Abstract
Background
Mechanical ventilation (MV) is a necessary life-saving measure for critically ill patients. Ventilator-associated events (VAEs) are potentially avoidable complications associated with MV that can double the rate of death. Oral care and oropharyngeal suctioning, although neglected procedures, play a vital role in the prevention of VAE.
Methods
A randomized controlled trial was conducted in the intensive care units to compare the effect of fourth hourly oropharyngeal suctioning with the standard oral care protocol on VAE among patients on MV. One hundred twenty mechanically ventilated patients who were freshly intubated and expected to be on ventilator support for the next 72 hours were randomly allocated to the control or intervention groups. The intervention was fourth hourly oropharyngeal suctioning along with the standard oral care procedure. The control group received standard oral care (i.e., thrice a day) and on-demand oral suctioning. On the 3rd and 7th days following the intervention, endotracheal aspirates were sent to rule out ventilator-associated pneumonia.
Results
Both groups were homogenous at baseline with respect to their clinical characteristics. The intervention group had fewer VAEs (56.7%) than the control group (78.3%) which was significant at P<0.01. A significant reduction in the status of “positive culture” on ET aspirate also been observed following the 3rd day of the intervention (P<0.001).
Conclusions
One of the most basic preventive strategies is providing oral care. Oropharyngeal suctioning is also an important component of oral care that prevents microaspiration. Hence, fourth-hourly oropharyngeal suctioning with standard oral care significantly reduces the incidence of VAE.
Keywords: critical illness, incidence, intensive care units, mechanical ventilators, randomized controlled trials, ventilator-associated pneumonia
INTRODUCTION
Mechanical ventilation (MV) is a necessary life-saving measure for critically ill patients. However, it is associated with considerable, yet preventable complications. MV is not a curative intervention; however, it supports the patient until he/she recovers the ability to breathe independently. Intubation with an endotracheal tube (ETT) keeps the glottis open, leaving only the inflated cuff to protect against aspiration of contaminated oral secretions and gastric contents into the lungs [1]. Therefore, MV predisposes the patient to an increased risk of ventilator-associated events (VAEs) due to aspiration, resulting in more complications and poor outcomes.
Among all potential complications of MV, ventilator-associated pneumonia (VAP) is considered the leading cause of mortality and is a potentially preventable iatrogenic illness. However, most of the diagnostic criteria for VAP are not objective or specific. In addition, VAP surveillance has limited accuracy. Hence, the Centers for Disease Control (CDC) developed a new surveillance definition of the VAE algorithm in January 2013 based on objective, streamlined, and potentially automatable criteria that identify a broad range of conditions and complications occurring in mechanically-ventilated adult patients [2,3].
There are three definition tiers within the VAE algorithm including ventilator-associated conditions (VACs), infection-related VAC (IVAC), and possible VAP (PVAP) [2]. However, the algorithm is not meant for clinical use in the management of mechanically ventilated patients. Instead, it was created as a surveillance system to improve the reliability of detecting VAP and other mechanical ventilator-associated complications quickly and easily [4]. A VAC is the first level among the three tiers. A VAC is defined by worsening respiratory status including hypoxemia based on an increase in daily minimum FiO2 ≥0.20 or positive end-expiratory pressure (PEEP) ≥3 cm H2O sustained for at least 2 calendar days following a baseline period (2 calendar days) of stability or improvement. An IVAC requires the VAC criteria, followed by an abnormal leukocyte count (≥12,000/≤4,000 cells/mm3) or temperature (>38 °C/<36 °C) along with treatment with any new antimicrobial agent. Further progression to the third level leads to probable or possible VAP, which is suggested by the presence of a pulmonary source of infection [5,6].
The use of comprehensive oral care protocols can significantly reduce VAP rates [6]. In 2001, Bergmans et al. [7] provided evidence that the key to preventing VAP is the prevention of bacterial colonization of the oropharynx. Therefore, the removal of the colonized accumulations from the oropharynx can prevent the development of VAP-related complications.
The development of VAEs can lead to a significant increase in ventilator days, hospital stays, days of antibiotics, and higher hospital morbidity and mortality compared to those of patients without VAEs [8,9]. Proper airway care is crucial to minimize the devastating side effects of an artificial airway and prevent VAE development. Therefore, the current study aims to assess the effectiveness of fourth hourly oropharyngeal suctioning with standard oral care on VAE development.
MATERIALS AND METHODS
A randomized controlled trial design was adopted for the study. Data were collected from seven intensive care units (ICU) at a tertiary care hospital in the public health sector of South India for a period of 8 months (January-August 2022).
Sampling and Randomization
The participants were selected using a convenience sampling technique. We anticipated a 20% reduction of VAP among patients undergoing oropharyngeal suctioning compared to the control group. With 80% power and a 5% level of significance, the sample size of 54 participants in each group was estimated using a comparison of two independent proportions. After considering a 10% attrition rate, a total of 120 participants were enrolled [10]. Adult patients who were ≥18 years, expected to be mechanically ventilated for at least the next 72 hours, on enteral feeding (nasogastric or orogastric tube feeding), and receiving an H2 receptor blocker/proton pump inhibitors were enrolled within 24 hours of intubation. Patients who were intubated for aspiration pneumonitis, reintubated, contraindicated for oral care, facial/oral surgeries, or who were receiving total parenteral nutrition were excluded from the study.
Stratified block randomization was used using the strata of sex (male, female) and Acute Physiology and Chronic Health Evaluation (APACHE) II score (≤25, >25). A random allocation sequence was generated by a statistician outside of the research team. Allocation concealment was performed using sequentially numbered opaque sealed envelopes (consort diagram) (Figure 1).
Figure 1.
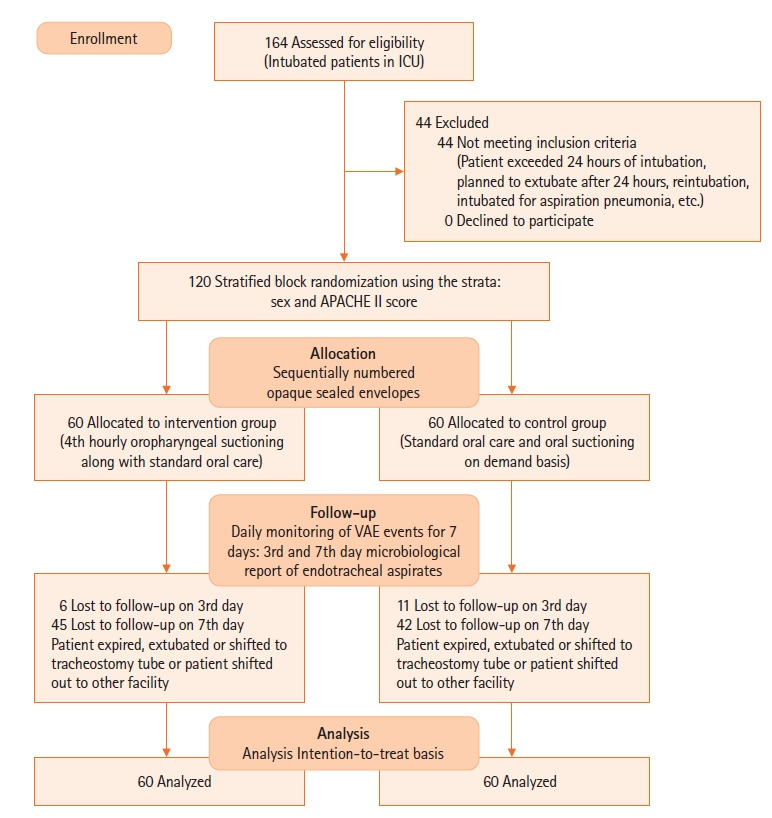
Consort diagram. ICU: intensive care unit; APACHE: Acute Physiology and Chronic Health Evaluation; VAE: ventilator-associated event.
Ethical Considerations
Permission was obtained for the current study from the Institute Nursing Research Monitoring Committee (CON/NRMC/M.Sc./2020/MSN/3) and the Institute Ethics Committee for human studies (CON/IEC/M.Sc./2020/MSN/3). The study was registered under Clinical Trial Registry India (CTRI/2022/01/039460). The procedures were performed in accordance with the ethical standards of the institution, as well as the Declaration of Helsinki (revised in 2013). After a brief explanation to the Legally authorized representative (LAR) of each enrolled patient regarding the study, informed consent was obtained from the LAR (as the patients were unable to give consent because of their critical condition and inability/unconsciousness). Patient data were stored confidentially. Confidentiality, the anonymity of the subjects, and the right to withdraw from the study were explained to the LAR before data collection.
Intervention and Data Collection
Before the intervention, the patient’s vital signs were assessed, including oxygen saturation, respiratory rate, and pulse rate. The ventilator parameters and oxygenation were also recorded. The patient was placed in the lateral position, and under a strict aseptic technique, a suction catheter was advanced through the mouth towards the trachea approximately 3–4 inches. Suction was then applied for a maximum of up to 10–15 seconds with a suction pressure of 100–120 mm Hg. A break of 30 seconds to 1 minute was provided before the next insertion. This procedure was repeated every fourth hour, along with oral care using chlorhexidine solution, for 7 days after enrollment, or until the patient got extubated or transferred to another setting. In contrast, the participants in the control group received oral suctioning when it was required and standard oral care (oral care with chlorhexidine thrice a day). Both groups received endotracheal suctioning every hour and when required as per unit protocol.
Each participant was classified as VAE-positive or -negative using the CDC algorithm [3]. The daily minimum FiO2 and PEEP were recorded after a period of sustainability for two days. Any value increase indicates worsening oxygenation, which reflects VAC. An IVAC was defined by a temperature >38 °C/<36 °C with or without a WBC count ≥12,000/≤4,000 cells/mm3 and a newly started antimicrobial agent with or without worsening of oxygenation. On day 3 and day 7, tracheal specimens were collected into sterile mucus traps from both groups following standard protocol in order to isolate any microorganisms or rule out VAP. Many participants were lost to follow-up because of extubation, placement of a tracheostomy, transfer out of the ICU, leaving against medical advice, or death. The day when VAC/IVAC/PVAP criteria were met was considered the time of VAE development. Throughout the trial, the investigator continuously ensured that the ETT cuff pressure was maintained between 25 and 30 mm Hg, and the head of the bed was elevated to 30°. Detection bias was avoided because the outcome assessment was performed by another investigator who was blinded to the study group.
Data Analysis
All statistical analyses were done using SPSS ver. 25.0 (IBM Corp.) on an intention-to-treat basis to reduce attrition bias. The categorical variables (such as sex, education, type of ETT, the incidence of VAE, etc.) were expressed as frequencies and percentages. The continuous data (such as age, body mass index, ETT cuff pressure, duration of intubation, etc.) were expressed as means with standard deviations, or medians with interquartile ranges. Comparison of the baseline characteristics between the groups was done using the chi-square test, Fisher’s exact test, Mann-Whitney U-test, and independent Student t-test. The number of VAE-positive participants was compared between the groups using the chi-square test or Fisher’s exact test. Kaplan-Meier survival analysis was performed to determine the time to VAE. Differences between the survival curves across each group were tested for significance by the log-rank statistic.
RESULTS
The study gathered data from a total of 120 participants from seven ICUs. The groups were homogeneous with regard to religion, nativity, occupation, and education. The mean ages in the intervention and control groups were 50.75 years and 48.85 years, respectively. In terms of sex, both groups had male preponderance. The majority of the participants were intubated with a size 7.5 ETT in both groups. The groups were similar with regard to the duration of intubation before enrollment (P=0.224), their APACHE II score (P=0.148), type of intubation (P=0.273), and type of suction (P=1.000) (Table 1).
Table 1.
Comparison of intubation and ventilation-related parameters between intervention and control groups (N=120)
| Variable | Intervention (n=60) | Control (n=60) | P-value |
|---|---|---|---|
| Size of ETT (mm) | 0.522a) | ||
| 6.5 | 0 | 1 (1.7) | |
| 7 | 7 (11.7) | 7 (11.7) | |
| 7.5 | 33 (55.0) | 28 (46.7) | |
| 8 | 20 (33.3) | 24 (40.0) | |
| Duration of hospital stay (day) | 3.0 (2.0–4.8) | 3.0 (2.0–4.0) | 0.593b) |
| Daily minimum FiO2 (%) | 40.0 (40.0–57.5) | 40.0 (40.0–60.0) | 0.827b) |
| Daily minimum PEEP (cm H2O) | 5.0 (5.0–6.0) | 5.0 (5.0–5.0) | 0.254b) |
| Type of intubation | 0.273c) | ||
| Emergency | 28 (46.7) | 34 (56.7) | |
| Elective | 32 (53.3) | 26 (43.3) | |
| Type of suction | 1.000c) | ||
| Open | 36 (60.0) | 36 (60.0) | |
| Closed | 24 (40.0) | 24 (40.0) | |
| DOI (before enrollment) (hr) | 12.8±6.4 | 14.2±6.5 | 0.224d) |
| APACHE II scored) | 16.0±6.1 | 16.8±6.9 | 0.500d) |
Values are presented as number (%), median (interquartile range), or mean±standard deviation.
ETT: endotracheal tube; FiO2: fraction of inspired oxygen; PEEP: positive end-expiratory pressure; DOI: duration of intubation; APACHE: Acute Physiology and Chronic Health Evaluation.
Fisher’s exact test;
Mann-Whitney U-test;
Chi-square test;
Independent Student t-test.
There was a significant difference in positive microbiological culture reports between the intervention group (48.3%) and control group (71.7%) following the 3rd day of the intervention (P<0.001). However, only a few participants (intervention, 9; control, 7) reached day 7, as most of them were either extubated, transitioned to a tracheostomy, transferred out to another setting or died (Table 2). The total duration of MV (in hours) was 83.5 (60.0–145.3) in the intervention group and 78.5 (59.3–139.0) in the control group, which was statistically non-significant. The number of days in the ICU was 9 (5.25–14.0) in the intervention group, and 7 (4.25–12.75) in the control group.
Table 2.
Microbiology profiles by day and group (N=120)
| Microbiology report | Intervention | Control | P-value |
|---|---|---|---|
| Day 3 | <0.001a) | ||
| Culture positive | 29 (48.3) | 43 (71.7) | |
| Culture negative | 25 (41.7) | 6 (10.0) | |
| Day 7 | 0.666b) | ||
| Culture positive | 8 (13.3) | 5 (8.3) | |
| Culture negative | 1 (1.7) | 2 (3.3) |
Values are presented as number (%).
Chi-square test;
Fisher’s exact test.
Fewer participants (56.70%) developed VAE in the intervention group than did in the control group (78.30%) (P=0.011). There were also fewer PVAP incidences in the intervention group (51.67%) than in the control group (71.67%), although the difference was not statistically significant (P=0.06). There was no statistically significant difference observed between VAC and IVAC. Although there was a trend for lower VAC cases in the intervention group as compared to in the control group, the number of IVAC events was similar in both groups (Table 3). There was no association between VAE and some of the selected clinical variables such as temperature, APACHE II score, random blood sugar, type of suction, type of intubation, etc. (Table 4).
Table 3.
Comparison of ventilator-associated events (N=120)
| Event | Intervention | Control | P-value |
|---|---|---|---|
| Ventilator-associated condition | 0.114a) | ||
| Yes | 1 (1.7) | 6 (10.0) | |
| No | 59 (98.3) | 54 (90.0) | |
| Infection-related ventilator-associated condition | 1.000b) | ||
| Yes | 9 (15.0) | 9 (15.0) | |
| No | 51 (85.0) | 51 (85.0) | |
| Possible ventilator-associated pneumonia | 0.069b) | ||
| Yes | 31 (51.7) | 43 (71.7) | |
| No | 29 (48.3) | 17 (28.3) | |
| Ventilator-associated event | 0.011b) | ||
| Yes | 34 (56.7) | 47 (78.3) | |
| No | 26 (43.3) | 13 (21.7) |
Values are presented as number (%).
Fisher’s exact test;
Chi-square test.
Table 4.
Association between ventilator-associated events and selected clinical parameters (N=120)
| Parameter | Ventilator-associated event |
P-value | |
|---|---|---|---|
| Present | Absent | ||
| Type of intubation | 0.446a) | ||
| Emergency | 41 (66.1) | 21 (33.9) | |
| Elective | 40 (69.0) | 18 (31.0) | |
| Type of suction | 0.359a) | ||
| Open | 50 (69.4) | 22 (30.6) | |
| Closed | 31 (64.6) | 17 (35.4) | |
| Duration of intubation before enrollment (hr) | 13.96±6.05 | 12.49±7.15 | 0.241b) |
| Duration of hospital stay (day) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) | 0.610c) |
| Daily minimum FiO2 (%) | 40.0 (40.0–60.0) | 40.0 (40.0–60.0) | 0.995c) |
| Daily minimum positive end-expiratory pressure (cm H2O) | 5.0 (5.0–5.50) | 5.0 (5.0–5.0) | 0.765c) |
| APACHE II score | 16.0 (12.0–21.0) | 16.0 (11.0–21.0) | 0.770c) |
| Body temperature (°C) | 36.7 (36.7–37.2) | 36.7 (36.7–37.2) | 0.456c) |
| Respiratory rate (/min) | 21.0 (18.0–27.0) | 20.0 (15.0–27.0) | 0.560c) |
| Random blood sugar (mg/dl) | 142.0 (111.5–206.5) | 152.0 (109.0–191.0) | 0.860c) |
| White blood cell (/mm3) | 15.0 (10.0–19.0) | 16.0 (12.0–21.0) | 0.257c) |
Values are presented as number (%), mean±standard deviation, or median (interquartile range).
APACHE: Acute Physiology and Chronic Health Evaluation.
Chi-square;
Independent Student t-test;
Mann-Whitney U-test.
The characterizations of the participants’ survival times are shown in Table 5 and Figure 2. The median time to VAE was 4 days (95% confidence interval [CI,] 3.780–4.220 days) in the intervention group and 4 days (95% CI, 3.677–4.323 days) in the control group. The Kaplan-Meier survival curves and log-rank test analysis also revealed no significant difference in the time to VAE (in days) based on the intervention.
Table 5.
Survival time (time to ventilator-associated events) of participants (N=120)
| Day to ventilator-associated events |
P-valuea) | ||||
|---|---|---|---|---|---|
| Median estimate | Standard error | 95% Confidence interval |
|||
| Lower | Upper | ||||
| Intervention | 4 | 0.112 | 3.780 | 4.220 | 0.770 |
| Control | 4 | 0.165 | 3.677 | 4.323 | |
Mantel-Cox log-rank test.
Figure 2.
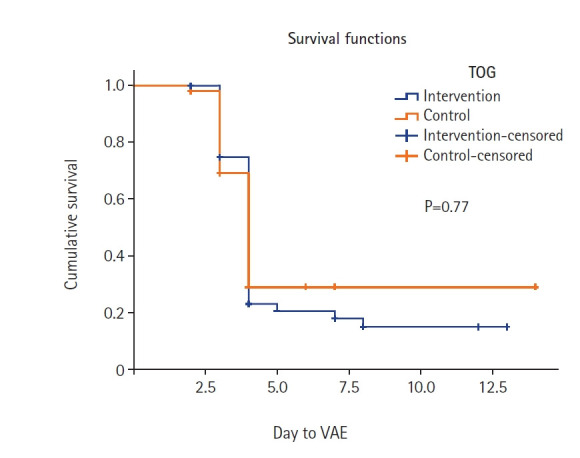
Kaplan-Meier survival curve. TOG: type of group; VAE: ventilator-associated event.
DISCUSSION
Aspiration of oral secretions one of the most common causes of pneumonia in mechanically ventilated patients [11]. In patients with oral ETT intubation, approximately 7.5 ml of secretions can accumulate in the oropharynx in 4 hours [1], which can be microaspirated. Therefore, removal of these secretions is necessary to prevent aspiration. The oral cavity is also an important source of bacteria; the ETT can act as a conduit from the oral cavity to the lung by which bacteria travel and cause VAP. Therefore, providing oral care to patients on MV can significantly reduce the relative risk of VAP development [12]. Despite its importance, oral care is commonly neglected in mechanically ventilated patients. Therefore, practicing an evidence-based oral care program can improve oral health, reduce the incidence of VAP statistically [10], and thereby lower the incidence of VAE.
One promising study which implemented the deep oropharyngeal suctioning to reduce oropharyngeal secretion pooling as the intervention (along with routine oral care with chlorhexidine), has identified decreased and incidence of aspiration and VAE [13]. Similarly, the current study also found that implementing fourth hourly oropharyngeal suctioning along with standard oral care reduces the incidence of development of VAE in the intervention group. The findings were consistent with the study conducted by Garcia et al. [6], who reported that suctioning every sixth hour along with other oral care measures can reduce the rate of VAP. In contrast to the present study findings, Atashi et al. [14] showed that deep mouth and throat suctioning every 4th, 6th, 8th, 12th hour along with other measures (depending upon the oral condition) did not decrease the incidence of VAP.
The CDC endorsed a new surveillance strategy based on VAE to assess complications in patients receiving MV. The VAE surveillance shifts the focus away from infectious etiologies like VAP toward other common complications related to a ventilator [3]. However, in this study, VAE surveillance corresponds to VAP surveillance because the data matched with the VAP rates assessed by the hospital infection control committee.
The APACHE II is a score that estimates the severity of disease and mortality of ICU patients. Studies show that the risk of developing VAP increases with an increase in the APACHE II score [15]. In contrast, our study and that of Nakahashi et al. [16] found that the APACHE II score had no association with the VAE and no-VAE groups. Previous studies have shown that the closed type of suctioning is associated with a lower risk of VAP development than is open suction [17,18]. In contrast, Hamishekar et al. [19] did not find significant differences in VAP development using closed or open suctioning. Similarly, the current study also did not find any difference in VAE events among the open and closed suctioning groups.
Oral care interventions such as oropharyngeal suctioning are performed by nurses to reduce ventilator complications. Many studies have been conducted to evaluate the relationship between oral care interventions and the prevention of VAP. However, few studies have evaluated the relationship between oral care interventions and VAE using the new surveillance protocol. VAE and VAP are two separate but related conditions; therefore, reducing VAP will ultimately minimize the incidence of VAE. A study assessing the effect of 4th hourly oropharyngeal aspiration on the incidence of VAP revealed a significant difference in VAP incidence in the intervention (14.89%) and control (39.58%) groups [20]. However, a surveillance study illustrates that oral care with chlorhexidine can lower the VAP rates, but is unlikely to minimize VAE [21]. In contrast with this, the current study reported a significant effect of 4th hourly oropharyngeal suctioning on VAE incidence, and a lower incidence of VAC and PVAP in the intervention group. The positivity of microbiological cultures from endotracheal aspiration on the 3rd day was significantly higher in the control group than it was in the intervention group (P<0.001). However, a similar study did not find a significant difference in the microbiology reports after the 3rd or 5th day of their intervention [14]. Yet Gershonovitch et al. [22] described that after oropharyngeal suctioning, oral care with 0.12% chlorhexidine, a thorough toothbrushing, and cleansing of the oral cavity, there were fewer cases of VAP in the intervention group (16.19%) than in the control group (25.9%) (P=0.084).
VAE are closely related to patient outcomes, such as the length of hospital stay, number of ventilator-free days, and mortality [23,24]. However, we found no significant difference in the length of ICU stay or MV duration between the groups. Moreover, the duration of MV and ICU stay in the intervention group was minimally longer than that of the control group. This finding might have been attributed to mortality events in both the groups and patients leaving against medical advice. Survival analysis also shows no difference in the time to the VAE between the groups. These findings may also be due to a lack of representation in terms of sample size, and loss to follow-up until the completion of the intervention period.
The limitations of the study include its smaller sample size, and performance at a single tertiary care center. Patient follow-up for mortality was not performed. Therefore, a large-scale multi-center study with long-term health outcomes may also be conducted for better generalization of the study findings for the proposed intervention.
VAE are the most common complications of MV. A patient’s normal defense mechanisms are compromised during mechanically ventilation, making the patient prone to complications related to ventilation itself. One of the most basic preventive strategies against these complications is to provide oral care and oropharyngeal suctioning in particular. We found that 4th hourly oropharyngeal suctioning along with standard oral care can be effectively reduce VAE.
KEY MESSAGES
▪ Oropharyngeal suctioning is an important component of the oral care protocol that can reduce the development of ventilator-associated events by preventing microaspiration.
▪ Fourth hourly oropharyngeal suctioning along with standard oral care was effective and is recommended to reduce the development of ventilator-associated events.
Acknowledgments
None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
AUTHOR CONTRIBUTIONS
Conceptualization: all authors. Methodology: all authors. Formal analysis: all authors. Data curation: KB, LR, MS, RM, HTL. Visualization: KB, LR, MS, HTL, RS. Project administration: LR. Writing–original draft: KB, LR, HTL. Writing–review & editing: all authors.
REFERENCES
- 1.Sole ML, Penoyer DA, Bennett M, Bertrand J, Talbert S. Oropharyngeal secretion volume in intubated patients: the importance of oral suctioning. Am J Crit Care. 2011;20:e141–5. doi: 10.4037/ajcc2011178. [DOI] [PubMed] [Google Scholar]
- 2.Burja S, Belec T, Bizjak N, Mori J, Markota A, Sinkovič A. Efficacy of a bundle approach in preventing the incidence of ventilator associated pneumonia (VAP) Bosn J Basic Med Sci. 2018;18:105–9. doi: 10.17305/bjbms.2017.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Department of Health and Human Services, Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2019. Ventilator-associated event (VAE) [cited 2023 Aug 1]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/10-vae_final.pdf. [Google Scholar]
- 4.Klompas M, Kleinman K, Murphy MV. Descriptive epidemiology and attributable morbidity of ventilator-associated events. Infect Control Hosp Epidemiol. 2014;35:502–10. doi: 10.1086/675834. [DOI] [PubMed] [Google Scholar]
- 5.Peña-López Y, Ramírez-Estrada S, Rello J. Ventilator-associated events: definitions and uses. Encyclopedia of Respiratory Medicine. 2022:523–9. [Google Scholar]
- 6.Garcia R, Jendresky L, Colbert L, Bailey A, Zaman M, Majumder M. Reducing ventilator-associated pneumonia through advanced oral-dental care: a 48-month study. Am J Crit Care. 2009;18:523–32. doi: 10.4037/ajcc2009311. [DOI] [PubMed] [Google Scholar]
- 7.Bergmans DC, Bonten MJ, Gaillard CA, Paling JC, van der Geest S, van Tiel FH, et al. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med. 2001;164:382–8. doi: 10.1164/ajrccm.164.3.2005003. [DOI] [PubMed] [Google Scholar]
- 8.Boyer AF, Schoenberg N, Babcock H, McMullen KM, Micek ST, Kollef MH. A prospective evaluation of ventilator-associated conditions and infection-related ventilator-associated conditions. Chest. 2015;147:68–81. doi: 10.1378/chest.14-0544. [DOI] [PubMed] [Google Scholar]
- 9.Muscedere J, Sinuff T, Heyland DK, Dodek PM, Keenan SP, Wood G, et al. The clinical impact and preventability of ventilator-associated conditions in critically ill patients who are mechanically ventilated. Chest. 2013;144:1453–60. doi: 10.1378/chest.13-0853. [DOI] [PubMed] [Google Scholar]
- 10.Bo H, He L, Qu J. Influence of the subglottic secretion drainage on the morbidity of ventilator associated pneumonia in mechanically ventilated patients. Zhonghua Jie He He Hu Xi Za Zhi. 2000;23:472–4. [PubMed] [Google Scholar]
- 11.Paju S, Scannapieco FA. Oral biofilms, periodontitis, and pulmonary infections. Oral Dis. 2007;13:508–12. doi: 10.1111/j.1601-0825.2007.1410a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fields LB. Oral care intervention to reduce incidence of ventilator-associated pneumonia in the neurologic intensive care unit. J Neurosci Nurs. 2008;40:291–8. doi: 10.1097/01376517-200810000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Sole ML, Talbert S, Yan X, Penoyer D, Mehta D, Bennett M, et al. Impact of deep oropharyngeal suctioning on microaspiration, ventilator events, and clinical outcomes: a randomized clinical trial. J Adv Nurs. 2019;75:3045–57. doi: 10.1111/jan.14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atashi V, Yousefi H, Mahjobipoor H, Bekhradi R, Yazdannik A. Effect of oral care program on prevention of ventilator-associated pneumonia in intensive care unit patients: a randomized controlled trial. Iran J Nurs Midwifery Res. 2018;23:486–90. doi: 10.4103/ijnmr.IJNMR_164_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sutiono AB, Arifin MZ, Adhipratama H, Hermanto Y. The utilization of APACHE II score to predict the incidence of ventilator-associated pneumonia in patients with severe traumatic brain injury: a single-center study. Interdiscip Neurosurg. 2022;28:101457. [Google Scholar]
- 16.Nakahashi S, Imai H, Imanaka H, Ohshimo S, Satou T, Shima M, et al. Ventilator-associated events: prevalence and mortality in Japan. J Thorac Dis. 2018;10:6942–9. doi: 10.21037/jtd.2018.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alipour N, Toulabi T, Manouchehrian N, Anbari K, Rahimi Bashar F. A comparison of the effect of open and close endotracheal suctioning on the hemodynamic status of patient with head trauma hospitalized in the intensive care unit. Evid Based Care. 2014;3:65–74. [Google Scholar]
- 18.Ebrahimi Fakhar H, Rezaei K, Kohestani HR. Effect of closed endotracheal suction on incidence of ventilator-associated pneumonia. Sci J Kurdistan Univ Med Sci. 2010;15:79–87. [Google Scholar]
- 19.Hamishekar H, Shadvar K, Taghizadeh M, Golzari SE, Mojtahedzadeh M, Soleimanpour H, et al. Ventilator-associated pneumonia in patients admitted to intensive care units, using open or closed endotracheal suctioning. Anesth Pain Med. 2014;4:e21649. doi: 10.5812/aapm.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi L, Guo X, Nie C, Lv X, Zhang M. Research on effects of oropharyngeal aspiration on incidence of ventilator-associated pneumonia in patients with cerebral hemorrhage in ICU. J Healthc Eng. 2022;2022:6433666. doi: 10.1155/2022/6433666. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Klompas M. Potential strategies to prevent ventilator-associated events. Am J Respir Crit Care Med. 2015;192:1420–30. doi: 10.1164/rccm.201506-1161CI. [DOI] [PubMed] [Google Scholar]
- 22.Gershonovitch R, Yarom N, Findler M. Preventing ventilator-associated pneumonia in intensive care unit by improved oral care: a review of randomized control trials. SN Compr Clin Med. 2020;2:727–33. doi: 10.1007/s42399-020-00319-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein Klouwenberg PM, van Mourik MS, Ong DS, Horn J, Schultz MJ, Cremer OL, et al. Electronic implementation of a novel surveillance paradigm for ventilator-associated events: feasibility and validation. Am J Respir Crit Care Med. 2014;189:947–55. doi: 10.1164/rccm.201307-1376OC. [DOI] [PubMed] [Google Scholar]
- 24.Bouadma L, Sonneville R, Garrouste-Orgeas M, Darmon M, Souweine B, Voiriot G, et al. Ventilator-associated events: prevalence, outcome, and relationship with ventilator-associated pneumonia. Crit Care Med. 2015;43:1798–806. doi: 10.1097/CCM.0000000000001091. [DOI] [PubMed] [Google Scholar]


