Abstract
Congenital ear anomalies affect 15 to 20% of neonates and can be categorized as either auricular deformations or malformations. Deformations involve a fully developed, albeit abnormally shaped, chondrocutaneous framework, which makes them amenable to correction with ear molding within the first few months of life. Malformations involve hypoplastic or fully absent auricular structures that require augmentation with alloplastic and/or autogenous reconstruction. The goal of this article is to outline the various auricular deformities and malformations, followed by a description of the latest clinical management options, both nonsurgical and surgical, by auricular anomaly.
Keywords: pediatric plastic surgery, ear anomalies, congenital ear, ear malformations
Congenital ear anomalies affect an estimated 15 to 20% of newborns. 1 Auricular anomalies can be broadly classified as deformations versus malformations. Deformations have a fully developed chondrocutaneous framework; however, there is distortion of the normal auricular architecture. 1 2 3 Auricular deformations are thought to be caused by external forces in utero or ex utero. 4 In contrast, auricular malformations are typically due to disrupted embryogenesis, resulting in deficient growth and absent structures of the ear. 4 Malformations include microtia, anotia, cryptotia, and preauricular anomalies. 2 5 While it is commonly reported that approximately 30% of ear deformations will self-resolve by 4 to 6 weeks of age, ear malformations generally do not display spontaneous improvement. 1 The embryological abnormalities contributing to auricular malformation may also result in hearing loss, developmental delays, and cosmetic issues leading to psychological and social morbidity. 4
Embryology
The pharyngeal or brachial arches are paired outgrowths on the ventrolateral embryo surface that give rise to the various structures of the head and neck. 6 7 The first and second pharyngeal arches house mesenchymal proliferations that drive external ear development. 4 7 These six prominences, or auricular hillocks, each compose an anatomical auricular component that fuse together throughout gestational weeks 5 through 20, forming the complete auricle ( Fig. 1 ). The first hillock develops into the tragus, and the second and third hillocks fuse to form the helix and cymba concha. Hillocks 4 and 5 form the antihelix. The sixth hillock forms the antitragus and the lobule. Disruption or underdevelopment of the aforementioned mesenchymal proliferations and vascular occlusion of a pharyngeal arch can thus result in a small, deformed, or even absent ear. Single and polygenic genetic mutations that disrupt embryological development have been linked to hereditary and sporadic cases of malformation. 7
Fig. 1.
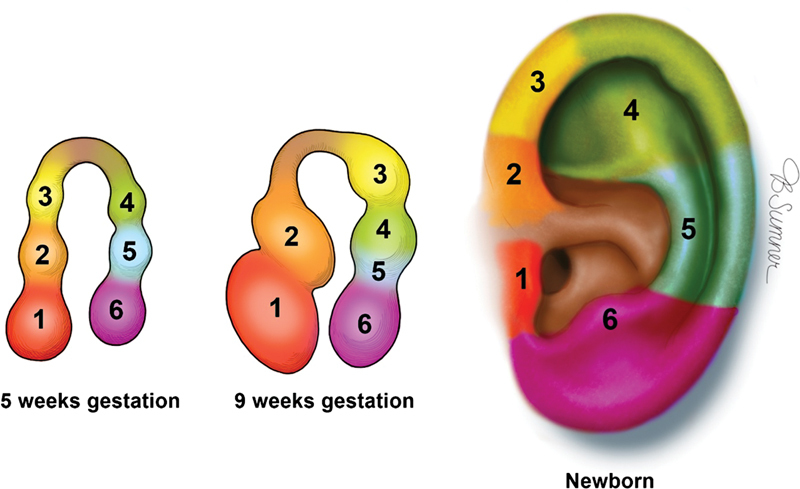
Auricular hillock contribution to each component of the neonatal auricle with permission from Texas Children's Hospital.
Auricular Anatomy
The external ear consists of an elastic cartilage covered with hairless skin, which is an important element of sound conduction and hearing, as well as craniofacial aesthetics ( Fig. 2 ). 4 8 The external ear is divided into the auricle, cartilaginous external ear canal, and the bony external ear that serves as foundation for the overlying auricle. Asymmetry between any of the external ear components, as well as the relation to the cranial vault, can result in an abnormal auricular appearance. Underneath the skin, the cartilaginous auricular framework is composed of three tiers: the helical–lobular complex, the antihelical–antitragal complex, and the conchal complex. The helix and lobule can be thought of as the most elevated portion of the ear, with the antihelix and tragus midlevel and the concha as the deepest part of the ear. 9 10 The lobule is the most caudal portion of the auricle and the only structure without an underlying cartilaginous framework. 10 11 The height of the adult ear is between 5.5 and 7.5 cm, with the width measuring approximately 55% of the height ( Fig. 3 ). 12 13 The long axis of the auricle is tilted posteriorly by approximately 20 degrees, with the superior aspect of the auricle on the same vertical plane as the eyebrow. 10 Normal projection of the ear from the mastoid to the helix at the superior third of the ear is 10 to 12 mm, 16 to 18 mm at the middle third, and 20 to 22 mm at the inferior third. The average auriculocephalic angle has been cited to be between 20 and 30 degrees. At birth, the ear is about 66% the size of the adult ear with the majority of auricular growth complete by age 5 to 7. 12
Fig. 2.
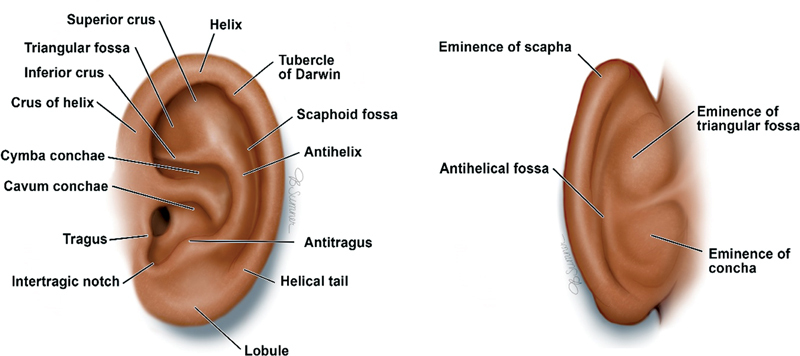
Normal anterior and posterior auricular anatomy. Reproduced with permission from Texas Children's Hospital.
Fig. 3.
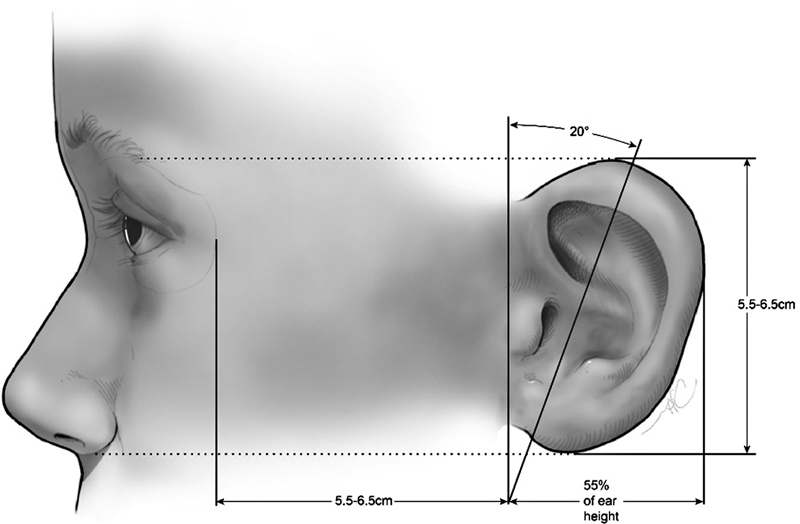
Auricular size relationship to the overall face. Reproduced with permission from Texas Children's Hospital.
Auricular Deformations
In auricular deformities, all auricular components are fully developed and present but have been distorted by an external force. The cartilage framework therefore has all necessary components of a normal appearing ear and is thus amenable to manual correction, such as ear molding, to regain a normal shape ( Table 1 ).
Table 1. Success of nonsurgical and surgical corrective modalities by ear anomaly.
| Molding/splinting | Surgery | |
|---|---|---|
| Deformities | ||
| Stahl's ear | ++ | * |
| Helical rim deformities | ++ | * |
| Constricted ear | ++ | * |
| Cryptotia | ++ | * |
| Prominent ear | + | * |
| Malformations | ||
| Microtia | + | ++ |
| Anotia | − | ++ |
+ Varying success depending on severity of anomaly, ++ Successful correction in majority of cases
− Not applicable (limited to prosthetics), *if not corrected with molding
Stahl's Ear
Stahl's ear affects the upper third of the ear and is characterized by an extraneous third crus in the auricular cartilage. The third crus is transversely oriented to the antihelix and extends to the helical rim, which causes an unfurling of the outer helix, along with a broadening of the scaphoid fossa. The result is an ear with a flat and pointed appearance, colloquially referred to as a “spock” ear ( Fig. 4 ). Stahl's ears are readily corrected with ear molding in the immediate neonatal period. Otherwise, if not molded, a Stahl's ear will need surgery for correction.
Fig. 4.
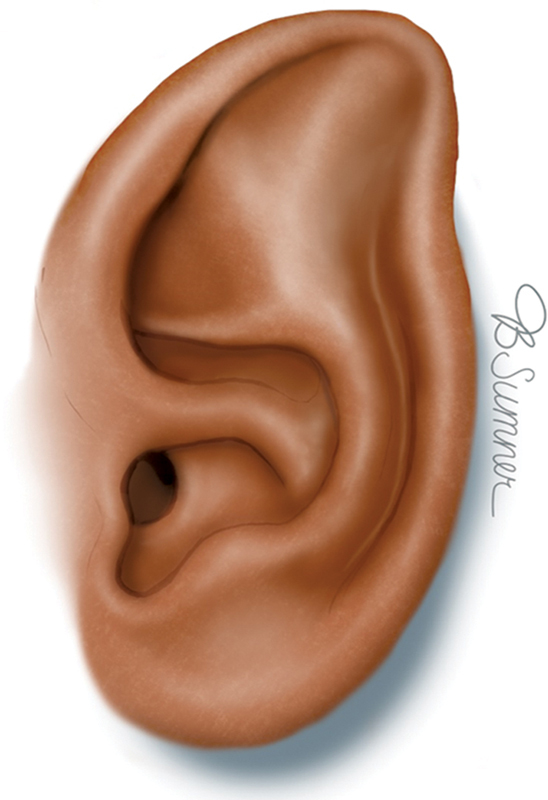
Stahl's ear. Reproduced with permission from Texas Children's Hospital.
Helical Rim Deformities
Helical rim deformity represents varying abnormalities that result in loss of the anatomic semicircular contour of the outer rim, which can be folded, irregular, or pleated ( Fig. 5 ). Abnormalities can occur anywhere along the circumference of the helical rim and is a more common deformity encountered.
Fig. 5.
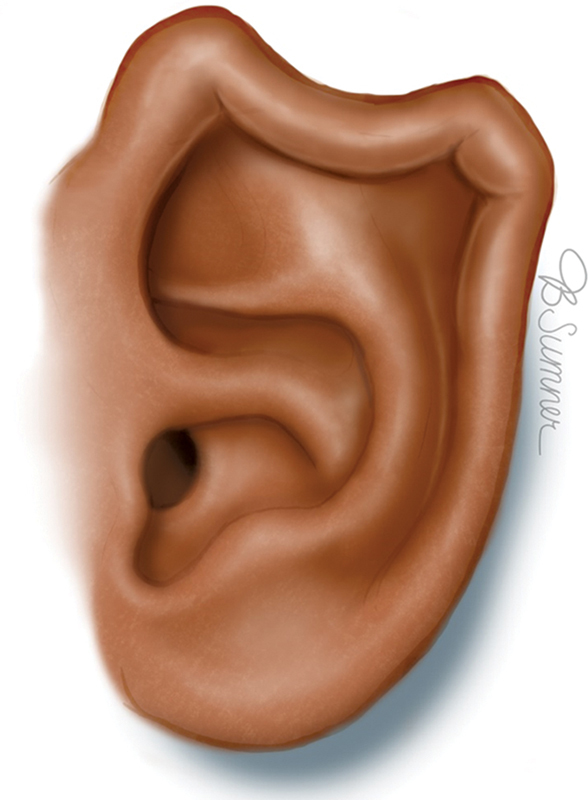
Helical rim deformity. Reproduced with permission from Texas Children's Hospital.
Constricted Ear
Constricted ear involves a spectrum of deformations in which the rim of the ear has a circumferentially tightened appearance due to abnormal chondrocutaneous distribution of the superior helix. The least constricted form, lidded or lop ear, involves the helix only, which is flattened or folded against its superior rim ( Fig. 6 ). The intermediate category involves both the helix and scapha, which are folded over their superior counterparts like a “hood” and can present with and without a deficiency of skin. 11 The most severe form of constriction, cup ear, has such an exaggerated auricular fold such that the ear takes on a tubular appearance. 9 11 All severities of constricted ear result in diminutive auricular height.
Fig. 6.
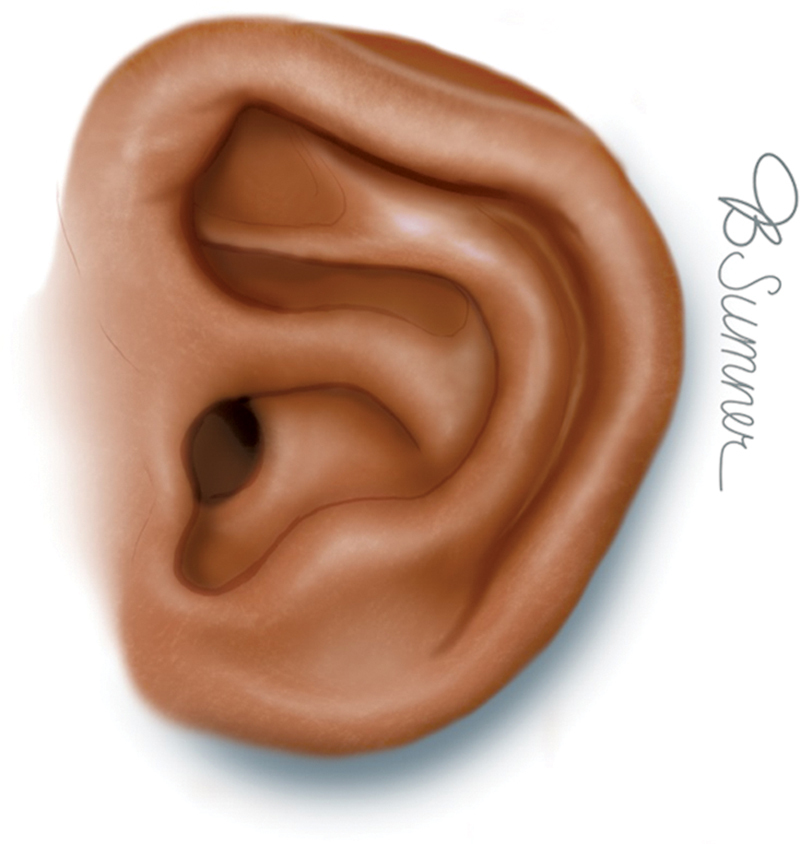
Constricted ear. Reproduced with permission from Texas Children's Hospital.
Cryptotia
In cryptotia, the superior third of the auricle is buried under the temporal scalp skin, resulting in a poorly defined auriculotemporal sulcus ( Fig. 7 ). 14 15 Cryptotia is marked by a distorted helix due to auricular cartilage deformity. Embedding of the auricle is thought to be due to abnormal insertion of the superior auricular muscle, while shortening of the auricular oblique or transverse muscles are responsible for the cartilage deformity seen in cryptotia. 11 13
Fig. 7.
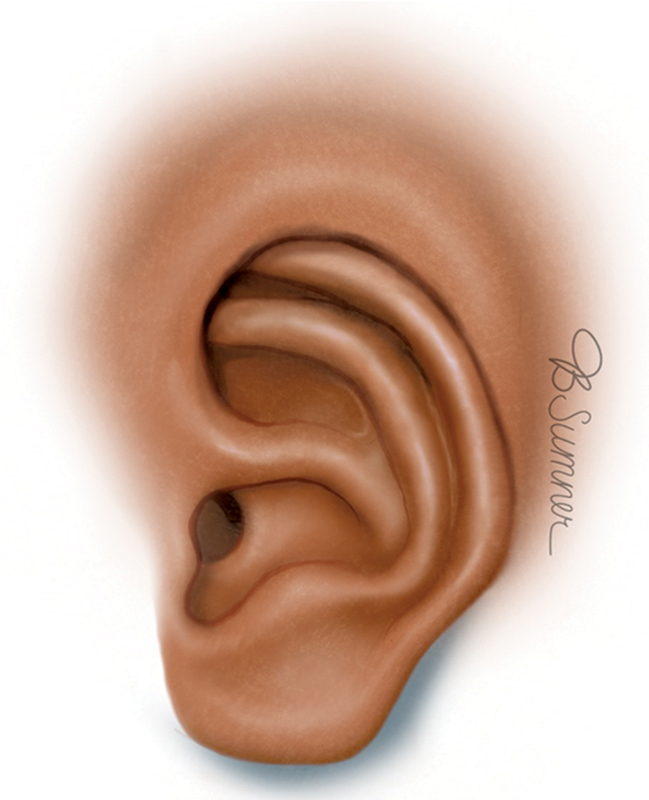
Cryptotia. Reproduced with permission from Texas Children's Hospital.
Prominent Ear
Prominent ear is a deformity in which the ear has an increased projection from the mastoid process, which can be objectively determined by an auriculocephalic angle greater than the normal 20 to 30 degrees or upper ear protrusion greater than approximately 2 cm in the matured ear ( Fig. 8 ). 16 17 Prominent ear deformities can have various contributing factors such as deepening of the conchal bowl, underdevelopment of the antihelix, exaggeration of the conchoscaphal angle, lobular protrusion, and underlying skeletal abnormalities. For adequate correction of prominent ear, it is vital to assess all contributing variables for protrusion in addition to addressing exacerbating factors such as macrotia. 16 17
Fig. 8.
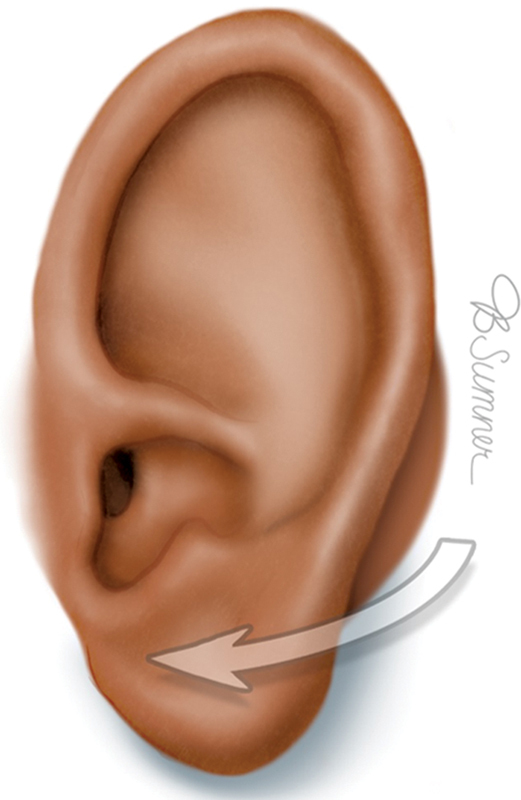
Prominent ear. Reproduced with permission from Texas Children's Hospital.
Nonoperative Treatment of Deformations
In 1984, Matsuo began popularizing the use of external compressive forces to reshape deformations in auricular architecture, now commonly referred to as ear molding. 18 High levels of circulating maternal estrogen in neonates results in relatively increased hyaluronic acid levels within cartilage, which manifests as greater pliability as compared with later stages in life. 18 19 20 21 Circulating maternal estrogen levels decrease with time, thus optimal correction with ear molding is associated with earlier intervention. While ear molding has traditionally been indicated for individuals under the age of 3 months, optimal results are seen when molding is initiated within the first 2 weeks of life. 1 18 20 Correction can still be seen if applied at 3 to 6 months of age; however, efficacy is significantly reduced. 22 23 24 Satisfactory rate for ear molding is between 90 and 100% when excluding prominent ear (80%) but declines when initiated beyond 60 days from birth. 25
Ear molding involves manually placing the ear in the desired position by the physician, which is then maintained by a splint secured to the head with either tape or a beanie. There are currently three splinting systems commercially available: EarWell, InfantEar, and EarBuddies. Splints are applied in the office, worn 24 hours a day, and adjusted by the physician typically every 2 weeks. The splint is maintained until the desired ear shape is reached or when the cartilage has lost pliability at around 3 months of age. Exact splinting duration varies based on the specific deformity, severity, and physician preference but typically lasts from 4 to 6 weeks. The most common complications are skin irritation and breakdown, suboptimal correction, and inadequate security of the device requiring multiple office visits for adjustments or replacement of the device. 1 26 Ear molding allows patients to participate in treatment much earlier, as patients are not candidates for surgical otoplasty until age 6, at which age they are at increased risk for psychosocial distress as they enter school.
Prominent ears have been shown to be most difficult out of all auricular deformities to correct by molding. 1 5 8 22 23 25 Mixed auricular deformities are also more resistant to corrective molding. 1 25 27 28 It is widely accepted that auricular malformations have a suboptimal response to molding due to insufficient skin and/or cartilage for manual correction; however, recent literature suggests that malformations can still result in significant parental and physician satisfaction with regard to improvement in the ear appearance at the conclusion of molding treatment. 19 27
Operative Treatment of Deformations
If molding is insufficient in correcting an auricular deformity, or if the patient presents for treatment beyond 3 months of age, surgical correction is indicated. The human ear reaches 90% of its adult width by age 1 and 87% of its adult length by age 5. 2 12 In order to reduce morbidity to the growing ear, otoplasty is usually not performed until age 6. Delay of surgical correction until school-age places children at risk for psychosocial morbidity from bullying from their peers. 9 11
Cryptotia
The goal of cryptotia repair is to unbury the cartilaginous auricular framework and recreate the auriculocephalic sulcus. 29 Should the cryptotia involve abnormalities of the superior, oblique, or transverse auricular muscles, the insertions are surgically released. Reconstruction of any deformed antihelical cartilage with a cartilage graft is performed, and a posteriorly based flap can be used to provide coverage. 29
Stahl's Ear
In order to correct the deformity, the anomalous crus is excised and may be used to construct the missing superior crus if deficient. If more cartilage is needed for superior crus construction, the conchal cartilage can be harvested. The transverse auricular muscle can be abnormal with Stahl's ear deformity and should be identified and dissected. Mustardé sutures are placed to recreate the antihelical and superior crus folding.
Helical Rim Deformities
Surgical correction is focused on helical rim cartilage reshaping. The deformed cartilage is exposed through a posterior incision along the rim and detached from the auricle. Excess cartilage is removed. Scoring is used to reshape the deformed cartilage, which is rotated 180 degrees, placed within the helical rim defect, and sutured into place.
Constricted Ear
Goals of constricted ear otoplasty include reduction of the conchoscaphal angle, creation of a defined antihelical fold, and elongation of the upper pole. An ellipse of conchal cartilage along the lateral conchal bowl is excised to reduce the conchoscaphal angle. Mustardé sutures are placed to define the antihelix and the upper helix is secured to the mastoid fascia to elongate the upper ear.
Prominent Ear
Correct identification of all contributory factors is vital for successful prominent ear correction. Each affected area must be addressed: the conchoscaphal angle, the conchal bowl, the antihelical fold, and the lobule ( Fig. 9 ). The surgeon must reestablish anatomical balance of the auricle while avoiding overcorrection and the inadvertent creation of unnatural contours. An ideal auricular outcome consists of 1.5 to 2.0 cm of protrusion from the scalp, visibility of the helix and antihelix with a smooth antihelical line, and an undisturbed postauricular sulcus. 13 15 16 30
Fig. 9.
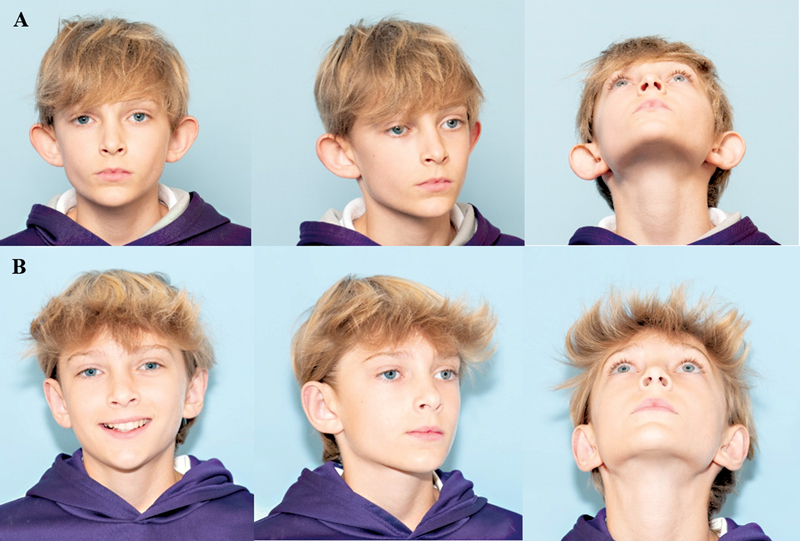
( A ) Patient with bilateral prominent ear. ( B ) Three months status post bilateral otoplasty with conchal bowl reduction, Furnas sutures, and anterior antihelical scoring. Reproduced with permission from Texas Children's Hospital.
The ear is manipulated by bending the unfurled helical rim posteriorly to denote where the antihelix naturally bends. The inflection lines of the new antihelical fold are marked on each side by puncturing the ear from the scapha to the concha with a 25-gauge needle impregnated with methylene blue dye. 31 32 The dyed marks denote where the Mustardé sutures will be placed to create the antihelical fold.
An ellipse is marked on the posterior auricle. The elliptical skin is dissected down to the cartilage. Mustardé sutures (typically 3–4 horizontal mattress sutures) are placed through and through connecting the methylene blue marks on either side to create the new antihelical fold ( Fig. 10 ). 31 32 Additionally, some surgeons elect to score the antihelix either posteriorly or anteriorly to weaken the cartilage, decreasing tension placed on sutures and possible reducing later recurrence.
Fig. 10.
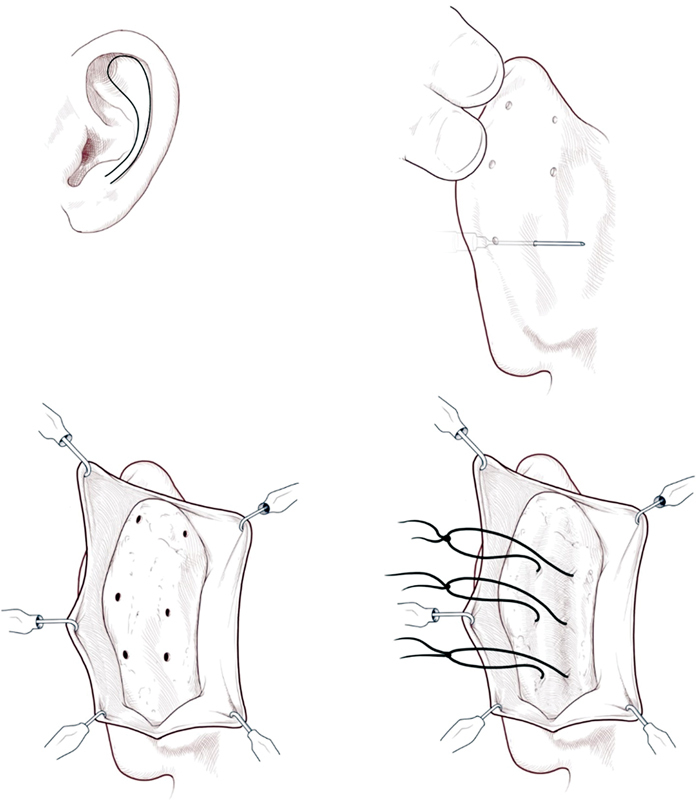
Mustardé suture technique for recreation of the antihelical fold. Reproduced with permission from Texas Children's Hospital.
Conchal prominence is addressed by removing a small wedge of cartilage from the lateral conchal bowl. Furnas sutures (2–3 conchomastoid sutures) are placed to position the conchal bowl closer to the head. 17 31
To address a prominent lobule, a diamond-shaped excision is added caudally to the postauricular incision. Excess cartilage influencing the prominence of the lobule can be removed. The skin within the elliptical and kite incisions are excised and the skin is closed primarily, which serves to pull the auricle and lobe closer to the head. A temporary bolster is placed to maintain the new antihelical fold and helical rim.
Immediate postoperative complications include hematoma, infection, and skin necrosis, which are reported to occur in less than 5% of patients. Long-term complications, which are more common, include recurrence, altered sensation, excessive scarring, suture extrusion, and an asymmetric or unsatisfactory auricular appearance. Recurrence rates following prominent ear otoplasty reported in the literature range from 4.8 to 11%, with reoperation rates ranging from 1.2 to 2.73%. 13 29 31 33 34 35
Malformations
Auricular malformations are the result of abnormal embryological development that results in absent or excess auricular components (skin and/or cartilage). Ear malformations can be associated with other syndromes affecting craniofacial structures such as microsomia, congenital facial palsy, Goldenhar syndrome, and Treacher Collins syndrome. 2 36 Anomalies of the middle ear ossicles can also be present in external ear malformations, which results in conductive hearing loss. Therefore, complete examination should involve radiographic and audiological assessment and may require interdisciplinary care. 37 While treatment is often pursued prior to the school-age years to mitigate the psychological effects of bullying, others propose postponing treatment until the child can be included in the reconstructive conversation, which is typically around 10 years old. 3 4 Prosthetic implants applied with adhesives can also serve as a temporary measure, while the child is old enough to decide on reconstruction. 37 38 Regardless, reconstructive timing should be a joint decision between the surgeon and family to make reconstructive decisions on timing that is best for that individual child.
The two most described malformations are microtia and anotia. Microtia is a small, malformed ear that is often associated with aural atresia, hearing loss, and craniofacial syndromes. 15 39 Anotia represents the severe end of the microtia spectrum, in which virtually no auricular components are present. Microtia is present in 1 to 10 per 10,000 births and more commonly affects males. 7 39 40 The malformation can be seen outside of any syndromic condition and is typically unilateral. 39 Associated craniofacial syndromes include craniofacial microsomia, Goldenhar syndrome, and Treacher Collins syndrome. 7 39 41 Facial nerve dysfunction is present in up to 15% of patients and should be evaluated by computed tomography before otologic surgery. 42
Nonsurgical reconstructive options include the use of a prosthesis. Surgical interventions include recreation of the ear utilizing either an alloplastic implant or autologous cartilage harvested from the rib. The goal of microtia/anotia reconstruction are a well-vascularized skin envelope, creation of an adequate three-dimensional cartilage framework, and anatomically sound location of ear placement. 37 39 42
Nonoperative Malformation Treatment
Prosthetic ears have the benefit of providing the closest replica of the contralateral ear and can be applied at any age by adhesive or osteointegration. 37 If the contralateral ear is normal, it is used as a template to recreate the microtic or anotic ear. If both ears are affected, the parent's ears may be used. 43 As adhesives can have unpredictable outcomes, osteointegration is typically preferred, although it involves a single-stage brief surgery for placement. 37 Any auricular skin and remnants are removed with osteointegration, which precludes any future autologous reconstruction. 37 42 Prosthetic reconstruction is not without cost burden and typically needs replacement every 5 years to account for the child's growth and normal wear and tear. 15 37 The implant requires regular cleaning and must be removed both at night and during contact sports, which can be psychologically jarring for the child, if witnessed by peers. 37
Operative Malformation Treatment
Timing of surgical reconstruction for microtia and anotia centers on balancing the risk for psychological distress experienced by the child with adequate delay to achieve sufficient cartilage maturation, which is typically around 6 to 7 years of age. 42 By this age, the contralateral ear will have reached the majority of its adult size and can reliably be used as a template for both size and shape of the reconstructed ear. 15 The underlying cartilaginous framework can be created with either an alloplastic implant or an autogenous rib cartilage graft.
Alloplastic
Alloplastic implants consist of porous high-density polyethylene (PHDPE), which is an inert substance easily integrated into overlying human tissue through collagen deposition and vascular ingrowth. 42 44 45 PHDPE is thermoplastic, as it molds and contours to its surroundings, and exerts minimal foreign body reactions in patients. 42 As it is still a foreign body, it has less absorptive recurrence compared with autologous rib cartilage and is more aesthetically accurate due to a precise manufacturing process. 46 47 PHDPE implantation can be performed at an earlier age, as it does not rely on autologous cartilage maturation; however, it may not be performed before 6 years old until the contralateral ear has reached the majority of its growth. 47 The reconstruction can be done as young as 3 years of age, but the surgeon must be aware of the potential for growth and inform the family that the new ear will be made slightly larger to accommodate for future growth of the contralateral ear.
Radiograph film is used to outline the size and shape contralateral ear and specifically mark its position relative to the oral commissure, nasal alar groove, orbitomalar groove, lateral canthus, and lateral brow. 47 The alloplastic implant has two component framework, consisting of a thin curled helical rim and a base outlining the shape of the conchal bowl and antihelix, which can be easily modified via scalpel by the surgeon. An ipsilateral temporoparietal fascia flap (TPFF) is raised to be later used for implant coverage. The remnant microtic cartilage is excised, with meticulous elevation of the thin anterior microtic skin flap, which can be later draped over the implant or used as a free skin graft. A postauricular incision is made and the TPFF is reflected inferiorly through the superior portion of the postauricular incision.
The two-piece framework is modified, using the radiographic film as a guide, and fused using cautery. 47 Two flat suction drains are placed so that one will be deep to the implant and the other in the posterior portion of the temporoparietal scalp donor site. The implant is placed with the proper orientation, axis, and projection on the mastoid and draped with the TPFF. The TPFF is loosely secured to the mastoid fascia via suture. The flat drains are placed to suction, which shrink–wraps the TPFF to the implant and prevents fluid accumulation from the donor site. The anterior remnant skin flap is used to cover the medial portion of the new ear. The lateral portion is covered using ipsilateral mastoid skin and/or a full-thickness skin graft taken from the contralateral postauricular sulcus. The posterior ear is covered by a skin graft ( Fig. 11 ). 47
Fig. 11.

( A ) Patient with left-sided lobular-type microtia. ( B ) Three years status post microtia repair with porous high-density polyethylene implant, temporoparietal fascia flap, and skin grafting. Reproduced with permission from Texas Children's Hospital.
Complications from implant-based reconstruction occur between 0 and 12% of the time and include implant extrusion, infection, skin flap ischemia/loss, and fracture. 47 48 49 Implant extrusion is the most common complication and requires partial or complete replacement of the implant. 48
Autogenous
Two main techniques utilized in autogenous reconstruction are those described by Nagata and Brent, both of which were adapted from Tanzer. 50 51 52 53 54 55 Autogenous reconstruction for external ear reconstruction utilize varying amounts of rib cartilage to create a new cartilaginous auricular framework, which is more resistant to infection and displays increased stability in response to trauma compared with alloplastic reconstruction ( Table 2 ). 42 There is increased morbidity as an additional incision site is created on the chest to harvest donor cartilage. Autogenous reconstruction involves multiple, staged procedures, so the child must undergo multiple surgeries to achieve a complete result.
Table 2. Rib contribution for Brent versus Nagata autologous microtia/anotia reconstruction.
| Rib | Brent | Nagata a |
|---|---|---|
| 5 th | – | Crescent shaped wedge to elevate framework |
| 6 th | Body of the framework | Framework base |
| 7 th | Body of the framework | Framework base |
| 8 th | – | Helix, crus helices |
| 9 th | – | Superior and inferior crus, antihelix |
| 11 th | Helical rim | – |
Leftover cartilage remnants create conchal bowl.
Nagata
The Nagata technique is a two-stage procedure that utilizes costal cartilage from the ipsilateral chest. As a relatively large amount of donor cartilage is required for Nagata reconstruction, children cannot undergo surgery until age 10 when the chest circumference measures at least 60 cm. 51
The first stage involves cartilage harvest, framework construction and placement, lobule transposition, and tragal reconstruction. 51 52 56 Ribs 6 through 9 are harvested en bloc, with 6 and 7 forming the framework base, 8 forming the helix and crus helices, and 9 forming the superior and inferior crus and antihelix. The conchal bowl is created from the leftover cartilage remnants. 51 56 The four levels of the reconstructed frame are secured together with wire sutures. A W incision is created over the auricular remnant, remnant cartilage is excised, and the cartilaginous framework is placed in the subcutaneous remnant pocket. The W incision creates three skin flaps from the anterior and posterior lobule and the tragus. The skin flaps are sutured closed and bolstered for 2 weeks. 52 56
The second stage of reconstruction occurs 6 months later and involves elevation of the framework from the mastoid. 52 53 56 Cartilage from rib 5 is harvested and carved into a crescent-shaped wedge that is placed beneath the auricular framework through an incision posterior to the helix. A TPFF is harvested and tunneled subcutaneously to the posterior ear to cover the dorsal aspect of the newly elevated framework and the mastoid. Advancement of the retroauricular skin and full-thickness skin grafts are used to cover any exposed areas. 52 53 56
Brent
The Brent technique is a 3 to 4 staged procedure that harvests costal cartilage from the contralateral chest. 54 55 57 Minimal costal cartilage is required in the Brent technique, and therefore, children can undergo reconstruction as early as age 5. 56 57
In the first reconstructive stage, 8 cm of rib cartilage is harvested. An ear template created from outline of the contralateral ear is used to guide how much cartilage to harvest. Ribs 6 and 7 are used for the body of the framework, and the first free-floating rib cartilage is used to construct the helical rim. The helix is secured to the auricular base construct with nylon horizontal mattress sutures. Next, details such as the antitragus and antihelix are added to the framework. A small incision is created anterior to the auricular remnant and remnant cartilage is removed, and the residual subcutaneous pocket is dissected posteriorly toward to mastoid. The new framework is placed into the subcutaneous pocket, which is closed. Residual cartilage is banked for later use.
Stage 2 is initiated 6 to 8 weeks later, involving transposition of the lobule into the proper position. Stage 3 involves elevation of the framework from the mastoid, by placing a piece of banked cartilage between the framework and the mastoid bone. An occipitalis fascia turnover flap covered with a split-thickness skin graft is used to cover the dorsal aspect of the framework. Stage 4 includes tragus construction using a composite graft from the contralateral concha cymba of the unaffected ear, which is covered with small skin graft. Any desired symmetrizing procedures of the contralateral auricle are performed during stage 4. 54 55 56 57
While the Nagata and Brent techniques are the most recognized methods of reconstruction, a multitude of modifications have been proposed. Ultimately, the autologous reconstructive technique selected is dependent upon shared decision-making with the family and surgeon preference. Regardless of method, complications from autologous reconstruction can be seen from the donor site and reconstructed auricular site. Donor site complications are the same for all autologous costal cartilage harvest and include pneumothorax, atelectasis, and scarring. 42 56 Auricular complications include hematoma, infection of the framework, and skin necrosis resulting from excess skin pocket tension. 15 42
Conclusion
Congenital auricular deformations and malformations are challenging due to complex anatomy and timing restraints for nonsurgical interventions. Clinical decision-making should be a bidirectional conversation between the plastic surgeon and the family, involving the child's wishes as much as appropriate for their age and understanding.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Byrd H S, Langevin C J, Ghidoni L A. Ear molding in newborn infants with auricular deformities. Plast Reconstr Surg. 2010;126(04):1191–1200. doi: 10.1097/PRS.0b013e3181e617bb. [DOI] [PubMed] [Google Scholar]
- 2.Joukhadar N, McKee D, Caouette-Laberge L, Bezuhly M. Management of congenital auricular anomalies. Plast Reconstr Surg. 2020;146(02):205e–216e. doi: 10.1097/PRS.0000000000006997. [DOI] [PubMed] [Google Scholar]
- 3.Matsuo K, Hayashi R, Kiyono M, Hirose T, Netsu Y. Nonsurgical correction of congenital auricular deformities. Clin Plast Surg. 1990;17(02):383–395. [PubMed] [Google Scholar]
- 4.Bhatti S L, Daly L T, Mejia M, Perlyn C. Ear abnormalities. Pediatr Rev. 2021;42(04):180–188. doi: 10.1542/pir.2019-0167. [DOI] [PubMed] [Google Scholar]
- 5.Daniali L N, Rezzadeh K, Shell C, Trovato M, Ha R, Byrd H S. Classification of newborn ear malformations and their treatment with the EarWell infant ear correction system. Plast Reconstr Surg. 2017;139(03):681–691. doi: 10.1097/PRS.0000000000003150. [DOI] [PubMed] [Google Scholar]
- 6.Bartel-Friedrich S. Congenital auricular malformations: description of anomalies and syndromes. Facial Plast Surg. 2015;31(06):567–580. doi: 10.1055/s-0035-1568139. [DOI] [PubMed] [Google Scholar]
- 7.Luquetti D V, Heike C L, Hing A V, Cunningham M L, Cox T C. Microtia: epidemiology and genetics. Am J Med Genet A. 2012;158A(01):124–139. doi: 10.1002/ajmg.a.34352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Wijk M P, Breugem C C, Kon M. A prospective study on non-surgical correction of protruding ears: the importance of early treatment. J Plast Reconstr Aesthet Surg. 2012;65(01):54–60. doi: 10.1016/j.bjps.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Ali K, Meaike J D, Maricevich R S, Olshinka A. The protruding ear: cosmetic and reconstruction. Semin Plast Surg. 2017;31(03):152–160. doi: 10.1055/s-0037-1604241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider A L, Sidle D M. Cosmetic otoplasty. Facial Plast Surg Clin North Am. 2018;26(01):19–29. doi: 10.1016/j.fsc.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Schultz K, Guillen D, Maricevich R S. Newborn ear deformities: early recognition and novel nonoperative techniques. Semin Plast Surg. 2017;31(03):141–145. doi: 10.1055/s-0037-1603958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farkas L G, Posnick J C, Hreczko T M. Anthropometric growth study of the ear. Cleft Palate Craniofac J. 1992;29(04):324–329. doi: 10.1597/1545-1569_1992_029_0324_agsote_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 13.Thorne C H, Wilkes G. Ear deformities, otoplasty, and ear reconstruction. Plast Reconstr Surg. 2012;129(04):701e–716e. doi: 10.1097/PRS.0b013e3182450d9f. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Sun Q, Wang Y et al. Cryptotia repair using a modified V-Y advancement flap with helix rounding technique. Aesthetic Plast Surg. 2023;47(02):640–646. doi: 10.1007/s00266-022-03050-y. [DOI] [PubMed] [Google Scholar]
- 15.Park C, Yoo Y S, Hong S T. An update on auricular reconstruction: three major auricular malformations of microtia, prominent ear and cryptotia. Curr Opin Otolaryngol Head Neck Surg. 2010;18(06):544–549. doi: 10.1097/MOO.0b013e32833fecb9. [DOI] [PubMed] [Google Scholar]
- 16.Janz B A, Cole P, Hollier L H, Jr, Stal S. Treatment of prominent and constricted ear anomalies. Plast Reconstr Surg. 2009;124(01):27e–37e. doi: 10.1097/PRS.0b013e3181aa0e9d. [DOI] [PubMed] [Google Scholar]
- 17.Furnas D W.Otoplasty for prominent ears Clin Plast Surg 20022902273–288., viii [DOI] [PubMed] [Google Scholar]
- 18.Matsuo K, Hirose T, Tomono T et al. Nonsurgical correction of congenital auricular deformities in the early neonate: a preliminary report. Plast Reconstr Surg. 1984;73(01):38–51. doi: 10.1097/00006534-198401000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Dinis J, Junn A, Long A et al. Non-surgical correction of congenital ear anomalies: a critical assessment of caretaker burdens and aesthetic outcomes. Aesthetic Plast Surg. 2022;46(02):898–906. doi: 10.1007/s00266-021-02610-y. [DOI] [PubMed] [Google Scholar]
- 20.Kenny F M, Angsusingha K, Stinson D, Hotchkiss J. Unconjugated estrogens in the perinatal period. Pediatr Res. 1973;7(10):826–831. doi: 10.1203/00006450-197310000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Ushiyama T, Ueyama H, Inoue K, Ohkubo I, Hukuda S. Expression of genes for estrogen receptors α and β in human articular chondrocytes. Osteoarthritis Cartilage. 1999;7(06):560–566. doi: 10.1053/joca.1999.0260. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi A A, Imani M T, Kardeh S, Karami M M, Kherad M. Non-surgical management of congenital auricular deformities. World J Plast Surg. 2016;5(02):139–147. [PMC free article] [PubMed] [Google Scholar]
- 23.Petersson R S, Recker C A, Martin J RK, Driscoll C LW, Friedman O. Identification of congenital auricular deformities during newborn hearing screening allows for non-surgical correction: a Mayo Clinic pilot study. Int J Pediatr Otorhinolaryngol. 2012;76(10):1406–1412. doi: 10.1016/j.ijporl.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 24.Yotsuyanagi T, Yokoi K, Urushidate S, Sawada Y. Nonsurgical correction of congenital auricular deformities in children older than early neonates. Plast Reconstr Surg. 1998;101(04):907–914. doi: 10.1097/00006534-199804040-00004. [DOI] [PubMed] [Google Scholar]
- 25.Feijen M MW, van Cruchten C, Payne P E, van der Hulst R RWJ. Non-surgical correction of congenital ear anomalies: a review of the literature. Plast Reconstr Surg Glob Open. 2020;8(11):e3250. doi: 10.1097/GOX.0000000000003250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan S LS, Lim G JS, Por Y C et al. Efficacy of ear molding in infants using the EarWell infant correction system and factors affecting outcome. Plast Reconstr Surg. 2019;144(04):648e–658e. doi: 10.1097/PRS.0000000000006057. [DOI] [PubMed] [Google Scholar]
- 27.Chang C S, Bartlett S P. A simplified nonsurgical method for the correction of neonatal deformational auricular anomalies. Clin Pediatr (Phila) 2017;56(02):132–139. doi: 10.1177/0009922816641368. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J L, Li C L, Fu Y Y, Zhang T Y. Newborn ear deformities and their treatment efficiency with EarWell infant ear correction system in China. Int J Pediatr Otorhinolaryngol. 2019;124:129–133. doi: 10.1016/j.ijporl.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Lin J, Sclafani A P. Otoplasty for congenital auricular malformations. Facial Plast Surg Clin North Am. 2018;26(01):31–40. doi: 10.1016/j.fsc.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Savetsky I L, Cohen J M, Avashia Y J, Byrd H S. Revisiting primary otoplasty: surgical approach to the prominent ear. Plast Reconstr Surg. 2021;148(01):28e–31e. doi: 10.1097/PRS.0000000000008105. [DOI] [PubMed] [Google Scholar]
- 31.Stewart K J, Lancerotto L. Surgical otoplasty: an evidence-based approach to prominent ears correction. Facial Plast Surg Clin North Am. 2018;26(01):9–18. doi: 10.1016/j.fsc.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Mustarde J C. The correction of prominent ears using simple mattress sutures. Br J Plast Surg. 1963;16:170–178. doi: 10.1016/s0007-1226(63)80100-9. [DOI] [PubMed] [Google Scholar]
- 33.Mandal A, Bahia H, Ahmad T, Stewart K J. Comparison of cartilage scoring and cartilage sparing otoplasty–a study of 203 cases. J Plast Reconstr Aesthet Surg. 2006;59(11):1170–1176. doi: 10.1016/j.bjps.2006.01.055. [DOI] [PubMed] [Google Scholar]
- 34.Szychta P, Stewart K J. Comparison of cartilage scoring and cartilage sparing techniques in unilateral otoplasty: a ten-year experience. Ann Plast Surg. 2013;71(05):522–527. doi: 10.1097/SAP.0b013e3182503c38. [DOI] [PubMed] [Google Scholar]
- 35.Chongchet V. A method of antihelix reconstruction. Br J Plast Surg. 1963;16:268–272. doi: 10.1016/s0007-1226(63)80120-4. [DOI] [PubMed] [Google Scholar]
- 36.Bennun R D, Mulliken J B, Kaban L B, Murray J E. Microtia: a microform of hemifacial microsomia. Plast Reconstr Surg. 1985;76(06):859–865. [PubMed] [Google Scholar]
- 37.Cubitt J J, Chang L Y, Liang D, Vandervord J, Marucci D D. Auricular reconstruction. J Paediatr Child Health. 2019;55(05):512–517. doi: 10.1111/jpc.14444. [DOI] [PubMed] [Google Scholar]
- 38.Federspil P A. The role of auricular prostheses (epitheses) in ear reconstruction. Facial Plast Surg. 2015;31(06):626–632. doi: 10.1055/s-0035-1568137. [DOI] [PubMed] [Google Scholar]
- 39.Bly R A, Bhrany A D, Murakami C S, Sie K CY. Microtia reconstruction. Facial Plast Surg Clin North Am. 2016;24(04):577–591. doi: 10.1016/j.fsc.2016.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luquetti D V, Leoncini E, Mastroiacovo P. Microtia-anotia: a global review of prevalence rates. Birth Defects Res A Clin Mol Teratol. 2011;91(09):813–822. doi: 10.1002/bdra.20836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw R D, Eid M A, Bleicher J et al. Current barriers in robotic surgery training for general surgery residents. J Surg Educ. 2022;79(03):606–613. doi: 10.1016/j.jsurg.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Cabin J A, Bassiri-Tehrani M, Sclafani A P, Romo T., III Microtia reconstruction: autologous rib and alloplast techniques. Facial Plast Surg Clin North Am. 2014;22(04):623–638. doi: 10.1016/j.fsc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava A, Hazra R, Kumar D. Bridging form and function: a bilateral auricular prosthesis. J Indian Prosthodont Soc. 2022;22(03):300–304. doi: 10.4103/jips.jips_546_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han K, Son D.Osseointegrated alloplastic ear reconstruction with the implant-carrying plate system in children Plast Reconstr Surg 200210902496–503., discussion 504–505 [DOI] [PubMed] [Google Scholar]
- 45.Spector M, Harmon S L, Kreutner A. Characteristics of tissue growth into proplast and porous polyethylene implants in bone. J Biomed Mater Res. 1979;13(05):677–692. doi: 10.1002/jbm.820130502. [DOI] [PubMed] [Google Scholar]
- 46.Lewin S, Bishop R, Woerner J E, Yates D. Three techniques for reconstruction of congenital microtia: porous implant ear reconstruction, auricular reconstruction using autologous rib, and osseointegrated craniofacial implants with auricular prosthesis. Atlas Oral Maxillofac Surg Clin North Am. 2022;30(01):113–128. doi: 10.1016/j.cxom.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Stephan S, Reinisch J. Auricular reconstruction using porous polyethylene implant technique. Facial Plast Surg Clin North Am. 2018;26(01):69–85. doi: 10.1016/j.fsc.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Constantine K K, Gilmore J, Lee K, Leach J., Jr Comparison of microtia reconstruction outcomes using rib cartilage vs porous polyethylene implant. JAMA Facial Plast Surg. 2014;16(04):240–244. doi: 10.1001/jamafacial.2014.30. [DOI] [PubMed] [Google Scholar]
- 49.Xing W, Wang Y, Qian J et al. Aesthetic auricular reconstruction in adult patients with rib cartilage calcification using a modified two-step technique. Aesthetic Plast Surg. 2018;42(06):1556–1564. doi: 10.1007/s00266-018-1206-y. [DOI] [PubMed] [Google Scholar]
- 50.Tanzer R C. Total reconstruction of the external ear. Plast Reconstr Surg Transplant Bull. 1959;23(01):1–15. doi: 10.1097/00006534-195901000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Nagata S.Modification of the stages in total reconstruction of the auricle: part I. grafting the three-dimensional costal cartilage framework for lobule-type microtia Plast Reconstr Surg 19949302221–230., discussion 267–268 [PubMed] [Google Scholar]
- 52.Nagata S.Modification of the stages in total reconstruction of the auricle: part II. grafting the three-dimensional costal cartilage framework for concha-type microtia Plast Reconstr Surg 19949302231–242., discussion 267–268 [PubMed] [Google Scholar]
- 53.Nagata S.Modification of the stages in total reconstruction of the auricle: part III. grafting the three-dimensional costal cartilage framework for small concha-type microtia Plast Reconstr Surg 19949302243–253., discussion 267–268 [PubMed] [Google Scholar]
- 54.Brent B. The correction of microtia with autogenous cartilage grafts: I. the classic deformity.? Plast Reconstr Surg. 1980;66(01):1–12. doi: 10.1097/00006534-198007000-00001. [DOI] [PubMed] [Google Scholar]
- 55.Brent B. The correction of microtia with autogenous cartilage grafts: II. atypical and complex deformities. Plast Reconstr Surg. 1980;66(01):13–21. doi: 10.1097/00006534-198007000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Olshinka A, Louis M, Truong T A. Autologous ear reconstruction. Semin Plast Surg. 2017;31(03):146–151. doi: 10.1055/s-0037-1603959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brent B.Auricular repair with autogenous rib cartilage grafts: two decades of experience with 600 cases Plast Reconstr Surg 19929003355–374., discussion 375–376 [PubMed] [Google Scholar]
- 58.Chen P, Yang J, Yang L et al. One-year outcomes of ear molding for infants with constricted ear. Plast Reconstr Surg. 2023;151(01):159–166. doi: 10.1097/PRS.0000000000009781. [DOI] [PMC free article] [PubMed] [Google Scholar]


