Abstract
There are several overlapping clinical practice guidelines in acute pancreatitis (AP), however, none of them contains suggestions on patient discharge. The Hungarian Pancreatic Study Group (HPSG) has recently developed a laboratory data and symptom-based discharge protocol which needs to be validated. (1) A survey was conducted involving all members of the International Association of Pancreatology (IAP) to understand the characteristics of international discharge protocols. (2) We investigated the safety and effectiveness of the HPSG-discharge protocol. According to our international survey, 87.5% (49/56) of the centres had no discharge protocol. Patients discharged based on protocols have a significantly shorter median length of hospitalization (LOH) (7 (5;10) days vs. 8 (5;12) days) p < 0.001), and a lower rate of readmission due to recurrent AP episodes (p = 0.005). There was no difference in median discharge CRP level among the international cohorts (p = 0.586). HPSG-protocol resulted in the shortest LOH (6 (5;9) days) and highest median CRP (35.40 (13.78; 68.40) mg/l). Safety was confirmed by the low rate of readmittance (n = 35; 5%). Discharge protocol is necessary in AP. The discharge protocol used in this study is the first clinically proven protocol. Developing and testifying further protocols are needed to better standardize patients’ care.
Subject terms: Gastroenterology, Medical research
Introduction
The incidence of acute pancreatitis (AP) is continuously increasing worldwide with an approximate annual incidence of 13–45 new cases per 100,000, meaning a 30% rise in the past 2 decades1,2. The disease itself, especially the severe form may lead to a prolonged hospital stay which can be associated with adverse patient outcomes and high hospital occupancy3. Moreover, longer hospital stay can result in higher costs4. The estimated annual total cost for AP admissions reached $2.2 billion, with a mean cost per hospitalization of $9870 based on a nationwide analysis in the United States5.
In order to achieve the best treatment for a disease, it is obvious that evidence-based guidelines need to be used6,7. The currently used evidence-based medicine guidelines in AP focuses on the diagnosis and management of AP, without clear recommendation on patient discharge8. Consequently, discharge decisions are made based on local experts’ onsite opinions leading to a variety of discharge approaches. A few years ago, the Hungarian Pancreatic Study Group (HPSG) developed a discharge protocol, but it has not been extensively tested and compared with other local protocols.
In this study, our aim was to conduct a widespread international survey and investigate the safety (readmission rate) and effectiveness (length of hospital stay) of the HPSG-protocol.
Methods and materials
International cohort
To assess the worldwide trends in patient discharge in AP we conducted a multicentre web-based survey by following the Checklist for Reporting of Survey Studies (CROSS)9. We sent a letter of invitation and a questionnaire to all members of the International Association of Pancreatology (IAP) in January 2021. The questionnaire’s main purpose was (i) to investigate the presence of any discharge protocol in AP and (ii) to understand the laboratory parameters and the clinical status of the patients upon discharge. There was no pre-testing period for the questionnaire. In case the collaborators confirmed their participation in the project, we sent a second email with further details and a pre-defined Excel sheet to collect data on gender, age, aetiology, length of hospitalization (LOH), mortality and severity of AP, discharge C-reactive protein (CRP) and 1-month readmission rate. Overall, the international data were collected retrospectively. The participants were asked to upload the completed Excel sheet to a private Google Drive folder. The participants did not have access to other collaborators’ datasets. The timeframe of the survey took two months. To avoid multiple participation, we carefully checked the participating centres, departments, and affiliations. In case of any questions, the first author was in charge of keeping in contact. The detailed questionnaire and pre-defined Excel sheet can be found in the supplementary documents (Fig. S1, Table S1). For the statistical analysis, we divided the international centres based on the presence of discharge protocol, creating an international protocol and an international non-protocol cohort, and compared the relevant clinical outcomes, such as LOH, discharge CRP value, and readmission rate.
The HPSG discharge protocol
In 2016, the HPSG developed a discharge protocol with specific and combined elements on clinical status, laboratory parameters, and therapy. The protocol was developed based on the C20 point of the IAP/APA and HPSG EBM guideline which indicated that oral feeding in predicted mild pancreatitis can be restarted as early as the intensity of abdominal pain and inflammatory markers have started to decline8. The protocol was as follows:
- Patient’s CRP level and either amylase or lipase levels were monitored every day.
- Once the patient’s abdominal pain resolved and
- Pancreatic enzyme levels showed a decreasing trend and
- CRP level started to decrease and
- there was no clinical condition that contraindicated oral feeding, the patient’s oral feeding with solid diet was immediately started.
- If, 24 h after oral refeeding,
- the patient has not developed any abdominal symptoms and
- the pancreatic enzyme level has decreased further and
- there were no other conditions or therapies (e.g., iv. antibiotics, endoscopic intervention) requiring hospitalisation and
- CRP level has
-
(i)fallen below 50 mg/l, the patient was discharged
-
(ii)further decreased but remained above 50 mg/l, both hospitalization and oral feeding were continued for an additional day
-
(i)
- If, after the additional 24 h of oral feeding (i.e., 48 h after refeeding was started)
- the patient has not developed any abdominal symptoms and
- the pancreatic enzyme level has decreased further and
- there was no clinical condition that contraindicated feeding and,
- CRP level has further decreased, the patient was discharged independently of the absolute CRP value.
The CRP value of 50 mg/l has been arbitrarily set based on previous clinical experience and related literature10–12. As the role of CRP at discharge in acute pancreatitis has not been previously investigated, this is the first time we tested its role and the safety of this cut off value.
Three of the 17 investigated centres used the above-mentioned discharge protocol in Hungary. Therefore, for data analysis, two groups of the Hungarian cohort were identified: (1) centres where the HPSG-discharge protocol was used (688 patients – Hungarian protocol cohort) and (2) where no discharge protocol was used (941 patients – Hungarian non-protocol cohort). A multicenter, multinational, prospective AP registry developed in 2013 by HPSG was used for data analysis and patients were enrolled during the period 2016–2019. Diagnosis of acute pancreatitis and its severity was defined based on the Atlanta “two of three” classification: abdominal pain, pancreatic enzyme elevation at least three times above the upper limit and morphological changes13.
Statistical analysis
Statistical analyses were performed by using R environment (R Core Team (2021), version 4.1.0). For descriptive statistics, the number of patients, mean, standard deviation (SD), minimum, median and maximum values were calculated for continuous variables and case number and percentage were calculated for categorical values. To determine statistical significance between two groups of independent samples, t-test was used for normally distributed data and the Mann–Whitney U and Mood’s test for non-normally distributed data. The association between categorical variables was calculated by the Chi-square test and Fisher’s exact test. “Pairwise Nominal Independence” post-hoc test (package: rcompanion) was conducted using Bonferroni correction for a 2-dimensional matrix of two categorical variables in which at least one dimension has more than two levels. Receiver operating characteristics (ROC) analysis was performed to assess the accuracy of the prediction of discharge CRP value in terms of 1-month readmission. The threshold of significance was p < 0.05.
Ethics
The study was approved by the Scientific and Research Ethics Committee of the Medical Research Council (22254e1/2012/EKU, 17787-8/2020/EÜIG). The study was performed in accordance with the Declaration of Helsinki and all patients provided written informed consent. Patients’ data of foreign centres were treated entirely anonymously.
Results
Basic characteristics of the international cohorts
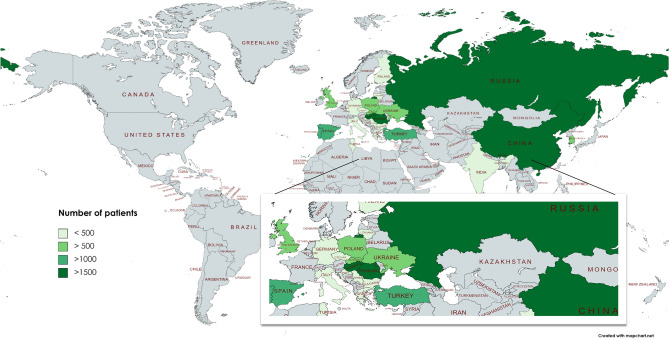
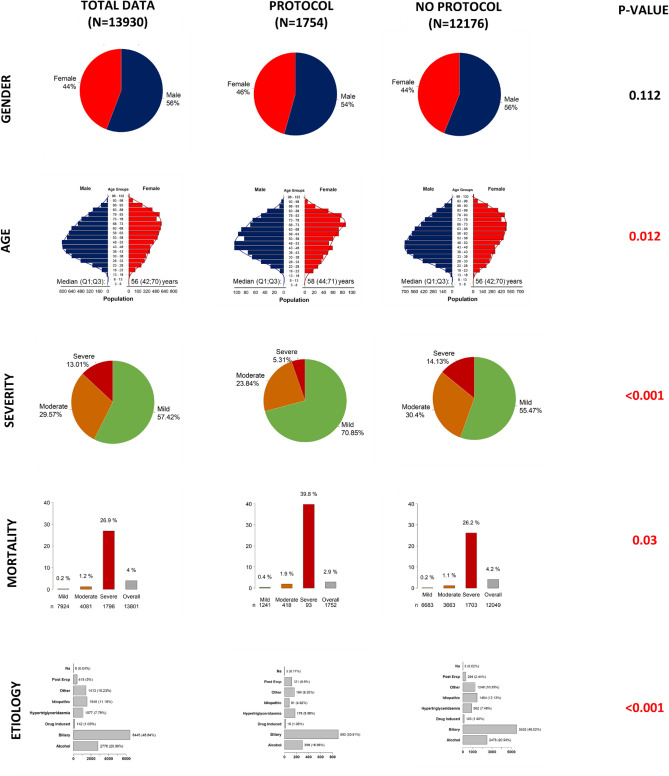
Overall, 13,930 cases from 3 continents, 23 countries, 56 centres participated in the survey and were analysed. Altogether 1754 (12.59%) cases belong to the international protocol group. The participating countries and the number of uploaded cases are illustrated on a colour-scaled map (Fig. 1) and listed in detail in the supplementary materials (Table S2). The median age was significantly lower in the non-protocol group (58 (Q1;Q3: 44;71) vs. 56 (Q1;Q3: 42;70) years, p = 0.012). Furthermore, in the non-protocol group the number of severe cases was significantly higher (14.1% vs. 5.3%) as well as the overall mortality rate (4.2% vs 2.9%, p = 0.03) (Fig. 2). Data quality can be seen in Table S4.
Figure 1.
Map of the participating countries in the survey and analysis. The map shows the number of patients provided for analysis in different shades of green. A darker shade indicates more patients included. Created by MapChart (https://mapchart.net/world.html).
Figure 2.
General characteristics of the international cohorts. Comparison of the protocol and non-protocol international cohorts. In terms of age, distribution of severity and etiology, and overall mortality there is a significant difference among the subcohorts (p < 0.05).
The majority (87.5%) of the international centres have no protocols to discharge patients in AP
According to our international survey, 87.5% (49/56) of the centres did not apply an AP discharge protocol. Notably, the protocols were moderately different from each other. Abdominal pain status was a part of every protocol, but for example, appetite was mentioned only in one case. Further details regarding the elements of discharge protocols are shown in Table S3.
Protocolized discharge strategy results in shorter length of hospitalization and
Patients discharged based on protocols have significantly shorter length of hospitalization (LOH) (7 (Q1;Q3: 5;10 days) vs. 8 (Q1;Q3: 5;12 days), p < 0.001) and lower rate of readmission due to RAP (2.8% vs. 3.9%). When separately analysing the cohorts based on severity, protocolized discharge decision still resulted in significantly shorter LOH both in the mild and moderate/severe cases (10 (Q1;Q3: 7;15 days) vs. 12 (Q1;Q3: 8;18 days)), p < 0.001) (Fig. S2).
There was no significant difference in the discharge CRP values between the groups (29.75 (9.26; 80.00) mg/l vs. 28.50 (11.80; 58.40) mg/l, p = 0.586) (Table 1). However, when separately analysing the patients based on severity, in the moderate/severe cases the discharge CRP was significantly higher 46.24 (16.65; 100.25) vs. 34.00 (15.70; 59.75) mg/l, p = 0.002) (Fig. S2).
Table 1.
Comparison of centres based on the presence of discharge protocol worldwide and in Hungary.
| International | Hungarian | |||||
|---|---|---|---|---|---|---|
| Protocol | No-protocol | p values | Protocol | No-protocol | p values | |
| Patient number | 1754 | 12,176 | NA | 688 | 941 | NA |
| Length of hospitalization | ||||||
| n (%not missing) | 1754 (100) | 12,146 (97.8) | 688 (100) | 920 (97.8) | ||
| mean (SD) | 8.55 (8.12) | 11.75 (14.30) | 8.20 (7.71) | 13.04 (15.80) | ||
| median (Q1; Q3) | 7 (5; 10.) | 8 (5;12) | < 0.0011 | 6 (5; 9) | 10 (7;15) | < 0.0011 |
| Discharge CRP | ||||||
| n (%not missing) | 1124 (64.1) | 8102 (67.2) | 688 (100) | 482 (51.2) | ||
| mean (SD) | 54.31 (61.99) | 48.61 (61.95) | 48.31 (46.38) | 47.41 (59.82) | ||
| median (Q1; Q3) | 29.75 (9.26, 80.00) | 28.50 (11.80, 58.40) | 0.5861 | 35.40 (13.78, 68.40) | 22.88 (8.80, 62.03) | 0.0031 |
| Readmission within 1 month | ||||||
| n (%not missing) | 1727 (98.4) | 11,829 (97.2) | 688 (100) | 609 (64.7) | ||
| readmission n (%) | 167 (9.7%) | 1101 (9.3%) | 0.6292 | 35 (5.09%) | 114 (19%) | < 0.0012 |
| Not pancreas related | 62 (3.6%) | 309 (2.6%) | 0.0052 | 12 (1.7%) | 67 (11%) | < 0.0012 |
| Complication of index AP | 39 (2.3%) | 275 (2.3%) | 4 (0.6%) | 24 (3.9%) | ||
| Recurrent episode of AP | 48 (2.8%) | 464 (3.9%) | 19 (2.7%) | 23 (3.8%) | ||
1Mood’s median test.
2Chi-squared test.
The table shows the comparison of centres with and with no discharge protocol, clearly describing that protocolized discharge results in shorter LOH, higher discharge CRP values and lower rate of readmission. LOH is expressed in days, while CRP in mg/l.
Safety and effectiveness of the HPSG-guided discharge protocol
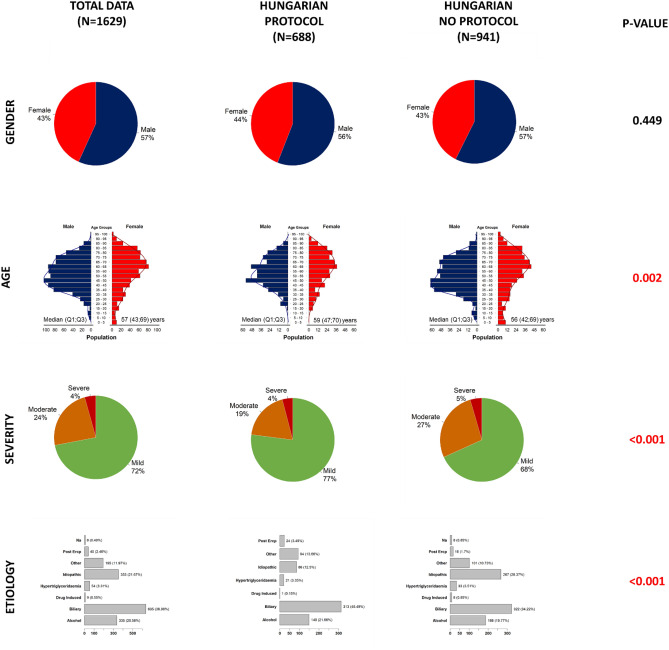
Overall, 688 patients were discharged with HPSG-protocol whereas 941 patients without it. The median age of the 2 subcohorts differed in terms of age (median (Q1;Q3): 59 (47;70) vs. (56 (42;69), severity (moderately severe cases: 19% in protocol vs 27% in non-protocol group) and the distribution of the aetiologies (Fig. 3). The median CRP value at discharge was shown to be significantly higher in the HPSG protocol group compared to the non-protocol Hungarian centres (35.40 (13.78; 68.40) vs. 22.88 (8.80; 62.03) mg/l, p = 0.003) (Table 1). This remarkable difference was also shown in the mild and moderate/severe cases separately (29.35 (12.22; 59.80 vs. 21.60 (8.33; 60.45) mg/l, p = 0.021 and 56.95 (23.17; 95.65) vs. 33.90 (10.55; 71.71), p < 0.001, respectively) (Fig S4). We also investigated the death rate within 1 month, based on the data of the Ministry for Home Affairs, 2 patients with serious comorbidities died before the 1-month follow-up visit. Data quality can be seen in Table S4.
Figure 3.
General characteristics of the Hungarian cohorts. Comparison of the protocol and non-protocol Hungarian cohorts. In terms of age, distribution of severity and etiology there is a significant difference among the subcohorts (p < 0.05).
The HPSG-developed discharge protocol was associated with a lower readmission rate vs non-protocolized discharge (5% vs. 19%)
In order to check the safety of the HPSG-protocol, patients were examined 1 month after discharge. Concerning the protocol-guided discharge, 45 out of 688 patients had elevated CRP value on the 1-month control visit compared to the discharge level (Figure S3). Nine (20%) had biliary tract inflammation (cholangitis, cholecystitis), 14 (31%) had recurrent episode, 6 (13%) had tumour-related complaints, whereas the remaining cases were mostly related non-GI diseases (rheumatoid arthritis, respiratory tract infection; 58%). Out of the 688 patients 35 (5%) were readmitted within 1 month. Among the readmitted patients 19 (54%) had recurrent episode of AP (alcohol induced: 47%, biliary: 26% CP/idiopathic: 26%), 4 (11%) had pseudocyst infection, 4 (11%) had cholecystitis/cholangitis. Five (14%) readmissions occurred due to tumour-related complaints, 2 (6%) other patients had IBD and gastroenteritis, and 1 (3%) was admitted because of trauma. In comparison, in the non-protocolized cohort, 179 of 941 (19%) patient were readmitted, mainly due to non-pancreas-related causes and index episode complication (59% vs. 21%) (Table 2).
Table 2.
Table of the readmission rates in the HPSG Protocol group.
| Readmitted | Non-readmitted | |||
|---|---|---|---|---|
| N (%) | discharge CRP* | N (%) | discharge CRP | |
| Overall | 35 (5) | 51.60 (19.95; 66.45) | 653 (95) | 35.10 (13.40, 68.70) |
| Related to AP etiology | 19 (54.29) | 38.70 (17.95; 57.2) | NA | |
| Complication of index admission | 4 (11.42) | 69.15 (66.23; 83.08) | NA | |
| Other causes | 12 (34.29) | 55.70 (27.8; 61.20) | NA | |
| Discharged < 50 mg/l CRP | 17 (48.57) | 18.60 (12.00; 38.60) | 448 (64.1) | 17.25 (8.18; 31.43) |
| Discharged > 50 mg/l CRP | 18 (51.43) | 66.45 (57.60; 76.60) | 205 (35.9) | 83.90 (63.35; 112.83) |
The table shows the number of readmitted patients due to certain causes. *Values are expressed in median (Q1;Q3). unit: mg/l. NA-not applicable, data are not available. Patients readmitted with pseudocyst infection had the highest median discharge CRP value.
Implementation of the new discharge protocol results in shorter hospital stay
One of the most relevant indices concerning the effectiveness is the LOH. Our cohort was shown to have significantly shorter LOH (6 (Q1;Q3: 5;9) days) compared to centres with no protocol either internationally (8 (Q1;Q3: 5;12) days) or nationally (10 (Q1;Q3:7;15) days) (Table 1). The difference in LOH in the Hungarian cohort was shown both in mild and moderate/severe cases when analysed separately (6 (5;7) vs. 9 (7;12.) days) (Fig. S4).
CRP value proved to be a poor prediction tool
We investigated whether the inflammatory biomarker CRP can predict readmission in AP. Discharge CRP has been identified as a poor prediction tool both in total and only in mild cases for readmission (AUC: 0.56 and 0.56 p = ns, respectively) (Fig. S4). In addition, readmission could not be predicted by the rate of decrease after the maximum CRP level (either investigated a 24 or a 48-h period). (p = 0.116, 0.208, respectively) (Fig. S5).
Discussion
In this study we tested the safety and effectiveness of discharge protocols in AP. We found that protocols significantly decrease the LOH and do not elevate the risk of readmissions. Protocolized discharge also resulted in higher discharge CRP values that may suggest, physicians are more confident in making discharge decisions in the presence of a protocol-based care.
Sheila Serra et al. showed that discharge patients with mild AP within 48 h is safe if the CRP level is below 15 mg/dl, the blood urea nitrogen change in 24 h interval is below 5 mg/dl and they tolerate oral intake11. An Australian study examined the possible risk factors which can lead to justified longer LOH than 2 days. Higher body temperature (> 38 °C), not tolerating oral diet by day 2, high pain score (VAS > 5), and high white blood cell level (> 18 G/L) were identified as risk factors. However, 87% of the admitted patients with mild AP could have been discharged at day 2 and transferred to outpatient clinic14. All these findings raise the question whether the vast majority of the patients do not require several days of hospitalization but an intensive outpatient follow-up. In other diseases, there were also positive results from the mindful patient discharge. Naureen et al. implemented a standardized, evidence-based discharge protocol when discharging patients with heart failure and consequently, it was shown that patient education can positively impact self-management after discharge resulting in shorter LOH and lower 30-day readmission rates15.
According to our results the protocol follower centres were identified to have lower 1-month readmission rate. This finding can be explained by the fact that these institutions most probably apply a strict etiology workup and follow additional AP-related guidelines, such as on-admission cholecystectomy or implement efficient patient education, thus lowering the number of recurrent or even the severe cases16,17. Furthermore, Whitlock at el. built up a model in which treatment with antibiotics, pain, pancreas necrosis, and gastrointestinal symptoms were identified as a risk factor for early (within 30 days) readmission18.
The proportion of severe cases in the non-protocol group is markedly higher, especially in the international cohort, despite the fact that it can be assumed that protocolized institutions operate as tertiary centres where a relatively large number of severe cases are transferred. However, we need to mention that since there is a higher proportion of moderate or severe cases in the non-protocol groups requiring antibiotic treatment, and having local or systemic complications, it could also contribute to the longer LOH and lower CRP level at discharge.
Of course, the prediction of possible readmission is of utmost importance and, therefore, we investigated whether CRP could be a reliable predictive tool. Unfortunately, CRP failed to be useful in this situation. CRP level as a prediction tool for readmission at discharge was investigated in several fields but not in AP. Acute heart failure patients discharged with elevated CRP value (> 10 mg/L) value, were shown to have a higher risk of mortality and readmission19,20. Furthermore, investigation of the delayed complications after esophagectomy showed that discharge patients with CRP level < 84 mg/L on day 7 proved to be a safe approach, however CRP trend itself could not predict delayed complications21. In our cohort, neither the absolute CRP value nor the degree of the decline showed a significant relationship with the 1-month readmission, supporting the theory that patient discharge should not depend on the current value or the volume of the decrease but rather on the direction of the tendency.
Strengths and limitations
The strength of our study is that we conducted an international survey including 23 countries from 3 continents and extensive data were collected. The data quality, especially in the HPSG registry analysis is remarkably high. However, we have to mention the limitations, such as the retrospective nature of the international cohort analysis. There is no information about the number of tertiary centres in our analysis, which can highly influence the number and characteristics of admitted patients. In the Hungarian cohort analyses, there might be slight differences in the way how the HPSG-discharge protocol was applied in different centres. Furthermore, the fact that those centres that apply protocols probably provide better patient care anyway.
Implication for practice and research
Implementing scientific data in the daily practice have high importance (6,7). The HPSG-discharge protocol can be immediately used in practice. Following an evidence-based discharge protocol will result in shorter LOH and thus, lower costs and also lower risk of hospital acquired infections. Is this the best possible protocol to implement? Probably not, therefore new protocols are warranted. Importantly, when additional individualized discharge protocols are possible, the individualized solution may lead to even better results. For the better assessment, randomized clinical trials are needed to be performed.
Conclusion
Using discharge protocols in AP shorten the hospital stay. The HPSG-protocol resulted in the shortest LOH and still did not increase the risk of readmittance. There is a particular need for evidence-based recommendations on discharge in guidelines.
Supplementary Information
Author contributions
R.N.: conceptualization, administration, writing the manuscript; K.O.: conceptualization, writing the manuscript; Z.S.: visualization, statistical support, A.P.: conceptualization, supervision, writing the original version; P.H.: conceptualization, supervision, writing the original version; Á.V., L.C., A.S.: supervision, prospective data collection and data quality control; J.B., P.S., R.H., S.G., I.S., J.C., G.P., A.I., N.F., P.K., T.N., A.M., B.N., J.H., B.B., M.V., I.T., J.N., Á.P., J.S., C.G., M.P., B.E., S.V., B.T., K.M., P.J.H., T.T., B.L., T.H., D.T., M.L., B.K., O.U., E.F., E.T.: prospective data collection and data quality control; N.S., V.P., A.Z., Y.Z., L.X., W.H., R.S., P.S., R.M., I.B., E.W., E.F., D.A., B.P., A.G., V.N., V.T., E.S., A.T., A.S., C.T., A.D., E.P., P.A., A.C., X.M., H.L., M.J., E.K., S.L., M.R., R.N., S.S., M.S., J.R., H.Z., E.M.-P., A.F., M.S.Y., V.M.-M., A.O., G.B., V.S., P.I., M.J., V.D.B., C.P., L.G., K.S., Y.L., T.G., M.F., V.M., M.K., S.K., G.B., A.K., M.T., T.E., N.M., E.C., J.O., Ł.N., S.G., A.F., M.G., D.I., E.U., D.D., R.I., A.G., L.D., W.H., Q.X., G.P., A.R., L.V., C.R., C.I., R.C., I.N., C.C., F.I., G.C., V.S., E.A., H.B., J.C., D.A., J.P., S.G., S.M., O.D., I.K., N.B., S.R., S.C., S.C., I.S., C.G., P.G., M.H., R.M., G.P., G.M., E.E., L.M., E.J., W.C., Q.Z., A.G., N.F., M.B., A.L., M.M., E.C., P.B., L.N., M.S., A.K., A.T., M.M., K.K., A.M., M.H., P.M., O.I., M.T., A.I., I.S., S.G., E.-R.M., V.S., P.P., Á.P.D., P.M.-H., O.E., Z.K., I.K., B.T.: provided retrospective data about the discharge protocols in acute pancreatitis in different centres. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by a project grant (TKP2021-EGA-23) of the Ministry of Innovation and Technology of Hungary to PH, by an NKFIH OTKA grant (K131996) to PH, by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (to AM), by the Project Grants (KA–2019–14, FK131864 to AM) and by the ÚNKP–22–5 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development and Innovation Fund (to AM). The project has received funding from the EU’s Horizon 2020 research and innovation program under grant agreement No. 739593. (to BCN). BCN has received funding from János Bolyai Research Grant (BO/00648/21/5) and the New National Excellence Program (UNKP-22-5-SZTE-585) and it was supported by the ÚNKP-22-4-II New national Excellence Program of the Ministry for Culture and Innovation from the Source of the National Research, Development and Innovation Fund (to KM).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-48480-z.
References
- 1.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Abd-Allah F, Abdulkader RS, Abdulle AM, Abebo TA, Abera SF, Aboyans V. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–1261. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gullo L, et al. Acute pancreatitis in five European countries: Etiology and mortality. Pancreas. 2002;24:223–227. doi: 10.1097/00006676-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Gódi S, et al. Centralized care for acute pancreatitis significantly improves outcomes. J. Gastrointest. Liver Dis. 2018;27:151–157. doi: 10.15403/jgld.2014.1121.272.pan. [DOI] [PubMed] [Google Scholar]
- 5.Fagenholz PJ, Fernández-del Castillo C, Harris NS, Pelletier AJ, Camargo CA., Jr Direct medical costs of acute pancreatitis hospitalizations in the United States. Pancreas. 2007;35:302–307. doi: 10.1097/MPA.0b013e3180cac24b. [DOI] [PubMed] [Google Scholar]
- 6.Hegyi P, Erőss B, Izbéki F, Párniczky A, Szentesi A. Accelerating the translational medicine cycle: The Academia Europaea pilot. Nat. Med. 2021;27:1317–1319. doi: 10.1038/s41591-021-01458-8. [DOI] [PubMed] [Google Scholar]
- 7.Hegyi P, et al. Academia Europaea position paper on translational medicine: The cycle model for translating scientific results into community benefits. J. Clin. Med. 2020;9:1532. doi: 10.3390/jcm9051532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Working Group IAP/APA Acute Pancreatitis Guidelines IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2013;13:e1–15. doi: 10.1016/j.pan.2013.07.063. [DOI] [PubMed] [Google Scholar]
- 9.Sharma A, et al. A consensus-based checklist for reporting of survey studies (CROSS) J. Gen. Intern. Med. 2021;36:3179–3187. doi: 10.1007/s11606-021-06737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tavernier C, et al. Assessing criteria for a safe early discharge after laparoscopic colorectal surgery. JAMA Surg. 2022;157:52–58. doi: 10.1001/jamasurg.2021.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serra Pla S, et al. Early discharge in Mild Acute Pancreatitis. Is it possible? Observational prospective study in a tertiary-level hospital. Pancreatol. Off. J. Int. Assoc. Pancreatol. 2017;17:669–674. doi: 10.1016/j.pan.2017.07.193. [DOI] [PubMed] [Google Scholar]
- 12.Plat VD, Voeten DM, Daams F, van der Peet DL, Straatman J. C-reactive protein after major abdominal surgery in daily practice. Surgery. 2021;170:1131–1139. doi: 10.1016/j.surg.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Banks PA, et al. Classification of acute pancreatitis—2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62:102–111. doi: 10.1136/gutjnl-2012-302779. [DOI] [PubMed] [Google Scholar]
- 14.Kumar VV, Treacy PJ, Li M, Dharmawardane A. Early discharge of patients with acute pancreatitis to enhanced outpatient care. ANZ J. Surg. 2018;88:1333–1336. doi: 10.1111/ans.14710. [DOI] [PubMed] [Google Scholar]
- 15.Naureen M, Rowe G. A standardized discharge protocol for heart failure patients to reduce hospital readmissions. J. Cardiovasc. Nurs. 2020;35:E113–E114. doi: 10.1097/JCN.0000000000000740. [DOI] [Google Scholar]
- 16.da Costa DW, et al. Same-admission versus interval cholecystectomy for mild gallstone pancreatitis (PONCHO): A multicentre randomised controlled trial. Lancet. 2015;386:1261–1268. doi: 10.1016/S0140-6736(15)00274-3. [DOI] [PubMed] [Google Scholar]
- 17.Párniczky A, et al. EPC/HPSG evidence-based guidelines for the management of pediatric pancreatitis. Pancreatology. 2018;18:146–160. doi: 10.1016/j.pan.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock TL, et al. A scoring system to predict readmission of patients with acute pancreatitis to the hospital within thirty days of discharge. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 2011;9:175–180. doi: 10.1016/j.cgh.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Nishimoto Y, et al. C-reactive protein at discharge and 1-year mortality in hospitalised patients with acute decompensated heart failure: An observational study. BMJ Open. 2020;10:e041068. doi: 10.1136/bmjopen-2020-041068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minami Y, Kajimoto K, Sato N, Hagiwara N. Effect of elevated C-reactive protein level at discharge on long-term outcome in patients hospitalized for acute heart failure. Am. J. Cardiol. 2018;121:961–968. doi: 10.1016/j.amjcard.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 21.Barrie J, et al. Predicting delayed complications after esophagectomy in the current era of early discharge and enhanced recovery. Am. Surg. 2020;86:615–620. doi: 10.1177/0003134820923314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.