Abstract
Background
Delirium occurs at high rates among patients in intensive care units and increases the risk of morbidity and mortality. The purpose of this study was to investigate the effects of environmental interventions on delirium.
Methods
This prospective cohort study enrolled 192 patients admitted to the surgical intensive care unit (SICU) during the pre-intervention (June 2013 to October 2013) and post-intervention (June 2014 to October 2014) periods. Environmental interventions involved a cognitive assessment, an orientation, and a comfortable environment including proper sleep conditions. The primary outcomes were the prevalence, duration, and onset of delirium.
Results
There were no statistically significant differences in incidence rate, time of delirium onset, general characteristics, and mortality between the pre-intervention and post-intervention groups. The durations of delirium were 14.4±19.1 and 7.7±7.3 days in the pre-intervention and post-intervention groups, respectively, a significant reduction (P=0.027). The lengths of SICU stay were 20.0±22.9 and 12.6±8.7 days for the pre-intervention and post-intervention groups, respectively, also a significant reduction (P=0.030).
Conclusions
The implementation of an environmental intervention program reduced the duration of delirium and length of stay in the SICU for critically ill surgical patients.
Keywords: critical care, delirium, intensive care unit, length of stay
INTRODUCTION
Delirium is a neuropsychiatric syndrome characterized by cognitive dysfunction, a decreased ability to maintain attention, and unorganized thinking owing to several factors [1]. The incidence rate of delirium is 20%–30% among patients hospitalized in the general ward and 36%–44% among older post-surgery patients [2,3]. However, the incidence rate among patients in intensive care units (ICUs) can approach 70%–90% [4-6]. Delirium has been associated with a prolonged hospital stay, more frequent complications, increased cost of care and duration of mechanical ventilator use, chronic impairment of cognitive function, and mortality [6-8].
Several studies reported that an estimated 30%–40% of delirium cases are preventable [2,9]. However, preventive measures are often not implemented because of advanced patient age, poor baseline cognition, and patient fragility. Inouye [1] identified four predisposing factors (cognitive impairment, severe illness, visual impairment, and dehydration) and five precipitating factors (polypharmacy, catheterization, use of restraints, malnutrition, and any iatrogenic event) associated with the development of delirium [6,10]. Their results suggest that patients with high baseline vulnerability can develop delirium in response to weak precipitants.
The most frequently debated factor that influences delirium onset is the ICU environment [2]. Crucial factors relevant to the development of delirium include (1) separation from family and acquaintances, (2) a mechanized environment for treatment, (3) noise, (4) bright lights, (5) insufficient sleep, (6) no guaranteed privacy, (7) an environment without windows, where the day is indistinguishable from the night, and (8) movement limitations owing to the numerous catheters required for monitoring and treatment [11].
In the ICU, the main treatment of delirium involves pharmacological intervention [12,13]. Non-pharmacological intervention requires a multi-disciplinary approach; therefore, implementing non-pharmacological interventions in ICU settings can be challenging. However, several studies have reported that non-pharmacological interventions can prevent delirium in ICU patients more effectively than pharmacological interventions [6,13-15]. Delirium frequently occurs in the surgical intensive care unit (SICU) and has been reported to have a negative effect on prognosis in several studies [16]. However, there are not many studies demonstrating the effectiveness of environmental interventions specifically in critically ill surgical patients, a group that experiences a high incidence of delirium. In the present study, we describe results of non-pharmacological environmental interventions targeting critically ill patients admitted to the SICU and investigated whether these interventions improved outcomes associated with delirium.
MATERIALS AND METHODS
Study Design
This prospective, pre-post intervention cohort study was performed to assess the impact of an environmental intervention program on critically ill surgical patients (such as those with postoperative trauma and sepsis) who were admitted to the SICU of Asan Medical Center, a tertiary academic teaching hospital with over 2,000 beds. The SICU has 14 beds and is attended by physicians (1 attending, 3 fellows) and registered nurses (staff to patient ratio, 1:2).
This study was approved by the Institutional Review Board of Asan Medical Center (No. 2014-0344). In the intervention group, we obtained written informed consent from the patients and/or the closest family member if a patient could not provide consent. In the control group, we collected data retrospectively and proceeded with a waiver of informed consent. This trial is registered at ClinicalTrials.gov (NCT04042649).
Study Population
The present study enrolled patients admitted to the SICU during the pre-intervention period (June 2013 to October 2013) and post-intervention period (June 2014 to October 2014). Environmental interventions had been applied in the SICU since March 2014, and a 3-month window was decided to consider trial and error in the early stages of the intervention protocol. Inclusion criteria were patients (1) 18 years or older, (2) who understood the purpose of this study and agreed to participate, and (3) who stayed in the SICU for at least 48 hours. Exclusion criteria were patients who (1) remained unresponsive (defined as a Richmond Agitation-Sedation Scale [RASS] score less than –4), (2) could not be assessed by the Confusion Assessment Method for the ICU (CAM-ICU) owing to severe visual or hearing disturbance, (3) had a history of severe psychiatric or neurologic deficits (including delirium before ICU admission), (4) required isolation due to transplantation or immunological compromise, (5) were discharged from the ICU within 48 hours, (6) were re-admitted to the ICU, (7) were younger than 18 years, and (8) were admitted to the SICU through another ICU, because patient transfer also affects delirium.
Delirium Diagnosis
The Society of Critical Care Medicine recommends using the CAM-ICU, developed for critically ill patients, to diagnose delirium [17]. The CAM-ICU has high reliability (93%–100%) and validity (98%–100%), as well as high internal validity [18,19]. The CAM-ICU can be easily administered to critically ill patients on mechanical ventilation [20]. The nurse in charge of each patient applied the CAM-ICU tool using the same method during the pre- and post-intervention periods and confirmed the development of delirium on each shift (three times per day). To increase accuracy, the presence of delirium was also confirmed by the head nurse. In addition, medical staff training was reinforced via quality improvement (QI) activities.
Delirium Prevention QI Program
The interdisciplinary QI team comprised SICU attending staff, a clinical nurse practitioner, an SICU nurse unit manager, and bedside registered nurses. First, environmental factors that could be improved were identified. During the post-intervention period, we conducted team education on the environmental intervention protocol, analyzed risk factors, and provided feedback on the subsequent outcomes (Figure 1). The environmental interventions were not conducted during the pre-intervention period. However, during the post-intervention period, they were implemented for all patients admitted to the SICU, regardless of delirium diagnosis, and intervention activities were reinforced based on a checklist for task performance. Environmental interventions were performed from the day after SICU admission to discharge from the SICU. The environmental intervention protocol was carried out as described in Figure 2. On the day after SICU admission, a calendar was placed at a site with easy visibility, while an accurate and clear-cut orientation was provided from time, place, and person on every shift. In addition, pictures of the family or close friends were posted, and patients listened to music or watched portable television. A call bell was installed within hands-reach for patients for whom oral communication was difficult owing to tracheal intubation or tracheostomy. Communication was improved by providing glasses and hearing aids to patients with visual and hearing impairments, respectively. A proper sleep environment to improve and promote sleep during the night was created by minimizing nonessential medical activities and providing earplugs and eye masks to be worn as desired [17].
Figure 1.
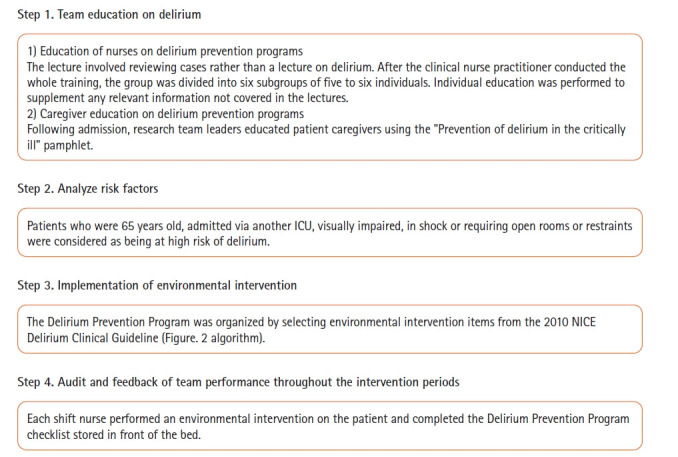
Delirium prevention quality improvement program. ICU: intensive care unit; NICE: National Institute for Health and Care Excellence.
Figure 2.
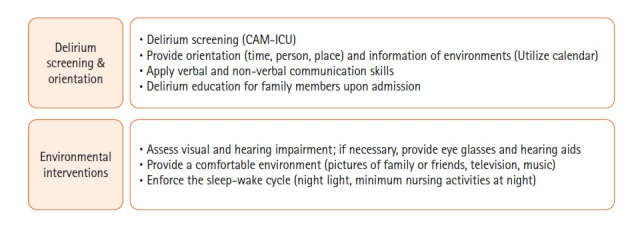
Environmental intervention protocol. CAM-ICU: Confusion Assessment Method for the intensive care unit.
Outcomes and Data Collection
The primary outcomes were prevalence, time to onset, and duration of delirium. Secondary outcomes were number of days of ventilator use, length of SICU stay, length of stay (LOS) at the hospital, rate of SICU readmission, and ICU and in-hospital mortality rates. The relevant data were collected through the electronic medical record system. Average sleep time was gathered through nursing records, while the history of sleep disorders and the use of sleeping pills were collected through medical records to determine whether patients had a prior history of treatment before admission to the SICU. Data from the pre-intervention period were collected retrospectively, while data from the post-intervention period were collected prospectively after obtaining IRB approval.
Statistical Analysis
All statistical analyses were performed using IBM SPSS 21.0 (IBM Corp.). The chi-square test and t-test were used to analyze the general characteristics and clinical outcomes of target groups. A two-sided P-value less than 0.05 was considered statistically significant.
RESULTS
Study Population and Characteristics
A total of 463 patients was admitted to the SICU during the study period. However, 271 patients were excluded based on the predefined criteria. We enrolled a total of 192 patients; 101 patients were in the pre-intervention (control) group without environmental interventions, and 91 patients were in the post-intervention (intervention) group with environmental intervention. During the study period, five patients in the pre-intervention group and three in the post-intervention group dropped out owing to sudden exacerbation of their general condition, with an RASS score of –4 or –5. The final analysis included 96 patients in the pre-intervention group and 88 in the post-intervention group. There were no significant differences between baseline measures of the two groups (Table 1). Of the final cohort, 69 (71.9%) patients in the pre-intervention group and 61 (69.3%) in the post-intervention group developed delirium (Figure 3). No significant differences were observed between the pre- and post-intervention groups in any general characteristics, including sex, age, severity, or ventilator use (Table 2).
Table 1.
General characteristics of included patients (n=184)
| Characteristics | Pre-intervention (n=96) | Post-intervention (n=88) | P-value |
|---|---|---|---|
| Male | 77 (80.2) | 61 (69.3) | 0.125 |
| Age (yr) | 62±14 | 64±16 | 0.334 |
| >65 yr | 61 (63.5) | 54 (61.4) | 0.879 |
| Average sleep time (hr) | 6.5±1.6 | 6.5±1.7 | 0.154 |
| Sleep disorder | 7 (7.3) | 6 (6.8) | 1.000 |
| Use of sleeping pills | 3 (3.1) | 6 (6.8) | 0.413 |
| Visual disturbance | 70 (72.9) | 54 (61.4) | 0.130 |
| Hearing disturbance | 8 (8.3) | 11 (12.5) | 0.493 |
| APACHE II score | 13.7±7.0 | 15.3±6.4 | 0.534 |
| Incidence of delirium | 69 (71.9) | 61 (69.3) | 0.750 |
Values are presented as number (%) or mean±standard deviation.
APACHE: Acute Physiology and Chronic Health Evaluation.
Figure 3.
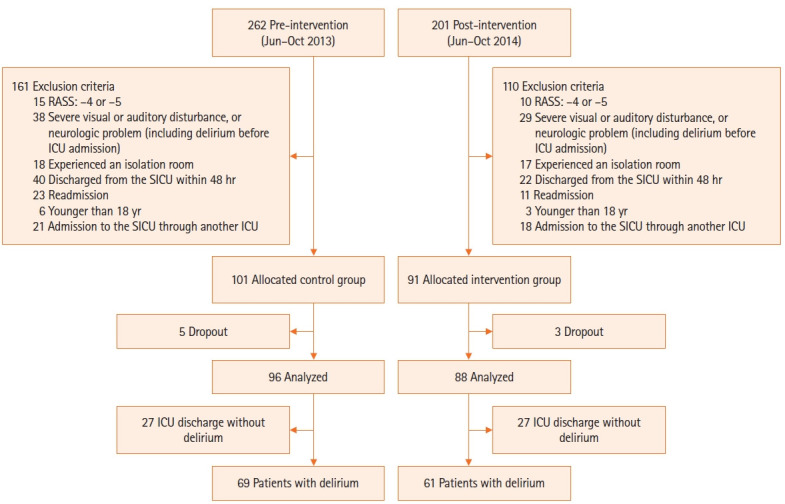
Flow diagram of study participants. RASS: Richmond Agitation-Sedation Scale; ICU: intensive care unit; SICU: surgical intensive care unit.
Table 2.
General characteristics of delirium patients (n=130)
| Characteristics | Pre-intervention (n=69) | Post-intervention (n=61) | P-value |
|---|---|---|---|
| Male | 57 (82.6) | 42 (68.9) | 0.066 |
| Age (yr) | 67±11 | 67±14 | 0.934 |
| >65 yr | 44 (63.8) | 43 (70.5) | 0.416 |
| Average sleep time (hr) | 6.4±1.4 | 6.5±1.5 | 0.601 |
| Sleep disorder | 5 (7.2) | 4 (6.6) | 0.877 |
| Use of sleeping pills | 2 (2.9) | 6 (9.8) | 0.100 |
| Visual disturbance | 52 (75.4) | 39 (63.9) | 0.156 |
| Hearing disturbance | 7 (10.1) | 7 (11.5) | 0.807 |
| Route of admission | 0.152 | ||
| Emergency room | 32 (46.3) | 26 (42.7) | |
| Operation room | 29 (42.0) | 27 (44.3) | |
| General ward | 8 (11.7) | 8 (13.1) | |
| Reason for admission | 0.865 | ||
| Postoperative monitoring | 26 (37.7) | 21 (34.4) | |
| Respiratory failure | 18 (26.1) | 16 (26.2) | |
| Sepsis | 19 (27.5) | 18 (29.5) | |
| Bleeding | 2 (2.9) | 3 (4.9) | |
| Others | 4 (5.8) | 3(4.9) | |
| APACHE II score | 15.3±6.8 | 17.4±5.9 | 0.066 |
| Mechanical ventilation | 65 (94.2) | 60 (98.4) | 0.219 |
| Use of restraint | 65 (94.2) | 58 (95.1) | 0.825 |
| Hemodialysis | 12 (17.4) | 7 (11.5) | 0.341 |
| Shock | 35 (50.7) | 36 (60.0) | 0.291 |
| Sedative drug | 64 (92.8) | 58 (95.1) | 0.581 |
| Use of benzodiazepine | 16 (23.2) | 9 (15.5) | 0.279 |
Values are presented as number (%) or mean±standard deviation.
APACHE: Acute Physiology and Chronic Health Evaluation.
Clinical Outcomes before and after Environmental Interventions
No significant difference was observed between the pre- and post-intervention groups regarding the prevalence of patients who developed delirium (71.9% vs. 69.3%, P=0.75). No difference in the time of delirium onset was observed between the groups (2.6±2.4 days vs. 2.1±1.8 days, P=0.242). However, the duration of delirium was 14.4±19.1 days for patients in the pre-intervention group and 7.7±7.3 days for those in the post-intervention group, a significant reduction (P=0.027). Regarding secondary outcomes, the number of days of ventilator use tended to be lower for patients in the post-intervention group than for those in the pre-intervention group (15.3±22.9 days vs. 9.8±11.7 days, P=0.088). The LOS in the SICU was 20.0±22.9 days for patients in the pre-intervention group and 12.6±8.7 days for patients in the post-intervention group, a significant reduction (P=0.030). However, no significant differences in LOS in the general ward or the ICU or in the in-hospital or 6-month mortality rates were detected between the groups (Table 3).
Table 3.
Clinical outcomes pre- and post-intervention (n=130)
| Characteristics | Pre-intervention (n=69) | Post-intervention (n=61) | P-value |
|---|---|---|---|
| Primary outcome | |||
| Time of delirium onset (day) | 2.6±2.4 | 2.1±1.8 | 0.242 |
| Duration of delirium (day) | 14.4±19.1 | 7.7±7.3 | 0.027 |
| Secondary outcome | |||
| Day of ventilator use | 15.3±22.9 | 9.8±11.7 | 0.088 |
| Length of ICU stay (day) | 20.0±22.9 | 12.6±8.7 | 0.030 |
| Length of GW stay (day) | 25.9±32.9 | 29.7±41.1 | 0.561 |
| Readmission to SICU | 11 (15.9) | 9 (14.7) | 0.549 |
| Length of hospital stay (day) | 52.0±42.6 | 46.8±43.3 | 0.435 |
| SICU mortality | 8 (11.6) | 9 (14.7) | 0.257 |
| In-hospital mortality | 9 (13.0) | 12 (19.7) | 0.248 |
| 6-Month mortality | 12 (17.4) | 12 (19.7) | 0.914 |
Values are presented as mean±standard deviation or number (%).
ICU: intensive care unit; GW: general ward; SICU: surgical intensive care unit.
DISCUSSION
The development of delirium predicts increases in the length of ICU and hospital stay, cost of care, and mortality. Therefore, many studies have aimed at preventing delirium. Our study revealed that environmental intervention could reduce the duration of delirium. We found that the mean duration of delirium after the environmental intervention was reduced from 14.4 to 7.7 days. As the duration of delirium decreased, the total number of days spent in the SICU decreased from 20.0 to 12.6 days. Pisani et al. [21] reported that longer durations of delirium resulted in higher 1-year mortality rates, with annual increases of 10%. In addition, Ely et al. [7] showed that delirium in ICU patients with mechanical ventilation is associated with increased 6-month mortality. Although our study did not detect any differences in mortality rates, shortening the duration of delirium could significantly reduce the days of ICU stay and the length of mechanical ventilation. Prolonged ICU stays can increase the risk of complications, including aspiration, pressure ulcers, ventilator-associated pneumonia, and post-intensive care syndrome [22]. As environmental intervention decreases ICU stay, it plays a crucial role in critical care.
Several studies have reported that the incidence of delirium can be reduced by environmental interventions that minimize the risk factors for delirium [5,6,23]. Vidán et al. [24] reported that non-pharmacologic interventions can reduce the incidence of delirium by 30%–40%. Similarly, in patients with a hip fracture, Björkelund et al. [25] documented a substantial reduction in the incidence of delirium (from 34% to 22%) with interventions such as hydration, oxygenation, analgesia, and optimization in the care environment. However, our results differ from those of other studies in a number of important aspects. In our study, the incidence of delirium was not significantly different before and after interventions. As with previous studies, for our patients, delirium developed within 2–3 days after SICU admission [3,26]. This short period was insufficient to observe the effect of environmental intervention on delirium onset. It is necessary to identify and minimize the risk factors associated with delirium onset, but there was a limitation in reducing incidence because most delirium occurred so soon after initial admission to the ICU.
Herein, the mean duration of delirium was 14 days, which is considerably longer than that reported in other studies (mean duration, 3–5 days) [3,27]. The duration of delirium depends on the severity of delirium and the composition of the ICU. In other studies, many patients were in the ICU for postoperative care after elective surgery; however, in our study, sepsis and respiratory failure patients accounted for most cases, which suggests that environmental intervention could substantially impact high-severity cases.
In addition to orientation and communication assistance, we focused on a proper sleep environment. Critically ill patients experience poor sleep, which worsens delirium. One study in the SICU found that patients only slept for 2 hours per day [28]. In the present study, a proper sleep environment was established by minimizing nonessential medical activities and providing earplugs and eye masks to be worn as desired. Before implementing the protocol, we performed radiography examinations, blood sampling, and weight measurements at night. After implementing the protocol, these tests were performed before sleep or in the morning to ensure a consistent sleep/wake cycle. However, there was no significant difference observed in the change in sleep duration between the pre-intervention and post-intervention groups. There was a limitation in appropriately assessing the quality of sleep. Future evaluation of sleep quality and additional analysis are necessary.
The main strength of our study is that specially trained nurses who were CAM-ICU educated examined the patients on every shift and verified delirium diagnoses three times per day. Early detection of delirium is an important factor in its treatment [17,29]. In previous reports, delirium was diagnosed once daily using the CAM-ICU or other diagnostic tools [3,27]. However, we performed the CAM-ICU thrice daily to improve the sensitivity of the delirium diagnosis; this increased sensitivity may positively affect the outcome of environmental interventions. Even before the intervention, delirium was evaluated using CAM-ICU, but the QI activities provided continuous education and increased validity and accuracy. Another strength of this study is that it was conducted only on critical ill surgical patients, who tend to have a high incidence of delirium. We prospectively analyzed the prevalence of delirium and the effectiveness of environmental interventions by comparing patients before and after intervention.
Nevertheless, there are some limitations to this study. First, use of the “before” and “after” design should be noted. No significant difference was observed between the characteristics of patients in the “before” and “after” groups. Second, our study was conducted at a single institution. As a relatively short-term study conducted within a single institution, it may have limitations in representing all critically ill patients. Additionally, the severity of the patients at our hospital may differ from those in other ICUs, making it challenging to generalize our findings. Also, the characteristics of the ICU, such as bed type being open-type or isolated-type, may have introduced bias. According to Zaal et al. [27], switching from an open-type ICU bed to a single-bed room can reduce the duration of delirium. Therefore, subgroup analysis may be required depending on the type of ICU bed. Finally, patient comorbidities may affect the occurrence of delirium and the LOS in the ICU, but correction for these confounding variables may not be sufficient. Future evaluation and additional analysis of risk factors related to delirium may be necessary.
Delirium is one of the main causes of prolonged ICU stay. Based on our findings, environmental intervention could be a useful tool for decreasing the duration of delirium in critically ill surgical patients. The tested environmental interventions are economical, safe, and effective. To implement an environmental intervention protocol, it is necessary to identify the risk factors of delirium and to introduce environmental changes that can be realized and adapted to the actual circumstances of each hospital.
HIGHLIGHTS
▪ Environmental interventions can help reduce the duration of delirium.
▪ Environmental interventions in the intensive care unit are safe and effective.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
None.
ACKNOWLEDGMENTS
None.
AUTHOR CONTRIBUTIONS
Conceptualization: all authors. Methodology: HJL, YJJ, SKH. Data curation: NJC. Supervision: SKH. Writing–original draft: HJL, YJJ. Writing–review & editing: NJC, SKH.
REFERENCES
- 1.Inouye SK. The dilemma of delirium: clinical and research controversies regarding diagnosis and evaluation of delirium in hospitalized elderly medical patients. Am J Med. 1994;97:278–88. doi: 10.1016/0002-9343(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 2.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5:210–20. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson TN, Raeburn CD, Tran ZV, Angles EM, Brenner LA, Moss M. Postoperative delirium in the elderly: risk factors and outcomes. Ann Surg. 2009;249:173–8. doi: 10.1097/SLA.0b013e31818e4776. [DOI] [PubMed] [Google Scholar]
- 4.Pisani MA, McNicoll L, Inouye SK. Cognitive impairment in the intensive care unit. Clin Chest Med. 2003;24:727–37. doi: 10.1016/s0272-5231(03)00092-3. [DOI] [PubMed] [Google Scholar]
- 5.Holt R, Young J, Heseltine D. Effectiveness of a multi-component intervention to reduce delirium incidence in elderly care wards. Age Ageing. 2013;42:721–7. doi: 10.1093/ageing/aft120. [DOI] [PubMed] [Google Scholar]
- 6.O’Hanlon S, O’Regan N, Maclullich AM, Cullen W, Dunne C, Exton C, et al. Improving delirium care through early intervention: from bench to bedside to boardroom. J Neurol Neurosurg Psychiatry. 2014;85:207–13. doi: 10.1136/jnnp-2012-304334. [DOI] [PubMed] [Google Scholar]
- 7.Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE, Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62. doi: 10.1001/jama.291.14.1753. [DOI] [PubMed] [Google Scholar]
- 8.Dammeyer JA, Mapili CD, Palleschi MT, Eagle S, Browning L, Heck K, et al. Nurse-led change: a statewide multidisciplinary collaboration targeting intensive care unit delirium. Crit Care Nurs Q. 2012;35:2–14. doi: 10.1097/CNQ.0b013e31823b1fec. [DOI] [PubMed] [Google Scholar]
- 9.Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, et al. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- 11.Arenson BG, MacDonald LA, Grocott HP, Hiebert BM, Arora RC. Effect of intensive care unit environment on in-hospital delirium after cardiac surgery. J Thorac Cardiovasc Surg. 2013;146:172–8. doi: 10.1016/j.jtcvs.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Serafim RB, Bozza FA, Soares M, do Brasil PE, Tura BR, Ely EW, et al. Pharmacologic prevention and treatment of delirium in intensive care patients: a systematic review. J Crit Care. 2015;30:799–807. doi: 10.1016/j.jcrc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Kang J, Lee M, Ko H, Kim S, Yun S, Jeong Y, et al. Effect of nonpharmacological interventions for the prevention of delirium in the intensive care unit: a systematic review and meta-analysis. J Crit Care. 2018;48:372–84. doi: 10.1016/j.jcrc.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Patel J, Baldwin J, Bunting P, Laha S. The effect of a multicomponent multidisciplinary bundle of interventions on sleep and delirium in medical and surgical intensive care patients. Anaesthesia. 2014;69:540–9. doi: 10.1111/anae.12638. [DOI] [PubMed] [Google Scholar]
- 15.Bounds M, Kram S, Speroni KG, Brice K, Luschinski MA, Harte S, et al. Effect of ABCDE bundle implementation on prevalence of delirium in intensive care unit patients. Am J Crit Care. 2016;25:535–44. doi: 10.4037/ajcc2016209. [DOI] [PubMed] [Google Scholar]
- 16.Ali MA, Hashmi M, Ahmed W, Raza SA, Khan MF, Salim B. Incidence and risk factors of delirium in surgical intensive care unit. Trauma Surg Acute Care Open. 2021;6:e000564. doi: 10.1136/tsaco-2020-000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJ, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–73. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 18.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 19.Lin SM, Liu CY, Wang CH, Lin HC, Huang CD, Huang PY, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32:2254–9. doi: 10.1097/01.ccm.0000145587.16421.bb. [DOI] [PubMed] [Google Scholar]
- 20.Luetz A, Heymann A, Radtke FM, Chenitir C, Neuhaus U, Nachtigall I, et al. Different assessment tools for intensive care unit delirium: which score to use? Crit Care Med. 2010;38:409–18. doi: 10.1097/CCM.0b013e3181cabb42. [DOI] [PubMed] [Google Scholar]
- 21.Pisani MA, Kong SY, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. Am J Respir Crit Care Med. 2009;180:1092–7. doi: 10.1164/rccm.200904-0537OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5:90–2. doi: 10.1515/jtim-2016-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faustino TN, Suzart NA, Rabelo RN, Santos JL, Batista GS, Freitas YS, et al. Effectiveness of combined non-pharmacological interventions in the prevention of delirium in critically ill patients: a randomized clinical trial. J Crit Care. 2022;68:114–20. doi: 10.1016/j.jcrc.2021.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Vidán MT, Sánchez E, Alonso M, Montero B, Ortiz J, Serra JA. An intervention integrated into daily clinical practice reduces the incidence of delirium during hospitalization in elderly patients. J Am Geriatr Soc. 2009;57:2029–36. doi: 10.1111/j.1532-5415.2009.02485.x. [DOI] [PubMed] [Google Scholar]
- 25.Björkelund KB, Hommel A, Thorngren KG, Gustafson L, Larsson S, Lundberg D. Reducing delirium in elderly patients with hip fracture: a multi-factorial intervention study. Acta Anaesthesiol Scand. 2010;54:678–88. doi: 10.1111/j.1399-6576.2010.02232.x. [DOI] [PubMed] [Google Scholar]
- 26.Ely EW, Siegel MD, Inouye SK. Delirium in the intensive care unit: an under-recognized syndrome of organ dysfunction. Semin Respir Crit Care Med. 2001;22:115–26. doi: 10.1055/s-2001-13826. [DOI] [PubMed] [Google Scholar]
- 27.Zaal IJ, Spruyt CF, Peelen LM, van Eijk MM, Wientjes R, Schneider MM, et al. Intensive care unit environment may affect the course of delirium. Intensive Care Med. 2013;39:481–8. doi: 10.1007/s00134-012-2726-6. [DOI] [PubMed] [Google Scholar]
- 28.Aurell J, Elmqvist D. Sleep in the surgical intensive care unit: continuous polygraphic recording of sleep in nine patients receiving postoperative care. Br Med J (Clin Res Ed) 1985;290:1029–32. doi: 10.1136/bmj.290.6474.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reade MC, Eastwood GM, Peck L, Bellomo R, Baldwin I. Routine use of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) by bedside nurses may underdiagnose delirium. Crit Care Resusc. 2011;13:217–24. [PubMed] [Google Scholar]


