Abstract
Plants have been used in various regions of the world to treat various medical conditions including male infertility. The review aims to evaluate the pharmacological effects of watermelon consumption in improving male fertility and sexual function. Watermelon is a popular fruit consumed around the world for its diverse nutritional and health-promoting qualities. This study showed the mechanism via which watermelon enhances male fertility as it was reported for improving semen quality, reversing erectile dysfunction, enhancing testicular redox status, as well as improving gonadotropin secretion. These activities have been linked to their constituents as it contains vitamins and phytochemicals such as phenols and certain flavonoids that contribute to their antioxidant properties. Watermelon has also been noted to possess antimicrobial, anti-helminthic, antioxidant, antidiabetic, anti-inflammatory, and antihypertensive properties that may contribute to its therapeutic use.
Keywords: male infertility, sperm, testis, testosterone, watermelon
INTRODUCTION
Infertility is characterized by the inability of a couple to become pregnant after a year of frequent intercourse without the use of contraceptives (Akhondi et al., 2019). Infertility can lead to stress, emotional pain, disappointment, frustration, fear, and anxiety for affected couples. Globally, over 50 million couples report being affected by fertility challenges, with about 30-50 % citing male reproductive disorders as the underlying issue (Fainberg & Kashanian, 2019; Olaolu et al., 2018). Major causes of male infertility include reduced semen quality, dysfunctional hypothalamic-pituitary-gonadal axis, infections, and endocrine disorders (Gunes et al., 2015; Iyiola et al., 2019; Kayode et al., 2020a; Musavi et al., 2018; Nwonuma et al., 2021; Olaolu & Rotimi, 2017; Pourmasumi et al., 2017). Potential treatment options include surgical interventions, hormone therapy, and specialized assisted reproductive technology (ART). Some examples of ART include intrauterine insemination (IUI), in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), gamete intrafallopian transfer (GIFT), zygote intrafallopian transfer (ZIFT), and third-party fertilization (donated eggs, sperm, uterus, and embryos) have all been used to treat male fertility issues in modern medicine (Musavi et al., 2018). Like all medical procedures, ART is associated with limitations of the application. Some examples of limitations of ART include mental and physical stress, surgical complications, and significant cost. Beyond this, patients may not qualify for specific therapies based on underlying medical conditions. These limitations lead patients to search for alternative interventions which are safe, affordable, and effective therapies. One category of alternative interventions that might be considered by patients is herbal remedies. Plants (herbs) have been used in various regions of the world to treat male infertility and a range of other medical conditions (Nwonuma et al., 2021). Many male fertility issues have been reported to be treated using herbal remedies, including improving sperm quality, sexual dysfunction, and erectile/ejaculatory problems. Celery, fennel, black seed, German chamomile, saffron, Vitex, fumitory, Oregano, and carrot are some of the plants that may promote male fertility (Dutta & Sengupta, 2018; Kayode et al., 2020b; Malviya et al., 2016; Olaolu et al., 2021; Vyas et al., 2018).
Ethnobotanical use of watermelon
Watermelon (Citrallus lanatus) is another potentially useful plant used for a variety of medical conditions. Concerning male fertility issues, it has also been reported to improve semen quality, erection, redox status, sexual function, and increase testosterone and gonadotropin secretion. Together, these features of watermelon may improve the fertility rate in male consumers (Khojasteh et al., 2016).
Watermelon is consumed for many reasons. Some of the features that make watermelon a popular fruit include its appealing color, distinctive flavor, and high water content, which can help to relieve thirst (Erhirhie & Ekene, 2013; Maoto et al., 2019; Renner et al., 2017). The fruit has a thick smooth outer rind [exocarp] and a pleasant, nutritious, juicy, fleshy interior [mesocarp and endocarp]. Sucrose and glucose make up about 20–40% of the total sugars in a mature watermelon, whereas fructose makes up 30–50% (Erhirhie & Ekene, 2013). The rind is normally tossed away; however, it is edible and can be eaten as a vegetable, fed to animals, or used as fertilizer (Ibrahim et al., 2017). In addition, watermelon rinds can be fermented or blended to be served as juice. Therapeutically, rinds have been administered in conditions involving alcohol intoxication and diabetes. When completely ripe or almost rotting, the fruits are consumed as a febrifuge. The fruit has high potassium content, making it more suitable for treating potassium deficiency and kidney stones (Deshmukh et al., 2015). Watermelon roots can be strongly purgative and therefore used as an emetic at high doses (Erhirhie & Ekene, 2013). The seeds may also have an antihypertensive property and be useful for reducing blood pressure. In addition, watermelon seeds have anti-helminthic properties and are occasionally prescribed for the treatment of helminthic infections. Experimentally, tapeworms and roundworms were observed to be inhibited by a fatty oil contained watermelon seeds, as well as aqueous or alcohol extracts. The seeds have also been used to extract tar, which is used to cure scabies and to tan the skin (Nasiru et al., 2019).
Watermelon consumption has rapidly gained popularity in developing countries and is highly recommended locally due to its numerous reported health benefits. The popularity of watermelon is related to, at least in part, the fact that it is fat-free, cholesterol-free, sodium-free, and high in minerals and phytochemicals (Mariya et al., 2020).
The chemical composition present in watermelon has been shown to decrease low-density lipoprotein (LDL) and high-density lipoprotein (HDL) in the cell membrane (Maoto et al., 2019). It has been suggested that watermelon could be used for weight loss (Hassan et al., 2011). In Northern Sudan, watermelon is used to treat burns, swelling, rheumatism, and gout, and as a laxative (Hassan et al., 2011). In Senegal, watermelon is used as a purgative, while in Nigeria, it is used to cure diarrhea and gonorrhea. Numerous epidemiological studies have reported its effectiveness in the treatment of cardiovascular disease (CVD) (Naz et al., 2014). As a result, watermelon consumption has been linked to a variety of therapeutic benefits, including a reduced risk of age-related neurodegenerative disorders and certain cancer types. Furthermore, watermelon is a good source of citrulline, a nonessential amino acid that is also a nitric oxide booster (Naz et al., 2014; Njoya et al., 2019; Oyelakin & Atilola, 2015).
Phytoconstituents of watermelon
Watermelon is a robust source of vitamins, minerals, and other important substances that may contribute to its pharmacologic applications. Vitamins provided through watermelon consumption include thiamine, riboflavin, niacin, and folate. Beyond this, vital electrolytes including potassium, magnesium, calcium, phosphorus, and iron are also found in watermelon. Together, these nutrients are known to function as cofactors for numerous cellular enzymes, play important roles in cell signaling, contribute to the maintenance of cellular architecture, and promote healthy cell differentiation (Aderiye et al., 2020). In addition, watermelon has a greater antioxidant content than other fruits including tomatoes, strawberries, and guavas (Maoto et al., 2019; Poduri et al., 2013). Carotenoids found in watermelons such as lycopene and β-carotene are responsible for the watermelon’s red and orange hues, respectively. Cucurbitacin, triterpenes, sterols, and alkaloids are bioactive chemicals found in watermelon (Zia et al., 2021). Taken together, these bioactive compounds have been linked as potentially therapeutic in issues related to male fertility. Given these inherent properties of watermelon, this review aimed to evaluate the effects of watermelon on male fertility, taking into account the risks and limitations of chemical medications and surgical methods.
The effect of watermelon on male fertility
The male reproductive system is particularly prone to structural and functional changes that result in male infertility. Therefore, there is a need to look for therapeutic options that can improve or mitigate the factors responsible for the change in the male organs.
Antioxidant effects on improving fertility
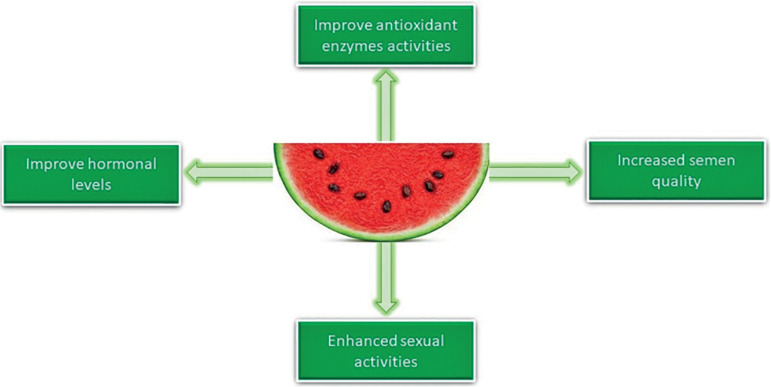
Watermelon contains lycopene, which is a powerful antioxidant. It also contains several phytochemicals including phenols and flavonoids, which could be responsible for its therapeutic role in reproductive pathophysiology (Figure 1; Table 1). One of the greatest known attributes of most flavonoids is their antioxidant properties (Akintunde & Thomas, 2021). Flavones and catechins are among the most potent flavonoids that protect the body against reactive oxygen species damage (Panche et al., 2016). Carotenoids found in watermelon including β-carotene, lutein, and lycopene provide the consumer a healthy boost in antioxidant levels. Experimentally, watermelon consumption has been associated with increased plasma antioxidant levels. One epidemiologic study in China associated the use of watermelon in the diet with a lower incidence of cancers (Panche et al., 2016).
Figure 1.
The therapeutic effect of watermelon on male fertility.
Table 1.
The effect of watermelon on male reproductive function.
| Reference | Animal used | Plant part used and form of extract | Route of administration | Dosage | Duration of Treatment | Effect of Watermelon |
|---|---|---|---|---|---|---|
| Onyeso et al. (2016) | Wistar rats | Seeds; methanolic extract | NM | 100 and 200 mg/kg | 30 days | *Increase in serum level of
testosterone *Increase the sperm motility, count, morphology, viability *Improved histological structure |
| Amedu & Idoko (2016) | Wistar rats | The inner part of the fruit | Intraperitoneally | Juice; NM | 8 weeks | *Increased level of
testosterone, FSH, and LH *Increased level of sperm count, motility, concentration, and morphology |
| Kolawole et al. (2017) | Wistar rats | Rind; Methanolic Extract | Orally | 200 mg/kg | 35 days | *Enhanced sperm count *Increased all reproductive hormone levels *non-significant increase in sperm motility, percentage of spermatocytes with normal morphology, and percentage of live spermatocytes, but decreased percentage of dead spermatocytes. |
| Khaki et al. (2014) | Wistar rats | Seed; hydro alcoholic extract | Oral gavage | 30 mg/kg | 28 days | *Serum testosterone was
enhanced *Enhanced sperm quality |
| Daramola et al. (2018) | Wistar rats | Fruit; aqueous extract | Orally | 100 and 200 mg/kg | 30 days | *Increased the
Follicle-stimulating hormone *Decreased the malondialdehyde level and increased the superoxide dismutase level. * Improved testicular histology |
| Akhuetie et al. (2018) | Wistar rats | Seed; crude powder and ethanolic extract | Orally | 200mg/kg | 28 days | *Reduced sperm morphological
abnormalities *Testicular histology showed numerous closely packed seminiferous tubules with normal architecture containing spermatogenic cells |
| Kolawole et al. (2019) | Wistar rats | Rind; Hydromethanolic Extract | Orally | 500 mg/kg | 42 days | *Increased sperm count and
reproductive hormone concentrations *increase in superoxide dismutase concentration and decreased malondialdehyde level |
| Pratama et al. (2021) | Wistar rats | Rind; ethanolic extract | Orally | 100, 200 and 400 mg/kg | 52 days | At 200 and 400 mg/kg, there was an increase in the number of Sertoli and spermatogenic cells. |
| Odo et al. (2021) | albino rats | Seed; ethanolic extract | Orally | 200 mg/kg | 8 weeks | *Increased testicular
weight *Increased Testosterone level, *Increased sperm motility, *Increased gonadal sperm, and extragonadal sperm reserves *Optimum histoarchitectural protection of the seminiferous tubules |
| Awaad & El Gamel (2020) | Sprague Dawley Strain | pulp and seed; methanol extract | Orally | 100 and 200 mg/kg | 28 days | *Increased Testosterone,
follicles stimulating hormone and luteinizing hormone in all
groups tested *Decrease in serum prolactin hormone *Improved testicular histoarchitecture |
| Onyinye & Emeka (2019) | adult Wistar rats | Seed; ethanolic extract | NM | 500 and 1000 mg/ kg | 14 days | *Enhanced libido *Increased mounting and intromission frequencies *Enhanced intromission and ejaculation latencies, *Decreased mounting latency and post-ejaculatory interval. *Serum testosterone and luteinizing hormones were increased. *improved histoarchitecture of the testes and hypothalamic sections. |
Khaki et al. (2013) suggested that the antioxidant properties of watermelon seeds could protect sperm DNA from free radicals, enhance blood-testis barrier integrity, and protect other essential components of the reproductive system from oxidation, ultimately improving sperm quality and, as a result, boost male fertility (Khaki et al., 2013; Oseni & Okoye, 2013).
Choudhary et al. (2015) also reported that watermelon contains a high vitamin C content. Some antioxidant vitamins, particularly tocopherol and ascorbic acid, have been found in the rind of watermelon (Johnson & Walcott, 2013). The presence of Vitamin C has been shown to preserve human sperm by neutralizing hydroxyl, superoxide, and hydrogen peroxide radicals, as well as inhibiting sperm agglutination. As a result, the presence of vitamin C is believed to aid in peroxidation, resulting in better sperm morphology and viability (Johnson & Walcott, 2013).
Many studies have reported the various antioxidant effects of watermelon on male reproductive processes. According to Kolawole et al. (2019), co-administration of nicotine and a hydromethanolic extract from watermelon rind resulted in improvements in semen quality as well as increased testosterone and gonadotropin secretion in male Wistar rats. The administration of watermelon was able to ameliorate the effect produced by nicotine on the male reproductive organ in male Wistar rats. This is consistent with the findings of Kolawole et al. (2017) and Daramola et al. (2018) who found that the watermelon extract reduced lead acetate and arsenic toxicity, respectively. Beyond these, Onyinye & Emeka (2019) reported that 200 mg/ kg of watermelon seed was able to reduce the toxicity induced by alloxan. The effect of reducing toxicity may lead to improved sperm motility, count, morphology, viability, and testosterone.
Khaki et al. (2013; 2014) found that daily injection of the hydro-alcoholic extract of watermelon seeds (30 mg/ kg daily for four weeks) enhanced sperm motility viability, and total count (Khaki et al., 2013; 2014). Phenols found in watermelon have also been proposed to be important in detoxifying the body. The phenol content of watermelon has been suggested to chelate arsenic and enhance its excretion from the body (Oseni & Okoye, 2013). Phenols might also activate endogenous antioxidant enzymes present in the epididymis (Oseni & Okoye, 2013). In this study, both 100 and 200 mg/kg of extract ameliorated the effects of arsenic on sperm viability and morphology (Daramola et al., 2018).
Other mechanisms of improved fertility
According to Onyinye & Emeka (2019), administration of watermelon ethanolic seed extract significantly improves sexual behavior as evidenced by increased mounting and intromission frequencies, ejaculation latency, and intromission latencies, as well as a shorter post-ejaculation interval. In addition, watermelon ethanolic seed extract supplementation was linked to an increase in serum sex hormones in male rats (Onyinye & Emeka, 2019). Following watermelon ethanolic seed extract treatment, testicular tubular size, compact seminiferous tubules, and the number of spermatozoa in the lumen of the seminiferous tubules increased significantly. These findings are consistent with those of Munglue et al. (2014), who found that the presence of pharmacologically active compounds in watermelon increased semen quality and sexual activity in male rats.
Watermelon seeds contain significant quantities of precursor substrates like arginine and citrulline, both of which are involved in the production of nitric oxide, a powerful vasodilator (Arise et al., 2016). Nitric oxide (NO) is an endogenous chemical that regulates the turgidity of penile erection by upregulating guanosine monophosphate (Aguayo et al., 2021). Therefore, the consumption of watermelon provides the user with sufficient nutrients to synthesize NO, given that all other mechanisms of production are intact. Beyond this, Munglue et al. (2014) discovered that watermelon extract had aphrodisiac properties in rat models. This might be related to the presence of citrulline, a phytonutrient in the seed (Munglue et al., 2014). Citrulline has the additional benefit of being converted to arginine, which has been shown to increase sperm count in males (Munglue et al., 2014).
Safety
The general belief is that natural or plant medicines may be safe mainly because of anecdotal usage without significant amounts of reported toxicities associated with their use. Despite this, generalizations can have potential safety implications for patients (Ezuruike & Prieto, 2014). Studies have indicated that many herbs used in traditional medicine may produce adverse effects. Data from Oyenihi et al. (2022) revealed that consumption of watermelon seed may lead to tissue-related toxicity specifically in the testes. This finding raises a safety concern for humans, especially chronic consumers. The consumption of these seeds at high doses could be detrimental to male reproductive function as evidenced by a significant decrease in sperm morphology in rats (Oyenihi et al., 2022). Additional dose-related studies and an extended experimental period are required to identify the safe dosing range for watermelon seed to maximize its potential health benefits.
Conflicting results have been reported for the impact of watermelon seed consumption on testes and sperm. A study by Ebeye et al. (2015) reported that the aqueous extracts at 100 and 300 mg/kg doses of watermelon seed led to dose-dependent testicular and Leydig cell hyperplasia, interstitial hydrolysis, and decreased spermatogenesis. In another earlier study, the hydro-alcoholic extract was administered at a 55 mg/kg dose and reported an improvement in sperm motility and viability (Khaki et al., 2013). Variations in the concentrations of tested chemicals could be responsible for these findings. Other constituent chemicals found within watermelon may also confound experimental outcomes. For example, saponins and certain alkaloids have been shown to interfere with spermatogenesis. The observed effects of the watermelon seed diet study may be mediated in part by these alkaloids and saponins amongst others. These compounds can elicit direct cell toxicity and reduce sperm quality via disturbances in Leydig cell function, vacuolation of the spermatozoa plasma membrane, and modification of ionic transport across the membrane (Singh et al., 2014). Cyanide and oxalate are some other potentially toxic antinutrients identified in watermelon seeds although at lower concentrations than other antinutrients. A study by Johnson & Walcott (2013) reported a significantly higher hydrocyanide concentration (HCN) (3-fold increase) in fresh watermelon seeds (1.47±0.03 mg/100 g of watermelon seed) than in the pulp and rind.
Future Research
In many parts of Africa (especially sub-Saharan Africa), Citron watermelon serves to combat food and nutrient insecurity. However, the consumption and utilization of this food source remain low, which could be attributed to the dearth of knowledge of its medicinal, nutritional, and pharmacological properties. There is a need to explore and further document the ethnopharmacological uses of watermelon as its utility is scarcely reported and under-exploited. Several phytochemicals have been identified in watermelon, although limited research has been focused on the identification, isolation, and quantification of specific phytochemicals. Under-researched chemicals with potential therapeutic value include cucurbitacins and related glycosides. Thus, there is a need to identify the various phytochemicals in watermelon to quantify and isolate their biological activities and mechanism of action for medicinal applications. Further studies are required in vivo and in vitro to form conclusions about the efficacy of the use of these chemicals, especially as it relates to male infertility. Increased evidence of utility would enable the reliable utilization of watermelon in the treatment of male infertility. Moreover, due to the limited number of clinical studies, there is no conclusive role for watermelon in fertility-related medical management. Clinical studies with large sample sizes, varied duration of administration, comparison with safe drugs, and the determination of the exact molecular mechanism are recommended.
CONCLUSION
Watermelon has played an important dietary and medicinal role throughout the history of mankind. However, the mechanism for its therapeutic actions on male reproduction remains unknown. The antioxidant properties of watermelon have been shown to improve sperm quality, male sexual dysfunction, and to improve testicle function.
ACKNOWLEDGMENT
The author appreciates Landmark University
REFERENCES
- Aderiye BI, David OM, Fagbohun ED, Faleye J, Olajide OM. Immunomodulatory and phytomedicinal properties of watermelon juice and pulp (Citrullus lanatus Linn): A review. GSC Biol Pharm Sci. 2020;11:153–165. doi: 10.30574/gscbps.2020.11.2.0079. [DOI] [Google Scholar]
- Aguayo E, Martínez-Sánchez A, Fernández-Lobato B, Alacid F. L-Citrulline: a non-essential amino acid with important roles in human health. Appl Sci. 2021;11:3293–3293. doi: 10.3390/app11073293. [DOI] [Google Scholar]
- Akhondi MM, Ranjbar F, Shirzad M, Behjati Ardakani Z, Kamali K, Mohammad K. Practical Difficulties in Estimating The Prevalence of Primary Infertility in Iran. Int J Fertil Steril. 2019;13:113–117. doi: 10.22074/ijfs.2019.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhuetie JO, Oyeyemi MO, Oloye AA. Studies on crude powder and ethanolic extract of Citrullus lanatus seeds: phytochemical analysis, effects on haemogram and some reproductive characteristics of male albino rats. Nig Vet J. 2018;39:133–142. doi: 10.4314/nvj.v39i2.5. [DOI] [Google Scholar]
- Akintunde OG, Thomas FC. Review of studies published on the medicinal importance of different parts of Citrullus lanatus in the last ten years. Bio-Research. 2021;19:1372–1387. doi: 10.4314/br.v19i2.10. [DOI] [Google Scholar]
- Amedu NO, Idoko UP. Dietary consumption of citrullus lanatus can ameliorate infertility potential of carica papaya seeds extract in male rats. Br Biotechnol J. 2016;11:1–9. doi: 10.9734/BBJ/2016/22805. [DOI] [Google Scholar]
- Arise RO, Yekeen AA, Ekun OE, Olatomiwa OJ. Protein Hydrolysates from Citrullus lanatus Seed: Antiradical and Hydrogen Peroxide-scavenging properties and kinetics of Angiotensin-I converting enzyme inhibition. Ceylon J Sci. 2016;45:39–52. doi: 10.4038/cjs.v45i2.7387. [DOI] [Google Scholar]
- Awaad EA, El Gamel AM. Effect of Citrullus Lanatus (watermelon) pulp and seeds extracts on fertility of experimental rats. Egypt J Nutr. 2020;35:1–31. doi: 10.21608/enj.2020.144754. [DOI] [Google Scholar]
- Choudhary BR, Haldhar SM, Maheshwari SK, Bhargava R, Sharma SK. Phytochemicals and antioxidants in watermelon (Citrullus lanatus) genotypes under hot arid region. Indian J Agric Sci. 2015;85:414–417. [Google Scholar]
- Daramola OO, Oyeyemi WA, Beka FU, Ofutet EA. Protective effects of aqueous extract of Citrullus lanatus fruit on reproductive functions and antioxidant activities in arsenic-treated male wistar rats. Afr J Biomed Res. 2018;21:65–72. [Google Scholar]
- Deshmukh CD, Jain A, Tambe MS. Phytochemical and pharmacological profile of Citrullus lanatus (THUNB) Biolife. 2015;3:483–488. doi: 10.17812/blj2015.32.18. [DOI] [Google Scholar]
- Dutta S, Sengupta P. Medicinal herbs in the management of male infertility. J Pregnancy Reprod. 2018;2:1–6. doi: 10.15761/JPR.1000128. [DOI] [Google Scholar]
- Ebeye O, Ekundina V, Odezi P, Iwalaye O. Histomorphological Effect of Aqueous Extract of Water Melon on The Testis of Adult Male Wistar rats. World J Pharm Pharm Sci. 2015;4:1477–1484. [Google Scholar]
- Erhirhie E, Ekene N. Medicinal values on Citrullus lanatus (watermelon): pharmacological review. Int J Res Pharm Biomed Sci. 2013;4:1305–1312. [Google Scholar]
- Ezuruike UF, Prieto JM. The use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerations. J Ethnopharmacol. 2014;155:857–924. doi: 10.1016/j.jep.2014.05.055. [DOI] [PubMed] [Google Scholar]
- Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000Res. 2019;8:F1000–F1000. doi: 10.12688/f1000research.17076.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunes S, Al-Sadaan M, Agarwal A. Spermatogenesis, DNA damage and DNA repair mechanisms in male infertility. Reprod Biomed Online. 2015;31:309–319. doi: 10.1016/j.rbmo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- Hassan LEA, Sirat HM, Yagi SMA, Koko WS, Abdelwahab SI. In vitro Antimicrobial activities of chloroformic, hexane and ethanolic extracts of Citrullus lanatus var. citroides (Wild melon) J Med Plant Res. 2011;5:1338–1344. doi: 10.5897/JMPR.9000741. [DOI] [Google Scholar]
- Ibrahim UK, Kamarrudin N, Suzihaque MUH, Hashib SA. Local fruit wastes as a potential source of natural antioxidant: an overview. IOP Conf Ser Mater Sci Eng. 2017;206:012040–012040. doi: 10.1088/1757-899X/206/1/012040. [DOI] [Google Scholar]
- Iyiola OA, Sulaiman AF, Sulaiman AA, Anifowoshe AT, Akolade JO, Adisa MJ, Otohinoyi DA, Rotimi DE, Batiha GES, Maimako RF, Adeyemi OS. Cypermethrin and chlorpyrifos raises serum urea level and causes abnormal sperm morphology in Wistar rats. Biointer Res Appl Chem. 2019;9:3969–3973. doi: 10.33263/BRIAC93.969973. [DOI] [Google Scholar]
- Johnson KL, Walcott RR. Quorum Sensing Contributes to Seed-to-Seedling Transmission of Acidovorax citrulli on Watermelon. J Phytopathol. 2013;161:562–573. doi: 10.1111/jph.12106. [DOI] [Google Scholar]
- Kayode OT, Rotimi DE, Kayode AAA, Olaolu TD, Adeyemi OS. Monosodium Glutamate (MSG)-Induced Male Reproductive Dysfunction: A Mini Review. Toxics. 2020a;8:7–7. doi: 10.3390/toxics8010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayode OT, Rotimi DE, Olaolu TD, Adeyemi OS. Ketogenic diet improves and restores redox status and biochemical indices in monosodium glutamate-induced rat testicular toxicity. Biomed Pharmacother. 2020b;127:110227–110227. doi: 10.1016/j.biopha.2020.110227. [DOI] [PubMed] [Google Scholar]
- Khaki A, Fathiazad F, Nouri M. Effects of watermelon seed extract (Citrullus vulgaris) on spermatogenesis in rat. Int J Women’s Health Reprod Sci. 2013;1:99–104. doi: 10.15296/ijwhr.2013.16. [DOI] [Google Scholar]
- Khaki A, Fathiazad F, Nouri M, Khaki AA. Effect of Citrullus lanatus seeds extracts on serum total testosterone in rat. Crescent J Medical Biol Sci. 2014;1:25–27. [Google Scholar]
- Khojasteh SMB, Khameneh RJ, Houresfsnd M, Yaldagard E. A review on medicinal plants used for improvement of spermatogenesis. Biol Med. 2016;8:4–4. doi: 10.4172/0974-8369.1000292. [DOI] [Google Scholar]
- Kolawole AT, Dapper VD, Eziuzo CI. Effects of the methanolic extract of the rind of Citrullus lanatus (watermelon) on some erythrocyte parameters and indices of oxidative status in phenylhydrazine-treated male Wistar rats. J Afr Ass Physiol Sci. 2017;5:22–28. [Google Scholar]
- Kolawole T, Adienbo O, Dapper V. Ameliorative Effects of Hydromethanolic Extract of Citrullus lanatus (Watermelon) Rind on Semen Parameters, Reproductive Hormones and Testicular Oxidative Status Following Nicotine Administration in Male Wistar Rats. Niger J Physiol Sci. 2019;34:83–90. [PubMed] [Google Scholar]
- Malviya N, Malviya S, Jain S, Vyas S. A review of the potential of medicinal plants in the management and treatment of male sexual dysfunction. Andrologia. 2016;48:880–893. doi: 10.1111/and.12677. [DOI] [PubMed] [Google Scholar]
- Maoto MM, Beswa D, Jideani AIO. Watermelon as a potential fruit snack. Int J Food Prop. 2019;22:355–370. doi: 10.1080/10942912.2019.1584212. [DOI] [Google Scholar]
- Mariya B, Haruna AH, Babangida AZ. Phytochemical Analysis of Some Selected Indigenous Fruits Collected from Lokogoma-Abuja, Nigeria. J Dis Med Plants. 2020;6:50–55. doi: 10.11648/j.jdmp.20200602.14. [DOI] [Google Scholar]
- Munglue P, Kupittayanant S, Kupittayanant P. Effect of watermelon (Citrullus lanatus) flesh extract on sexual behavior of male rats. Chiang Mai Univ J Nat Sci. 2014;13:519–527. doi: 10.12982/CMUJNS.2014.0054. [DOI] [Google Scholar]
- Musavi H, Tabnak M, Sheini FA, Bezvan MH, Amidi F, Abbasi M. Effect of garlic (Allium sativum) on male fertility: a systematic review. J Herbmed Pharmacol. 2018;7:306–312. doi: 10.15171/jhp.2018.46. [DOI] [Google Scholar]
- Nasiru A, Oluwasegun A, Ume O. Antibacterial studies, physicochemical properties and fatty acid composition of fixed oil from Citrullus lanatus seed cultivated in South-South Nigeria. J Pharmacogn Phytochem. 2019;8:3691–3694. [Google Scholar]
- Naz A, Butt MS, Sultan MT, Qayyum MM, Niaz RS. Watermelon lycopene and allied health claims. EXCLI J. 2014;13:650–660. [PMC free article] [PubMed] [Google Scholar]
- Njoya HK, Erifeta GO, Okwuonu CU, Elendu ZE. Estimation of some phytoconstituents in the aqueous extract of the endocarp, seeds and exocarp of watermelon (Citrullus lanatus) fruit. J Pharmacogn Phytochem. 2019;8:4750–4757. [Google Scholar]
- Nwonuma CO, Osemwegie OO, Irokanulo EO, Alejolowo OO, Kayode OT, Olaolu TD, Ada AS, Rotimi DE, Maimako RF, Adedayo AS, Ojo OA. Comparative effects of combined use of alcohol with cannabis and tobacco on testicular function in rats. Toxicol Res (Camb) 2021;10:761–770. doi: 10.1093/toxres/tfab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odo RI, Uchendu CN, Okeke SE. Protective effects of Citrullus lanatus seed ethanol extract on aluminum chloride-induced testosterone, testicular and hematological changes in an experimental male rat model. Vet Res Forum. 2021;12:7–13. doi: 10.30466/vrf.2020.104327.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaolu T, Rotimi DE. Evaluation of the effect of Cissus populnea aqueous root infusion on some testicular function indices and serum hormones of male rats. Nig J Biotech. 2017;34:105–116. doi: 10.4314/njb.v34i1.14. [DOI] [Google Scholar]
- Olaolu TD, Rotimi DE, Olaolu AP. Effect of alcohol infusion of Cissus populnea root on testicular function and serum hormone of male Wistar rats. Asian Pac J Reprod. 2018;7:117–122. doi: 10.4103/2305-0500.233572. [DOI] [Google Scholar]
- Olaolu T, Ajibola D, Rotimi D, Akpor O. Effect of Co-administration of Agnus castus Aqueous Leaf Extract and Cadmium Chloride on Testicular Function Indices. Jundishapur J Nat Pharm Prod. 2021;16:e99042–e99042. doi: 10.5812/jjnpp.99042. [DOI] [Google Scholar]
- Onyeso G, Nkpaa K, Eche-Goerge A. Hydromethanolic extracts of Citrullus lanatus seeds and caffeine improved reproductive hormones and ovarian histology of lead-acetate poisoned female rats. J Basic Appl Res Int. 2016;15:23–30. [Google Scholar]
- Onyinye AV, Emeka AG. Citrullus lanatus ethanolic seed extract improved male sexual behavior in rats via enhancement of sexual hormone and hypothalamic-pituitary-gonadal pathway. J Krishna Inst Med Sci Univ. 2019;8:96–107. [Google Scholar]
- Oseni O, Okoye VI. Studies of Phytochemical and Antioxidant properties of the fruit of watermelon (Citrullus lanatus).(Thunb.) J Pharm Biomed Sci. 2013;27:508–514. [Google Scholar]
- Oyelakin A, Atilola O. Biochemical Analysis and Nutritional Components of Watermelon (Citrullus lanatus) J Sustain Dev. 2015;12:129–131. [Google Scholar]
- Oyenihi OR, Afolabi BA, Oyenihi AB, Ojo GB. Toxicity assessment of watermelon seed supplemented diet in rats. Drug Chem Toxicol. 2022;45:1891–1898. doi: 10.1080/01480545.2021.1894699. [DOI] [PubMed] [Google Scholar]
- Panche AN, Diwan AD, Chandra SR. Flavonoids: an overview. J Nutr Sci. 2016;5:e47–e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduri A, Rateri DL, Saha SK, Saha S, Daugherty A. Citrullus lanatus ‘sentinel’ (watermelon) extract reduces atherosclerosis in LDL receptor-deficient mice. J Nutr Biochem. 2013;24:882–886. doi: 10.1016/j.jnutbio.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourmasumi S, Sabeti P, Rahiminia T, Mangoli E, Tabibnejad N, Talebi AR. The etiologies of DNA abnormalities in male infertility: An assessment and review. Int J Reprod Biomed. 2017;15:331–344. doi: 10.29252/ijrm.15.6.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratama API, Susilowati S, Maslachah L, Ratnani H, Suprayogi TW. The effect of watermelon (Citrullus lanatus) rind ethanolic extract on the number of Leydig, Sertoli, and spermatogenic cells of rat (Rattus novergicus) exposed to heat. Ovozoa. 2021;10:7–11. doi: 10.20473/ovz.v10i1.2021.7-11. [DOI] [Google Scholar]
- Renner SS, Sousa A, Chomicki G. Chromosome numbers, Sudanese wild forms, and classification of the watermelon genus Citrullus, with 50 names allocated to seven biological species. Taxon. 2017;66:1393–1405. doi: 10.12705/666.7. [DOI] [Google Scholar]
- Singh K, Dubey BK, Tripathi AC, Singh AP, Saraf SK. Natural male contraceptive: phytochemical investigation and anti-spermatogenic activity of Pistia stratiotes Linn. Nat Prod Res. 2014;28:1313–1317. doi: 10.1080/14786419.2014.900772. [DOI] [PubMed] [Google Scholar]
- Vyas N, Gamit K, Raval M. Male infertility: A major problem worldwide and its management in Ayurveda. Pharm Sci Monit. 2018;9:446–469. [Google Scholar]
- Zia S, Khan MR, Shabbir MA, Aadil RM. An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends Food Sci Technol. 2021;114:275–291. doi: 10.1016/j.tifs.2021.05.039. [DOI] [Google Scholar]