
This is a single-centered, prospective observational study. Patients presenting with soft tissue infections of the limbs to the emergency department from November 2020 to October 2021 were included in this study. The clinical findings and blood parameters for the LRINEC score were collected and the score was calculated. Based on clinical suspicion of NF, patients underwent debridement and had a tissue biopsy to confirm the diagnosis. The data obtained was analyzed using SPSS version 24 and MS Excel. The AUC curve was used to calculate a cutoff, sensitivity, specificity, positive predictive value, and negative predictive values for the LRINEC score based on our study.
Results:
Forty-five patients with 28 males and 17 females were included. The average age was 53.667 years within a range of 19–79 years. Among them 44.4% of the patients had NF and 66.6% had other minor forms of soft tissue infections. The ROC curve obtained a cutoff value of greater than or equal to 6, with an AUC of 0.751. At this cut of value study showed a sensitivity of 85% with a specificity of 52%. Similarly, positive predictive value was found to be 58.62%, negative predictive values of 81.25%, and overall accuracy of 66.67% in early diagnosis of NF.
Conclusion:
In conclusion, our study showed that the LRINEC score can be a reliable tool for the early diagnosis of NF in an ED setting. This scoring system is best to be used to rule out NF.
Keywords: LRINEC score, Necrotizing fasciitis, NF, NSTIs
Introduction
Highlights
Necrotizing fasciitis is a disease with high morbidity and mortality.
Early diagnosis is the key point in management of the condition.
The Laboratory Risk Indicator for Necrotizing Fasciitis score is useful in predicting necrotizing fasciitis in an emergency setting.
Necrotizing fasciitis (NF) is a progressive, fulminant bacterial infection of subcutaneous tissue that spreads rapidly through the fascial planes causing extensive tissue destruction1. It spreads from subcutaneous tissue along superficial and deep fascial planes facilitated by bacterial enzymes and toxins. The infection causes vascular occlusion, ischemia, and tissue necrosis along with the damage of superficial nerves leading to characteristic localized anesthesia.
Even with advances in management modalities, globally, the mortality is still high today, ranging from 19 to 30% for all affected NF sites, including the neck, trunk, perineum, and extremities2–6. Early diagnosis of NF is critical for carrying out aggressive surgical debridement and decreasing the mortality and morbidity of NF patients. However, it is a clinical challenge to distinguish NF from other non-necrotizing soft-tissue infections in the emergency department (ED).
Although many diagnostic adjuncts have been developed to assist with the prompt and accurate diagnosis of NF, the diagnosis of NF primarily relies on clinical suspicion7. These diagnostic tools include soft tissue ultrasonography, enhanced computed tomography (CT), MRI, laboratory tests, and scoring systems. Plain radiography had poor sensitivity to rule out NF8–10. CT and MRI can recognize the subtle signs of NF, but will invariably delay the definitive surgical intervention9,11,12. Abnormal biochemical tests may aid in the diagnosis of NF but are not specific since these abnormal changes may also be seen in other causes of infection or inflammation9,10.
The Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score was developed as a tool for distinguishing NF from other soft tissue infections by Wong et al.8. It consists of six laboratory tests including white blood cell (WBC) count, hemoglobin, sodium, glucose, creatinine, and C-reactive protein. The maximum score is 13, and a score of greater than or equal to 6 is suspicious of NF with a probability of 50–75%, whereas a score of greater than or equal to 8 is strongly predictive of NF with a probability of more than 75%8,13. This score has shown robust performance in an initial retrospective external validation. However, in recent validation studies with retrospective chart reviews, the LRINEC score has had inadequate sensitivity in diagnosing NF, ranging from 36 to 83%2,10,13,14.
The LRINEC score varied markedly depending on the affected part of the body, with limbs scoring 6, groin 6.8, and chest/trunk 7.315. This reflects the fact that patients with NF involving different body parts may have unique characteristics. The main objective of this study was to prospectively evaluate the performance of the LRINEC score for the early diagnosis of patients presenting with NF and differentiate it from other soft tissue infections like cellulitis in the ED.
Methods
This was a prospective observational study which was done in the Department of Plastic Surgery and General Surgery in one of the tertiary centers of Nepal. This study was registered in ResearchRegistry.com. Ethical approval was provided by the institutional review committee (Approval No. - 1546–11 E2 077/078). The work has been reported in line with the strengthening the reporting of cohort, cross-sectional, and case–control studies in surgery (STROCSS) criteria16.
All patients older than 18 years, attending the ED with STI of extremities who were admitted for treatment under the Department of General Surgery and the Department of Plastic Surgery and Burns were included from November 2020 to October 2021. Similarly, those patients who attended the ED with STI of the extremities and were treated with IV antibiotics for more than 48 h in the ED were included as well. Those patients who underwent debridement for the same episode of NSTI and who took antibiotics for greater than 48 h before presenting to the ED were excluded.
Considering prevalence of NF to be 39.2 per 1000 population per year (admission data from the same institute in the previous year), sensitivity of LRINEC score of 85.7% from the study of Gard S et al., expected drop out of 10% and CI of 95%, sample size was calculated to be 45. Sample size was calculated using formula n= zα 2×SN (100- SN)/(d2×Prevalence)
The LRINEC score was calculated for all patients presenting to the ED with STI of the extremities within 24 h of presentation. These patients were classified as low (<6), intermediate6,7 and high-risk (>7) for the onset of NF based on their score. In each category, patients with infections were managed appropriately. Patients were selected for surgical intervention based on the clinical suspicion of NF or abscess formation. Intraoperative findings and/or biopsy-proven diagnosis of NF was compared with the LRINEC score at presentation. The tissue was sent for culture and biopsy in case of surgical intervention. Patients were followed till mortality or discharged from the hospital.
A LRINEC score of greater than or equal to 6 was considered as the cutoff for a diagnosis of NF and this was compared with the result of intraoperative findings and/or tissue biopsy report to obtain positive and negative predictive values (NPV) of the LRINEC score as a diagnostic test. A 2×2 table made for analysis and sensitivity, specificity, positive predictive value (PPV), and NPV was obtained. Likelihood ratios for positive and negative tissue biopsy were also calculated. The ROC curve was used to calculate the cutoff LRINEC value in our study.
Statistical analysis was done using Statistical Package for Social Science (SPSS) version 24.00. A P-value of 0.05 was considered statistically significant. The independent student t-test was used to calculate the significance of continuous variables. Pearson’s χ 2-test was used for categorical data. The area under the receiver operating characteristic (AUROC) curves was used to examine the performance characteristics of the LRINEC score and calculate a cutoff value.
Results
A total of 45 patients who presented with soft tissue infections of extremities suspected of NF or cellulitis to our hospital were included in the study. The mean and SD of the age of patients was 53.67±17.17 years within a range of 19–79 years. Maximum patients were in the age group greater than 70 years. Out of 45 patients, 28 (62.2%) were male and 17 (37.8%) were female.
The most frequent presentation was painful swelling (82.2%) followed by ulceration/gangrene (31.1%). Black leathery discoloration and fever were most predictive of NF at presentation. Lower extremity was more frequently involved than upper extremities (84.44 vs. 15.56%). 31 cases (68.68%) had soft tissue infections of unknown origin and the remaining 14 cases (31.11%) were attributed injury as a cause; history of trauma (animal bite, surgery, RTA, and other minor injuries), and IV drug use. Regarding co-morbid conditions, diabetes mellitus was the most common comorbidity (17 cases). Other conditions included systemic hypertension (six cases), peripheral vascular disease (four cases), hypothyroidism (two cases) and, Leprosy (one case).
Among the 45 patients, 16 (35.5%) were categorized as low-risk, 17 (37.78%) were categorized as intermediate-risk and 12 (26.67%) were high-risk for NF based on the LRINEC score. Among the study groups, final diagnosis of NF proven in biopsy was observed in 66.67% of high-risk, 52.9% of the intermediate-risk group, and only 18.75% among the low-risk patients based on LRINEC score.
The CRP, CBC, creatinine, and RBS values increased with the increase in the risk of NF based on the LRINEC score. Hemoglobin and sodium values decreased with the risk. The average CRP value was greater than or equal to 15 mg/dl, WBC value was 15000–25000/mm 3, Hb less than 11 g/dl, Na less than 135 mEq/l, Creatinine greater than 141, RBS greater than 10 in high-risk cases. The duration of IV antibiotics and hospital stay increased with increased risk based on the LRINEC score Table 1.
Table 1.
LRINEC score parameters with risk category.
| Parameter | Low-risk | Intermediate risk | High-risk |
|---|---|---|---|
| CRP (mg/l) | 20.13±26.51 | 34.63±22.52 | 62.45±27.22 |
| WBC (x10³/mm³) | 12851.88±50443.66 | 10918.24±4842.57 | 16400±6555.08 |
| Hb (g/dl) | 13.32±2.47 | 11.188±1.99 | 10.08±2.82 |
| Na (mEq/l) | 135.06±3.51 | 135.47±4.39 | 129±6.87 |
| Creat (umol/l) | 93.32±29.27 | 134.46±133.91 | 203±97.49 |
| RBS (mmol/l) | 8.10±5.60 | 6.63±3.51 | 11.01±4.99 |
| Duration of IV antibiotics (Days) | 7.44±6.35 | 15.68±13.21 | 18.92±11.68 |
| Duration of hospital stay (Days) | 10.06±10.72 | 19.18±18.26 | 21.75±12.27 |
The average duration of IV antibiotics use was 21.50±12.947 days for those who had NF and 7.28±4.50 days for biopsy negative patients. The average duration of stay was 26.55±16.497 days for the tissue positive cases and 8.68±6.793 days for those who did not have NF. CRP and WBC showed a significant difference between NF and No NF cases, (P-value 0.019 and 0.031) Table 2.
Table 2.
Mean LRINEC score parameters compared with the final diagnosis.
| Parameter | No NF | NF | P |
|---|---|---|---|
| CRP (mg/dl) | 27.688±27.8254 | 48.400±28.9025 | 0.019 |
| WBC (x1000/mm³) | 11.4416±4.3905 | 15.1000±6.6125 | 0.031 |
| Hb (g/dl) | 11.912±2.9797 | 11.325±2.3283 | 0.474 |
| Creat (umol/l) | 130.628±121.2074 | 147.915±83.4781 | 0.590 |
| Na (mEq/l) | 135.16±4.394 | 132.20±6.092 | 0.065 |
| RBS (mmol/l) | 8.592±5.6203 | 7.980±4.0657 | 0.985 |
Among the 45 patients, 34 patients had done tissue c/s. 41.17% showed no growth in culture, 29.41% had a single organism and multiple organisms were isolated in 29.14% as well. Ninety percent of patients who showed polymicrobial growth also had NF. The most commonly isolated organism was Escherichia coli (25%) followed by Staph aureus and Acinetobacter spp. (18% each). Seventy-one percent of the patients underwent surgical intervention, 31.25% in the low-risk group, and 34.37% in intermediate and high-risk groups.
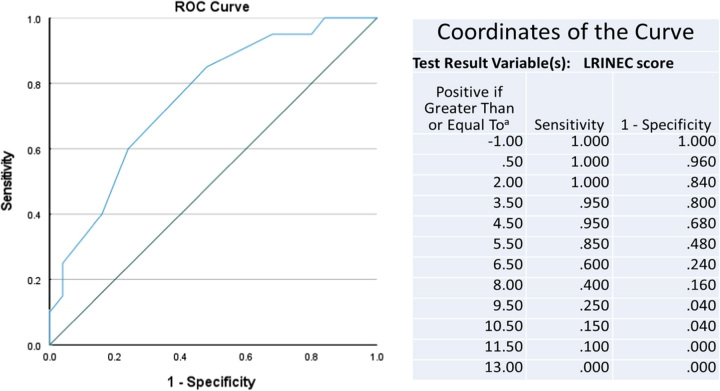
The value of the LRINEC score of 6 had a sensitivity of 85% and specificity of 52% with an accuracy of 66.67% in predicting NF. We obtained a P-value of 0.004 with a 95% CI for LRINEC greater than or equal to 6 which is statistically significant. The ROC curve for the study gave a cutoff value LRNEC score of 6 with an AUC of 0.751 (Fig. 1).
Figure 1.
ROC curve for LRINEC score based on the current study.
Discussion
Necrotizing soft tissue infections are fatal progressive infectious processes, most prevalent among diabetic patients, impoverished obese diabetic patients and IV drug users with a varied spectrum of clinical features associated with severe sepsis. The associated systemic inflammatory response syndrome in the setting of sepsis causes changes in the biochemical parameters in a predictable manner.
The LRINEC score is a measure of these changes and predicts the presence of NF. In this prospective study of 45 patients with suspected NSTIs of extremities in TUTH, Nepal the ROC curve was used to calculate a cutoff value of greater than or equal to 6, which is in agreement with most of the papers published on the LRINEC score. The largest retrospective validation study (NF group: 233, severe cellulitis group: 1394) by Liao et al. 201217 showed that the AUROC curve of the LRINEC score for NF was 0.779, this is similar to our obtained value of 0.751 with a P-value of 0.004.
Our study showed a sensitivity of 85% with a specificity of 52%. We calculated PPV of 58.62%, NPV of 81.25%, and overall accuracy of 66.67% in early diagnosis of NF. Our data corresponds well with a literature review by Abdallah et al. 201918 which reported the LRINEC score had a variable sensitivity ranging from 43.2 to 80%, while 57–64% for PPV and 42–86% NPV. Regarding the accuracy of detecting NF early, various retrospective studies and meta-analyses have shown variable results. Wong et al. 8 first described the use of the LRINEC score for early diagnosis of NF in 2004. They carried out a retrospective observational study of 145 patients with NF and 309 patients with abscess or cellulitis. They obtained a PPV of 92 and NPV of 96% with a LRINEC score cutoff of greater than or equal to 6.
A prospective cohort study conducted in Thailand from December 2013 to December 2015 by Sirikurnpiboon19 included 164 patients diagnosed with NF and the other 103 were confirmed as cases of cellulitis. The results showed that for patients who had a duration of symptoms greater than 8 h, the optimal cutoff LRINEC score of greater than 4 was effective in predicting NF. The sensitivity was 85.42%, specificity was 75.31%, PPV was 67.21%, NPV was 89.71%, and accuracy was 79.07%. Their results were fairly similar to the results we obtained from our study.
Another study conducted by Gargand Resident 201920 retrospectively analyzed 35 cases admitted to Darbhanga Medical College and Hospital from September 2017 to February 2019 with an initial diagnosis of NF. Out of these, 14 patients had biopsy-proven NF. Taking LRINEC Score greater than or equal to 6 as a cutoff, they obtained a sensitivity of 85.7%, specificity of 67%, PPV of 63.2% and NPV of 87.5% for diagnosing NF. The second-largest retrospective validation study for LRINEC score by Neeki et al. 21 (NF group: 47, severe cellulitis group: 948) revealed that the sensitivity with an LRINEC score greater than or equal to 6 was 36%, and the specificity was 89%.
A systematic review by Bechar et al. 201722 to identify articles reporting the use of the LRINEC score and the incidence of NF with a random-effects model and 95% CI, included 16 studies with 846 patients. This review concluded that the LRINEC score was a useful clinical determinant in the diagnosis and surgical treatment of patients with NF, with a statistically positive correlation between the LRINEC score and a true diagnosis of NF.
Our study showed a significant difference in only CRP and WBC values (P-value 0.019 and 0.031). Neeki et al. 201721 and Hsiao et al. 20202 found a significant difference among all the variables except hemoglobin values. However, Narasimhan et al. 201823 and Sirikurnpiboon et al. 19 found a significant difference among all the variables.
The main fallacy with the use of the LRINEC score is that different co-morbid conditions such as diabetes and CKD will give a high LRINEC score in the absence of any inflammation. Furthermore, TLC and CRP may be elevated in any condition that causes inflammation regardless of the clinical features of NF.
Since NF is a clinical diagnosis that relies primarily on clinical judgement, the LRINEC score seems to have a less important role in the diagnostic process. However, the LRINEC score can give an extra edge in early diagnosis for those with equivocal clinical suspicion. The scoring should not be the only factor for deciding early surgical intervention as this may lead to misdiagnosing some cases. The LRINEC score must be used in adjunction with clinical judgement for the best results.
We are aware of the limitations of our study as it was a single-center observational study consisting of only a limited patient pool. All the cases were selected based on clinical suspicion and further compared by intra-op findings and biopsy results after undergoing debridement. The cases did not undergo CT or MRI or percutaneous biopsy. Secondly, we only included patients with clinical suspicion of NF in the extremities and established literature has shown that the LRINEC score varied significantly depending on the body part affected. To further establish the validity, a multicenter study involving a large study sample is needed.
Conclusion
In conclusion, our study showed that the LRINEC score can be a reliable tool for early diagnosis of NF in an ED setting which is robust and easily obtained. It can be used as an adjunct in the management of soft tissue infections especially in secondary care hospitals and may prevent delayed referrals to tertiary care cares for urgent care. It is also helpful in high-volume tertiary care centers with large turnover in the ED where the decision for intervention must be made at the earliest. However, the LRINEC score should not be the only indicator for early surgical intervention. It must be used with caution to further impress the clinical findings (Table 3).
Table 3.
2×2 table of No cases based on LRINEC score.
| LRINEC score | <6 | ≥6 | P |
|---|---|---|---|
| NF | 3 | 17 | 0.004 |
| No NF | 13 | 12 |
Ethical approval
Ethical approval was taken from Institutional Review Committee, Institute of Medicine, Kathmandu, Nepal. (Reference no: 154(6-11) E2 077/078).
Consent
Written informed consent was obtained from the patients for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Sources of funding
No funding was done.
Author contribution
I.A., S.D., S.G., and A.K.: were involved in study design, data collection, analysis and interpretation, and writing manuscript; R.K., P.P., P.N., P.N., and R.D.K.: were involved in data analysis and manuscript revision; J.M.S.: was involved in data analysis and final manuscript editing. All authors approved the final submission.
Conflicts of interest disclosure
None.
Research registration unique identifying number (UIN)
Name of the registry: Research registry.
Unique identifying number or registration ID: researchregistry9321.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.researchregistry.com/browsetheregistry#home/registrationdetails/64c12c40b428ff0028d9cca0/
Guarantor
Suman Dahal, Department of General surgery, Tribhuvan University Teaching Hospital, Institute of Medicine, Kathmandu, Nepal. Tel: +977 9817883095. E-mail: drdahalsurgeon@gmail.com
Data availability statement
Will be available on request from the concerned authority.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Published online 1 November 2023
Contributor Information
Ibrahim Adhil, Email: ibrahim91adhil@gmail.com.
Suman Dahal, Email: drdahalsurgeon@gmail.com.
Sushil Gyawali, Email: drsushilgyawali@gmail.com.
Prashansha Neupane, Email: neupane.prashansha@gmail.com.
Ashok Kharel, Email: kharelashok001@gmail.com.
Priyanka Neupane, Email: priyankaneupane365@gmail.com.
Prarthana Pachhai, Email: aries.pachhai@gmail.com.
Rabi Khadka, Email: khadka543210@gmail.com.
Raj D. Khatiwada, Email: rajdeep_456@hotmail.com.
Jayan M. Shrestha, Email: jayanshrestha11@yahoo.com.
References
- 1.Davoudian P, Flint NJ. Necrotizing fasciitis. Continuing education in anaesthesia. Crit Care Pain 2012;12:245–250. [Google Scholar]
- 2.Hsiao CT, Chang CP, Huang TY, et al. Prospective validation of the laboratory risk indicator for necrotizing fasciitis (LRINEC) score for necrotizing fasciitis of the extremities. PLoS One 2020;15:e0227748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nordqvist G, Walldén A, Brorson H, et al. Ten years of treating necrotizing fasciitis. Infect Dis 2015;47:319–325. [DOI] [PubMed] [Google Scholar]
- 4.Hua C, Sbidian E, Hemery F, et al. Prognostic factors in necrotizing soft-tissue infections (NSTI): a cohort study. J Am Acad Dermatol 2015;73:10061012.e8. [DOI] [PubMed] [Google Scholar]
- 5.Kapp DL, Rogers M, Hermans MHE. Necrotizing fasciitis: an overview and 2 illustrative cases. Int J Lower Extrem Wounds 2018;17:295–300. [DOI] [PubMed] [Google Scholar]
- 6.Corona PS, Erimeiku F, Reverté-Vinaixa MM, et al. Necrotising fasciitis of the extremities: implementation of new management technologies. Injury 2016;47(Suppl 3):S66–S71. [DOI] [PubMed] [Google Scholar]
- 7.Stevens DL, Bryant AE. Necrotizing soft-tissue infections. In: Longo DL, editor. New England J Med 2017;377:2253–2265. [DOI] [PubMed] [Google Scholar]
- 8.Wong C-H, Khin L-W, Heng K-S, et al. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med 2004;32:1535–1541. [DOI] [PubMed] [Google Scholar]
- 9.Hakkarainen TW, Kopari NM, Pham TN, et al. Necrotizing soft tissue infections: review and current concepts in treatment, systems of care, and outcomes. Curr Problems Surg 2014;51:344–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hodgins N, Damkat-Thomas L, Shamsian N, et al. Analysis of the increasing prevalence of necrotising fasciitis referrals to a regional plastic surgery unit: a retrospective case series. J Plastic, Reconst Aesthetic Surg 2015;68:304–311. [DOI] [PubMed] [Google Scholar]
- 11.Carbonetti F, Cremona A, Carusi V, et al. The role of contrast enhanced computed tomography in the diagnosis of necrotizing fasciitis and comparison with the laboratory risk indicator for necrotizing fasciitis (LRINEC). La Radiologia Medica 2016;121:106–121. [DOI] [PubMed] [Google Scholar]
- 12.Ali SZ, Srinivasan S, Peh WCG. MRI in necrotizing fasciitis of the extremities. Br J Radiol 2014;87:1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong C-H, Khin L-W. Clinical relevance of the LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score for assessment of early necrotizing fasciitis. Crit Care Med 2005;33:1677. [DOI] [PubMed] [Google Scholar]
- 14.Harasawa T, Kawai-Kowase K, Tamura J, et al. Accurate and quick predictor of necrotizing soft tissue infection: Usefulness of the LRINEC score and NSTI assessment score. J Inf Chemoth 2020;26:331–334. [DOI] [PubMed] [Google Scholar]
- 15.Kim KT, Kim YJ, Won Lee J, et al. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology 2011;259:816–824. [DOI] [PubMed] [Google Scholar]
- 16.Mathew G, Agha R.for the STROCSS Group . STROCSS 2021: strengthening the reporting of cohort, cross-sectional and case-control studies in surgery. Int J Surg 2021;96:106165. [DOI] [PubMed] [Google Scholar]
- 17.Liao C-I, Lee Y-K, Su Y-C, et al. Validation of the laboratory risk indicator for necrotizing fasciitis (LRINEC) score for early diagnosis of necrotizing fasciitis. Tzu Chi Med J 2012;24:73–76. [Google Scholar]
- 18.Abdullah M, McWilliams B, Khan SU. Reliability of the Laboratory Risk Indicator in Necrotising Fasciitis (LRINEC) score. Surgeon 2019;17:309–318. [DOI] [PubMed] [Google Scholar]
- 19.Sirikurnpiboon S, Sawangsangwattana T. Early Diagnosis of Necrotizing Fasciitis using Laboratory Risk Indicator of Necrotizing Fasciitis (LRINEC) Score. J Med Assoc Thai 2017;100 (Suppl 1):S192–S199. [PubMed] [Google Scholar]
- 20.Garg S, Resident AJ. Laboratory risk indicator in necrotizing fasciitis (lrinec) score-an early diagnostic tool for necrotizing fasciitis. JMSCR 2017;7:905–909. [Google Scholar]
- 21.Neeki MM, Dong F, Au C, et al. Evaluating the laboratory risk indicator to differentiate cellulitis from necrotizing fasciitis in the emergency department. West J Emerg Med 2017;18:684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bechar J, Sepehripour S, Hardwicke J, et al. Laboratory risk indicator for necrotising fasciitis (LRINEC) score for the assessment of early necrotising fasciitis: a systematic review of the literature. Ann Royal Coll Surg Engl 2017;99:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Narasimhan V, Ooi G, Weidlich S, et al. Laboratory risk indicator for necrotizing fasciitis score for early diagnosis of necrotizing fasciitis in Darwin. ANZ J Surg 2018;88:E45–E49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Will be available on request from the concerned authority.