Abstract
Neurofilament light chain (NFL), as a measure of neuroaxonal injury, has recently gained attention in alcohol dependence (AD). Aldehyde dehydrogenase 2 (ALDH2) is the major enzyme which metabolizes the alcohol breakdown product acetaldehyde. An ALDH2 single nucleotide polymorphism (rs671) is associated with less ALDH2 enzyme activity and increased neurotoxicity. We examined the blood NFL levels in 147 patients with AD and 114 healthy controls using enzyme-linked immunosorbent assay and genotyped rs671. We also followed NFL level, alcohol craving and psychological symptoms in patients with AD after 1 and 2 weeks of detoxification. We found the baseline NFL level was significantly higher in patients with AD than in controls (mean ± SD: 264.2 ± 261.8 vs. 72.1 ± 35.6 pg/mL, p < 0.001). The receiver operating characteristic curve revealed that NFL concentration could discriminate patients with AD from controls (area under the curve: 0.85; p < 0.001). The NFL levels were significantly reduced following 1 and 2 weeks of detoxification, with the extent of reduction correlated with the improvement of craving, depression, and anxiety (p < 0.001). Carriers with the rs671 GA genotype, which is associated with less ALDH2 activity, had higher NLF levels either at baseline or after detoxification compared with GG carriers. In conclusion, plasma NFL level was increased in patients with AD and reduced after early abstinence. Reduction of NFL level corroborated well with the improvement of clinical symptoms. The ALDH2 rs671 polymorphism may play a role in modulating the extent of neuroaxonal injury and its recovery.
Keywords: Alcohol dependence, Neurofilament light chain, ALDH2, rs671, Detoxification
Introduction
Alcohol drinking is the leading risk factor for disability and premature loss of life globally [1]. 18.4% of the adult population worldwide reported heavy episodic drinking in the preceding month, and alcohol dependence (AD), affecting 63.5 million of people, is the most prevalent disorder of substance dependence [45]. Excessive alcohol intake causes toxicity and severe complications in multiple organ systems and poses an ongoing crisis in public health [8]. Evaluating alcohol-related toxicity is important in patient care service because the information could not only direct professionals in making clinical decisions but also provide patients with relevant assessment results in the discussion of motivational enhancement.
Chronic and heavy alcohol consumption results in neurotoxicity and profound damage to the brain [29, 54, 55]. Although neuronal cell loss is observed in some regions of prefrontal cortex in individuals with AD, the white matter, which is mainly composed of axons surrounded by myelins, is more vulnerable to alcohol toxicity [7, 38, 55]. The disproportionate loss of cerebral white matter relative to cerebral cortex suggests that a major neurotoxic effect of alcohol in the central nervous system is axonal degeneration [18]. In addition, the white matter microstructural deficits have been related to the pathophysiology of AD [19] and the severity of alcohol problems [43]. Given that the brain imaging facilities are not universally available for healthcare systems, it is desirable to look for a reliable and alternative diagnostic indicator to evaluate and monitor neurotoxicity in AD patients.
Neurofilaments (NF) are the most abundant neuron-specific cytoskeletal proteins in myelinated axons to enable effective high-velocity axonal conduction [53]. Upon neuroaxonal injury, the NF are released extracellularly, allowing their detection in the cerebrospinal fluid (CSF) and peripheral blood, with levels proportional to the extent of axonal damage or neurodegeneration [35]. Neurofilament light chain (NFL) is a type of NF that could be quantified from peripheral blood with its level positively correlated with the level in the CSF [24]. Recent research has consistently documented that NFL could reliably reflect the subcortical large-caliber axonal degeneration or neuronal damage and serve a nonspecific predictor or diagnostic biomarker of neurodegenerative neuroaxonal damage in neuropsychiatric disorders [3, 35]. The relevance of NFL has also been addressed in the field of addiction science, such as opioid dependence [25], ketamine dependence [41] or AD [39]. Li et al. observed the NFL levels were correlated with neuropsychological dysfunction and while matter lesions and suggested that NFL was a potential neurotoxic indicator for AD. However, up to now, it is unclear whether the NFL level will be normalized after discontinuation of the substance.
Alcohol is metabolized by alcohol dehydrogenase to a toxic and reactive metabolite, acetaldehyde, which is further oxidized to acetate, mainly by aldehyde dehydrogenase 2 (ALDH2), an enzyme present in the mitochondrial matrix of cells [10]. Acetaldehyde derived from alcohol can cause DNA mutation and cellular damage [2, 21] and impairs axonal transport and cytoskeletal properties in the nervous system [37]. A common Asian-specific ALDH2 single nucleotide polymorphism (SNP), rs671 (G to A nucleotide change), results in an amino acid substitution from glutamic acid to lysine at position 504 (Glu504Lys) and causes a dramatic loss of ALDH2 enzyme activity and accumulation of acetaldehyde level after alcohol consumption, with manifestation of facial flushing, palpitations, nausea, muscle weakness and headache [28] . Worldwide, ~540 million East Asians are estimated to have ALDH2 deficiency caused by the rs671 missense mutation [6]. ALDH2 deficiency has also been associated with increased risk of neurotoxicity to the nervous system that is particularly rich in mitochondrial content [11]. Based on these observations, Rs671 A allele carriers with AD might have more pronounced neuroaxonal injury and would be reflected by higher blood level of NFL.
Since alcohol withdrawal is a cardinal phase in AD treatment because the patients are more prone to relapse during this period [30], understanding the alterations of NFL level during early abstinence would help to characterize the course of neurobiological changes during this key phase. In this study, we aimed to compare the blood NFL level between treatment-seeking patients with AD and healthy controls at the time of recruitment and followed the changes in NFL level after one- and two-week alcohol withdrawal. Also, we explored the possibility of using NFL as a biomarker to distinguish AD from non-AD patients and the correlation with clinical variables. Finally, the genetic effect of the common ALDH2 rs671 alcohol metabolizing variant on NFL level was also determined. We hypothesized that the NFL level would be increased in patients with AD compared to healthy controls and reduced after cessation of alcohol use, and the effect is contributed by ALDH2 rs671 polymorphism. We believe the determination of the novel indicator of NFL and the genetic status of ALDH2 could provide a better understanding of the neuroaxonal disturbances induced by chronic alcohol use and provide better diagnostic accuracy and prognostic assessment in the future, especially for the large East Asian populations, which carry very distinct genetic polymorphisms in alcohol metabolism as opposed to the Non-East Asian populations [23].
Methods
Study participants
This research followed the ethical standards described in the declaration of Helsinki and received the approval from the institutional review board at Taipei City Psychiatric Center (TCPC) (IRB No: TCHIRB-10701109) and National Health Research Institutes (Zhunan, Taiwan) (IRB No: EC1070102). Patients with AD were recruited from the inpatient ward of the Department of Addiction Sciences in TCPC from September 2018 to February 2020. The inclusion criteria were: (1) age being between 20 to 65 years; (2) fulfilling the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria for alcohol dependence. The exclusion criteria were: (1) with a history of schizophrenia, bipolar disorder, or major depressive disorders with psychotic features, or having been treated with antipsychotics, mood stabilizers (including valproic acid and carbamazepine); (2) with a concurrent non-nicotine substance abuse or dependence; (3) with significant systemic or physical illnesses such as infectious, autoimmune, or cardiovascular disease and diabetes mellitus: (4) showing a history of neurological conditions such as primary seizure disorder, cerebrovascular disease, apoplexy, Parkinson’s disease, dementia, polyneuropathy other than alcoholic polyneuropathy. Following an initial clinical interview to ascertain their psychiatric diagnoses, the patients underwent a physical examination and urine toxicology test to screen for illicit drug use and to exclude other substance use disorders. All eligible patients received a comprehensive description of the study and were enrolled after a written informed consent was obtained.
Healthy control participants
The control group was recruited from the Health Examination Center of Taipei City Hospital, Jen-Ai Branch, Taipei, Taiwan. The control participants had to meet the following inclusion criteria: (1) no known physical or psychiatric illnesses identified in the interview and with normal results for routine laboratory tests; (2) not meeting the diagnostic criteria for any substance (except nicotine) use disorder, including alcohol, in the past; (3) alcohol consumption less than once per month in the preceding year with drinking amount less than 20 g for males and 10 g for females.
Clinical assessments of active alcohol dependent patients
We measured severity of alcohol dependence by using the Chinese version of Severity of Alcohol Dependence Questionnaire (SADQ) [14] and smoking history information by self-reported smoking status (never vs. ever smoker) and pack-years (average number of packs of cigarettes they smoked each day multiplied by the total number of years they have smoked) [44]. Alcohol craving rated by Penn Alcohol Craving Scale (PACS) [22], depressive symptoms by the Chinese version of the 21-item Beck Depression Inventory (BDI) [42], and anxiety symptoms by the 21-item Beck Anxiety Inventory (BAI) [9], were measured at baseline and end of week 1 and week 2, respectively.
Biochemical assays
Venous blood samples were obtained between 8 and 9 a.m. after overnight fasting. Additional samples were collected for those who were enrolled after 1 week and 2 weeks of detoxification. Laboratory panels for chronic alcohol consumption including mean corpuscular volume (MCV), aspartate-aminotransferase (AST), alanine-aminotransferase (ALT), gamma-glutamyltransferase (γ-GT), and total bilirubin (Bil-T) were measured by an automated system. The blood specimens were centrifuged, and serum samples were rapidly frozen and stored at −80°C until analysis for NFL.
Plasma NFL assay
The concentration of neurofilament light (NFL) in plasma was measured using commercially available quantitative ELISA kit (OKCD01380; Aviva Systems Biology, San Diego, CA,) based on standard sandwich enzyme-linked immunosorbent assay technology. An antibody specific for NFL has been pre-coated with 100 μl of standards or diluted samples being added, incubated, and removed. A biotinylated detector antibody specific for NEFL is added. Avidin-Peroxidase Conjugate is then added and unbound conjugate is washed away. An enzymatic reaction is produced through the addition of TMB substrate which is catalyzed by HRP generating a blue color product that changes to yellow after adding acidic stop solution. The intensity of the color was measured by using a SpectraMax M2e microplate reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm. The NFL concentration was calculated based on a standard curve, which was linearized by plotting the log of the human NFL concentrations between 1.56 and 100 pg/ml versus the log of the O.D. and the best fit line can be determined by regression analysis. The intra-assay coefficient of variations was 7.2 %.
ALDH2 rs671 (G/A) genotyping
Genotyping was performed with the Axiom Genome-Wide TWB 2.0 Array, which was specially designed to identify disease-related SNPs with Taiwanese. Genotype calling was carried out using Genotyping Console 4.0 with default parameters (http://www.affymetrix.com). The allele frequency distribution of rs671 did not violate Hardy-Weinberg’s equilibrium test (p = 1.00 in control and p = 0.23 in AD patients).
Statistical analysis
Statistical analyses were conducted by the SAS software, Version 9.4 (SAS Institute, Inc., Cary, NC) and statistical figures were plotted by GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA). Continuous variables were examined for normality and homogeneity of variance with non-normal distribution confirmed by the Kolmogorov-Smirnov test. Mann-Whitney U test was used to test the differences between two continuous variables and Chi-square test was used for the contingency tables. Bivariate correlation variables were calculated by Spearman’s correlation coefficient and by linear regression analysis for considering the covariate with age. The variables in multiple linear regression analysis, which had a p value less than 0.1 in Table 2 of Spearman’s correlation, were considered as predictors of NFL levels. The receiver operating characteristic (ROC) curves for the NFL levels were analyzed to distinguish AD and controls. The optimal cutoff was selected using the Youden Index [5]. We performed mixed models of repeated-measures ANOVA, adjusting for pack-years of smoking, to determine the differences in NFL levels as well as clinical variables at week 0, week 1 and week 2 alcohol detoxification. The repeated measured correlations (rmCORR) were used to calculate the within-individual relations in paired measures assessed at week 0, week 1 and week 2 with R package [4]. Because less than no patients with AD and 7% of controls had the rs671 (AA) homozygous genotype, we combined the rs671 (AG) heterozygous and rs671 (AA) homozygous genotypes for the genetic analysis. Statistical significance was determined when p value < 0.05.
Table 2.
Spearman’s correlation analysis and multiple linear regression analysis for plasma NFL levels with clinical variables in alcohol dependent patients.
| Variable | N | Spearman's correlation |
Multiple linear regression analysis * |
|||||
|---|---|---|---|---|---|---|---|---|
| r | p value | b | S.E. | Partial r2 | p value | VIF | ||
| Age | 147 | 0.305 | <0.001 | 6.96 | 4.51 | 0.027 | 0.13 | 1.31 |
| Sex [Male: 286.3 ± 278.4 (N=121); Female: 161.2 ± 121.7 (N=26)] | - | 0.049 m | −6.84 | 96.72 | 0.077 | 0.94 | 1.65 | |
| Tobacco smoking | ||||||||
| Pack-years of smoking | 139 | 0.153 | 0.07 | −1.29 | 1.77 | 0.001 | 0.47 | 1.47 |
| Alcohol drinking variables | ||||||||
| Age of first drink, years old | 146 | 0.065 | 0.44 | |||||
| Duration of alcohol dependence (years) | 145 | 0.156 | 0.06 | 2.14 | 4.25 | 0.016 | 0.62 | 1.60 |
| Average drinking amount in the past one month (gm of pure ethanol/day) | 104 | 0.315 | 0.001 | 0.73 | 0.32 | 0.098 | 0.026 | 1.23 |
| Psychological assessment | ||||||||
| SADQ | 84 | 0.368 | <0.001 | 9.44 | 3.51 | 0.125 | 0.010 | 1.46 |
| PACS | 145 | 0.294 | <0.001 | 9.49 | 4.63 | 0.047 | 0.046 | 1.17 |
| BDI | 145 | 0.035 | 0.68 | |||||
| BAI | 145 | 0.136 | 0.10 | 6.54 | 3.93 | 0.030 | 0.10 | 1.04 |
Abbreviations: SADQ: Severity of Alcohol Dependence Questionnaire total score; PACS: Penn Alcohol Craving Score; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
The variables were included when the p value of spearman’s correlation less than 0.1.
b: regression coefficient; S.E.: standard error of regression coefficient; VIF: Variance Inflation Factor.
Mann-Whitney U test used for sex.
Bold in p value indicates a value ≦ 0.05.
Results
The demographic and clinical characteristics of participants
A total of 147 patients with AD and 114 age- and sex-matched controls were recruited (Table 1). Compared to control group, the AD group had a significantly higher percentage of smokers and pack-years (p < 0.001). NFL levels were significantly increased by 3.7 folds in patients with AD (264.2 ± 261.8 vs. 72.1 ± 35.6 pg/ml, p < 0.001), and the increase remained significant after using age and smoking pack-years as the covariate in ANCOVA analysis (p < 0.001).
Table 1.
Baseline clinical characteristics in alcohol dependent (AD) and controls.
| Variable | AD group | Control group | p value |
|---|---|---|---|
| N = 147 | N = 114 | ||
| Age, mean ± SD | 44.9 ± 9.2 | 43.1 ± 10.6 | 0.13 |
| Gender, N (%) | 0.98 | ||
| Male | 121 (82.3) | 94 (82.5) | |
| Female | 26 (17.7) | 20 (17.5) | |
| Smokers, N (%) | 124 (84.9) | 12 (10.5) | <0.001 |
| Pack-years of smoking, mean ± SD | 23.1 ± 22.6 (N=139) | 0.8 ± 3.2 (N=111) | <0.001 |
| Alcohol drinking variables, mean ± SD | |||
| Age of first drink, years old | 16.9 ± 5.8 (N=146) | ||
| Duration of alcohol dependence (yrs) | 16.7 ± 10.3 (N=145) | ||
| Average drinking amount in the past one month (gm of pure ethanol/day) | 167.0 ± 121.3 (N=104) | ||
| Biochemial data, mean ± SD | |||
| AST (10-39) (U/L) | 61.3 ± 58.4 (N=116) | ||
| ALT (7-42) (U/L) | 38.2 ± 32.9 (N=110) | ||
| Bil-T (0.2-1.2) (mg/dL) | 1.2 ± 3.7 (N=102) | ||
| γ-GT (5-61) (U/L) | 258.3 ± 321.8 (N=104) | ||
| MCV (80-100) (fL) | 92.9 ± 7.4 (N=113) | ||
| Psychological assessment, mean ± SD | |||
| SADQ (3-49) | 23.7 ± 10.7 (N=84) | ||
| PACS (3-30) | 21.8 ± 7.7 (N=145) | ||
| BDI (0-57) | 19.2 ± 12.1 (N=145) | ||
| BAI (0-52) | 10.9 ± 9.4 (N=145) | ||
| NFL (pg/ml), mean ± SD | 264.2 ± 261.8 | 72.1 ± 35.6 | <0.001/<0.001 adj |
| ALDH2 rs671 genotype, N (%) | <0.001 | ||
| GG | 113 (76.9) | 62 (54.4) | |
| GA | 34 (23.1) | 44 (38.6) | |
| AA | 0 (0.0) | 8 (7.0) | |
| ALDH2 rs671 allele frequency, N† (%) | <0.001 | ||
| G allele | 260 (88.4) | 168 (73.7) | |
| A allele | 34 (11.6) | 60 (26.3) |
Data are presented as mean ± SD (N) or N (%).
Allelic number.
Mann-Whitney U test used for continuous variables; Chi-square test used for categorical variable.
ANCOVA using age and pack-years of smoking as the covariate.
The number in ‘pack-years of cigarette smoking’ (N) considered non-smokers to be zero.
Abbreviations: SD, Standard Deviation; AST: aspartate transaminase; ALT: alanine transaminase; Bil-T: total bilirubin; γ-GT: gamma-glutamyl transferase; SADQ: Severity of Alcohol Dependence Questionnaire total score; PACS: Penn Alcohol Craving Score; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
Bold in p value indicates a value ≦ 0.05.
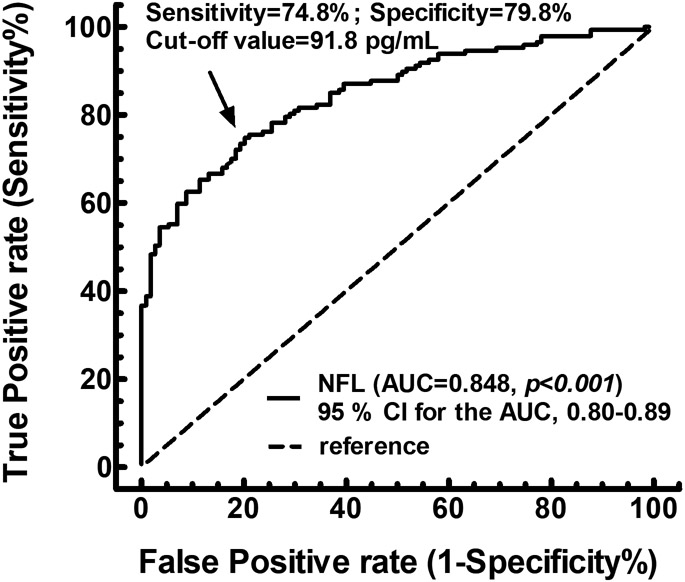
The ROC curve for NFL to distinguish patients with AD from controls
The ROC curve analyses revealed that an optimal cut-off value of NFL at 92 pg/mL significantly differentiate AD from control, reaching a sensitivity of 74.8 %, specificity of 79.8 %, and Area Under the Curve (AUC) of 0.85 with a 95% confidence interval (CI) of 0.80- 0.89 (p < 0.001) (Fig. 1).
Fig. 1.
The ROC curve for plasma NFL levels to distinguish patients with alcohol dependence from controls. The cut-off of 91.8 pg/mL was observed with a sensitivity of 74.8% and specificity of 79.8% with AUC of 0.85 (p < 0.001)
AUC: area under the curve. CI: confidence interval
Correlation of NFL levels with clinical variables
The Spearman’s correlation analysis showed the NFL level was correlated with age, sex, pack-years of smoking, duration of AD, average alcohol consumption amount in the past month, and SADQ and PACS scores (Spearman’s r value shown in Table 2). The multiple linear regression analysis demonstrated that NFL levels were significantly positively correlated with alcohol consumption in the past month (p = 0.026), and SADQ (p = 0.010) and PACS (p = 0.046) scores after adjustment. We also found that NFL levels were significantly correlated with liver function parameters (AST [Spearman’s r = 0.688, p < 0.001], ALT [r = 0.365, p < 0.001], γ-GT [r = 0.654, p < 0.001] after adjusted for age) (Supplementary Table 1).
Changes of NFL level after one- and two-week of detoxification and the correlations with clinical variables
NFL levels were significantly reduced after one week of alcohol detoxification and the levels remained non-altered from week 1 to week 2 (repeated-measures ANOVA, p < 0.001) (Table 3) although the levels remained higher than those in controls (p < 0.001) (Supplementary Fig. 1a). Craving, depression, and anxiety severity were also significantly reduced throughout the 2 weeks of detoxification (repeated-measures ANOVA, p < 0.001, respectively). The rmCORR showed that the reduction in NFL levels correlated significantly with the reduction of craving, depression, and anxiety symptoms (all p < 0.001), suggesting that the decrease in NFL correlates with symptom amelioration.
Table 3.
The alterations of NFL levels after one- (week 1) and two-week (week 2) detoxification and the correlations with changes of clinical variables using repeated measures analysis.
| Variable | Week 0 | Week 1 | Week 2 | rANOVA | rmCorr with NFL levels | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD | n | Mean ± SD | n | Mean ± SD | n | p value | r | p value | |
| NFL (pg/ml) | 264.2 ± 261.8 | 147 | 182.4 ± 163.5 | 85 | 170.8 ± 154.6 | 79 | <0.001 a**,b** | ||
| PACS | 21.8 ± 7.7 | 145 | 8.7 ± 7 | 86 | 7.1 ± 6 | 80 | <0.001 a**,b**,c* | 0.523 | <0.001 |
| BDI | 19.2 ± 12.1 | 145 | 13.1 ± 10.4 | 86 | 10.1 ± 8.9 | 80 | <0.001 a**,b**,c** | 0.459 | <0.001 |
| BAI | 10.9 ± 9.4 | 145 | 5.9 ± 8 | 86 | 4.1 ± 5 | 80 | <0.001 a**,b**,c** | 0.359 | <0.001 |
rANOVA: The mixed models of repeated-measures ANOVA, adjusting for pack-years of smoking.
rmCorr: repeated measures correlations.
Multiple comparison:
Week 0 vs. Week 1
Week 0 vs. Week 2
Week 1 vs. Week 2.
p <0.05
p <0.01.
Abbreviations: SD, Standard Deviation; PACS: Penn Alcohol Craving Score; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
Bold in p value indicates a value ≦ 0.05.
Association of ALDH2 rs671 genotypes with NFL levels
Genotype frequencies of rs671 and A allele frequency are shown in Table 1. The comparison of clinical profile between GG and GA groups in patients with AD is shown in Table 4. There was a greater proportion of GA genotype in males. GA group had higher AST, γ-GT, and MCV values as well as craving severity than the GG group. Also, the GA group had significantly higher NFL levels than GG group at baseline (p = 0.014) and week 1 (p = 0.029). At the end of week 2, GA group still manifested near-significant higher NFL levels than GG group (p = 0.059). There were also similar results in G- and A- alleles of rs671 (Table 4-1). This suggests GA carriers and A allele might suffer from more severe alcohol-related toxicity, including neuroaxonal toxicity, than GG carriers and G allele. The comparison of NFL levels between control and GG and AG groups at baseline, week 1, and week 2 is shown in Supplementary Fig. 1B, 1C and 1D.
Table 4.
Comparison of clinical characteristics between ALDH2 rs671 GG and AG group in patients with AD.
| Variable | GG | GA | p value |
|---|---|---|---|
| N = 113 | N = 34 | ||
| Age, mean ± SD | 44.9 ± 9.3 | 44.9 ± 9.3 | 0.90 |
| Gender, N (%) | 0.04 | ||
| Male | 89 (78.8) | 32 (94.1) | |
| Female | 24 (21.2) | 2 (5.9) | |
| Smokers, N (%) | 92 (82.1) | 32 (94.1) | 0.09 |
| Pack-years of smoking, mean ± SD | 21.7 ± 22.7 (N=107) | 27.9 ± 21.7 (N=32) | 0.06 |
| Alcohol drinking variables, mean ± SD | |||
| Age of first drink, years old | 16.8 ± 5.4 (N=112) | 17.1 ± 7.3 | 0.22 |
| Duration of alcohol dependence (yrs) | 16.7 ± 10.7 (N=111) | 16.5 ± 9.2 | 0.91 |
| Average drinking amount in the past one month (gm of pure ethanol/day) | 162.7 ± 125.9 (N=79) | 180.5 ± 106.9 (N=25) | 0.29 |
| Biochemical data, mean ± SD | |||
| AST (U/L) (10-296) | 54.7 ± 52.3 (N=90) | 84.1 ± 72.5 (N=26) | 0.006 |
| ALT (U/L) (6-175) | 40.4 ± 36.2 (N=86) | 30.5 ± 14.2 (N=24) | 0.83 |
| Bil-T (mg/dL) (0.2-38) | 1.3 ± 4.2 (N=78) | 1.1 ± 1.0 (N=24) | 0.43 |
| γ-GT (U/L) (11-1490) | 219.5 ± 291.2 (N=80) | 387.7 ± 386.8 (N=24) | 0.02 |
| MCV (fL) (66.7-114.1) | 92.0 ± 6.6 (N=87) | 96.0 ± 9.0 (N=26) | 0.006 |
| Psychological assessment, mean ± SD | |||
| SADQ (3-49) | 23.1 ± 11.5 (N=61) | 25.1 ± 8.3 (N=23) | 0.21 |
| PACS (3-30) | 21.0 ± 7.9 (N=111) | 24.3 ± 6.6 | 0.03 |
| BDI (0-57) | 19.2 ± 12.9 (N=111) | 19.1 ± 9.3 | 0.76 |
| BAI (0-52) | 10.9 ± 9.9 (N=111) | 10.8 ± 7.6 | 0.63 |
| NFL (pg/ml), mean ± SD | |||
| Week 0 (baseline) | 237.8 ± 245.9 | 351.8 ± 296.1 | 0.01 |
| Week 1 | 172.7 ± 177.4 (N=62) | 208.5 ± 117.7 (N=23) | 0.03 |
| Week 2 | 165.0 ± 167.6 (N=59) | 187.7 ± 109.3 (N=20) | 0.06 |
Data are presented as mean ± SD (N).
Mann-Whitney U test used for continuous variables; Chi-square test used for categorical variable.
The number in ‘pack-years of cigarette smoking’ (N) considered non-smokers to be zero.
Abbreviations: SD, Standard Deviation; AST: aspartate transaminase; ALT: alanine transaminase; Bil-T: total bilirubin; γ-GT: gamma-glutamyl transferase; SADQ: Severity of Alcohol Dependence Questionnaire total score; PACS: Penn Alweecohol Craving Score; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory.
Bold in p value indicates a value ≦ 0.05.
Table 4-1.
Comparison of NFL levels between G and A alleles of ALDH2 rs671 in patients with AD.
| Variable | G | A | p value |
|---|---|---|---|
| N = 260 | N = 34 | ||
| NFL (pg/ml), mean ± SD | |||
| Week 0 (baseline) | 252.7 ± 254.8 | 351.8 ± 296.1 | 0.02 |
| Week 1 | 178.3 ± 169.0 (N=147) | 208.5 ± 117.7 (N=23) | 0.04 |
| Week 2 | 168.3 ± 159.7 (N=138) | 187.7 ± 109.3 (N=20) | 0.08 |
Data are presented as mean ± SD (N: allelic number).
Mann-Whitney U test used for continuous variables.
Bold in p value indicates a value ≦ 0.05.
Discussion
In this study, we demonstrated that blood NFL levels increased following chronic and heavy alcohol consumption. NFL levels were correlated with drinking amount, AD severity, craving, and pertinent biochemical data (AST, ALT, γ-GT, MCV). A cut-off level of NFL 91.8 pg/mL could optimally distinguish AD with an AUC up to 0.85. Even within a week after alcohol consumption was stopped, NFL levels were reduced in patients with AD after alcohol discontinuation but remained higher than the healthy controls. The magnitude of reduction correlated with the improvement of craving, depression, and anxiety severity. In addition, among patients with AD, those with ALDH2 deficient rs671 GA genotype had higher NFL levels before and after early abstinence than those with GG genotype. Taken together, these results suggest that patients with AD are vulnerable to enhanced neuroaxonal injury but this injury can we reduced greatly even after a short-term of alcohol discontinuation. In addition, the genetic effect of ALDH2 may play an important role in neurotoxicity caused by chronic alcohol intake. Our results also indicated that blood NFL levels may serve as a useful diagnostic indicator for AD and its clinical outcomes.
Our observation of heightened NFL levels in patients with AD is in line with the recent work by Li et al [39]. In that study, NFL levels were found to be elevated in AD patients and those with higher NFL levels had more serious sleep and depression problems, white matter lesions, and neuropsychological dysfunction. Additionally, using ROC analysis, NFL levels can discriminate AD patients from healthy controls (with a sensitivity 84% and specificity 94% and AUC (0.92). Our ROC analysis also yielded similar sensitivity (75%), specificity (80%) and AUC (0.85). The lower sensitivity and specificity in our study might be due to the conventional NFL ELISA assay that we used in this study vs. the Single Molecule Arrays (Simoa) NFL ELISA assay used in Li’s study, with the latter shown to be approximately 50-fold more sensitive than the conventional ELISA [49]. Nevertheless, the similar findings support the feasibility of using blood NFL levels to predict AD-induced central nervous system injury. Our findings further found that NFL levels correlated well with clinical variables associated with AD. In agreement with this correlation, previous publications indicated a relationship between white matter microstructural deficits, and severity of alcohol-related problems [43] and alcohol consumption amount [17]. These observations collectively suggested that patients with AD have severer neuroaxonal injury and blood NFL levels could serve a potential indicator for AD.
The NFL levels in our patients decreased significantly after one week of detoxification but reduced levels remained unaltered from week 1 to week 2. The limited amelioration of neuronal injury was also reported in some reports which showed that markers of neuronal damage decreased in early withdrawal but did not normalize [26, 32, 33]. In line with that observation, white matter microstructural abnormalities were found to be persistent during abstinence [19]. Whether blood NFL levels will continue to decrease after a prolonged period of abstinence should be clarified in future studies. Furthermore, in our rmCORR analysis, the reduction in blood NFL levels significantly correlated with the improvement of clinical symptoms (craving, depression, and anxiety) during withdrawal period. It is therefore possible that blood NFL levels could be used not only to detect the severity of AD-related symptoms, but also to monitor clinical progress in patients under treatment.
It is well-established that ALDH2 deficiency caused by the rs671 missense mutation has a strong positive effect on alcohol avoidance due to the unpleasant feelings of the alcohol flushing syndrome. Carriers of the ALDH2 GA and AA genotypes consume less alcohol in general and are less likely to become alcohol dependent [46, 47]. Consistent with these studies, our participants with the either GA or AA genotype only accounted for 23.1% of patients with AD vs. 45.6% of the health control group (Table 1). In this study, patients harbored the GA genotype displayed higher blood NFL levels than those with the GG genotype at baseline and after early abstinence, implying that the enhanced AD-related neuroaxonal injury in GA carriers was related to alcohol-derived acetaldehyde toxicity. This observation is accordance with current knowledge that A-allele carriers are more susceptible to neurotoxicity [13, 40, 52]. Also, in line with the significant correlation of NFL level and blood biochemical data of heavy drinking, GA carriers displayed higher craving, AST, γ-GT, and MCV values than GG carriers. In other words, the health risks of high alcohol consumption for GA carriers are greater relative to GG carriers
A few mechanisms may help explain the relationship between increased neuroaxonal injury and increased NFL release in patients with AD. First, oxidative stress could cause proteolysis and abnormal structural changes in neurofilament which contributes to increased NFL release [15, 36]. Oxidative damage to nucleic acid is also known to be correlated with increased NFL level in the cerebrospinal fluid [20]. Patients with AD are reported to suffer from heightened oxidative stress [33] and oxidative DNA damage [12, 32], hence may likely to have increased release of NFL. Second, Neuroaxonal injury has been reported to be associated with increased inflammatory activity and ensuing cytokine dysregulation in neurodegenerative diseases [50]. The subsequent damaging pathways involve the generation of antibodies against the myelin sheath and may result in ultimate loss of myelin [56].. Patients with AD are reported to have increased inflammatory cytokines or chemokines [34, 51], which might result in a perturbation of neuroaxonal integrity and subsequent increased levels of released NFL. The attenuated immunocytokines after early abstinence observed previously [34] supports our observation that NFL release was reduced after alcohol discontinuation. Third, alcohol may act as an N-methyl-D-aspartate (NMDA) receptor antagonist. The excitotoxicity mediated by NMDA up-regulation following long-tern alcohol exposure has longed been associated with brain damage [31]. In vitro experiment showed neurofilaments were rapidly lost from axons in response to NMDA excitotoxic insult and the neurofilament degeneration could be blocked by NMDA antagonists [16]. Although a direct link of NMDA up-regulation and NFL level has not been established yet in the literature, we speculate that NMDA excitotoxicity secondary to AD may at least in part contribute to axonal damage.
Our study has several limitations. Brain imaging data were not available, precluding us a more direct way to confirm a causal relationship between blood NFL elevation and macrostructural or microstructural axonal pathology. Second, we followed patients with AD only for two weeks after detoxification; changes of NFL levels over time in drinking population, e.g., from initially phase of problem drinking to AD or the potential reversibility of neurotoxicity after a long-term abstinence, is unknown. Third, the number of ALDH2 rs671 genotyping derived from our cohort was relatively small, thus the association of A-allele with elevation of NFL levels needs to be confirmed by a larger sample size. Fourth, tobacco smoking often cooccurs in alcohol dependent patients. Although we are not aware of any research investigating the effect of smoking on NFL levels, some evidence has indicated that tobacco smoking can cause toxicity in the brain, in particular affecting white matter [27, 48]. Only a small proportion of controls were tobacco smokers. In order to overcome the potential bias, we used multiple linear regression and showed no significant correlation between pack-years of smoking and NFL levels. Nevertheless, we cannot exclude the effect of smoking on NFL levels in our patients.
In conclusion, we demonstrated the blood NFL levels were higher in patients with AD than healthy controls and correlated well with the severity of AD, craving and drinking-related biochemistry markers. After withdrawal, the increased NFL levels, although not normalized, were reduced, with the extent of NFL level reduction correlated with the improvement of psychological symptoms. These correlation data and ROC results support the possibility of using blood NFL levels for the detection of AD and monitoring of clinical outcome in patients with AD. Interestingly, we found that AD patients with the ALDH2 GA genotype tend to have a more severe neurotoxicity. This finding may indicate a need to genotype AD patients for ALDH2 when using blood NFL as a potential neurotoxic indicator for clinical diagnosis, evaluation of alcohol-induced neuroaxonal injury and monitoring of treatment outcome. Also, given the common phenotype of GA genotype is facial flushing following alcohol consumption, it is possible individuals with this experience might suffer from more severe neuroaxonal injury.
Supplementary Material
Acknowledgement
We thank the National Center for Genomic Medicine at Academia Sinica for their assistance in genotyping.
Funding
This work was supported by grants from National Health Research Institutes in Taiwan (NP-111-SP-01 and NP-111-PP-03) (Y-L Liu); Ministry of Science and Technology (109-2314-B-532-004 & 110-2314-B-532-005-MY3) (M-C Huang); Taipei City Government (TPECH 11001-62-003 & 11101-62-029) (M-C Huang), and Taipei City Hospital (TPCH 108-057, 110-61 & 111-56) (M-C Huang), Taiwan. C-H Chen and D Mochly-Rosen are supported in part by NIH AA11147 to D. Mochly-Rosen. The funding organization did not have any involvement in the content and writing of the manuscript.
Footnotes
Conflict of interest
Daria Mochly-Rosen and Che-Hong Chen hold patents related to Alda-1 for activation of ALDH2. One of the patents is licensed to Foresee Pharmaceuticals, a company that D. M.-R. consults. However, these authors do not own stocks of the company and none of this research is supported by the company.
References
- 1.Alcohol GBD, Drug Use C (2018) The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet Psychiatry 5:987–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V, Group WHOIAfRoCMW (2007) Carcinogenicity of alcoholic beverages. Lancet Oncol 8:292–293 [DOI] [PubMed] [Google Scholar]
- 3.Bacioglu M, Maia LF, Preische O, Schelle J, Apel A, Kaeser SA, Schweighauser M, Eninger T, Lambert M, Pilotto A, Shimshek DR, Neumann U, Kahle PJ, Staufenbiel M, Neumann M, Maetzler W, Kuhle J, Jucker M (2016) Neurofilament light chain in blood and csf as marker of disease progression in mouse models and in neurodegenerative diseases. Neuron 91:494–496 [DOI] [PubMed] [Google Scholar]
- 4.Bakdash JZ, Marusich LR (2017) Repeated measures correlation. Front Psychol 8:456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bangdiwala SI, Haedo AS, Natal ML, Villaveces A (2008) The agreement chart as an alternative to the receiver-operating characteristic curve for diagnostic tests. J Clin Epidemiol 61:866–874 [DOI] [PubMed] [Google Scholar]
- 6.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A (2009) The alcohol flushing response: An unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med 6:e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhler M, Mann K (2011) Alcohol and the human brain: A systematic review of different neuroimaging methods. Alcohol Clin Exp Res 35:1771–1793 [DOI] [PubMed] [Google Scholar]
- 8.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J (2019) Alcohol use disorders. Lancet 394:781–792 [DOI] [PubMed] [Google Scholar]
- 9.Che HH, Lu ML, Chen HC, Chang SW, Lee YJ (2006) Validation of the chinese version of the beck anxiety inventory. Formosan Journal of Medicine 10:447–454 [Google Scholar]
- 10.Chen CH, Ferreira JC, Gross ER, Mochly-Rosen D (2014) Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol Rev 94:1–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CH, Joshi AU, Mochly-Rosen D (2016) The role of mitochondrial aldehyde dehydrogenase 2 (aldh2) in neuropathology and neurodegeneration. Acta Neurol Taiwan 25:111–123 [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CH, Pan CH, Chen CC, Huang MC (2011) Increased oxidative DNA damage in patients with alcohol dependence and its correlation with alcohol withdrawal severity. Alcohol Clin Exp Res 35:338–344 [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Huang W, Cheng CH, Zhou L, Jiang GB, Hu YY (2019) Association between aldehyde dehydrogenase-2 polymorphisms and risk of alzheimer's disease and parkinson's disease: A meta-analysis based on 5,315 individuals. Front Neurol 10:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng WJ, Huang M, Huang P, Gau YF, Chen CH (2009) The chinese version of the severity of alcohol dependence questionnaire: Reliability and factor structure. Taiwanese Journal of Psychiatry 23:159–166 [Google Scholar]
- 15.Chiasson K, Lahaie-Collins V, Bournival J, Delapierre B, Gelinas S, Martinoli MG (2006) Oxidative stress and 17-alpha- and 17-beta-estradiol modulate neurofilaments differently. J Mol Neurosci 30:297–310 [DOI] [PubMed] [Google Scholar]
- 16.Chung RS, McCormack GH, King AE, West AK, Vickers JC (2005) Glutamate induces rapid loss of axonal neurofilament proteins from cortical neurons in vitro. Exp Neurol 193:481–488 [DOI] [PubMed] [Google Scholar]
- 17.Daviet R, Aydogan G, Jagannathan K, Spilka N, Koellinger PD, Kranzler HR, Nave G, Wetherill RR (2022) Associations between alcohol consumption and gray and white matter volumes in the uk biobank. Nat Commun 13:1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de la Monte SM (1988) Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol 45:990–992 [DOI] [PubMed] [Google Scholar]
- 19.De Santis S, Bach P, Perez-Cervera L, Cosa-Linan A, Weil G, Vollstadt-Klein S, Hermann D, Kiefer F, Kirsch P, Ciccocioppo R, Sommer WH, Canals S (2019) Microstructural white matter alterations in men with alcohol use disorder and rats with excessive alcohol consumption during early abstinence. JAMA Psychiatry 76:749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellis RJ, Moore DJ, Sundermann EE, Heaton RK, Mehta S, Hulgan T, Samuels D, Fields JA, Letendre SL (2020) Nucleic acid oxidation is associated with biomarkers of neurodegeneration in csf in people with hiv. Neurol Neurophysiol Neurosci 7:e902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eriksson CJ (2001) The role of acetaldehyde in the actions of alcohol (update 2000). Alcohol Clin Exp Res 25:15S–32S [DOI] [PubMed] [Google Scholar]
- 22.Flannery BA, Volpicelli JR, Pettinati HM (1999) Psychometric properties of the penn alcohol craving scale. Alcohol Clin Exp Res 23:1289–1295 [PubMed] [Google Scholar]
- 23.Gaetani L, Blennow K, Calabresi P, Di Filippo M, Parnetti L, Zetterberg H (2019) Neurofilament light chain as a biomarker in neurological disorders. J Neurol Neurosurg Psychiatry 90:870–881 [DOI] [PubMed] [Google Scholar]
- 24.Gaiottino J, Norgren N, Dobson R, Topping J, Nissim A, Malaspina A, Bestwick JP, Monsch AU, Regeniter A, Lindberg RL, Kappos L, Leppert D, Petzold A, Giovannoni G, Kuhle J (2013) Increased neurofilament light chain blood levels in neurodegenerative neurological diseases. PLoS One 8:e75091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Sevilla JA, Ventayol P, Busquets X, La Harpe R, Walzer C, Guimon J (1997) Marked decrease of immunolabelled 68 kda neurofilament (nf-l) proteins in brains of opiate addicts. Neuroreport 8:1561–1565 [DOI] [PubMed] [Google Scholar]
- 26.Girard M, Malauzat D, Nubukpo P (2019) Serum inflammatory molecules and markers of neuronal damage in alcohol-dependent subjects after withdrawal. World J Biol Psychiatry 20:76–90 [DOI] [PubMed] [Google Scholar]
- 27.Gogliettino AR, Potenza MN, Yip SW (2016) White matter development and tobacco smoking in young adults: A systematic review with recommendations for future research. Drug Alcohol Depend 162:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harada S, Agarwal DP, Goedde HW, Tagaki S, Ishikawa B (1982) Possible protective role against alcoholism for aldehyde dehydrogenase isozyme deficiency in japan. Lancet 2:827. [DOI] [PubMed] [Google Scholar]
- 29.Harper C (2009) The neuropathology of alcohol-related brain damage. Alcohol Alcohol 44:136–140 [DOI] [PubMed] [Google Scholar]
- 30.Heilig M, Egli M, Crabbe JC, Becker HC (2010) Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addict Biol 15:169–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holmes A, Spanagel R, Krystal JH (2013) Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl) 229:539–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang MC, Chen CC, Pan CH, Chen CH (2014) Comparison of oxidative DNA damage between alcohol-dependent patients with and without delirium tremens. Alcohol Clin Exp Res 38:2523–2528 [DOI] [PubMed] [Google Scholar]
- 33.Huang MC, Chen CH, Peng FC, Tang SH, Chen CC (2009) Alterations in oxidative stress status during early alcohol withdrawal in alcoholic patients. J Formos Med Assoc 108:560–569 [DOI] [PubMed] [Google Scholar]
- 34.Huang MC, Chung RH, Lin PH, Kuo HW, Liu TH, Chen YY, Chen ACH, Liu YL (2022) Increase in plasma ccl11 (eotaxin-1) in patients with alcohol dependence and changes during detoxification. Brain Behav Immun 99:83–90 [DOI] [PubMed] [Google Scholar]
- 35.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, Barro C, Kappos L, Comabella M, Fazekas F, Petzold A, Blennow K, Zetterberg H, Kuhle J (2018) Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol 14:577–589 [DOI] [PubMed] [Google Scholar]
- 36.Kim NH, Jeong MS, Choi SY, Hoon Kang J (2004) Oxidative modification of neurofilament-l by the cu,zn-superoxide dismutase and hydrogen peroxide system. Biochimie 86:553–559 [DOI] [PubMed] [Google Scholar]
- 37.Koike H, Sobue G (2006) Alcoholic neuropathy. Curr Opin Neurol 19:481–486 [DOI] [PubMed] [Google Scholar]
- 38.Konrad A, Vucurevic G, Lorscheider M, Bernow N, Thummel M, Chai C, Pfeifer P, Stoeter P, Scheurich A, Fehr C (2012) Broad disruption of brain white matter microstructure and relationship with neuropsychological performance in male patients with severe alcohol dependence. Alcohol Alcohol 47:118–126 [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Duan R, Gong Z, Jing L, Zhang T, Zhang Y, Jia Y (2021) Neurofilament light chain is a promising biomarker in alcohol dependence. Front Psychiatry 12:754969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin CY, Yu RL, Wu RM, Tan CH (2019) Effect of aldh2 on sleep disturbances in patients with parkinson's disease. Sci Rep 9:18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu YL, Bavato F, Chung AN, Liu TH, Chen YL, Huang MC, Quednow BB (2021) Neurofilament light chain as novel blood biomarker of disturbed neuroaxonal integrity in patients with ketamine dependence. World J Biol Psychiatry 22:713–721 [DOI] [PubMed] [Google Scholar]
- 42.Lu ML, Che HH, Chang S, Shen WW (2002) Reliability and validity of the chinese version of the beck depression inventory-ii. Taiwanese Journal of Psychiatry 16:301–310 [Google Scholar]
- 43.Monnig MA, Yeo RA, Tonigan JS, McCrady BS, Thoma RJ, Sabbineni A, Hutchison KE (2015) Associations of white matter microstructure with clinical and demographic characteristics in heavy drinkers. PLoS One 10:e0142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S (1994) The validity of self-reported smoking: A review and meta-analysis. Am J Public Health 84:1086–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, Giovino GA, West R, Hall W, Griffiths P, Ali R, Gowing L, Marsden J, Ferrari AJ, Grebely J, Farrell M, Degenhardt L (2018) Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 113:1905–1926 [DOI] [PubMed] [Google Scholar]
- 46.Quillen EE, Chen XD, Almasy L, Yang F, He H, Li X, Wang XY, Liu TQ, Hao W, Deng HW, Kranzler HR, Gelernter J (2014) Aldh2 is associated to alcohol dependence and is the major genetic determinant of "daily maximum drinks" in a gwas study of an isolated rural chinese sample. Am J Med Genet B Neuropsychiatr Genet 165B:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun Y, Chang S, Wang F, Sun H, Ni Z, Yue W, Zhou H, Gelernter J, Malison RT, Kalayasiri R, Wu P, Lu L, Shi J (2019) Genome-wide association study of alcohol dependence in male han chinese and cross-ethnic polygenic risk score comparison. Transl Psychiatry 9:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swan GE, Lessov-Schlaggar CN (2007) The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev 17:259–273 [DOI] [PubMed] [Google Scholar]
- 49.Wang X, Cohen L, Wang J, Walt DR (2018) Competitive immunoassays for the detection of small molecules using single molecule arrays. J Am Chem Soc 140:18132–18139 [DOI] [PubMed] [Google Scholar]
- 50.Williams T, Zetterberg H, Chataway J (2021) Neurofilaments in progressive multiple sclerosis: A systematic review. J Neurol 268:3212–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yen CH, Ho PS, Yeh YW, Liang CS, Kuo SC, Huang CC, Chen CY, Shih MC, Ma KH, Sung YF, Lu RB, Huang SY (2017) Differential cytokine levels between early withdrawal and remission states in patients with alcohol dependence. Psychoneuroendocrinology 76:183–191 [DOI] [PubMed] [Google Scholar]
- 52.Yu RL, Tan CH, Lu YC, Wu RM (2016) Aldehyde dehydrogenase 2 is associated with cognitive functions in patients with parkinson's disease. Sci Rep 6:30424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yuan A, Rao MV, Veeranna, Nixon RA (2017) Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 9:a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zahr NM, Kaufman KL, Harper CG (2011) Clinical and pathological features of alcohol-related brain damage. Nat Rev Neurol 7:284–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zahr NM, Pfefferbaum A (2017) Alcohol's effects on the brain: Neuroimaging results in humans and animal models. Alcohol Res 38:183–206 [PMC free article] [PubMed] [Google Scholar]
- 56.Zindler E, Zipp F (2010) Neuronal injury in chronic cns inflammation. Best Pract Res Clin Anaesthesiol 24:551–562 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.