Abstract
The hippocampus is a uniquely infolded allocortical structure in the medial temporal lobe that consists of the microstructurally and functionally distinct subregions: subiculum, cornu ammonis, and dentate gyrus. The hippocampus is a remarkably plastic region that is implicated in learning and memory. At the same time it has been shown that hippocampal subregion volumes are heritable, and that genetic expression varies along a posterior to anterior axis. Here, we studied how a heritable, stable, hippocampal organisation may support its flexible function in healthy adults. Leveraging the twin set-up of the Human Connectome Project with multimodal neuroimaging, we observed that the functional connectivity between hippocampus and cortex was heritable and that microstructure of the hippocampus genetically correlated with cortical microstructure. Moreover, both functional and microstructural organisation could be consistently captured by anterior-to-posterior and medial-to-lateral axes across individuals. However, heritability of functional, relative to microstructural, organisation was found reduced, suggesting individual variation in functional organisation may be explained by experience-driven factors. Last, we demonstrate that structure and function couple along an inherited macroscale organisation, suggesting an interplay of stability and plasticity within the hippocampus. Our study provides new insights on the heritability of the hippocampal of the structure and function within the hippocampal organisation.
Keywords: Hippocampus, Hippocampal subfields, Gradients, Heritability, Microstructure, Functional connectivity
1. Introduction
The hippocampal formation in the medial temporal lobe is involved in numerous functions such as episodic memory (Battaglia et al., 2011, Milner et al., 1998, Squire, 1992), spatial navigation (Burgess et al., 2002), emotional reactivity (Phelps, 2004) and stress resilience (Franklin et al., 2012, Pruessner et al., 2010, Lupien et al., 2009). It is a region highly susceptible to disorder in various neurological and neuropsychiatric conditions, such as schizophrenia (Lieberman et al., 2018), posttraumatic stress disorder (Karl et al., 2006), temporal lobe epilepsy (Bernhardt et al., 2016), and Alzheimer’s disease (Iglesias et al., 2015). Having a three layered allocortex, the hippocampal formation consists of multiple subfields, or zones, starting at the subiculum (SUB) and moving inward to the hippocampus proper; the cornu ammonis (CA), and dentate gyrus (DG) (Palomero-Gallagher et al., 2020, Wisse et al., 2017, Yushkevich et al., 2015, van Strien et al., 2009). These subfields have unique microstructure (Yushkevich et al., 2015, van Strien et al., 2009) and participate differently in the hippocampal circuitry (de Flores et al., 2017), likely implicating different contributions to function (Hodgetts et al., 2017, Berron et al., 2016, Neunuebel and Knierim, 2014). Beyond the internal hippocampal wiring, anatomical projections to isocortical targets vary based on the position within the hippocampal formation (Plachti et al., 2019, Strange et al., 2014). Thus, the intrinsic organisation of the hippocampus relates to its connectivity to the rest of the brain. For example, tracer studies in rodents have shown that the ventral hippocampus is anatomically connected to the olfactory regions, prefrontal cortex, and amygdala, while the dorsal hippocampus is connected to the retrosplenial cortex, mammillary bodies, and anterior thalamus (Bienkowski et al., 2018, Cenquizca and Swanson, 2007). This ventral-dorsal transition in rodents may relate to an anterior-posterior (A-P) axis in humans (Kharabian Masouleh et al., 2020, Nordin et al., 2018). Conversely, hippocampal infolding aligns with a medial-lateral (M-L) axis followed by the subfields, suggesting another transitional axis driven by intracortical microstructure (van Strien et al., 2009, Maass et al., 2015). Thus, the hippocampal formation features two major axes, one from anterior to posterior segments, and the other along its infolding from SUB via CA to DG.
Hippocampal organisational axes can be described using gradients (Margulies et al., 2016). This framework enables continuous representations of the high-dimensional inter-regional patterns, unrestricted by the traditional network boundaries (Bayrak et al., 2019, Langs et al., 2016). Along each gradient axis, voxels/vertices sharing similar connectivity patterns are situated close to each other, whereas those most divergent are at opposite ends of the respective axis (Krienen and Sherwood, 2017). Using this method, hippocampal organisational axes observed in the structure of the hippocampus have been reported to be paralleled by the functional organisation of the hippocampus, as measured in vivo using functional MRI (Genon et al., 2021, Li et al., Vos de Wael et al., 2018). Hippocampal gradients were further associated with its microstructural organisation (Vos de Wael et al., 2018), as well as performance on memory recollection (Przeździk et al., 2019) and pattern separation tasks (Li et al.), suggesting a link between functional organisation of the hippocampus, its structure, and behavioural variability. At the same time, whether hippocampal functional and microstructural organisation axes vary according to genetic factors or rather adapt flexibly as a function of environment is incompletely understood.
There is ample evidence that individual variation on the organization of the hippocampal formation is shaped by both genetic and environmental factors. Indeed, various studies in non-human mammals have indicated unique plasticity of the hippocampal formation associated with its different subfields and underlying microstructural variations (McEwen, 1999, Bannerman et al., 2014). Though studies on genetic and environmental impact on individual variation of hippocampal structure and function in humans are limited relative to work in animal models, various studies have reported subfield heritability (Whelan et al., 2016, Elman et al., 2019, van der Meer et al., 2020). Moreover, genome-wide studies identified single-nucleotide polymorphisms (SNP) associated with hippocampal volumes (Zhao et al., 2019, Hibar et al., 2017, 2015, Stein et al., 2012) showing, in part, unique SNPs for each subfield and furthermore associated with neuropathology of schizophrenia (Maller et al., 2012, Warland et al., 2020). Importantly, heritability provides an estimate to what extent genetic and environmental factors may impact a given trait as it provides a, sample specific, upper limit on the amount of variance explained by genetic factors. This is particularly relevant given that the individual variation in hippocampus is likely also partly explained by non-genetic factors. For example, the unique plasticity of hippocampal formation is associated with its different subfields and, amongst others, affected by the hormonal levels and stress responses (McEwen, 1999, Bannerman et al., 2014). Such environmentally-induced plasticity has also been shown in humans, such as variation in hippocampal structure due to variation hormonal status (Barth et al., 2016, Zsido, et al.) and stress levels (Kim et al. 2015). A second way to reconcile the notion of plasticity and stability reported in the hippocampus is by means of the structural model (Barbas, 2015). This model links isocortical cytoarchitecture and associated connectivity patterns to regional variations in plasticity and stability (Valk et al., 2022, Suárez et al., 2020, Baum et al., 2020, Paquola et al., 2019). In the isocortex, structure-function coupling has been shown to progressively decrease along an axis from unimodal to transmodal regions (Valk et al., 2022, Suárez et al., 2020, Baum et al., 2020, Paquola et al., 2019). Such uncoupling is paralleled by reductions in genetic control from unimodal to transmodal regions (Valk et al., 2022). It is possible that the coupling of microstructure and function in the hippocampus shows meaningful variation along its large-scale axes, and helps to further understand the interrelation between plasticity and stability, or genetic and environmental factors, within this allocortical structure.
Here, we studied to what extent hippocampal function and structure is heritable and shows a genetic correlation with the cortex. To do so, we leveraged the multimodal dataset of the Human Connectome Project (Van Essen et al., 2013) to sample resting state functional time series as well as intensity (as a proxy for intracortical myelination (Glasser and Van Essen, 2011)) in both the hippocampal subfields and isocortex. Our method of choice was the connectivity gradients approach across all hippocampal subfields rather than network parcellation approaches. Connectivity gradients situate brain areas in a continuous fashion based on their functional connectome patterns, whereas the network parcellations draw sharp boundaries (Krienen and Sherwood, 2017). Indeed, recent work has indicated that the structure of hippocampal subfields is governed by shared genetic factors that differ along anterior-posterior axis but are shared among subfields (Whelan et al., 2016, Elman et al., 2019, van der Meer et al., 2020). Diffusion map embedding (Coifman and Lafon, 2006) was used to describe the gradients with the largest axes of variance in functional connectivity and microstructural covariance (Kharabian Masouleh et al., 2020, Vos de Wael et al., 2018). The twin set-up of the HCP data enabled us to quantify both the heritability of these intrinsic functional and microstructural representations, as well as the genetic coupling between hippocampal and isocortical microstructural profiles. Last, we studied the shared organisation of hippocampal function and structure to describe spatial co-variation of structure-function associations along genetic hippocampal organisational axes. We performed extensive robustness analysis to assess the stability of our findings. Overall, these analyses will help to further understand the relationship between the genetic basis of hippocampal organisation and its flexible functional role.
2. Results
2.1. Hippocampal-isocortical functional connectivity is heritable (Fig. 1)
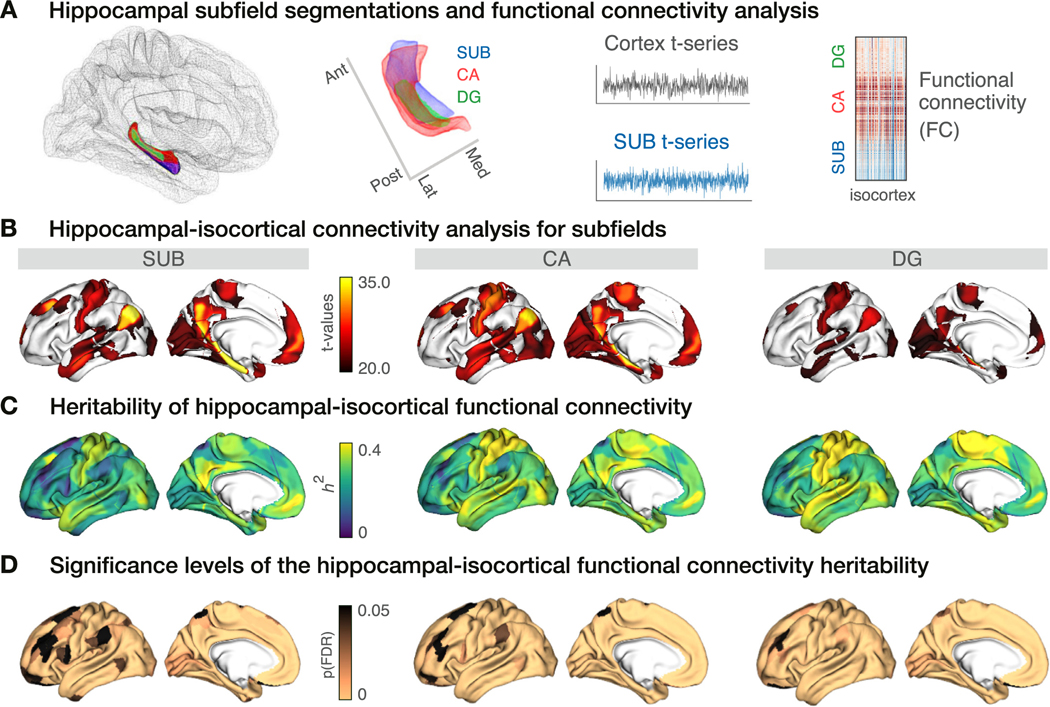
Fig. 1. Hippocampal-isocortical functional connectivity and its heritability.
A. Hippocampal subfield surfaces were automatically delineated using SurfPatch (Caldairou et al., 2016): subiculum (SUB, blue), CA1–3 (CA, red), and CA4-DG (DG, green). rs-fMRI time series were extracted along the individual subfields and correlated with the time series of the isocortex to obtain the functional connectivity (FC). B. Isocortex-wide FC of SUB (left), CA (middle), and DG (right). Isocortex-wide findings were thresholded at t > 20 to represent the highest connections. C. Heritability () scores of the subfield-isocortical functional couplings throughout the cortex. D. Significance levels of the scores from panel C. Significance level was reported with the multiple comparison corrected p-values (p(FDR)). Copper colour denotes pFDR < 0.05 and black colour pFDR ≥ 0.05.
Hippocampal subfields i.e. subiculum (SUB), CA1–3 (CA), and CA4-DG (DG), were delineated automatically using SurfPatch, a previously validated surface-based subfield segmentation algorithm (Caldairou et al., 2016) (Fig. 1A). Resting-state (rs) fMRI time series were extracted along subfield surfaces and isocortical parcels (Glasser Atlas of 360 areas (Glasser et al., 2016)) and correlated to estimate functional connectivity (FC). After quality assessment (Fig. S1A), n = 709 participants (395 women, mean ± SD age = 28.7 ± 3.7 y, 176 monozygotic twins, 178 siblings without twin status and 355 participants without familial relatedness, HCP S900 data release (Van Essen et al., 2013)) were included.
Hippocampal time series were averaged along each subfield (SUB: 1200 × 1, CA: 1200 × 1 and DG: 1200 × 1) and correlated with the isocortical time series (360 × 1200) for each subject. The resulting subfield-to-cortex FC measures (360 × 1) were labeled to the conte69 surface (Van Essen et al., 2012). Subfield-isocortical FC measures were mapped using linear and mixed effects models in BrainStat and thresholded at t > 20 to indicate highest connections (https://github.com/MICA-MNI/BrainStat) (Fig. 1B). Though overall patterns look similar across subfields, comparing connectivity we found each subfield to also have unique connectivity patterns (Supplementary Material). The strongest connections were found in the default-mode, somatomotor, visual and limbic areas, across subfields. To evaluate the heritability of isocortical FC, we ran SOLAR (Sequential Oligogenic Linkage Analysis Routines, SOLAR, v8.5.1) (Almasy and Blangero, 1998) heritability analysis on the mean subfield-to-isocortex FC measures (360 × 1) for every isocortical vertex across all subjects (n = 709) (Fig. 1C). Heritability scores () indicated that heritability of SUB-isocortex FC was the highest in regions part of sensorimotor (mean score: ) and default mode () networks (Fig. S1B). A similar heritability profile was observed for CA-isocortex FC, with highest heritability in sensorimotor (), default mode () and dorsal attention () networks. For the DG-isocortex FC, compared to SUB and CA, we observed a higher heritability in the sensorimotor () and ventral attention () networks. The significance level of the scores were assessed using a likelihood ratio test (p-values) and then corrected for multiple comparisons using FDR (pFDR) (Fig. 1D). Throughout most of the cortical parcels, the heritability was found to be significant, with an increasing number of significant parcels from SUB towards CA and DG.
2.2. Hippocampal functional organisation is moderately heritable (Fig. 2)
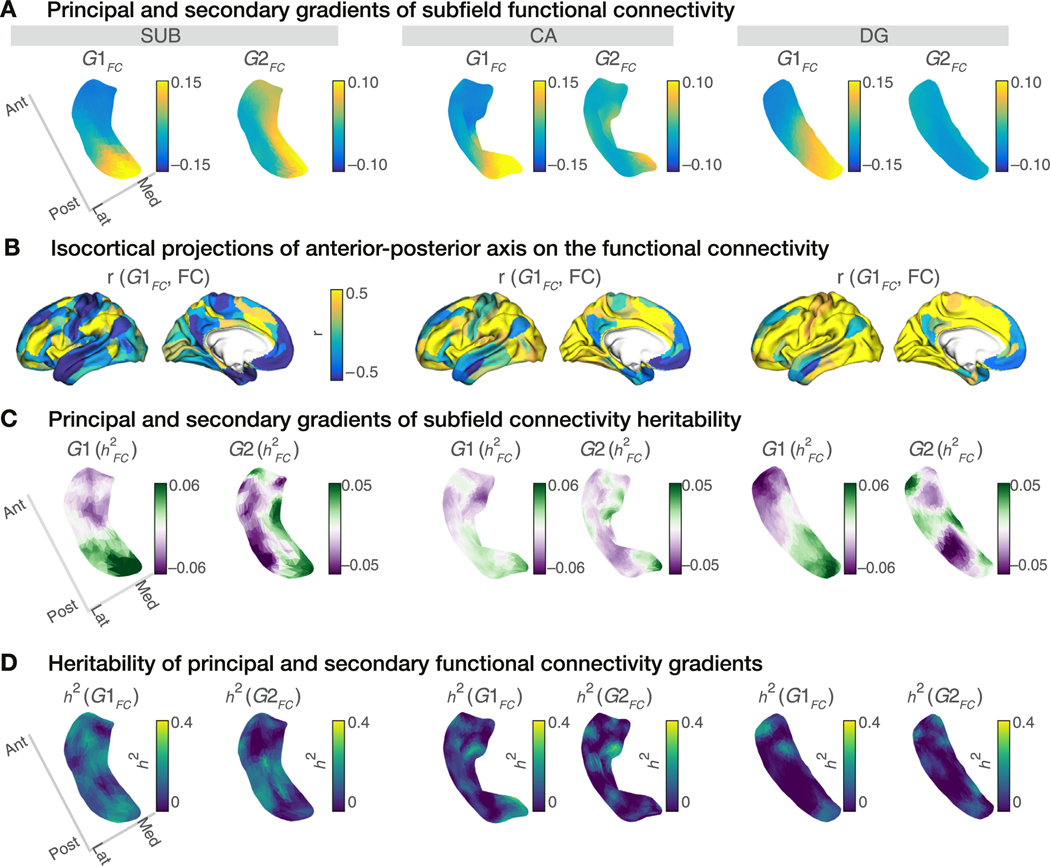
Fig. 2. Topological representations of hippocampal functional organisation and their heritability.
A. Connectivity gradients of subfield-isocortical FC for SUB (left), CA (middle) and DG (right). Gradient 1 () depicts an anterior-posterior (A-P) connectivity axis, whereas Gradient 2 () displays a medial-lateral axis. B. Variations in hippocampal-isocortical FC across the projected on the isocortex (Pearson’s r-values). Lower r-values (blue) indicate FC similarity between the anterior subfield portions and isocortex, whereas higher r-values (yellow) that of the posterior subfield portions and isocortex. C. Subfield-isocortical FC-heritability decomposed into its gradient representations. Primary gradient of FC-heritability depicts an A-P separation of the profiles for all subfields. D. Heritability of primary and secondary functional gradient loadings ( and ) for each subfield vertex. Heritability analysis was run on and from Panel A and revealed a score for gradient loadings for each vertex.
Previous studies have reported strong heritability in hippocampal subfield volumes (Whelan et al., 2016, Elman et al., 2019, van der Meer et al., 2020) (Supplementary Table T4). Here, we aimed to evaluate whether the functional organisation within hippocampal subfields was heritable as well. To do so, we first constructed topographic gradients of the hippocampal FC patterns using unsupervised dimension reduction (Vos de Wael et al., 2018, Coifman and Lafon, 2006) (Fig. 2A). Replicating previous work (Vos de Wael et al., 2018), the principal subfield gradient () presented an A-P axis across hippocampal subfields and explained 42.6% of the variance, whereas the second subfield gradient () described a M-L axis and explained 15.4% of the variance (Fig. S2A). Anterior hippocampal subfield portions (blue in Fig. 2A) were functionally coupled to sensorimotor, default mode and limbic networks (Fig. 2B, Fig. S2B). Posterior hippocampal subfield portions (yellow in Fig. 2A) were functionally more connected to fronto-parietal, salience, dorsal attention and visual networks (Fig. 2B, Fig. S2B).
Next, we computed the hippocampal-isocortical FC-heritability () for every subfield vertex and then decomposed the FC-heritability onto its gradients ( and ) (Fig. 2C). Performing the gradient decomposition on the FC-heritability, we aimed to probe a potential organisational axis underlying the heritability patterns of hippocampal-isocortical FC. The principal heritability gradient, , depicted an A-P trajectory in profiles for all the subfields. We observed almost identical organisational axes stretching from anterior to posterior subfield portions for (Fig. 2A) and (Fig. 2C), although their gradient score loadings were different. However, the secondary heritability gradient, , traversed the M-L axis for SUB, similar to , but did not reveal a clear pattern for CA and DG.
We further obtained the heritability of the A-P and M-L functional gradients ( and ) themselves, as represented in Fig. 2A, and , respectively, to assess whether individual variations in local gradient loadings were heritable (Fig. 2D). For all subfields was found to be modest to low (SUB: mean: 0.14, range: [0, 0.29]; CA: mean: 0.08, range: [0, 0.32]; DG mean: 0.05, range: [0, 0.27]). Also the second gradient’s heritability, , was found to be modest to low for all subfields (SUB: mean: 0.09, range: [0, 0.30], CA: mean: 0.06, range: [0, 0.39], and DG: mean: 0.06, range: [0, 0.23]). Moreover, the heritability strength of both functional gradients did not show a clear spatial pattern (Fig. S2C).
2.3. Hippocampal microstructure is highly heritable and shows genetic correlation with the isocortex along its intrinsic organisational axes (Fig. 3)
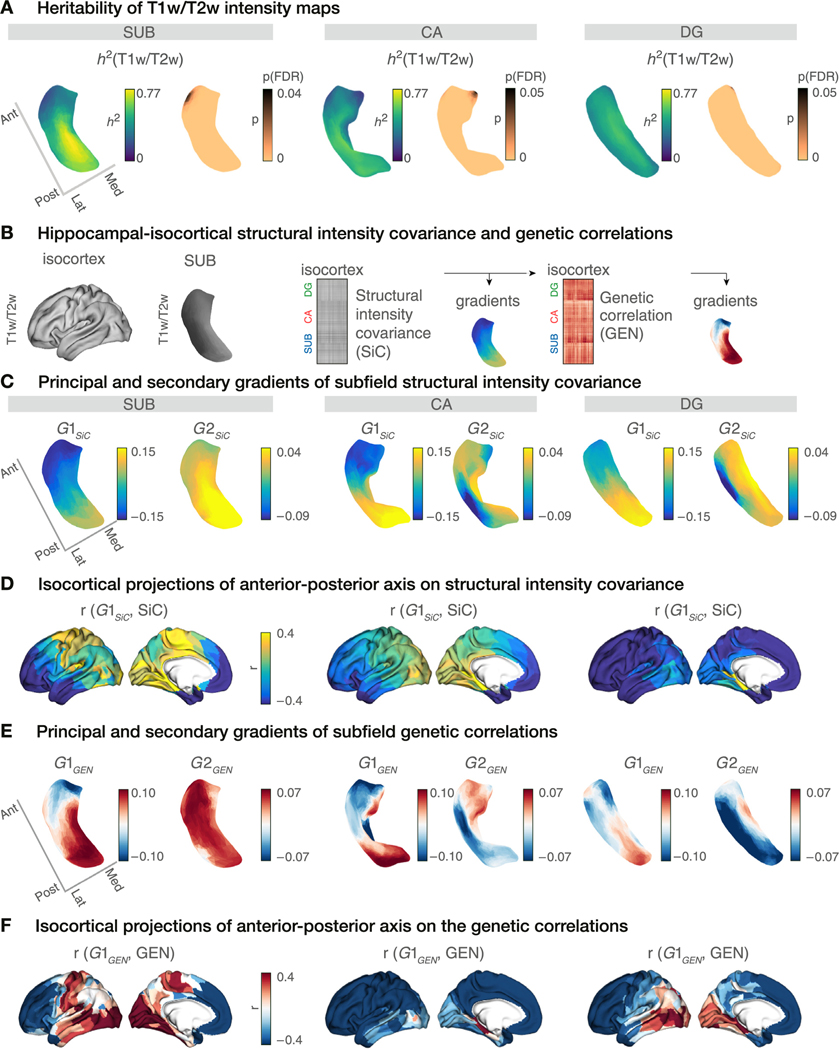
Fig. 3. Hippocampal microstructural organisation and its heritability.
A. Heritability of subfield profiles and its significance levels. maps were strongly heritable across all subfields. p-values were reported after multiple comparison corrections using FDR (copper colour denotes pFDR < 0.05, black pFDR ≥ 0.05). B. Hippocampal-isocortical structural intensity covariance (SiC) was assessed by correlating hippocampal and isocortical intensity maps across participants and subfields. Shared genetic variations in intensity maps were assessed by conducting a genetic correlation (GEN) analysis on the SiC. Both SiC and GEN matrices were then decomposed into their gradient representations, separately. C. Gradients of SiC for SUB (left), CA (middle), and DG (right). represents an anterior-posterior (A-P) axis for all subfields, whereas reflects the differential axis of local transitions for individual subfields. D. Variations in SiC across its projected on the isocortex (Pearson’s r-values). Lower r-values (blue) indicate SiC similarity between the anterior subfield portions and isocortex, whereas higher r-values (yellow) that of the posterior subfield portions and isocortex. E. Gradients of GEN for SUB (left), CA (middle), and DG (right). represents an A-P axis for all subfields, whereas reflects the differential axis of local transitions for individual subfields. F. Variations in GEN across its projected on the isocortex (Pearson’s r-values). Lower r-values (dark blue) depict shared genetic influence between anterior subfield portions and isocortex and higher r-values (red) that of posterior subfield portions and isocortex.
Having shown that functional connectivity of hippocampal subfields is heritable, but intrinsic functional organisation of subfields is less so, we aimed to evaluate the heritability of hippocampal subfield structure. To do so, we utilised individual intensity maps to probe microstructure in vivo (Fig. S3). Local maps were highly heri- table across all subfields, reaching up to for SUB (mean ± SD = 0.44 ± 0.15 for SUB, 0.41 ± 0.12 for CA, and 0.43 ± 0.07 for DG) (Fig. 3A). Multiple comparison corrections using FDR reported significant heritability scores across almost all subfield vertices. By adjusting for the mean as a covariate in the heritability model, we found similar heritability patterns for individual subfields, and both hemispheres (Fig. S4). This indicates that the heritability of subfields was present beyond any mean intensity variation across individuals.
To evaluate the spatial similarity between local microstructure and functional gradients, we quantified the group-level association between the and for subfields (Fig. S5A). maps had the highest correlation with for the SUB (Pearson’s r = 0.93, p-value after spatial autocorrelation correction (Burt et al., 2020); ), and less with the other subfields (CA: r = 0.23, , DG: r = −0.01, ). Furthermore, individual-level and correlations were found to be significantly positive across participants for SUB (median , p < 0.005, one-tailed Wilcoxon signed-rank test) and CA (, p < 0.005), however not for DG (, p < 0.005) (Fig. S5B). We also computed the heritability of individual-level correlations and found them marginally heritable in SUB and CA but not DG (SUB: and p = 0.030, CA: and p = 0.011, DG: and p = 0.5). We further quantified the group-level association between the and (Fig. S5C), that did not result in high associations (SUB: r = 0.20 and , CA: r = 0.12 and , DG: r = 0.37 and ). Last, we evaluated whether functional or microstructural axes of hippocampal formation was influenced by age and sex differences (Fig. S6, S7). Age and sex effects were both observed to be significant along the subfield measures, whereas age factor alone was reported to affect subfield FC measures more predominantly compared to the sex effects.
Then, we evaluated whether there is a genetic correlation between microstructure of hippocampal subfields and that of the isocortex, to probe whether potential co-variation of hippocampal and isocortical microstructure is governed by shared genetic factors. We first correlated vertex-wise subfield and parcel-wise isocortical maps across all participants (n = 709), resulting in a structural intensity covariance (SiC) matrix (Fig. 3B). Using gradient decomposition, we evaluated intrinsic axes of covariance/genetic correlation within subfields based on their correspondence with isocortical microstructure (Vos de Wael et al., 2020). The principal gradient of SiC () revealed an A-P organisational axis across all the subfields (Fig. 3C). We observed a high similarity between and profiles (SUB: r = 0.88, CA: r = 0.86, and DG: r = 0.88, for all subfields). The second gradient of SiC () did not represent a converging organizational pattern for the subfields. Evaluating the pattern of correlation between subfield-isocortex SiC and , we could assess how hippocampal and isocortical regions spatially relate to each other in terms of their microstructural similarity (Fig. 3D). Anterior hippocampal portions (blue in Fig. 3C) shared more microstructural similarity with the anterior isocortex, in particular anterior frontal and temporal cortex, while the posterior hippocampal portions (yellow in Fig. 3C) were related to visual cortex, lingual and fusiform areas, and sensorimotor cortex for SUB, and visual cortex, lingual and fusiform areas for CA. For the DG, we observed less divergent patterns of subfield-isocortical similarity between its anterior and posterior portions, with anterior portions relating to all of the isocortex except for visual, lingual and fusiform areas which showed a positive relation to posterior parts of DG.
Given that the hippocampal microstructure is highly heritable and shows heritable functional connectivity with the isocortex, we next evaluated whether hippocampal and isocortical microstructure are governed by shared genetic factors. Therefore, we computed genetic correlations (GEN) of the SiC between hippocampus and isocortex (Almasy and Blangero, 1998). Here, assigning the SiC as our phenotype of interest, GEN analysis aimed to identify shared genetic processes underlying the microstructural similarity of hippocampus and isocortex. A high GEN score indicates that the microstructural phenotype of subfield and isocortex are influenced by the same set of genes (Almasy et al., 1997). Performing a gradient decomposition on the GEN measures, we observed highly similar gradients as in the SiC (Fig. 3E). The principal gradient of the GEN () again displayed an A-P axis for all the subfields (Fig. 3E). Also showed spatial similarity with (SUB: r = 0.67, CA: r = 0.41, DG: r = 0.75, and for all subfields). The second gradient () did not reveal a consistent organisational axis within each subfield, but rather varied between subfields. Analogous to SiC, we then investigated the correlation between hippocampal-isocortical GEN variations and (Fig. 3F). Indeed, patterns were largely mirroring those observed in SiC, indicating that the structural intensity covariance between hippocampus and isocortex is largely concordant with genetic patterning.
2.4. Coupling of hippocampal subfield function and microstructure (Fig. 4)
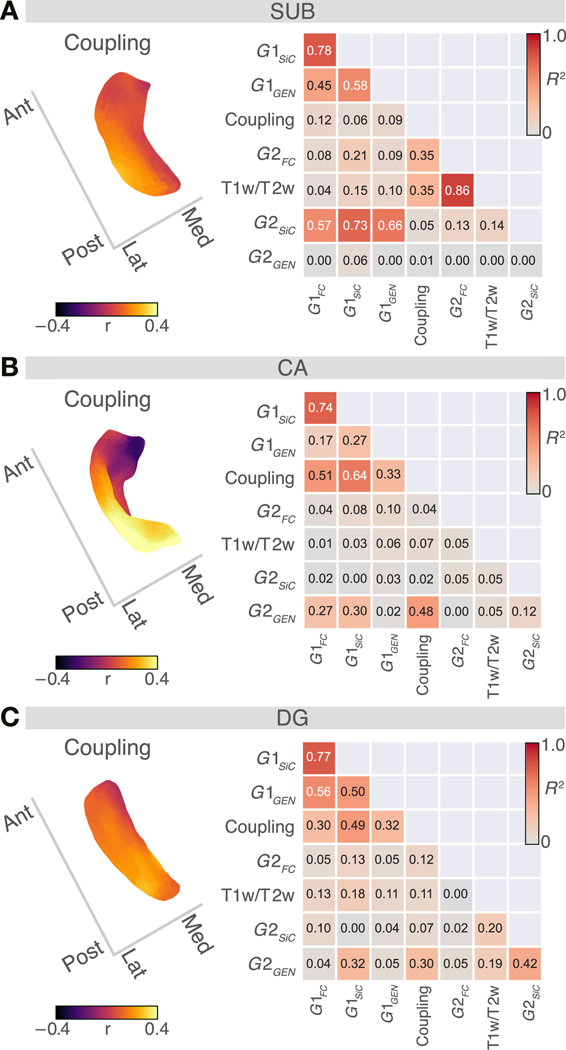
Fig. 4. Hippocampal structural-functional coupling maps and associations among organisational axes.
A. SUB: Subfield vertex-wise coupling map between hippocampal-isocortical functional connectivity (FC) and structural intensity covariance (SiC) (upper panel). Higher coupling values (Pearson’s r) denote an association between FC and SiC, whereas lower coupling values display a dissociation between them. Spatial similarity between hippocampal organisational axes (, , , coupling and maps) is denoted by the coefficient of determination . High values (red) indicate a strong spatial alignment between the organisational axes, whereas low values (grey) an unalignment. Panels B. and C. display coupling maps and values for CA and DG, respectively.
Last, we studied the shared organisation of hippocampal function and structure to evaluate whether regions with similar microstructure in hippocampus and isocortex also show a functional connection as predicted by the structural model. To do so, we computed the coupling of microstructure covariance and functional connectivity between the subfield and isocortex at each vertex of the subfields. Second, to probe whether the similarity of microstructure and functional profiles varied along the respective subfields’ intrinsic functional and structural axes, we computed the degree of hippocampal organisational axes’ similarity using the coefficient of determination () (Fig. 4). For SUB, we found a dominant pattern of A-P axes shared by , , and . However, the M-L axes, reflecting variation in local and , best described the coupling between microstructure and function (), with lateral regions showing moderately positive coupling and medial regions showing low coupling. For CA, coupling rather followed a posterior (high) to anterior (low) pattern, corresponding to , , and (). Last, for DG we found moderate variation in coupling, which showed a spatial relation to , , and (). Here, posterior regions showing increased and anterior regions showing decreased coupling between microstructure covariance and functional connectivity.
3. Discussion
The hippocampus is a densely interconnected region where stability and plasticity coincide. Building on emerging work describing the coalescence of anterior-to-posterior and medial-to-lateral gradients of function and microstructure in the hippocampal formation in vivo (Plachti et al., 2019, Vos de Wael et al., 2018, DeKraker et al., 2021, Paquola et al., 2020), we describe the heritability of hippocampal functional and structural organisation as well as its genetic relationship with the isocortex. First, we found that functional connectivity of hippocampal subfields to isocortex was heritable. However, intrinsic functional organisation of hippocampal subfields showed only marginal heritability. At the same time, we found that spatial variations in subfield intensity maps, serving as a marker for myelin-related microstructure (Glasser and Van Essen, 2011), were heritable and topographically related to local subfield functional organisation. Exploring the covariance of local hippocampal and isocortex microstructure, we found they consistently followed an A-P axis, with anterior subfield regions relating to anterior frontal and temporal cortex, whereas posterior subfield regions relating to visual and inferior temporal areas. These patterns were genetically correlated, indicating that microstructure of the subfields underlie shared genetic influences with the isocortex. Last, evaluating the similarity by the structure-function coupling in the hippocampal subfields along intrinsic hippocampal axes, we found lateral/posterior regions to be highly coupled, whereas anterior/medial regions were uncoupled. In sum, our work illustrates the heritability of hippocampal microstructural and functional organisation, and genetic correlation with the isocortex.
To study the heritability of subregional functional and microstructural organisation of the hippocampal formation, we automatically segmented the hippocampal formation via a subfield and surface-based approach (SUB, CA, and DG) (Caldairou et al., 2016), which has been previously validated in both healthy individuals and those with hippocampal pathology (Bernhardt et al., 2016). Such surface- based approaches improve anatomical alignment across individuals (DeKraker et al., 2021). In the current work, we could replicate previously established hippocampal-cortical FC organisation across subfields. To preserve within-subfield interactions, we implemented the gradients approach on an across-all-subfields functional connectome (FC). This way, we obtained low-dimensional representations of hippocampal connectivity gradients in a purely connectome-driven and continuous fashion. Although connectome gradients were obtained across-all-subfields once, they still represented subfield variations. For instance, the primary gradient emphasised A-P transitions (long axis (Strange et al., 2014, Brunec et al., 2018, Chase et al., 2015, Poppenk et al., 2013)) in all subfields, whereas the secondary gradient depicted M-L separations (transverse axis (Plachti et al., 2019, Maass et al., 2015, Henriksen et al., 2010)) predominantly for SUB, and indeed not for CA and DG. Also, the gradient decomposition of the FC-heritability itself delivered A-P profiles across all the subfields that highly resemble the A-P distribution of molecular hippocampal gradients as shown by Vogel et al., 2020. Second, as in previous work, the M-L axis was found to align strongly with the microstructural proxy, particularly for the SUB and to a lesser extent in CA. In sum, we could replicate previous work (Vos de Wael et al., 2018) and again observe that specialisation of the long axis was preserved in all subfields, whereas the transverse axis indicated a link between intrinsic FC and microstructure, particularly in SUB.
Extending previous work describing mean axes of microstructural and functional organisation of hippocampal subfields, we investigated whether individual variation in hippocampal organisation was partly attributable to genetic factors. Previous molecular genetics studies have shown that hippocampal subregions as well as whole-hippocampal volumes exhibit strong heritability (Whelan et al., 2016, Elman et al., 2019, van der Meer et al., 2020). Moreover, genome-wide studies identified single-nucleotide polymorphisms (SNP) associated with hippocampal volumes (Zhao et al., 2019, Hibar et al., 2017, 2015, Stein et al., 2012) showing, in part, unique SNPs for each subfield and furthermore associated with neuropathology of schizophrenia (Maller et al., 2012, Warland et al., 2020). Here, we extended this work by studying the heritability of subtle variations of microstructure and function within subfield surfaces, as well as their link to the isocortex. We observed highest heritability within the subfield microstructure proxy () and lowest for the A-P and M-L functional hippocampal gradients. Indeed, the heritability of both functional gradients was moderate to low, indicating that individual variation within functional gradients did not vary strongly as a function of genetic proximity of individuals. The variance explained by the functional gradient along the M-L axis, although they are topographically meaningful, was fairly low. This could reduce the ability to detect a significant heritability. At the same time, we found that heritability of subfield-isocortical FC was again organised along an A-P axis, indicating that anterior and posterior portions of hippocampal subfields have distinct and heritable relations with the isocortex. Moreover, we found that the functional M-L gradient correlated strongly with microstructure along the hippocampal subfield surfaces, which, in turn, was highly heritable. It is possible that the intrinsic, heritable, structural axes within the hippocampus scaffold a more flexible functional organisation. Indeed, environmentally induced brain changes may be interpreted as a degree of aberration from the heritability, i.e. the less heritable a brain region/metric, the larger the potential environmental influence (Valk et al., 2022, Arnatkeviciute et al., 2021, Haak and Beckmann, 2019). It is thus possible that the low heritability of functional organisation of subfields reflects these variations, attributable to environmental effects and associated with hippocampal plasticity.
intensity measures require careful consideration while interpreting the microstructure. is a proxy for the myelination degree rather than being a direct biomarker for the cortical microstructure. However, although a direct relationship between cortical myelin and measures has not yet been fully explained, there is subtle evidence for the intensity revealing the myelin differentiation (Glasser et al., 2016, Assem et al., 2020, Ganzetti et al., 2014, Glasser and Van Essen, 2011). Of note, the intensity also reflects the iron- and water-density, cytological variations such as dendritic arborization, cell size and cell density (Burt et al., 2018, Lorio et al., 2016, Stüber et al., 2014). Previous work examining the gradients of the microstructural profile covariances built upon measures showed that 1) the intensity contrasts follow an A-P topography embedded in the cortex (Paquola et al., 2019), and 2) this topography resembles a differentiation of mean myelin content from sensory towards fronto-polar areas (Vogt and Vogt, 1928). Also, the values were shown to follow R1 changes in HCP S900 sample (Vos de Wael et al., 2018), which is the inverse of quantitative T1 relaxation times (R1 = 1/qT1) and were furthermore projected in a difference dataset including healthy controls and epilepsy patients to explain the association between microstructural damage and a neuropathology (Bernhardt et al., 2018).
Overall, our observations on heritability of functional and structural axes, functional connectivity with the cortex and genetic correlation between hippocampal microstructure and isocortical structure illustrate how inherited genetic factors contribute to the hippocampal formation and association with the cortex at the level of the individual. Heritability is the proportion of variance in a population of a trait explained by inherited genetic variants. It is a measure that provides an estimate of the upper limit of how well we can predict a trait based on the genetic profile of an individual. As such the measurement of heritability provides a first estimate to what extent genetic and environmental factors may impact a given trait. Importantly, heritability is a feature of the population and not of an individual. Moreover, heritability is reduced when there is measurement error, but also as a function of non-genetic factors impacting variation across individuals. Consequently, the observed reduced heritability of hippocampal functional organisation may not mean that the hippocampus is weakly influenced by the genetic information, but rather that individual variation could not be accounted for by heritable effects evaluated in our twin model. Rather it means that individual differences in functional gradients may be largely explained by non-genetic factors, such as variations of cognitive state, stress-levels or neuroendocrine levels (Kim et al., 2015, McEwen, 1999). Recent work in functional connectomics has indicated that the intrinsic FC in the isocortex may reflect more trait-like than state-like features (Gratton et al., 2018). Nevertheless, it is still possible that the captured variability in hippocampal functional organisation may reflect more state-like features, also taking into account that the hippocampus is a region that is considered particularly plastic (Cooper et al., 2018). Next to effects on cognitive state on hippocampal function, hippocampal structure and function have been reported to be susceptible to variation hormonal status (Barth et al., 2016, Zsido, et al.) and stress (Kim et al., 2015, McEwen, 1999), factors that likely differ between twins at a given time point. In line with these observations, we found subtle co-variations between age and sex and hippocampal functional and structural organisation (Kim et al., 2015, McEwen, 1999), possibly suggestive of long-term hormonal plasticity effects as well as experience-driven plasticity effects. Future work may expand on our heritability analyses by studying in more detail how environmental factors may impact hippocampal organisation in humans. For instance, feeding machine learning algorithms with heritability measures and hippocampal connecome gradients, a prediction of the behavioral capacity and therefore the individual differences could be possible. Also, evaluating the genetic impact on hippocampal dysregulation, we could understand hippocampus associated neuropathologies better. This would enhance detecting diseases at early stages and moreover suggest employing prophylactic treatments accordingly.
As the internal wiring of the hippocampus relates to its connectivity to the rest of the brain, we evaluated the genetic relationship between subfield surface microstructure and isocortical microstructure. To do so, we probed the covariance between hippocampal subfields and isocortex microstructure (structural intensity covariance, SiC), and their genetic correlation. SiC emphasises the morphological similarity among brain regions, with high covariance between two regions across individuals indicating these regions share maturational and genetic trajectories (Alexander-Bloch et al., 2013). Although the decomposed SiC and genetic correlation measure originated from the maps, its low dimensional components depicted similar spatial organisation to that of the functional maps. The primary covariance gradient revealed an A-P axis for all the subfields, which was mirrored by a highly similar gradient based only on the genetic correlation between local subfield and isocortical microstructure. This indicates a distinction between microstructure of anterior and posterior regions of hippocampal subfields based on its genetic similarity with the isocortex, which was found to be mirrored in its functional organisation in the current sample. Regions in anterior parts of the subfields showed a genetic similarity with anterior frontal and temporal cortex, whereas those in posterior parts of the subfields showed a genetic similarity with posterior occipital-temporal regions. Earlier studies have presented an isocortex-wide A-P topography derived from cortical thickness morphology (Valk et al., 2020), microstructural profile covariance (Paquola et al., 2019), and grey matter volumes (Kharabian Masouleh et al., 2020). The isocortical A-P topography resembles a frontal-polar differentiation of myelin density (Burt et al., 2018, Cahalane et al., 2014) and shows spatial similarity with a cortical functional gradient traversing between the transmodal to unimodal axis (Cahalane et al., 2014, Fornito et al., 2019). In line with our observation that morphometric similarity of hippocampus and isocortex is genetically determined (Alexander-Bloch et al., 2019, Chen et al., 2013), the concordance of genetic similarity between the A-P subfield axis and A-P isocortical axis has been previously reported using transcriptomic data (Vogel et al., 2020). Thus, the internal, heritable, organisation of hippocampal subfield microstructure has a genetic correlation with isocortex, which spatially co-varies with its functional organisation.
Beyond similarities, we also observed differences in subfield-isocortical genetic associations, both in the primary and secondary covariance and genetic correlation gradient. For example, we found a clear differentiation between genetic relationships of hippocampus and isocortex along the anterior-posterior axes with posterior regions of CA, showing only little association with temporal-occipital regions, and SUB showing a clear distinction between anterior subfield regions and its correspondence to anterior frontal/temporal cortex and posterior subfield regions and its correspondence to temporo-occipital and sensory regions. Moreover, the second genetic correlation gradient varied strongly between SUB, CA, and DG, suggesting to vary rather as a function of subfield infolding. This may relate to the subfield specific neurodevelopmental trajectories. For example, the CA - Ammon’s Horn - is one of the first brain regions to develop in the prenatal period (Whelan et al., 2016, Taupin, 2007). Conversely, the SUB extends its maturation towards the postnatal period (Jabès et al., 2011). Finally, DG maturation exceeds the postnatal period (Insausti et al., 2010), possibly underscoring posterior parietal associations. Thus, timing of pre- and post-natal development may be reflected in the genetic similarity patterning between subfields and their association with the isocortex. Non-genetic factors such as lifestyle impact the brain and behavior, also referred to as an experience-dependent plasticity in neuroanatomy (van Praag et al., 2000). Such factors may also modulate hippocampal structure and functional development. For example, a recent study using genome-wide DNA methylation sequencing - a technique to mark gene activation patterns associated with cellular aging - showed that mice retained a ‘younger’ dentate gyrus under stimulating living conditions than those from low-stimulus environments (Zocher et al., 2021). A direct translation of animal findings on hippocampal neurogenesis is not possible, however, there has also been evidence for human hippocampal development affected by early-life experiences (Smith and Pollak, 2020). Chronic stress exposure in early childhood is linked to reduced hippocampal volume (Hanson et al., 2015, Teicher et al., 2018) and associated with behavioral deficits such as poor learning processes (Gorka et al., 2014). Detecting the ratios in veterans, another study depicted a higher myelin content following posttraumatic stress disorder in young adults (Chao et al., 2015).
Lastly, to understand whether hippocampal function and structure covary along genetic organisational axes, we assessed local coupling maps of structural and functional subfield-isocortical profiles. Evaluating differences between structural and functional organisation, we found that coupling between structure and function was highest in posterior/medial portions of the hippocampal subfields, whereas anterior portions were uncoupled. Moreover, the coupling of hippocampal microstructure and function shows covariation with intrinsic functional and structural axes that we showed to align with genetic correlation to the isocortex. In particular, in the hippocampus, we found that posterior regions have a predominant structural and functional association with unimodal cortical regions whereas anterior regions are linked to transmodal cortex, similar to previous reports (Paquola et al., 2020, Vogel et al., 2020). Thus, mirroring observations in the cortex (Valk et al., 2020, Baum et al., 2020), it may be that portions of the hippocampus associated with posterior/unimodal regions show more similarity between structure and function than those related to transmodal areas such as anterior frontal and temporal cortex. Functionally, the anterior hippocampus has been reported to participate in associative memory processing (Buzsáki and Tingley, 2018), in which DMN is also involved and known to be integrating with parietal and temporal lobes for episodic memory retrieval (Wagner et al., 2005). Conversely, the posterior hippocampus is suggested to be a mediator for spatial memory encoding (Fanselow and Dong, 2010), in which parietal cortices (Save et al., 1992) and attention and salience networks are recruited (Corbetta and Shulman, 2011). Together, the divergence observed in subfield heritability of functional organisation and structure may reflect a differentiable role of heritable and experience-dependent variance across individuals, with more uncoupled portions of the hippocampus enabling more flexible forms for cognitive processing, an important hypothesis for future studies to examine.
Overall, though the sample size of the current work was sufficient to investigate effects of heritability above , maximum likelihood models used to model heritability in the current work improve their estimates with larger samples. Thus, future studies using comparable neuroimaging methods in larger samples may help gaining further insights into the heritability of hippocampal microstructural and function. Moreover, the present approach is limited to hippocampal functional connectivity with the isocortex, although hippocampus is highly likely connected with the subcortex such as amygdala (Phelps, 2004) and nucleus accumbens (Kahn and Shohamy, 2013). Future studies might reveal other large-scale topographic patterns emerging from hippocampus to subcortex connectivity and search whether these patterns have heritable correspondence. Also, the microstructural covariance between hippocampus and isocortex was restricted by the ratio as an indirect in vivo marker of myelin. Instead, including a more direct myelin biomarker (Weiskopf et al., 2013) and combining it with other microstructural features such as cortical thickness, surface area, folding and gyrification elucidate a more detailed hippocampal structure (DeKraker et al., 2020). Additionally, the within subfield variation should be further clarified with the subfield gene distribution profiles to emphasise whether subfields are distinct in terms of gene expression levels (Vogel et al., 2020). Identifying the subfield specific genes and therefore the protein transcription might clarify the functional and structural properties governed by the genetic information. Moreover, heritability is not a static marker but rather varies across samples and age-ranges, therefore, future studies may evaluate changes in heritability as a function of age in additional samples.
In sum, we showed that hippocampal subfields are organised along heritable posterior-to-anterior and medial-to-lateral axes which show a genetic link to isocortical functional and structural organisation. Though the current work focussed on functional organisation described by gradient 1 and 2, together explaining 58% of eigenvariance in the functional connectome, future work may gain increased insights of hippocampal organisation by studying also more subtle patterns crossing A-P and M-L axes. As another potential implication of gradients, one may evaluate the association between maturational axes in cortical structure and divergent functional profiles along the hippocampal formation. This may provide an important step to better understand how the anatomy of the hippocampus supports its unique and versatile function.
4. Materials and methods
4.1. Participants
We leveraged the HCP S900 data release (Van Essen et al., 2013) with n = 898 subjects with resting-state fMRI sessions and high-resolution structural images. Participant recruitment procedures and informed consent forms, including consent to share de-identified data, were previously approved by the Washington University Institutional Review Board as part of the HCP. The current research complies with all relevant ethical regulations as set by The Independent Research Ethics Committee at the Medical Faculty of the Heinrich-Heine-University of Duesseldorf (study number 2018–317).
The quality assurance (QA) was based on the following exclusion criteria: i) subjects with anatomical anomalies or tissue segmentation errors listed in the HCP issues (n = 47), ii) subjects with missing four resting-state fMRI scan sessions (n = 69), iii) subjects with poor hippocampal subfield segmentation quality (n = 42), and iv) subjects whose functional connectome (FC) differed from the group level FC (n = 31), as a necessity for the gradient analysis (see section Functional Connectivity and Gradients). For the QA step iii), we first segmented hippocampal subfields: subiculum (SUB), CA1–3 (CA), and CA4-DG (DG) along the structural images using a patch-based surface algorithm (Surf-Patch) (Caldairou et al., 2016). Then, all subfield delineations underwent a visual inspection by Dr. Sofie Valk (SLV), Şeyma Bayrak (ŞB), and Reinder vos de Wael (RW). There remained n = 709 participants (395 women, mean ± SD age = 28.7 ± 3.7 y) accessible for our study. Among the 709 participants included in this study, there were 176 monozygotic twins, 178 siblings without twin status and 355 participants without familial relatedness. All QA steps and analysis scripts used in this study are available at https://github.com/CNG-LAB/cngopen/tree/main/hippocampus.
4.2. Neuroimaging Data Acquisition and Preprocessing
Details of the HCP neuroimaging protocol and processing pipelines are available at (Glasser et al., 2013). In brief, we extracted T1-weighted (T1w) and T2-weighted (T2w) images available in the HCP initiative, which were all acquired on a 3T Siemens Skyra scanner. T1w images were acquired using a three-dimensional magnetization prepared rapid gradient-echo (3D-MPRAGE) sequence (0.7 mm isotropic voxels, matrix = 320 × 320, 256 sagittal slices, TR = 2400 ms, TE = 2.14 ms, TI = 1000 ms, flip angle = 8°, iPAT = 2). Two T2w images were acquired with identical geometry (TR = 3200 ms, TE = 565 ms, variable flip angle; iPAT = 2). Resting-state fMRI images were acquired using a multi-band accelerated 2D-BOLD echo-planar imaging (EPI) sequence (2 mm isotropic voxels, matrix = 104 × 90, 72 sagittal slices, TR = 720 ms, TE = 33 ms, flip angle = 52°, mb factor = 8, 1200 volumes/scan). The fMRI data was collected at two sessions (1, 2) and in two phase encoding directions at each session (left-right [LR] and right-left [RL]), resulting in four resting-state fMRI datasets in total ([LR1], [RL1], [LR2], [RL2]).
Preprocessing steps for the structural MRI images included gradient nonlinearity correction, brain extraction, distortion correction and co-registration of T1w and T2w images using rigid body transformations. Then, an intensity nonuniformity correction was performed using T1w and T2w contrasts (Glasser and Van Essen, 2011) and subcortical structures were segmented using FSL FIRST (Patenaude et al., 2011). Subsequently, preprocessed images were nonlinearly registered to the MNI152 template and cortical surfaces were reconstructed with FreeSurfer v5.3.0-HCP (Dale et al., 1999, Fischl, Sereno and Dale, 1999a, Fischl et al., 1999b). Finally, the individual cortical surfaces were registered to the Conte69 template (Van Essen et al., 2012) using MSMA11 (Glasser et al., 2016).
Preprocessing of rs-fMRI images included corrections for the gradient nonlinearity, head motion and distortion. The images were then aligned to the T1w space using rigid-body and boundary-based registrations together (Greve and Fischl, 2009). The transformation matrices from this alignment step and that of the earlier T2w to T1w alignment were concatenated and applied to the rs-fMRI images at a single interpolation step to warp rs-fMRI images to the MNI152. Further processing in MNI152 space included bias field removal, whole brain intensity normalisation, high pass filtering (> 2000 s FWHM) and noise removal with the ICA-FIX procedure (Salimi-Khorshidi et al., 2014).
4.3. Hippocampus Subfield Segmentations
We used the SurfPatch algorithm (Caldairou et al., 2016) to automatically delineate the hippocampal subfields of all participants: subiculum (SUB), CA1–3 (CA), and CA4-DG (DG). The automated delineation was carried out on the minimally processed T1w neuroimaging data in the MNI152 template space, using a validated multi-template surface-patch algorithm (Caldairou et al., 2016). SurfPatch was previously trained on another brain dataset of a multi-contrast and sub-millimetric MRI data at 3 Tesla, which included manual hippocampal subfield delineations (Kulaga-Yoskovitz et al., 2015). In previous work, Caldairou et al., 2016 have reported the association between accuracy and T1-image resolution. Overall, higher associations between manual and automated volumes are found based on sub-millimeter T1 as compared to millimeter level T1, yet differences in correlation are marginal (Caldairou et al., 2016). Dice overlap between automated and manual segmentations were above 81.10 ± 3.86 %. The current work used T1 maps with a resolution of 0.7 mm isotropic voxel, and projected functional maps of 2.0 mm isotropic voxel.
SurfPatch performs a spherical harmonic shape parametrization and point distribution model of the surfaces (Styner et al., 2006). The medial sheet representations of hippocampal subfields were generated by running through each subfield’s core using a Hamilton-Jacobi approach (Kim et al., 2014) to minimise partial volume effects due to feature sampling. Furthermore, the spherical harmonic parametrization was propagated from the outer shell to the medial sheet for a better match of vertex-correspondence across individuals based on shape-inherent information. Resultant CA surfaces consisted of 10242 vertices and both DG and SUB surfaces of 5762 vertices. Next, CA, DG, and SUB surfaces were further downsampled to 2048, 1024, and 1024 vertices, respectively. All subfield segmentations generated by the SurfPatch underwent a visual inspection and are available upon request. The visual inspection reports from the previously published Vos de Wael et al., 2018 were included for n = 399 subjects, and Dr. Sofie Valk (SLV) and Şeyma Bayrak (ŞB) inspected further the remaining n = 499 subjects in the S900 data release.
4.4. Isocortex and Subfield Time Series
We mapped medial sheet meshes and volumetric resting-state fMRI data to native T1w space. Time series were sampled at each hippocampal and cortical mid-thickness vertex (Glasser et al., 2016). Hippocampal surface features were smoothed using a Gaussian diffusion kernel with 5 mesh units as FWHM in all subfields and isocortex. Sampling was carried out in a native T1w space to minimise the interpolation. Cortical time series were averaged within a previously established multi-modal parcellation scheme of the Glasser Atlas of 360 areas (180 regions per hemisphere) (Glasser et al., 2016). Surface-based time series were smoothed using a Gaussian diffusion kernel with 5 mesh units as FWHM.
4.5. Functional Connectivity
For every participant separately (n = 740), we computed the linear correlation coefficients between isocortex-wide time series (360 × 1200) and hippocampal subfield time series for SUB (1024 × 1200), CA (2048 × 1200), and DG (1024 × 1200). This resulted in a isocortex wide functional connectivity (FC) map (360 × 1) for every subject and subfield. We obtained group-level reference FC maps for every subfield by averaging individual FC maps across participants. We further profiled the similarity of individual FC maps to the reference FC maps by means of simple correlation (Fig. S1A). Participants with a lower degree of similarity (r < 0.45) to the reference map were excluded (n = 31). Finally, the FC map of the isocortex to each hippocampal subfield for the remaining 709 participants was mapped using linear and mixed effects models in BrainStat (https://github.com/MICA-MNI/BrainStat).
4.6. Maps and Structural Intensity Covariance
To study microstructural features of the hippocampus, we used the ratio of T1- over T2-weighted () image intensities. We resampled native images to the MNI152 space and mapped them to hippocampal subfield surfaces (SUB, CA, DG) using Connectome Workbench (v1.4.2, volume-warpfield-resample and volume-to-surface-mapping tools) (Marcus et al., 2011). To assess the quality of intensities projected on the hippocampal subfields, we obtained the mean intensity distributions of all participants for potential outlier detection (Fig. 2A, Fig. S3C). We computed the structural intensity covariance (SiC) by correlating hippocampal and cortical intensity maps resulting in 1384 × 1384 matrix for SUB, 2408 × 2408 matrix for CA, and 1384 × 1384 matrix for DG.
4.7. Heritability and Genetic Correlation
Heritability and genetic correlation analysis were conducted with the Sequential Oligogenic Linkage Analysis Routines (SOLAR, v8.5.1, http://www.solar-eclipse-genetics.org/). SOLAR employs a maximum likelihood variance-decomposition approach optimised to perform genetic analyses in pedigrees of arbitrary size and complexity (Almasy and Blangero, 1998, Kochunov et al., 2019). SOLAR models genetic proximity by covariance between family members (Almasy and Blangero, 1998, Kochunov et al., 2019).
In brief, heritability (i.e. narrow-sense heritability ) is defined as the proportion of the phenotypic variance () in a trait that is attributable to the additive effects of genes (), i.e. . SOLAR estimates heritability by comparing the observed phenotypic covariance matrix with the covariance matrix predicted by kinship (Almasy and Blangero, 1998, Kochunov et al., 2019). Significance of the heritability estimate was tested using a likelihood ratio test where the likelihood of a restricted model (with constrained to zero) is compared with the likelihood of the estimated model. Twice the difference between the log likelihoods of these models yields a test statistic, which is asymptotically distributed as a 50:50 mixture of a variable with 1 degree-of-freedom and a point mass at zero (Almasy and Blangero, 1998, Kochunov et al., 2019). We quantified the heritability of i) hippocampal-isocortical functional connectivity patterns, ii) hippocampal subfield gradients, and iii) intensity maps. We included covariates in all heritability analyses including , , , and . Quantitative variables are mean-adjusted in SOLAR, avoiding collinearity for age squared effects.
To estimate if variations in intensity maps between hippocampus and isocortex were influenced by the same genetic factors, a genetic correlation analysis was conducted. Genetic correlations indicate the proportion of variance that determines the extent to which genetic influences on one trait are shared with genetic influences on another trait (e.g. pleiotropy). In SOLAR, the phenotypic correlation () was decomposed through bivariate polygenic analyses to estimate genetic () and environmental () correlations using the following formula: , where and are the heritability estimates of the vertex-based values in hippocampus and isocortex ( Almasy et al., 1997, Glahn et al., 2010 ). The significance of these correlations was determined (similar to heritability analyses) by likelihood ratio tests comparing a model in which was estimated with a model in which was constrained to zero (no shared genetic effect) and constrained to 1 (complete pleiotropy) (Almasy et al.,1997, Glahn et al., 2010).
4.8. Hippocampal-Isocortical Functional Connectivity Heritability
Hippocampal time series were averaged across subfield vertices, yielding mean time series for SUB: (1 × 1200), CA: (1 × 1200), and DG: (1 × 1200) and for every subject. The subfield mean time series were then correlated with that of the cortex (360 × 1200). This approach resulted in mean subfield-to-isocortex FC matrices (mean SUB-isocortex: (360 × 1), CA-isocortex: (360 × 1), DG-isocortex: (360 × 1)). Having saved the mean subfield-isocortical FC for every subject (n = 709) and every cortical vertex (360), we ran SOLAR heritability analysis along Glasser vertices FC values (709 × 360). SOLAR runs the heritability analysis for each vertex by taking the familial relatedness (HCP pedigree files) into account, ie. whether an FC value of the given Glasser vertex is heritable. At the end, having the heritability scores for 360 Glasser parcels, we labeled them to conte69 template (Van Essen et al., 2012) for the cortex visualizations in Fig. 1C.
4.9. Connectivity Gradients
Using the diffusion embedding algorithm, we generated low-dimensional representations of hippocampal-cortical FC, namely the gradients. For every participant separately (n = 709), we computed the linear correlation coefficients between isocortex-wide time series 360 × 1200 and hippocampal subfield time series for SUB (1024 × 1200), CA (2048 × 1200), and DG (1024 × 1200) to identify the FC matrices. Subfield-to-isocortex FC measures were concatenated across the subfields, yielding a hippocampal-isocortical FC map (4096 × 360) for every subject. Averaging FC matrices across all subjects (n = 709), we obtained a group level FC matrix. We used BrainSpace (Vos de Wael et al., 2020) to derive connectivity gradients from the group-level FC matrix using diffusion map embedding (normalised angle kernel, 90th percentile thresholding for the sparsity, and diffusion time estimation of ) (Coifman and Lafon, 2006), similarly to those identified by Vos de Wael et al., 2018.
Along each single gradient (4096 × 1), hippocampus vertices that share similar connectivity patterns have similar embedding values. The first and second gradients explained 58% of the total variance in the subfield FC map (Fig. S2A). Having validated the gradient representations of hippocampal subfields at the group-level, we computed the individual-level gradients for every participant. Subsequently, individual gradients were aligned to the group-level gradients using Procrustes alignment to be scaled onto a common embedded connectivity space.
4.10. Gradients of Subfield Functional Connectome Heritability
To probe the organisational axis of heritability scores itself, we derived the gradient decomposition of FC-heritabilities along subfields. We computed 1) the subfield-to-cortex FC matrix, 2) the heritability of this FC matrix (), and 3) the gradient decomposition of the FC-heritability ( and ). FC was obtained for each subject (n = 709) and subfield (SUB-cortex: 1024 ×360, CA-cortex: 2048 ×360, and DG-cortex: 1024 ×360), separately. We ran SOLAR heritability analysis for every FC value from every subfield vertex to cortical vertex, resulting in heritability of FC, (, for SUB: (1024 ×360), CA: (2048 ×360) and DG: (1024 ×360). We then concatenated these data arrays across subfields, that yielded a matrix of size (4096 ×360). This heritability matrix was then decomposed into its gradients, resulting in the primary and secondary gradients of FC-heritability, and , respectively.
4.11. Structure-Function Coupling along Subfields
To measure “coupling”, we evaluated the degree of spatial overlap between hippocampal microstructural intensity covariance (SiC) and functional connectivity (FC) measures per subfield vertex. The SiC was computed by correlating the measures between hippocampus and cortex across all subjects, which yielded a covariance matrix of size (4096 × 360). The FC was computed by correlating the time series between hippocampus and cortex, which yielded a connectivity matrix of size (4096 × 360). For every subfield vertex, we obtained the SiC array from that vertex to all cortical vertices (360 × 1), and FC array from the same vertex to all cortical vertices (360 × 1). We then correlated these two arrays (Pearson’s r) to quantify the degree of spatial overlap between the SiC and FC profiles. This degree of spatial overlap was then called as “coupling”, ie. a higher coupling value indicates a high overlap between SiC and FC measures for a subfield vertex, whereas a low coupling (or “uncoupling”) depicts a low overlap. Similar approaches have been used by Valk et al., 2022, Vázquez-Rodríguez et al., 2019.
Supplementary Material
Acknowledgements
We would like to thank the various contributors to the open access databases that our data was downloaded from. Funding: HCP data were provided by the Human Connectome Project, Washington University, the University of Minnesota, and Oxford University Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/21-1), the National Institute of Mental Health (R01-MH074457), the Helmholtz Portfolio Theme “Supercomputing and Modeling for the Human Brain” and the European Union’s Horizon 2020 Research and Innovation Program under Grant Agreement No. 785907 (HBP SGA2). R.V. was supported by the Richard and Ann Sievers award. S.L.V. was supported by Max Planck Gesellschaft (Otto Hahn award). B.C.B. acknowledges support from the SickKids Foundation (NI17-039), the National Sciences and Engineering Research Council of Canada (NSERC; Discovery-1304413), CIHR (FDN154298), Azrieli Center for Autism Research (ACAR), an MNI-Cambridge collaboration grant, and the Canada Research Chairs program. Last, this work was funded in part by Helmholtz Association’s Initiative and Networking Fund under the Helmholtz International Lab grant agreement InterLabs-0015, and the Canada First Research Excellence Fund (CFREF Competition 2, 2015-2016) awarded to the Healthy Brains, Healthy Lives initiative at McGill University, through the Helmholtz International BigBrain Analytics and Learning Laboratory (HIBALL). M.D.H. was funded by the Max Planck Society and the German Ministry of Education and Research.
Footnotes
Credit authorship contribution statement
Şeyma Bayrak: Methodology, Conceptualization, Writing – review & editing, Visualization. Reinder Vos de Wael: Methodology, Data curation. H. Lina Schaare: Conceptualization, Writing – review & editing. Meike D. Hettwer: Writing – review & editing. Benoit Caldairou: Software. Andrea Bernasconi: Data curation. Neda Bernasconi: Data curation. Boris C. Bernhardt: Data curation, Conceptualization, Writing – review & editing. Sofie L. Valk: Supervision, Conceptualization, Writing – review & editing.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.neuroimage.2022.119656.
Declaration of Competing Interest
The authors declare no conflict of interest.
Data availability
All data, analyzed in this manuscript, were obtained from the open-access HCP young adult sample (http://www.humanconnectome.org/) (Van Essen et al., 2013). The raw data may not be shared by third parties due to ethics requirements, but can be downloaded directly via the above weblink.
References
- Alexander-Bloch A, Raznahan A, Bullmore E, Giedd J, 2013. The convergence of maturational change and structural covariance in human cortical networks. J. Neurosci 33, 2889–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch AF, et al. , 2019. Human cortical thickness organized into genetically-determined communities across spatial resolutions. Cereb. Cortex 29, 106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Blangero J, 1998. Multipoint quantitative-trait linkage analysis in general pedigrees. Am. J. Hum. Genet 62, 1198–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasy L, Dyer TD, Blangero J, 1997. Bivariate quantitative trait linkage analysis: pleiotropy versus co-incident linkages. Genet. Epidemiol 14, 953–958. [DOI] [PubMed] [Google Scholar]
- Arnatkeviciute A, et al. , 2021. Genetic influences on hub connectivity of the human connectome. Nat. Commun 12, 4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assem M, Glasser MF, Van Essen DC, Duncan J, 2020. A domain-general cognitive core defined in multimodally parcellated human cortex. Cereb. Cortex 30, 4361–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, et al. , 2014. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci 15, 181–192. [DOI] [PubMed] [Google Scholar]
- Barbas H, 2015. General cortical and special prefrontal connections: principles from structure to function. Annu. Rev. Neurosci 38, 269–289. [DOI] [PubMed] [Google Scholar]
- Barth C, et al. , 2016. In-vivo Dynamics of the Human Hippocampus across the Menstrual Cycle. Sci. Rep 6, 32833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battaglia FP, Benchenane K, Sirota A, Pennartz CMA, Wiener SI, 2011. The hippocampus: hub of brain network communication for memory. Trends Cogn. Sci 15, 310–318. [DOI] [PubMed] [Google Scholar]
- Baum GL, et al. , 2020. Development of structure-function coupling in human brain networks during youth. Proc. Natl. Acad. Sci. U. S. A 117, 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrak Ş, et al. , 2019. The impact of ischemic stroke on connectivity gradients. Neuroimage Clin 24, 101947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, et al. , 2016. The spectrum of structural and functional imaging abnormalities in temporal lobe epilepsy. Ann. Neurol 80, 142–153. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, et al. , 2018. Preferential susceptibility of limbic cortices to microstructural damage in temporal lobe epilepsy: a quantitative T1 mapping study. NeuroImage 182, 294–303. [DOI] [PubMed] [Google Scholar]
- Berron D, et al. , 2016. Strong Evidence for pattern separation in human dentate gyrus. J. Neurosci 36, 7569–7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowski MS, et al. , 2018. Integration of gene expression and brain-wide connectivity reveals the multiscale organization of mouse hippocampal networks. Nat. Neurosci 21, 1628–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunec IK, et al. , 2018. Multiple scales of representation along the hippocampal antero-posterior axis in humans. Curr. Biol 28, 2129–2135 e6. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J, 2002. The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. [DOI] [PubMed] [Google Scholar]
- Burt JB, et al. , 2018. Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography. Nature Neuroscience 21, 1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt JB, Helmer M, Shinn M, Anticevic A, Murray JD, 2020. Generative modeling of brain maps with spatial autocorrelation. Neuroimage 220, 117038. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Tingley D, 2018. Space and time: the hippocampus as a sequence generator. Trends Cogn. Sci 22, 853–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahalane DJ, Charvet CJ, Finlay BL, 2014. Modeling local and cross-species neuron number variations in the cerebral cortex as arising from a common mechanism. Proc. Natl. Acad. Sci. U. S. A 111, 17642–17647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldairou B, et al. , 2016. A surface patch-based segmentation method for hippocampal subfields. Med. Image Comput. Comput.-Assist. Intervent. – MICCAI 2016 379–387. doi: 10.1007/978-3-319-46723-8_44. [DOI] [Google Scholar]
- Cenquizca LA, Swanson LW, 2007. Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Res. Rev 56, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Tosun D, Woodward SH, Kaufer D, Neylan TC, 2015. Preliminary evidence of increased hippocampal myelin content in veterans with posttraumatic stress disorder. Front. Behav. Neurosci 9, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, et al. , 2015. Evidence for an anterior-posterior differentiation in the human hippocampal formation revealed by meta-analytic parcellation of fMRI coordinate maps: focus on the subiculum. Neuroimage 113, 44–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-H, et al. , 2013. Genetic topography of brain morphology. Proc. Natl. Acad. Sci. U. S. A 110, 17089–17094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coifman RR, Lafon S, 2006. Diffusion maps. Appl. Comput. Harmon. Analy 21, 5–30. [Google Scholar]
- Cooper C ’iana, Moon HY, van Praag H, 2018. On the run for hippocampal plasticity. Cold Spring Harb. Perspect. Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL, 2011. Spatial neglect and attention networks. Annu. Rev. Neurosci 34, 569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- de Flores R, et al. , 2017. Intrinsic connectivity of hippocampal subfields in normal elderly and mild cognitive impairment patients. Hum. Brain Mapp 38, 4922–4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKraker J, Köhler S, Khan AR, 2021. Surface-based hippocampal subfield segmentation. Trend. Neurosci 44, 856–863. [DOI] [PubMed] [Google Scholar]
- DeKraker J, Lau JC, Ferko KM, Khan AR, Köhler S, 2020. Hippocampal subfields revealed through unfolding and unsupervised clustering of laminar and morphological features in 3D BigBrain. Neuroimage 206, 116328. [DOI] [PubMed] [Google Scholar]
- Elman JA, et al. , 2019. Genetic architecture of hippocampal subfields on standard resolution MRI: How the parts relate to the whole. Hum. Brain Mapp 40, 1528–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Dong H-W, 2010. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 65, 7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM, 1999a. Cortical surface-based analysis. NeuroImage 9, 195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM, 1999b. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp 8, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Arnatkevičiūtė A, Fulcher BD, 2019. Bridging the gap between connectome and transcriptome. Trend. Cogn. Sci 23, 34–50. [DOI] [PubMed] [Google Scholar]
- Franklin TB, Saab BJ, Mansuy IM, 2012. Neural mechanisms of stress resilience and vulnerability. Neuron 75, 747–761. [DOI] [PubMed] [Google Scholar]
- Ganzetti M, Wenderoth N, Mantini D, 2014. Whole brain myelin mapping using T1- and T2-weighted MR imaging data. Front. Hum. Neurosci 8, 671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Bernhardt BC, La Joie R, Amunts K, Eickhoff SB, 2021. The many dimensions of human hippocampal organization and (dys)function. Trend. Neurosci 44, 977–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, et al. , 2010. Genetic control over the resting brain. Proc. Natl. Acad. Sci. U. S. A 107, 1223–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, et al. , 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, et al. , 2016. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC, 2011. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci 31, 11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Van Essen DC, 2011. Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci 31, 11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka AX, Hanson JL, Radtke SR, Hariri AR, 2014. Reduced hippocampal and medial prefrontal gray matter mediate the association between reported childhood mal-treatment and trait anxiety in adulthood and predict sensitivity to future life stress. Biol. Mood Anxiety Disord 4, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C, et al. , 2018. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron 98, 439–452 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve DN, Fischl B, 2009. Accurate and robust brain image alignment using boundary-based registration. Neuroimage 48, 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haak KV, Beckmann CF, 2019. Plasticity versus stability across the human cortical visual connectome. Nat. Commun 10, 3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, et al. , 2015. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol. Psychiatry 77, 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen EJ, et al. , 2010. Spatial representation along the proximodistal axis of CA1. Neuron 68, 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, et al. , 2015. Common genetic variants influence human subcortical brain structures. Nature 520, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, et al. , 2017. Novel genetic loci associated with hippocampal volume. Nat. Commun 8, 13624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts CJ, et al. , 2017. Ultra-high-field fMRI reveals a role for the subiculum in scene perceptual discrimination. J. Neurosci 37, 3150–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, et al. , 2015. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: application to adaptive segmentation of in vivo MRI. NeuroImage 115, 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Cebada-Sánchez S, Marcos P, 2010. Postnatal development of the human hippocampal formation. Adv. Anatomy, Embryol. Cell Biol doi: 10.1007/978-3-642-03661-3. [DOI] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P, 2011. Postnatal development of the hippocampal formation: a stereological study in macaque monkeys. J. Comp. Neurol 519, 1051–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Shohamy D, 2013. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus 23, 187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, et al. , 2006. A meta-analysis of structural brain abnormalities in PTSD. Neurosci. Biobehav. Rev 30, 1004–1031. [DOI] [PubMed] [Google Scholar]
- Kharabian Masouleh S, Plachti A, Hoffstaedter F, Eickhoff S, Genon S, 2020. Charac- terizing the gradients of structural covariance in the human hippocampus. Neuroimage 218, 116972. [DOI] [PubMed] [Google Scholar]
- Kim EJ, Pellman B, Kim JJ, 2015. Stress effects on the hippocampus: a critical review. Learn. Memory 22, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, et al. , 2014. Multivariate hippocampal subfield analysis of local MRI intensity and volume: application to temporal lobe epilepsy. Med. Image Comput. Comput. Assist. Interv 17, 170–178. [DOI] [PubMed] [Google Scholar]
- Kochunov P, et al. , 2019. Homogenizing estimates of heritability among solar-eclipse, OpenMx, APACE, and FPHI software packages in neuroimaging data. Front. Neuroinform 13, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Sherwood CC, 2017. Gradients of connectivity in the cerebral cortex. Trend. Cognit. Sci 21, 61–63. [DOI] [PubMed] [Google Scholar]
- Kulaga-Yoskovitz J, et al. , 2015. Multi-contrast submillimetric 3 Tesla hippocampal subfield segmentation protocol and dataset. Sci. Data 2, 150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langs G, et al. , 2016. Identifying shared brain networks in individuals by decoupling functional and anatomical variability. Cereb. Cortex 26, 4004–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q. et al. Human brain function during pattern separation follows hippocampal and neocortical connectivity gradients. doi: 10.1101/2020.06.22.165290. [DOI] [Google Scholar]
- Lieberman JA, et al. , 2018. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Molecul. Psychiatry 23, 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorio S, et al. , 2016. Neurobiological origin of spurious brain morphological changes: a quantitative MRI study. Hum. Brain Mapp 37, 1801–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Maass A, Berron D, Libby LA, Ranganath C, Düzel E, 2015. Functional subregions of the human entorhinal cortex. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller JJ, et al. , 2012. Hippocampal volumetrics in treatment-resistant depression and schizophrenia: the devil’s in de-tail. Hippocampus 22, 9–16. [DOI] [PubMed] [Google Scholar]
- Marcus DS, et al. , 2011. Informatics and data mining tools and strategies for the human connectome project. Front. Neuroinform 5, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, et al. , 2016. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. U. S. A 113, 12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, 1999. Stress and hippocampal plasticity. Annu. Rev. Neurosci 22, 105–122. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER, 1998. Cognitive neuroscience and the study of memory. Neuron 20, 445–468. [DOI] [PubMed] [Google Scholar]
- Neunuebel JP, Knierim JJ, 2014. CA3 retrieves coherent representations from degraded input: direct evidence for CA3 pattern completion and dentate gyrus pattern separation. Neuron 81, 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordin K, et al. , 2018. Structural whole-brain covariance of the anterior and posterior hippocampus: associations with age and memory. Hippocampus 28, 151–163. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Kedo O, Mohlberg H, Zilles K, Amunts K, 2020. Multimodal mapping and analysis of the cyto- and receptorarchitecture of the human hippocampus. Brain Struct. Funct 225, 881–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola C, et al. , 2019. Microstructural and functional gradients are increasingly dissociated in transmodal cortices. PLoS Biol 17, e3000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquola C, et al. , 2020. Convergence of cortical types and functional motifs in the human mesiotemporal lobe. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M, 2011. A Bayesian model of shape and appearance for subcortical brain segmentation. NeuroImage 56, 907–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, 2004. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol 14, 198–202. [DOI] [PubMed] [Google Scholar]
- Plachti A, et al. , 2019. Multimodal parcellations and extensive behavioral profiling tackling the hippocampus gradient. Cereb. Cortex 29, 4595–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L, 2013. Long-axis specialization of the human hippocampus. Trend. Cogn. Sci 17, 230–240. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, et al. , 2010. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations - 2008 Curt Richter Award Winner. Psychoneuroendocrinology 35, 179–191. [DOI] [PubMed] [Google Scholar]
- Przeździk I, Faber M, Fernández G, Beckmann CF, Haak KV, 2019. The functional organisation of the hippocampus along its long axis is gradual and predicts recollection. Cortex 119, 324–335. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, et al. , 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage 90, 449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Save E, Poucet B, Foreman N, Buhot M-C, 1992. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav. Neurosci 106, 447–456. [PubMed] [Google Scholar]
- Smith KE, Pollak SD, 2020. Early life stress and development: potential mechanisms for adverse outcomes. J. Neurodev. Disord 12, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, 1992. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol. Rev 99, 195–231. [DOI] [PubMed] [Google Scholar]
- Stein JL, et al. , 2012. Identification of common variants associated with human hippocampal and intracranial volumes. Nat. Genet 44, 552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI, 2014. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci 15, 655–669. [DOI] [PubMed] [Google Scholar]
- Stüber C, et al. , 2014. Myelin and iron concentration in the human brain: a quantitative study of MRI contrast. NeuroImage 93, 95–106. [DOI] [PubMed] [Google Scholar]
- Styner M, et al. , 2006. Framework for the statistical shape analysis of brain structures using SPHARM-PDM. Insight J. 242–250. [PMC free article] [PubMed] [Google Scholar]
- Suárez LE, Markello RD, Betzel RF, Misic B, 2020. Linking structure and function in macroscale brain networks. Trend. Cogn. Sci 24, 302–315. [DOI] [PubMed] [Google Scholar]
- Taupin P, 2007. The Hippocampus: Neurotransmission and Plasticity in the Nervous System. Nova Publishers. [Google Scholar]
- Teicher MH, et al. , 2018. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage 169, 443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, et al. , 2020. Shaping brain structure: Genetic and phylogenetic axes of macroscale organization of cortical thickness. Sci. Adv 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valk SL, et al. , 2022. Genetic and phylogenetic uncoupling of structure and function in human transmodal cortex. Nat. Commun 13, 2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer D, et al. , 2020. Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol. Psychiatry 25, 3053–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, et al. , 2013. The WU-Minn Human Connectome Project: an overview. Neuroimage 80, 62–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T, 2012. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex 22, 2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 2000. Neural consequences of enviromental enrichment. Nat. Rev. Neurosci 1, 191–198. [DOI] [PubMed] [Google Scholar]
- van Strien NM, Cappaert NLM, Witter MP, 2009. The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci 10, 272–282. [DOI] [PubMed] [Google Scholar]
- Vázquez-Rodríguez B, et al. , 2019. Gradients of structure-function tethering across neo-cortex. Proc. Natl. Acad. Scie. U. S. A 116, 21219–21227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel JW, et al. , 2020. A molecular gradient along the longitudinal axis of the human hippocampus informs large-scale behavioral systems. Nat. Commun 11, 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C, Vogt O, 1928. Die Grundlagen und die Teildisziplinen der mikroskopischen Anatomie des Zentralnervensystems. Nervensystem 448–477. doi: 10.1007/978-3-642-66443-4_8. [DOI] [Google Scholar]
- Vos de Wael R, et al. , 2018. Anatomical and microstructural determinants of hippocampal subfield functional connectome embedding. Proc. Natl. Acad. Sci. U. S. A 115, 10154–10159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos de Wael R, et al. , 2020. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Commun Biol 3, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL, 2005. Parietal lobe contributions to episodic memory retrieval. Trends Cogn. Sci 9, 445–453. [DOI] [PubMed] [Google Scholar]
- Warland A, Kendall KM, Rees E, Kirov G, Caseras X, 2020. Schizophrenia-associated genomic copy number variants and subcortical brain volumes in the UK Biobank. Mol. Psychiatry 25, 854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiskopf N, et al. , 2013. Quantitative multi-parameter mapping of R1, PD(*), MT, and R2(*) at 3T: a multi-center validation. Front. Neurosci 7, 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan CD, et al. , 2016. Heritability and reliability of automatically segmented human hippocampal formation subregions. Neuroimage 128, 125–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisse LEM et al. A Harmonized Segmentation Protocol for Hippocampal and Parahippocampal Subregions: Why Do We Need One and what are the Key Goals? (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yushkevich PA, et al. , 2015. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage 111, 526–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, et al. , 2019. Heritability of Regional Brain Volumes in Large-Scale Neuroimaging and Genetic Studies. Cereb. Cortex 29, 2904–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zocher S, Overall RW, Lesche M, Dahl A, Kempermann G, 2021. Environmental enrichment preserves a young DNA methylation landscape in the aged mouse hippocampus. Nat. Commun 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsido RG et al. Longitudinal 7T MRI reveals volumetric changes in subregions of human medial temporal lobe to sex hormone fluctuations. doi: 10.1101/2022.05.02.490281. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, analyzed in this manuscript, were obtained from the open-access HCP young adult sample (http://www.humanconnectome.org/) (Van Essen et al., 2013). The raw data may not be shared by third parties due to ethics requirements, but can be downloaded directly via the above weblink.