Abstract
Background
Reduction in distal vascular volume in acute pulmonary embolism (PE) is a significant predictor of 30- and 90-day mortality. The likely cause of this is pulmonary arterial obstruction. The effect of pharmacomechanical catheter-directed thrombolysis (PM-CDT) on the occlusions of these pulmonary artery (PA) branches is not known.
Objectives
The RESCUE study evaluated PM-CDT with the Bashir endovascular catheter in patients with acute intermediate-risk PE. This analysis assessed PA occlusions using core laboratory data before and after PM-CDT therapy.
Methods
The baseline and 48-hour post-treatment contrast-enhanced chest computed tomography angiography of PE patients with right ventricular dilatation enrolled in the RESCUE trial were used. The primary analysis was the change in the number of segmental and proximal PA branches with total or subtotal (>65%) occlusions after 48 hours compared to baseline using McNemar’s test.
Results
A total of 107 patients enrolled across 18 United States sites comprised this analysis. At 48 hours post-PM-CDT, the number of segmental PA branches with total or subtotal occlusions decreased from 40.5% to 11.7% (P < 0.0001). Proximal PA branch total or subtotal occlusions decreased from 28.7% to 11.0% (P < 0.0001). The reduction in segmental artery occlusions correlated significantly with the magnitude of reduction in right ventricular/left ventricular ratio (correlation coefficient of 0.287 [95% CI: 0.102-0.452]; P = 0.0026), whereas that in the proximal PA arteries did not (correlation coefficient of 0.132 [95% CI: 0.059-0.314] P = 0.173).
Conclusions
PM-CDT with the Bashir catheter was associated with a significant reduction in total and subtotal occlusion of segmental and proximal PAs.
Key words: catheter-directed therapy, fibrinolysis, pulmonary embolism, Refined Modified Miller Index, RV/LV ratio
Central Illustration
Reduced blood volume through the distal pulmonary vasculature is associated with significantly higher 30- and 90-day cardiovascular mortality in patients with acute pulmonary embolism (PE).1 This is most likely due to decreased arterial inflow and pulmonary arterial (PA) obstruction from acute thromboembolism and vasoconstriction.2 This acute vascular obstruction occurs as a result of the occlusion of not only the proximal pulmonary arteries but also the more distal smaller segmental branches.3 Advances in imaging technology and post-processing software facilitate the analysis of flow and perfusion through these distal smaller vessels. In addition, PA occlusions have been shown to be a predictor of chronic thromboembolic pulmonary hypertension (CTEPH) and chronic thromboembolic disease (CTED) in these patients.4,5
Several contemporary studies of catheter-based treatment of acute PE have shown a reduction in PA obstruction at 48 hours post-treatment by core lab assessment.6, 7, 8, 9 However, the magnitude of this reduction has been quite modest, particularly with mechanical thrombectomy devices, despite impressive post-procedural clot specimens.10,11 The Bashir endovascular catheter (Thrombolex, Inc) is a novel pharmacomechanical infusion catheter consisting of an expandable basket of 6 nitinol-reinforced infusion limbs that was engineered to maximize thrombus reduction.6 The recently published RESCUE12 (Recombinant tPA by Endovascular Administration for the Treatment of Submassive PE Using CDT for the Reduction of Thrombus Burden) trial showed a 35.9% reduction in PA obstruction using the Refined Modified Miller Index (RMMI), the largest reduction of all published catheter studies with core lab measurement, with similar doses of tissue plasminogen activator (tPA). In the present analysis of the RESCUE trial, we evaluated the change in the proximal and segmental PAs with total and subtotal occlusions on CTA before and at 48 hours after pharmacomechanical catheter-directed thrombolysis (PM-CDT) by the Bashir endovascular catheter in patients with acute intermediate-risk PE.
Methods
Study design and population
This report is based on the RESCUE trial data set, which was a prospective, multicenter, single-arm study to assess the safety and efficacy of PM-CDT with the Bashir endovascular catheter in patients with acute intermediate-risk PE.12 The primary study reported the effect of PM-CDT on right ventricular/left ventricular (RV/LV) ratio and RMMI, while in this analysis, we evaluated the effect of PM-CDT on the total and subtotal occlusions of the PA and its branches. The study was sponsored by the NHLBI (grant #R44HL151032-03), the Commonwealth of Pennsylvania and Thrombolex Inc. It was conducted under an Investigational Device Exemption approved by the Food and Drug Administration. Institutional review board approval was obtained at all sites, and informed consent was obtained from every patient. The study was monitored by a clinical research organization (EMINENCE Clinical Research, Inc) and an independent data safety monitoring board. The analyses of the CT scans were performed by the Core Laboratory (NAMSA/SYNTACTX, Inc): The metrics analyzed included measurements of RV/LV ratios and PA obstruction as measured by the RMMI.
The RESCUE trial enrolled patients aged 18 to 75 years with a filling defect in at least one main or lobar PA as determined on CTA, right ventricular-to-left-ventricular diameter (RV/LV) ratio >0.9, and symptom duration <14 days. The detailed inclusion and exclusion criteria were previously published.12 The Bashir catheter and the procedural techniques have previously been described in detail.6 The total recombinant-tissue plasminogen activator (r-tPA) dose administered was 7 mg in unilateral and 14 mg in bilateral PE patients.
Endpoints
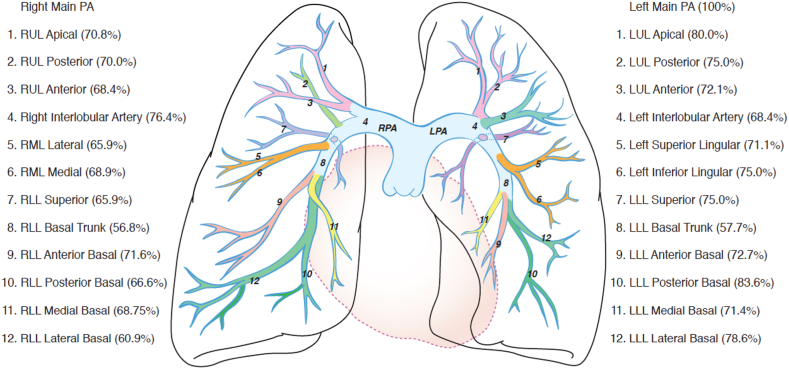
The primary endpoint for this analysis was the change in the number of segmental PA branches with total or subtotal occlusions before and at 48 ± 8 hours after PM-CDT with the Bashir catheter. We also evaluated the change in the number of proximal PA branches with total or subtotal occlusions (Figure 1 shows the branches and their names in anteroposterior and lateral views). The PA obstruction was measured by the core lab (NAMSA/SYNTACTX Inc) using the RMMI,13 which is a refinement of the Modified Miller scoring system. They assessed the degree of obstruction in 10 segmental arteries to the right lung and 10 to the left lung and assigned scores of 0 (no obstruction), 0.5 (1%-33%), 1 (34%-66%), 1.5 (67%-99%), and 2 (total obstruction) to each of these segmental arteries. A cumulative score was calculated by adding the scores for all arteries. Total scores can range from 0 to a maximum of 40 (20 per lung). The proximal PA branch scores were ascribed based on the number of segmental arteries that arise from that proximal artery as long as the highest possible scores are ascribed to each branch. For purposes of this discussion, we focused on total (100%) and subtotal (67%-99%) occlusions. We also evaluated the association between the reduction in the total and subtotal occlusions in the segmental and proximal PA branches with the improvement in RV/LV ratios at 48 hours after PM-CDT. The scoring of these vessels was performed on CTAs at baseline and 48 hours after treatment. The changes in RV/LV ratio were measured by a dedicated, imaging core laboratory using anonymized chest CTA studies at baseline and 48 hours after treatment. The RV/LV ratio was measured using the reformatted 4-chamber view. Troponin and brain-type natriuretic peptide (BNP) levels were assessed before and after PM-CDT.
Figure 1.
Magnitude of Reduction of Total and Subtotal Occlusions After Pharmacomechanical Catheter-Directed Thrombolysis With the Bashir Endovascular Catheter
This is a diagrammatic representation of the percent reduction in total and subtotal occlusions in various branches of the right and left pulmonary arteries after pharmacomechanical catheter-directed thrombolysis with the Bashir endovascular catheter. LLL = left lower lobe; LUL = left upper lobe; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Data analysis
Data were summarized using descriptive statistics. Results for continuous variables were compared with baseline using a McNemar’s test. All reported P values were 2-sided, and a P value < 0.05 was considered statistically significant. The correlation of change in total and subtotal occlusions with RV recovery was assessed by a Pearson correlation (Fisher’s Z transformation) test. The change in the RV/LV ratio, the RMMI scores, and patient-level changes in the total and subtotal occlusions were assessed by using a paired t-test. Statistical analyses were performed by biostatisticians from Pharma Lex. using SAS statistical software version 9.4 (SAS Institute).
Results
A total of 109 patients were enrolled across 18 U.S. sites. All patients met the criteria for acute intermediate-risk PE as defined by European Society of Cardiology guidelines. Two patients did not have a follow-up CTA at 48 hours; therefore, a total of 107 evaluable patients were used for this analysis. A total of 102 patients (93.6%) had bilateral PE. Ninety-eight (89.9%) patients had intermediate high-risk PE with an elevated troponin and/or BNP level. A total of 211 Bashir catheters were placed in the 109 enrolled patients: 2 each in 102 patients with bilateral PEs and 1 each in 7 patients with unilateral PEs. All patients received a pulse spray of 2 mgs of r-tPA into each lung, followed by 5 mg over 5 hours for a total of 7 mg of r-tPA for unilateral and 14 mg for bilateral PEs, respectively. The median catheter-placement time was 15 ± 14 minutes, and the total procedure time was 54 ± 28 minutes.
Primary and secondary outcomes
One major bleeding event (0.92%) occurred within 72 hours, and the same patient had a device-related left common iliac vein thrombosis while off anticoagulation. There was 1 non-PE-related death (0.92%) within 30 days. There were no intracranial bleeds. Additional procedural characteristics, safety outcomes, and study sites have been previously described.12 There was no significant effect of the procedure times on improvement in RV/LV ratio or RMMI scores. The median procedure time was numerically longer in patients who had adverse events, but it was not statistically significant because of very low numbers (64 minutes vs 53 minutes: P = 0.59).
At 48 hours after Bashir catheter therapy, the RV/LV ratio decreased from 1.66 ± 0.45 to 1.10 ± 0.24 (P < 0.0001; a reduction of 0.56 ± 0.41: 95% CI: 0.48-0.64; 33.3% reduction). The median baseline and 24-hour follow-up levels of biomarkers in the RESCUE trial were BNP 253 ± 1,950 pg/mL to 165 ± 763 pg/mL and troponin baseline 0.13 ± 2.1 ng/mL to 0.08 ± 3.03 ng/mL. PA obstruction as measured by the RMMI decreased from 22.42 ± 3.93 to 14.35 ± 4.77 (P < 0.0001, a reduction of 8.05 ± 3.89; 95% CI: 7.3-8.8; 35.9% reduction).
Effect on total and subtotal occlusions
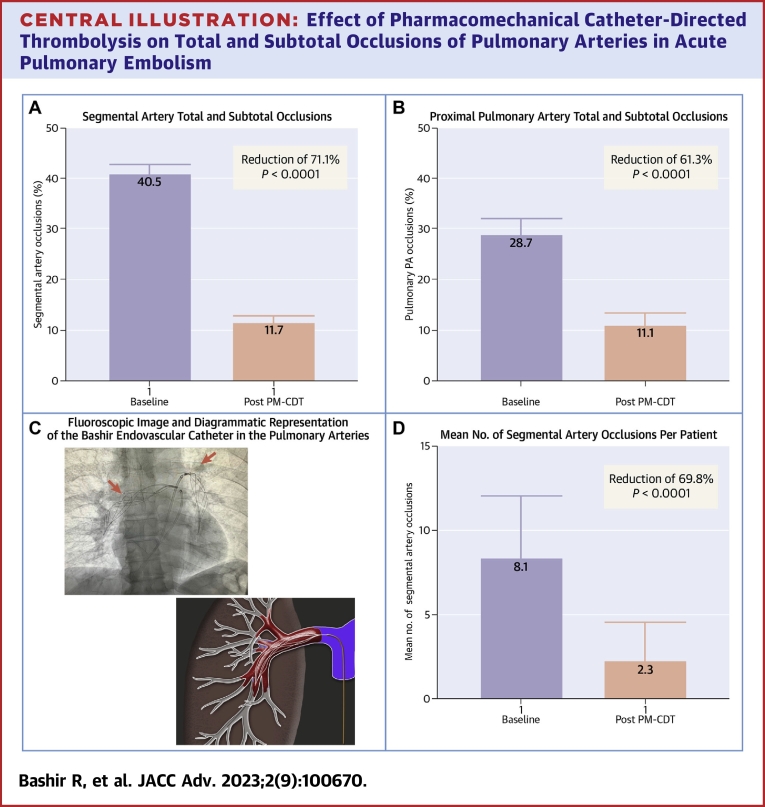
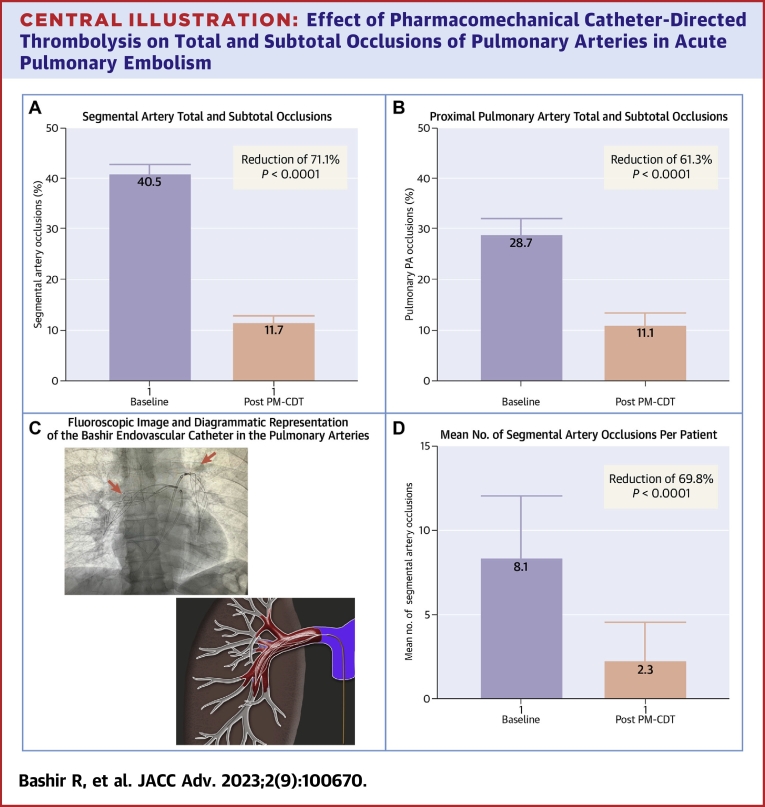
At 48 hours after Bashir catheter therapy, the number of segmental PA branches that had total or subtotal occlusions decreased from 40.5% (95% CI: 38.4%-42.6%) to 11.7% (95% CI: 11.7%-13.1%) (P < 0.0001, a 71.1% reduction) (Central Illustration A). This reduction was also noted in the proximal PA branches with a reduction from 28.7% (95% CI: 25.5%-32.1%) to 11.1% (95% CI: 8.9%-13.6%) (P < 0.0001; a 61.3% reduction) (Central Illustration B). These improvements were seen not only in the PA branches where the device was placed but also in the branches that were remote from the location of the infusion basket (Figure 1) On a per patient basis, the average number of segmental arteries that were totally or sub-totally occluded at baseline was 8.1 out of the 20 (or 40.5%) and 2.3 (or 11.5%) at 48 hours, a 69.8% reduction (P < 0.0001) (Central Illustration D). Central Illustration C shows a fluoroscopic image of the 2 Bashir endovascular catheters in the right and left pulmonary arteries (red arrow) along with the diagrammatic representation of the expanded Bashir catheter within the thrombus. 11 segmental arteries (0.5%) had new total occlusions at 48 hours, of which 8 had a subtotal occlusion at baseline, whereas the other 3 also had thrombus; however, they were <65% occluded. The distribution of the thrombus in various segmental arteries is shown in Tables 1 and 2.
Central Illustration.
Effect of Pharmacomechanical Catheter-Directed Thrombolysis on Total and Subtotal Occlusions of Pulmonary Arteries in Acute Pulmonary Embolism
Computed tomographic angiography parameters before and after Bashir catheter-directed PM-CDT. (A) Reduction in the total and subtotal occlusions of the segmental pulmonary artery branches. (B) Reduction in the total and subtotal occlusions of the proximal pulmonary artery branches. (C) Fluoroscopic image of the 2 Bashir endovascular catheters in the right and left pulmonary arteries (red arrows) along with the diagrammatic representation of the expanded catheter within the thrombus. (D) Reduction in the mean number of total and subtotal segmental artery occlusions per patient. PM-CDT = pharmacomechanical catheter-directed thrombolysis.
Table 1.
Segmental Artery Occlusion Analysis: Change in Number of Totally Occluded and Subtotally Occluded Segmental Arteries From Baseline to Post-PM-CDT
| Total Occlusions |
Total and Subtotal Occlusions |
All Arteries | |||
|---|---|---|---|---|---|
| Baseline | Post PM-CDT | Baseline | Post PM-CDT | ||
| Total of segmental arteries | 114 | 33 | 867 | 250 | 2,140 |
| Artery | |||||
| RUL apical | 3 | 2 | 48 | 14 | 107 |
| RUL posterior | 3 | 1 | 30 | 9 | 107 |
| RUL anterior | 7 | 3 | 38 | 12 | 107 |
| RML middle lobe medial | 8 | 0 | 45 | 14 | 107 |
| RML lateral | 11 | 2 | 44 | 15 | 107 |
| RLL superior | 5 | 1 | 35 | 12 | 107 |
| RLL posterior basal | 2 | 0 | 66 | 22 | 107 |
| RLL lateral basal | 3 | 0 | 64 | 25 | 107 |
| RLL anterior basal | 10 | 2 | 53 | 15 | 107 |
| RLL medial basal | 6 | 2 | 48 | 15 | 107 |
| LUL apical | 1 | 1 | 25 | 5 | 107 |
| LUL posterior | 5 | 2 | 20 | 5 | 107 |
| LUL anterior | 6 | 3 | 43 | 12 | 107 |
| LLL (lingula) superior lingula | 5 | 4 | 38 | 11 | 107 |
| LLL (lingula) inferior lingula | 6 | 3 | 28 | 7 | 107 |
| LLL superior | 7 | 2 | 20 | 5 | 107 |
| LLL posterior basal | 7 | 1 | 55 | 9 | 107 |
| LLL lateral basal | 7 | 1 | 56 | 12 | 107 |
| LLL anterior basal | 5 | 2 | 55 | 15 | 107 |
| LLL medial basal | 7 | 1 | 56 | 16 | 107 |
LLL = left lower lobe; LUL = left upper lobe; PM-CDT = pharmacomechanical catheter-directed thrombolysis; RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe.
Table 2.
Proximal Pulmonary Artery Occlusion: Change in Number of Totally Occluded and Subtotally Occluded Proximal Pulmonary Arteries From Baseline to Post PM-CDT
| Total Occlusions |
Total and Subtotal Occlusions |
All Arteries | |||
|---|---|---|---|---|---|
| Baseline | Post PM-CDT | Baseline | Post PM-CDT | ||
| Total of the major arteries | 3 | 3 | 215 | 83 | 749 |
| Major artery | |||||
| Main pulmonary artery | 0 | 0 | 0 | 0 | 107 |
| Right pulmonary artery | 0 | 0 | 0 | 0 | 107 |
| Right interlobar artery | 0 | 0 | 34 | 8 | 107 |
| Right lower lobe basal trunk | 0 | 1 | 88 | 38 | 107 |
| Left pulmonary artery | 0 | 0 | 1 | 0 | 107 |
| Left interlobar artery | 1 | 0 | 19 | 6 | 107 |
| Left lower lobe basal trunk | 2 | 2 | 73 | 31 | 107 |
PM-CDT = pharmacomechanical catheter-directed thrombolysis.
The magnitude of the reduction in the total and subtotal occlusions of the segmental arteries was associated with the magnitude of RV recovery as measured by the reduction in RV/LV ratio, (P = 0.0026: 95% CI: 0.102-0.452) with a correlation co-efficient of 0.287. The magnitude of the reduction in the total and subtotal occlusions of the proximal pulmonary arteries was not associated with the RV recovery as measured by the reduction in RV/LV ratio (P = 0.173: 95% CI: −0.059 to 0.314) with a correlation co-efficient of 0.132.
Discussion
This prospective single-arm study showed that Bashir catheter-directed PM-CDT was not only associated with improvement in RV/LV ratio and PA obstruction (as measured by RMMI) but also with a significant reduction in total and subtotal occlusions of the segmental (71% relative and 29% absolute reduction) and proximal PAs (61% relative and 17.6% absolute reduction) at 48 hours. The study also showed that reduction in occlusions in the segmental PAs correlated with the right ventricular recovery. Conversely, reduction in proximal PA occlusions did not correlate with RV recovery.
Our analysis demonstrated a 71.1% relative reduction in occlusions of segmental arteries and 61% in proximal PAs at 48 hours after PM-CDT. Prior studies suggest that the presence of total occlusions and intense inflammatory response that these occlusive thrombi produce in the vessel walls of the pulmonary arteries may play a role in the development of CTEPH or CTED in patients with acute PEs.4,5 Whether reduction in such occlusions with PM-CDT could reduce the risk of post-PE syndrome or chronic pulmonary vascular obstructive disorders, like CTEPH and CTED, remains to be seen.
The correlation of reduction in occlusions of the segmental branches, but not the proximal ones, with the improvement in RV/LV ratios is another important finding of this analysis. This is consistent with the observation from the SEATLE 3D study, which showed that the reduction in the distal vascular volumes was associated with RV recovery as compared to the proximal vascular volumes.2 In view of the potential risks of distal embolization from mechanical thrombectomy, this improvement in distal perfusion and reduction in total and subtotal occlusions of segmental PA should not be assumed with these devices. This highlights the need for evaluation of outcomes that not only focus on proximal thrombus burden but also on the assessment of distal vascular occlusions and perfusion in acute PE clinical trials.
The reduction in PA obstruction seen with the Bashir catheter-directed PM-CDT was not only seen in the vessels where the device was placed but also in vessels distant from the device. This may be related to the effect of endogenous and exogenous fibrinolytics, overall improvement in hemodynamics, and recirculation of non-thrombus bound rt-PA. One of the major advantages of CDT is the effective restoration of alveolar perfusion with thrombolysis in contrast to pure mechanical thrombectomy, which may fragment thrombus and lead to distal vessel occlusions due to embolization, which is frequently seen angiographically.14 This may be responsible for some on-table cases of respiratory failures seen with pure mechanical thrombectomy.14,15
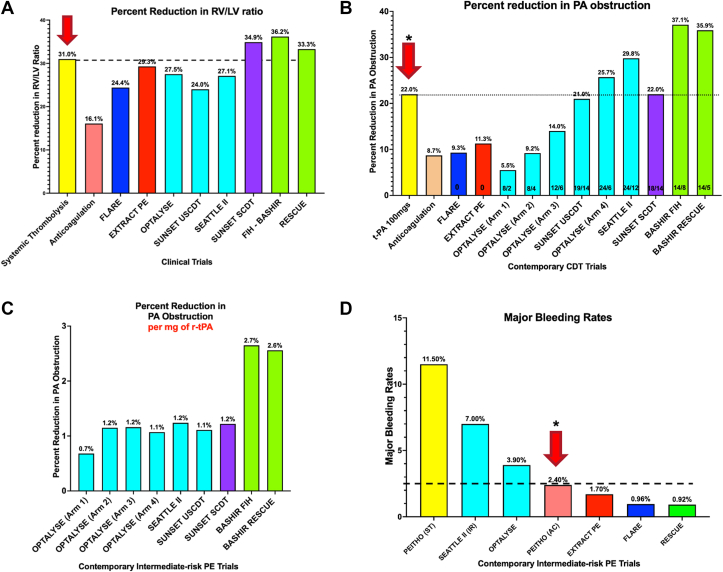
The RESCUE trial results show that PM-CDT with the Bashir catheter is improving both elements of the acute PE-related pathophysiology by restoring RV function and relieving the PA obstruction. The reduction in the RV/LV ratio was like that noted with full-dose intravenous thrombolytics (100 mg of rt-PA) (Figure 2A), while the improvement in PA obstruction was much greater than that seen with 100 mg of rt-PA (Figure 2B). The thrombolytic efficiency of 1 mg of rt-PA was more than double when compared to that in CDT studies using conventional single-lumen infusion catheters (Figure 2C). All these effects were seen with a safety similar to anticoagulation alone (Figure 2D), which could make this therapy feasible for a much broader set of acute PE patients in large and small hospitals around the globe.
Figure 2.
Safety and Efficacy of Pharmacomechanical Catheter-Directed Thrombolysis With Bashir Endovascular Catheter (Core Laboratory Data)
(A) Reduction in right ventricular/left ventricular (RV/LV) ratio across various acute PE trials.6,7,9, 10, 11, 12,16, 17, 18 (B) Percent reduction in pulmonary artery obstruction across various contemporary CDT trials.6,7,9, 10, 11, 12,18,19 (C) Percent reduction in pulmonary artery obstruction per mg of t-PA in contemporary catheter-directed thrombolysis trials.6,7,9,12,18 (D) Major bleeding rates in intermediate risk PE trials.9, 10, 11, 12,16,18 CDT = catheter-directed thrombolysis; PE = pulmonary embolism.
Study limitations
There are some limitations to this study. This is a study of the acute effect of Bashir catheter-directed PM-CDT on total and subtotal occlusions of the PA but does not have long-term clinical follow-up and outcomes. The reduction of these obstructions in both the proximal and segmental arteries, with only a modest dose of r-tPA, is very encouraging, but the long-term benefit needs to be validated in larger studies. This is a single-arm study without any comparison group of anticoagulation alone, mechanical thrombectomy, or systemic fibrinolysis. Another limitation of this analysis is that it does not extend past the segmental vessels, and so implications for the microvasculature are not known. In addition, all contemporary PE trials including the RESCUE trial have used CTA imaging that was not gated, which may introduce some bias in the measurement of RV/LV ratio. Also, this was not the primary efficacy analysis of the RESCUE trial and therefore should be viewed as hypothesis-generating.
Conclusions
This core laboratory-assessed study showed that Bashir catheter–directed PM-CDT is associated with a significant reduction in the number of PA branches with total or subtotal occlusions. This effect was seen with a low dose of r-tPA with a short infusion time. Future studies are needed to assess the direct effect of a reduction in segmental PA occlusions on pulmonary vascular volumes and clinical outcomes, including exercise tolerance, quality of life, CTED/CTEPH, and mortality.
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: Reduction in distal vascular volume in patients with acute PE is a significant predictor of 30- and 90-day mortality. The likely cause of this reduction is PA obstruction. PM-CDT with the Bashir endovascular catheter was associated with a significant reduction in total and subtotal occlusion of segmental and proximal pulmonary arteries. The decrease in occlusions in the segmental PAs correlated with the right ventricular recovery, while the reduction in proximal PA occlusions did not correlate with RV recovery.
TRANSLATIONAL OUTLOOK: The assessment of segmental and proximal PA occlusions should be carefully studied in future randomized controlled trials of PE therapies, such as CDT, PM CDT, mechanical thrombectomy, or anticoagulation alone.
Funding support and author disclosures
The study was sponsored by NHLBI, Commonwealth of Pennsylvania and Thrombolex, Inc. Dr Sista has received research grant support from the National Heart, Lung, and Blood Institute (NHLBI); is an unpaid member of the Scientific Advisory Board of Thrombolex, Inc; is an unpaid member of the Clinical Events Committee for the APEX-AV trial (Angiodynamics); and is an unpaid advisory board member of Abbott. Dr Piazza has received research grant support from EKOS, a BTG International Group company, Bayer, the Bristol Myers Squibb/Pfizer Alliance, Daiichi-Sankyo, Portola, and Janssen; and consulting fees from Amgen, Pfizer, Boston Scientific, and Thrombolex, Inc. Dr Firth is a Chief Scientific Officer for Thrombolex, Inc. Dr Comerota is an unpaid member of the Scientific Advisory Board of Thrombolex, Inc. Dr Bashir is the Ex-Officio Chief Medical Consultant for Thrombolex, Inc. Dr Rosenfield is a member of the Scientific Advisory Board or consultant for Abbott Vascular, Access Vascular, Boston Scientific-BTG, Volcano-Philips, Surmodics, Cruzar Systems, Magneto, Summa Therapeutics, and University of Maryland; an unpaid member of the Scientific Advisory Board of Thrombolex, Inc; has received grants from NIH and Boston Scientific; has equity from Access Vascular, Accolade, Contego, Endospan, Embolitech, Eximo, JanaCare, PQ Bypass, Primacea, MD Insider, Shockwave, Silk Road, Summa Therapeutics, Cruzar Systems, Capture Vascular, Magneto, Micell, and Valcare; and is a board member of VIVA Physicians, a not-for-profit 501c3, and National PERT Consortium, a not for profit 501c3. Dr Rali is a consultant Viz.AI, Telling.AI, Thrombolex, and Inari; and on Speakers Bureau for Janssen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors would like to acknowledge the invaluable assistance of NAMSA (SYNTACTX) (New York City, New York, USA) and EMINENCE Clinical Research Inc (Colorado Springs, Colorado, USA) in the conduct of this study.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Minhas J., Nardelli P., Hassan S.M., et al. Loss of pulmonary vascular volume as a predictor of right ventricular dysfunction and mortality in acute pulmonary embolism. Circ Cardiovasc Imaging. 2021;14 doi: 10.1161/CIRCIMAGING.120.012347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahaghi F.N., San José Estépar R., Goldhaber S.Z., et al. Quantification and significance of pulmonary vascular volume in predicting response to ultrasound-facilitated, catheter-directed fibrinolysis in acute pulmonary embolism (SEATTLE-3D) Circ Cardiovasc Imaging. 2019;12 doi: 10.1161/CIRCIMAGING.119.009903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piazza G. Off the beaten path: the need for innovation in medical therapy to improve outcomes in acute pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11:10–12. doi: 10.1093/ehjacc/zuab100. [DOI] [PubMed] [Google Scholar]
- 4.Lorenz G., Saeedan M.B., Bullen J., et al. CT-based biomarkers for prediction of chronic thromboembolic pulmonary hypertension after an acute pulmonary embolic event. AJR Am J Roentgenol. 2020;215:800–806. doi: 10.2214/AJR.19.22541. [DOI] [PubMed] [Google Scholar]
- 5.Bender M., Aggarwal A., Mix D., et al. Cellular, molecular, and enzymatic signatures of thrombi are vascular bed-dependent. bioRxiv. Published online August 12, 2022 doi: 10.1101/2022.08.11.503688. [DOI] [Google Scholar]
- 6.Sista A.K., Bhatheja R., Rali P., et al. First-in-human study to assess the safety and feasibility of the Bashir endovascular catheter for the treatment of acute intermediate-risk pulmonary embolism. Circ Cardiovasc Interv. 2021;14 doi: 10.1161/CIRCINTERVENTIONS.120.009611. [DOI] [PubMed] [Google Scholar]
- 7.Avgerinos E.D., Jaber W., Lacomis J., et al. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE trial. J Am Coll Cardiol Intv. 2021;14:1364–1373. doi: 10.1016/j.jcin.2021.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuo W.T., Banerjee A., Kim P.S., et al. Pulmonary embolism response to fragmentation, embolectomy, and catheter thrombolysis (PERFECT): initial results from a Prospective Multicenter Registry. Chest. 2015;148:667–673. doi: 10.1378/chest.15-0119. [DOI] [PubMed] [Google Scholar]
- 9.Piazza G., Hohlfelder B., Jaff M.R., et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the SEATTLE II study. J Am Coll Cardiol Intv. 2015;8:1382–1392. doi: 10.1016/j.jcin.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Tu T., Toma C., Tapson V.F., et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. J Am Coll Cardiol Intv. 2019;12:859–869. doi: 10.1016/j.jcin.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 11.Sista A.K., Horowitz J.M., Tapson V.F., et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. J Am Coll Cardiol Intv. 2021;14:319–329. doi: 10.1016/j.jcin.2020.09.053. [DOI] [PubMed] [Google Scholar]
- 12.Bashir R., Foster M., Iskander A., et al. Pharmacomechanical catheter-directed thrombolysis with the Bashir endovascular catheter for acute pulmonary embolism: the RESCUE study. J Am Coll Cardiol Intv. 2022;15:2427–2436. doi: 10.1016/j.jcin.2022.09.011. [DOI] [PubMed] [Google Scholar]
- 13.Ouriel K., Ouriel R.L., Lim Y.J., Piazza G., Goldhaber S.Z. Computed tomography angiography with pulmonary artery thrombus burden and right-to-left ventricular diameter ratio after pulmonary embolism. Vascular. 2017;25:54–62. doi: 10.1177/1708538116645056. [DOI] [PubMed] [Google Scholar]
- 14.Giri J., Sista A.K., Weinberg I., et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019;140:e774–e801. doi: 10.1161/CIR.0000000000000707. [DOI] [PubMed] [Google Scholar]
- 15.Benfor B., Haddad P., Bohle K., Atkins M.D., Lumsden A.B., Peden E.K. Cardiovascular collapse during mechanical thrombectomy for acute pulmonary embolism and the role of extracorporeal membrane oxygenation in patient rescue. J Vasc Surg Venous Lymphat Disord. 2023;11:978–985.e3. doi: 10.1016/j.jvsv.2023.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Meyer G., Vicaut E., Danays T., et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402–1411. doi: 10.1056/NEJMoa1302097. [DOI] [PubMed] [Google Scholar]
- 17.Fasullo S., Scalzo S., Maringhini G., et al. Six-month echocardiographic study in patients with submassive pulmonary embolism and right ventricle dysfunction: comparison of thrombolysis with heparin. Am J Med Sci. 2011;341:33–39. doi: 10.1097/MAJ.0b013e3181f1fc3e. [DOI] [PubMed] [Google Scholar]
- 18.Tapson V.F., Sterling K., Jones N., et al. A randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. J Am Coll Cardiol Intv. 2018;11:1401–1410. doi: 10.1016/j.jcin.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Goldhaber S.Z., Agnelli G., Levine M.N., et al. Reduced dose bolus alteplase vs conventional alteplase infusion for pulmonary embolism thrombolysis: an international multicenter randomized trial. Chest. 1994;106:718–724. doi: 10.1378/chest.106.3.718. [DOI] [PubMed] [Google Scholar]