Abstract
Enpatoran is a selective inhibitor of toll‐like receptors 7 and 8 (TLR7/8) that potentially targets pro‐inflammatory pathways induced by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). A phase II study conducted in Brazil, the Philippines, and the USA during the early pandemic phase assessed the safety and efficacy of enpatoran in patients hospitalized with COVID‐19 pneumonia (NCT04448756). A total of 149 patients, who scored 4 on the World Health Organization's (WHO) 9‐point ordinal severity scale, were randomized 1:1:1 and received enpatoran 50 mg (n = 54) or 100 mg (n = 46), or placebo (n = 49) twice daily (b.i.d.) for 14 days plus standard of care. The primary objectives were safety and time to recovery (WHO 9‐point scale ≤3). Clinical deterioration (WHO 9‐point scale ≥ 5) was a key secondary objective. Treatment‐emergent adverse events (TEAEs) were comparable across groups (56.5%–63.0%). Treatment‐related TEAEs were numerically higher with enpatoran 50 mg (14.8%) than 100 mg (10.9%) or placebo (8.2%). Serious TEAEs were numerically lower with enpatoran (50 mg 9.3%, 100 mg 2.2%) than placebo (18.4%). The primary efficacy objective was not met; median time to recovery was 3.4–3.9 days across groups, with placebo‐treated patients recovering on average faster than anticipated. Clinical deterioration event‐free rates up to Day 7 were 90.6%, 95.6%, and 81.6% with enpatoran 50 mg, 100 mg, and placebo, respectively. Enpatoran was well tolerated by patients acutely ill and hospitalized with COVID‐19 pneumonia. Positive signals in some secondary end points suggested potential beneficial effects, supporting further evaluation of enpatoran in patients with hyperinflammation due to infection or autoimmunity.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Enpatoran is a dual toll‐like receptor 7 and 8 (TRL7/8) inhibitor shown to target pro‐inflammatory pathways that are activated in autoimmune disorders such as lupus and are induced by single‐stranded RNA viruses such as SARS‐CoV‐2.
WHAT QUESTION DID THIS STUDY ADDRESS?
We hypothesized that intervention with enpatoran at a critical point in the development of COVID‐19 may prevent the hyperinflammation and cytokine storm associated with severe disease.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
In this randomized, exploratory phase II study, enpatoran up to 100 mg twice daily for 14 days was well tolerated by patients hospitalized with COVID‐19 pneumonia. Despite a numerical trend towards higher recovery rates in both enpatoran groups compared with placebo, the primary efficacy end point of the time to recovery from Day 1 through Day 28 was not met. However, there was some evidence that enpatoran provided measurable treatment effects, particularly with the highest enpatoran dose tested, based on secondary and exploratory objectives. A trend towards decreased likelihood of clinical deterioration was observed in the overall study population, and the time to recovery was improved in patients with high interferon‐gene signature scores at baseline.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
To our knowledge, this is the first study to clinically evaluate a TLR7/8 inhibitor in an infectious disease. Although the primary efficacy end point was not met, the results provide important safety data and show potential beneficial effects that support further evaluation of TLR7/8 inhibitors such as enpatoran in patients with hyperinflammation due to infection or autoimmunity.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is a single‐stranded RNA (ssRNA) virus that causes coronavirus disease 2019 (COVID‐19). 1 In the early stages of infection, SARS‐CoV‐2 proteins suppress the production and downstream signaling of type I interferon (IFN), a key part of the antiviral response that protects against viral dissemination. 2 , 3 This enables unrestrained replication of SARS‐CoV‐2, which later promotes high levels of IFN production and the overstimulation of inflammatory cytokines (cytokine storm) leads to serious illness in certain susceptible individuals. 1 , 4 , 5 While corticosteroids are a broad strategy used to treat hyperinflammation in patients with COVID‐19, targeted treatments are needed to further improve patient outcomes. Drugs that target the production of cytokines, including interleukin‐6 (IL‐6), tumor necrosis factor (TNF), and Janus kinase inhibitors, have been tested in clinical trials with varied results. 5
Toll‐like receptors 7 and 8 (TLR7/8) reside in the endosome of certain immune cells and trigger innate and adaptive immune responses following detection of ssRNA viruses. 6 TLR7 activation results in the secretion of type I IFN and other cytokines, B‐cell activation, and antibody production. 7 , 8 The activation of TLR8 in cells of myeloid origin (monocytes, macrophages, and neutrophils) leads to the production of cytokines and the activation of host protective mechanisms such as NETosis. 7 , 9
Targeting TLR7/8 for the treatment of severe COVID‐19 is supported by several studies. First, TLR7 and TLR8 expression is increased in nasopharyngeal epithelial cells from patients with symptomatic COVID‐19 compared with that in patients with COVID‐19 without clinical symptoms as well as SARS‐CoV‐2‐negative controls (healthy individuals, those with symptoms similar to that of COVID‐19, and hospitalized patients). 10 Expression of TLR7 and TLR8 correlates with the level of inflammation and oxygen saturation, suggesting that their hyperactivation may be linked to the severity of COVID‐19 symptoms and the need for supportive care in hospital. 10 Second, blood and lung samples from patients hospitalized in the intensive care unit (ICU) with COVID‐19 show hallmarks of TLR7 hyperactivation. 11 Finally, ssRNAs from the related SARS‐CoV were identified that induce the release of pro‐inflammatory cytokines through TLR7 and TLR8. 12
Enpatoran, a highly selective and potent dual TLR7/8 inhibitor with potential to modulate innate and adaptive immune processes, is being developed as a novel oral treatment for autoimmune disorders including systemic and cutaneous lupus erythematosus. 13 , 14 Enpatoran blocked both synthetic and natural TLR7/8 ligands in vitro, including microRNAs and Alu RNA, and inhibited RNA‐induced cytokine secretion in human peripheral blood mononuclear cells and whole blood. 13 , 15 In the first‐in‐human study in healthy participants, enpatoran up to 200 mg once daily for 14 days was well tolerated. 14 Pharmacokinetic (PK) and pharmacodynamic (PD) data from this study were used in population PK/PD model‐based simulations to inform dose selection for clinical trials. 16 A phase I ethno‐bridging study demonstrated no ethnic differences in PK, PD, or safety between healthy Japanese and Caucasian participants across a range of single enpatoran doses, supporting the inclusion of Asian participants in clinical trials. 17
Preclinical and early clinical studies demonstrated that enpatoran targets the pro‐inflammatory pathways induced by ssRNA viruses such as SARS‐CoV‐2, inhibiting IL‐6 release more strongly than IFN‐α. 13 , 14 , 16 It was therefore hypothesized that intervention with enpatoran at a critical point in COVID‐19 development, following progression to pneumonia but before mechanical ventilation, may prevent the hyperinflammation and cytokine storm associated with severe COVID‐19. The objective of this exploratory phase II study was to assess the safety and efficacy of enpatoran in hospitalized patients with COVID‐19 pneumonia.
METHODS
Study design and ethics
This exploratory, randomized, double‐blind, placebo‐controlled phase II study (ANEMONE, NCT04448756) was conducted between July 29, 2020 and August 16, 2021 in Brazil, the Philippines, and the USA in accordance with international guidelines, including the Belmont Report 18 and International Council for Harmonisation (ICH) E6 (R2). Patients received enpatoran 50 mg, enpatoran 100 mg, or placebo tablets orally twice daily (b.i.d.) for 14 days in addition to standard of care (SoC), with monitoring to Day 28 and safety follow‐up to Day 60 (Figure S1). The enpatoran doses were selected based on PK/PD modeling and simulations of early clinical data. 16
The study was conducted in two parts; Part A was an assessment of safety planned in 15 patients (5 per group), before expanding in Part B to a full clinical evaluation planned in an additional 135 patients (45 per group), for a total of 150 patients (Figure S1). An independent data monitoring committee (IDMC) provided ongoing surveillance of patient safety during the study and recommended proceeding to Part B following review of available safety data from Part A.
The study was conducted in accordance with the principles of the Declaration of Helsinki, applicable Good Clinical Practice guidelines from the ICH, and applicable local laws and regulations. Ethics approval was obtained from institutional review boards and ethics committees at all participating sites. Study participants provided written informed consent before enrollment.
Study participants
Patients were eligible if they were aged 18–75 years, tested positive for SARS‐CoV‐2 as established by a nucleic acid amplification test, polymerase chain reaction, antigen test, or another commercial or public health assay (based on locally accepted guidelines) less than 10 days prior to randomization, and were hospitalized with chest imaging consistent with COVID‐19 pneumonia. Patients were not on mechanical ventilation and had SpO2 < 94% in room air and PaO2/FiO2 ≥ 150 with a maximum FiO2 of 0.4. Based on these eligibility criteria, all patients were expected to score 4 on the World Health Organization's (WHO) 9‐point ordinal severity scale at enrollment. 19
Patients with clinically significant cardiovascular disease, or history of uncontrolled illness within the past 3 months, were excluded. Patients were to receive the local SoC but could not receive antimalarials or other immunomodulating drugs. Corticosteroids initiated prior to randomization at a maximum dose of 40 mg prednisone‐equivalent dose per day, and antivirals (e.g., remdesivir) that were part of the SoC for the local hospital, were permitted. Following commencement of the study, the protocol was updated to include SARS‐CoV‐2 vaccination in the exclusion criteria.
Interventions
Enpatoran, which has been characterized previously, 13 , 14 , 16 and matching placebo tablets were identical in physical appearance. Four tablets (4 placebo; 2 placebo and 2 enpatoran 25 mg; or 4 enpatoran 25 mg) were taken orally every 12 h (within a 2‐h window). Patients who entered the ICU were able to receive study treatment orally or by nasogastric tube. If a patient was discharged prior to treatment completion, they self‐administered at home.
Randomization and blinding
Patients were enrolled by study investigators and centrally allocated to either enpatoran 50 mg b.i.d., enpatoran 100 mg b.i.d., or placebo b.i.d. in a 1:1:1 ratio using an Interactive Web Response System and a computer‐generated randomization list. In Part A, patients were stratified by the presence/absence of obstructive lung disease. In Part B, patients were stratified based on: (i) use of corticosteroids at a prednisone‐equivalent daily dosage of ≤15 mg or >15 mg within 48 h prior to randomization; (ii) use of antiviral therapy, including convalescent plasma, within 48 h prior to randomization; and (iii) country.
The study was double‐blinded. For patients who received treatment via nasogastric tube, an independent pharmacist may have been unblinded to prepare tablets for administration. The IDMC received partially unblinded data, provided by an independent statistician who was not involved with the study.
Outcomes
The primary objectives were: (i) to assess the safety of enpatoran compared with placebo through Day 60; and (ii) to evaluate the time to recovery (WHO 9‐point scale score of ≤3) from Day 1 up to Day 28 with enpatoran compared with placebo. Prespecified secondary end points included the time to clinical deterioration (WHO 9‐point scale score of ≥5) assessed from Day 1 to Day 28, clinical status (WHO 9‐point scale) and all‐cause mortality through Day 60, and modulation of inflammatory biomarkers to Day 28. Exploratory end points included assessment of the PK of enpatoran by sparse sampling on Days 1, 3, and 7. A post‐hoc exploratory analysis was performed to evaluate the time to recovery for patients with high versus low IFN‐gene signature (IFN‐GS) scores at baseline.
The primary efficacy objective was defined initially as the proportion of patients who were alive and not requiring supplemental oxygen at Day 14. This was based on data from the initial phase of the pandemic, when patients hospitalized with COVID‐19 pneumonia receiving SoC had a median duration of hospitalization and a median time to clinical improvement of 16 days. 20 It was later reported that remdesivir shortened the time to recovery for patients hospitalized with COVID‐19; median time to recovery was 10 days for patients who scored 4–7 on the WHO's 9‐point scale at baseline, and 5 days for those who scored 4 at baseline. 21 Remdesivir was approved subsequently by the Food and Drug Administration (FDA) in October 2020. Considering the rapidly changing SoC for COVID‐19, the primary end point was updated (while study staff and investigators were still blinded) to the time to recovery from Day 1 up to Day 28; this end point would capture an earlier discharge or shorter duration of hospitalization compared with the beginning of the pandemic.
Study assessments
All patients were evaluated during hospitalization at least once daily. Patients discharged from hospital had study visits, either by telephone or at the study site, on Days 3, 5, 7, 10, 14, 21, 28, 44, and 60. Clinical score was recorded daily during hospitalization, at the time of hospital discharge, and on study visits following discharge up to Day 60, using the WHO 9‐point ordinal scale that captures information such as hospital discharge, ICU stay, and mechanical ventilation. Adverse events (AEs) and vital signs were recorded each day at approximately the same time throughout hospitalization and on study site visits following discharge through Day 60. AEs of special interest (AESIs) included serious and opportunistic infections, seizures, clinically significant arrythmias, and serotonin syndrome.
Whole blood was collected for measurement of enpatoran plasma concentrations and/or serum biomarkers and cytokines while the patients remained in hospital. In Part A, PK was assessed pre‐dose and 1, 2, 6, and 12 h post‐dose on Day 1, and 1, 2, and 6 h post‐dose on Day 7. In Part B, PK was assessed pre‐dose and 1–2, 4–6, and 8–12 h post‐dose on Day 1, and 1–2 and 4–6 h post‐dose on Days 3 and 7. Inflammatory biomarkers C‐reactive protein (CRP), ferritin, and D‐dimer were tested during screening and on Days 1, 3, 7, 10, at early termination/hospital discharge, and at the end of the treatment (Day 14) and surveillance (Day 28) periods. Normal biomarker levels are <10 mg/L for CRP, 30–400 μg/L (men) or 13–150 μg/L (women) for ferritin, and <0.5 mg/L for D‐dimer. 22
IFN‐GS scores were evaluated at baseline using DxTerity's IFN‐I Test (DxTerity, California, USA), which evaluates the expression of HERC5, IFI27, IFIT1, and RSAD2. The cut‐off for high and low IFN‐GS was −0.5; this was predefined and based on expression profiles in lupus patients and healthy controls (data on file).
Statistical analysis
The total sample size was set at 150 patients. For the time to recovery from Day 1 up to Day 28, 150 patients at a 1:1:1 ratio would allow the detection of an individual hazard ratio (HR) between 2.0 and 1.6 with a respective power of 86% and 51%, under the assumption of piecewise constant hazards, controlling the overall one‐sided type I error of 0.025 with a Sidak correlation for multiplicity.
Baseline characteristics and safety data were summarized using descriptive statistics. Statistical testing of efficacy data was considered exploratory due to the exploratory nature of the study, and results are presented with no adjustment of type I error for multiplicity. Time to recovery and time to clinical deterioration were estimated via Kaplan–Meier (KM) analysis, presented with two‐sided 95% confidence intervals (CIs). The effect of each dose level compared with placebo was evaluated using a stratified log‐rank test. Strata were defined by: (i) use of corticosteroids at a prednisone‐equivalent daily dose of ≤15 mg or >15 mg within 48 h prior to randomization; (ii) use of antiviral therapy and/or convalescent plasma (presence or absence) within 48 h prior to randomization; and (iii) country. Time to recovery was defined as time from Day 1 to first occurrence of WHO 9‐point ordinal scale of ≤3. Time to clinical deterioration was defined as time from Day 1 to first occurrence of WHO 9‐point ordinal scale of ≥5.
Estimation of the effect of each treatment dose compared with placebo was planned to be based on HR from a stratified Cox regression model, with terms for treatment group, use of corticosteroids at a prednisone‐equivalent daily dosage of ≤15 mg or >15 mg within 48 h prior to randomization, and country. However, because the proportional hazards assumption was not met, only KM estimates and nominal one‐sided p values from stratified log‐rank tests are presented.
All patients who received at least one dose of study intervention, referred to as the safety analysis set, were included in the efficacy and safety analyses and analyzed according to the actual treatment received. The PD analysis set comprised all patients who received at least one dose of active treatment, with at least one post‐baseline serum biomarker assessment, and with no relevant protocol deviations or other events that may have influenced PD. The PK analysis set comprised all patients who received at least one dose of active treatment, with at least one quantifiable serum concentration of enpatoran, and without any relevant protocol deviations or factors that may have influenced PK.
RESULTS
Patient demographics
Of the 200 patients enrolled across 17 sites, 149 were randomized and received treatment (safety analysis set), 54 received enpatoran 50 mg b.i.d., 46 received enpatoran 100 mg b.i.d., and 49 received placebo (Figure 1). The majority (88%) of patients completed the full course of treatment. The median (quartile [Q]1–Q3) duration of treatment was 13.4 (13.3–13.5), 13.5 (13.4–13.5), and 13.4 (13.3–13.5) days in the enpatoran 50 mg b.i.d., enpatoran 100 mg b.i.d., and placebo groups, respectively. Treatment compliance was similar across groups; the respective proportions of patients who received 80%–110% of the assigned study treatment were 91% (n = 49), 91% (n = 42), and 88% (n = 43). Two patients received treatment via nasogastric tube (placebo n = 1; 50 mg b.i.d. n = 1).
FIGURE 1.
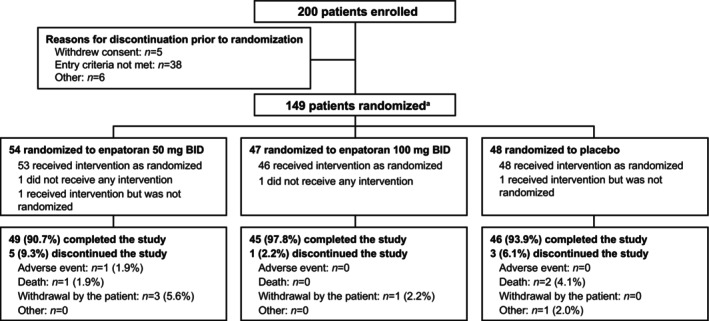
Patient disposition. Efficacy and safety analyses were according to the actual treatment received: enpatoran 50 mg b.i.d. n = 54, enpatoran 100 mg b.i.d. n = 46, and placebo n = 49 (safety analysis set). b.i.d., twice daily. a151 patients were eligible to be randomized; however, 2 patients received intervention but were not randomized (enpatoran 50 mg n = 1; placebo n = 1).
Patient demographics were balanced across groups (Table 1). Median age was 50 (range: 21–75) years, 65.8% were male, the majority were White (45.0%) or Asian (27.5%), and 64.4% were Hispanic or Latino. The mean ± SD body mass index (BMI) was 30.5 ± 6.0 kg/m2 and 46.3% of patients had a BMI ≥30 kg/m2.
TABLE 1.
Patient demographics and baseline disease characteristics (safety analysis set).
|
Parameter |
Total | Enpatoran | Enpatoran | Placebo |
|---|---|---|---|---|
| (N = 149) | 50 mg b.i.d. (n = 54) | 100 mg b.i.d. (n = 46) | (n = 49) | |
| Age | ||||
| Mean ± SD (years) | 49.6 ± 12.9 | 51.0 ± 12.3 | 49.2 ± 12.4 | 48.4 ± 14.1 |
| <60 years, n (%) | 115 (77.2) | 40 (74.1) | 38 (82.6) | 37 (75.5) |
| Sex, n (%) | ||||
| Male | 98 (65.8) | 35 (64.8) | 32 (69.6) | 31 (63.3) |
| Female | 51 (34.2) | 19 (35.2) | 14 (30.4) | 18 (36.7) |
| Body mass index | ||||
| Mean ± SD (kg/m2) | 30.5 ± 6.0 | 31.7 ± 6.9 | 30.2 ± 5.0 | 29.4 ± 5.8 |
| ≥30 kg/m2, n (%) | 69 (46.3) | 26 (48.1) | 24 (52.2) | 19 (38.8) |
| Missing, n (%) | 5 (3.4) | 1 (1.9) | 3 (6.5) | 1 (2.0) |
| Race, n (%) | ||||
| Asian | 41 (27.5) | 16 (29.6) | 12 (26.1) | 13 (26.5) |
| Black or African American | 14 (9.4) | 7 (13.0) | 4 (8.7) | 3 (6.1) |
| Native Hawaiian or Other Pacific Islander | 1 (0.7) | 0 | 0 | 1 (2.0) |
| White | 67 (45.0) | 20 (37.0) | 25 (54.3) | 22 (44.9) |
| Other a | 26 (17.4%) | 11 (20.4) | 5 (10.9) | 10 (20.4) |
| Ethnicity – Hispanic or Latino, n (%) | ||||
| Yes | 96 (64.4) | 38 (70.4) | 29 (63.0) | 29 (59.2) |
| Missing | 3 (2.0) | 1 (1.9) | 2 (4.3) | 0 |
| Country, n (%) | ||||
| Brazil | 108 (72.5) | 39 (72.2) | 34 (73.9) | 35 (71.4) |
| Philippines | 35 (23.5) | 12 (22.2) | 11 (23.9) | 12 (24.5) |
| USA | 6 (4.0) | 3 (5.6) | 1 (2.2) | 2 (4.1) |
| Tobacco use, n (%) | ||||
| Never used | 115 (77.2) | 42 (77.8) | 36 (78.3) | 37 (75.5) |
| Former user | 30 (20.1) | 10 (18.5) | 10 (21.7) | 10 (20.4) |
| Current user | 4 (2.7) | 2 (3.7) | 0 | 2 (4.1) |
| Mean ± SD days since | ||||
| COVID‐19 symptoms onset b | 10.2 ± 3.6 | 9.8 ± 3.7 | 10.3 ± 3.2 | 10.5 ± 4.0 |
| COVID‐19 diagnosis c | 4.8 ± 4.0 | 5.1 ± 5.4 | 4.2 ± 2.9 | 5.1 ± 3.0 |
| COVID‐19 symptoms, n (%) | ||||
| Cough | 129 (86.6) | 46 (85.2) | 40 (87.0) | 43 (87.8) |
| Dyspnea | 109 (73.2) | 40 (74.1) | 31 (67.4) | 38 (77.6) |
| Fever | 99 (66.4) | 33 (61.1) | 33 (71.7) | 33 (67.3) |
| Fatigue/malaise | 70 (47.0) | 23 (42.6) | 27 (58.7) | 20 (40.8) |
| Myalgia | 60 (40.3) | 24 (44.4) | 15 (32.6) | 21 (42.9) |
| Loss of appetite | 33 (22.1) | 14 (25.9) | 11 (23.9) | 8 (16.3) |
| Loss of smell | 26 (17.4) | 11 (20.4) | 9 (19.6) | 6 (12.2) |
| Diarrhea | 25 (16.8) | 9 (16.7) | 7 (15.2) | 9 (18.4) |
| Loss of taste | 24 (16.1) | 9 (16.7) | 9 (19.6) | 6 (12.2) |
Abbreviations: b.i.d., twice daily; COVID‐19, coronavirus disease 2019; SD, standard deviation.
Includes mixed race and not reported.
Defined as: date of first administration of study treatment – date of symptoms onset (which was patient‐reported).
Defined as: date of first administration of study treatment – date of diagnosis (based on testing performed by the hospital).
The mean ± SD time since COVID‐19 symptoms onset and diagnosis was 10.2 ± 3.6 and 4.8 ± 4.0 days, respectively, and there were no meaningful differences between groups (Table 1). Half (50.3%) of the patients had at least one COVID‐19 comorbidity at study entry; the most common comorbidities were hypertension (39.6%) and diabetes (23.5%) (Table S1 ). All patients received at least one concomitant medication, including systemic corticosteroids (89.3%), antithrombotic agents (87.9%), antibiotics (71.8%), and antivirals (22.8%; Table S2 ). Mean IFN‐GS scores (50 mg b.i.d. –0.84; 100 mg b.i.d. –0.74; placebo –0.91) and the proportion of patients with high IFN‐GS scores at baseline (50 mg b.i.d. 35%; 100 mg b.i.d. 43%; placebo 35%) were reasonably consistent across treatment groups.
Safety
A total of 88 (59.1%) patients reported treatment‐emergent AEs (TEAEs), the incidence of which was comparable between treatment groups (Table 2). The proportion of patients who reported treatment‐related TEAEs was numerically higher with enpatoran 50 mg (n = 8, 14.8%) than enpatoran 100 mg (n = 5, 10.9%) or placebo (n = 4, 8.2%). Both enpatoran groups (50 mg b.i.d. 3.7%; 100 mg b.i.d. 0%) had a lower incidence of Grade ≥3 TEAEs compared with the placebo group (10.2%); no patient in the enpatoran 100 mg b.i.d. group reported a TEAE of Grade ≥3. The proportion of patients in the enpatoran groups (50 mg b.i.d. 9.3%; 100 mg b.i.d. 2.2%) reporting serious TEAEs was numerically lower than the placebo group (18.4%). Two patients in the placebo group died due to AEs of sepsis and one patient in the enpatoran 50 mg b.i.d. group died due to an AE of COVID‐19 worsening; none of these were considered treatment related.
TABLE 2.
Overview of treatment‐emergent adverse events (safety analysis set).
| Total | Enpatoran 50 mg b.i.d. | Enpatoran 100 mg b.i.d. | Placebo | |
|---|---|---|---|---|
| Number (%) of patients | (N = 149) | (n = 54) | (n = 46) | (n = 49) |
| Any TEAE | 88 (59.1) | 34 (63.0) | 26 (56.5) | 28 (57.1) |
| Grade ≥3 TEAE | 7 (4.7) a | 2 (3.7) | 0 | 5 (10.2) |
| Grade ≥4 TEAE | 3 (2.0) b | 1 (1.9) | 0 | 2 (4.1) |
| Treatment‐related TEAE | 17 (11.4) | 8 (14.8) | 5 (10.9) | 4 (8.2) |
| Serious TEAE | 15 (10.1) c | 5 (9.3) | 1 (2.2) | 9 (18.4) |
| TEAE leading to death | 3 (2.0) b | 1 (1.9) | 0 | 2 (4.1) |
| TEAE leading to treatment discontinuation | 9 (6.0) d | 4 (7.4) | 1 (2.2) | 4 (8.2) |
Note: TEAEs are defined as events that start or worsen any time on or after the first dose of study treatment. TEAEs were graded according to NCI‐CTCAE Version 5.0. Events were considered to be serious TEAEs if, in the view of the investigator or sponsor, they were life‐threatening, required hospitalization (initial or prolonged), or resulted in congenital anomaly, disability, or death.
Abbreviations: b.i.d., twice daily; TEAE, treatment‐emergent adverse event.
Two were considered treatment‐related (placebo n = 1, 50 mg b.i.d. n = 1).
None were considered treatment‐related.
One (placebo group) was considered treatment‐related.
Four were considered treatment‐related (50 mg b.i.d. n = 3 [alanine aminotransferase increased n = 1, transaminases increased n = 1, mental disorder n = 1], 100 mg b.i.d. n = 1 [nausea]).
The most frequently reported TEAEs, occurring in ≥10% of patients overall, were in the System Organ Classes of gastrointestinal disorders (15.4%), investigations (14.1%), metabolism and nutrition disorders (14.1%), and infections and infestations (13.4%; Table 3). The most common TEAEs (reported by ≥5% of patients) in the enpatoran 50 or 100 mg b.i.d. groups were constipation, COVID‐19 worsening, diarrhea, hypokalemia, transaminase increase (Table 3), rash (50 mg b.i.d. 0%; 100 mg b.i.d. 6.5%; placebo 2.0%), and sinus bradycardia (50 mg b.i.d. 1.9%; 100 mg b.i.d. 6.5%; placebo 0%).
TABLE 3.
Summary of treatment‐emergent adverse events that were reported by ≥10 of patients at the System Organ Class level (safety analysis set).
| Number (%) of patients | Total | Enpatoran 50 mg b.i.d. | Enpatoran 100 mg b.i.d. | Placebo |
|---|---|---|---|---|
| (N = 149) | (n = 54) | (n = 46) | (n = 49) | |
| GI disorders | 23 (15.4) | 15 (27.8) | 4 (8.7) | 4 (8.2) |
| Constipation | 8 (5.4) | 6 (11.1) | 0 | 2 (4.1) |
| Diarrhea | 4 (2.7) | 4 (7.4) | 0 | 0 |
| Nausea | 4 (2.7) | 0 | 2 (4.3) | 2 (4.1) |
| Dyspepsia | 2 (1.3) | 1 (1.9) | 1 (2.2) | 0 |
| Hematochezia | 2 (1.3) | 2 (3.7) | 0 | 0 |
| Investigations | 21 (14.1) | 7 (13.0) | 5 (10.9) | 9 (18.4) |
| Alanine aminotransferase increased | 8 (5.4) | 1 (1.9) | 1 (2.2) | 6 (12.2) |
| Transaminases increased | 5 (3.4) | 3 (5.6) | 1 (2.2) | 1 (2.0) |
| Oxygen saturation decreased | 2 (1.3) | 0 | 0 | 2 (4.1) |
| Metabolism and nutrition disorders | 21 (14.1) | 8 (14.8) | 6 (13.0) | 7 (14.3) |
| Hypokalemia | 8 (5.4) | 3 (5.6) | 4 (8.7) | 1 (2.0) |
| Hyperglycemia | 3 (2.0) | 1 (1.9) | 1 (2.2) | 1 (2.0) |
| Hyperuricemia | 2 (1.3) | 0 | 1 (2.2) | 1 (2.0) |
| Hypocalcemia | 2 (1.3) | 1 (1.9) | 0 | 1 (2.0) |
| Infections and infestations | 20 (13.4) | 9 (16.7) | 2 (4.3) | 9 (18.4) |
| COVID‐19 worsening | 9 (6.0) | 5 (9.3) | 0 | 4 (8.2) |
| Sepsis | 2 (1.3) | 0 | 0 | 2 (4.1) |
Note: TEAEs were coded using MedDRA Version 24.0. Primary System Organ Classes in which ≥10% of patients overall reported events are included, and TEAEs that were reported by at least two patients in total are shown. Investigations included laboratory tests and other medical investigations.
Abbreviations: b.i.d., twice daily; COVID‐19, coronavirus disease 2019; GI, gastrointestinal; TEAE, treatment‐emergent adverse event.
Four patients (2.7%) reported AESIs. Two patients (3.7%) in the enpatoran 50 mg b.i.d. group had clinically significant arrythmias of intermittent tachycardia and prolonged QTc interval. Two patients (4.1%) in the placebo group experienced concurrent infection with sepsis that was not deemed to be treatment‐related and led to the death of the patients. The TEAE of intermittent tachycardia was considered to be treatment‐related. There were no cases of seizure or serotonin syndrome during the study, and no other AESIs were identified.
There were no clinically meaningful findings in vital sign measurements, electrocardiogram (ECG) measurements, or other observations related to enpatoran safety.
Efficacy
Primary outcome
The primary efficacy end point of the time to recovery from Day 1 through Day 28 was not met, despite a numerical trend towards higher recovery rates in both enpatoran groups (50 mg b.i.d. n = 48, 88.9%, p = 0.054; 100 mg b.i.d. n = 42, 91.3%, p = 0.107) compared with the placebo group (n = 37, 75.5%). The median time to recovery was similar across groups (3.4–3.9 days), with a lack of differentiation until Day 6 (Figure 2). The KM‐estimated cumulative recovery rates up to Day 14 (95% CI) were 88.7% (78.6, 95.4), 93.3% (83.6, 98.3), and 78.7% (66.2, 89.0) in the enpatoran 50 mg b.i.d., enpatoran 100 mg b.i.d., and placebo groups, respectively.
FIGURE 2.
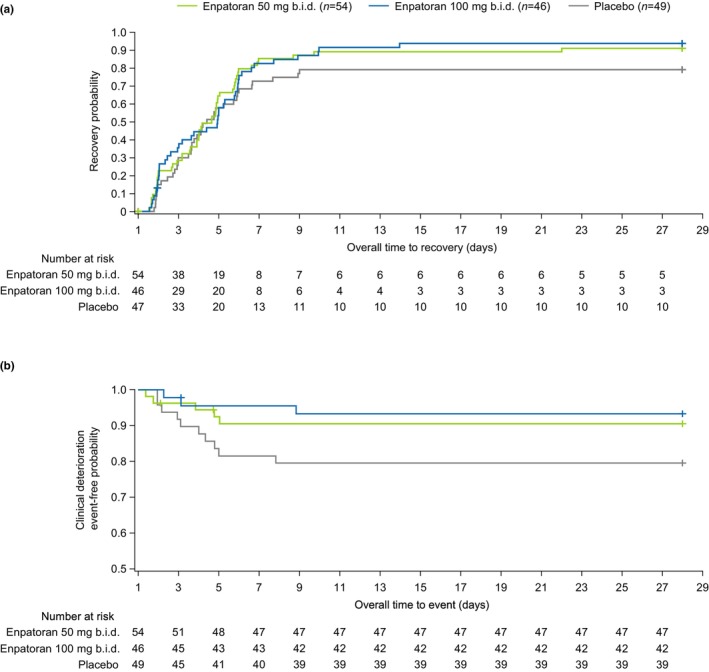
Cumulative distribution function of (a) time to recovery and (b) time to clinical deterioration. Time to recovery (the time from Day 1 to first occurrence of WHO 9‐point ordinal scale of ≤3) was not improved despite a numerical trend towards higher recovery rates in both enpatoran groups. Recovery rates from Day 1 through Day 28 were 75.5% (n = 37/49) with placebo compared with 88.9% (n = 48/54) with 50 mg b.i.d. and 91.3% (n = 42/46) with 100 mg b.i.d. (p = 0.054 and 0.107 vs. placebo, respectively). The event‐free rates for clinical deterioration (the time from Day 1 to first occurrence of WHO 9‐point ordinal scale of ≥5) were higher in the enpatoran groups compared with placebo. Clinical deterioration rates Day 1 through Day 28 were 20.4% (n = 10/49) with placebo compared with 9.3% (n = 5/54) with 50 mg b.i.d. and 6.5% (n = 3/46) with 100 mg b.i.d. (p = 0.0390 and 0.0249 vs. placebo, respectively). Kaplan–Meier curves are shown. Safety analysis set, N = 149. Two patients in the placebo group had SpO2 ≥94% at the first timepoint and were therefore not counted as at risk in the time to recovery analysis. b.i.d., twice daily.
Secondary outcomes
There was a trend towards reduced likelihood of clinical deterioration from Day 1 through Day 28 in the enpatoran groups (50 mg b.i.d. 9.3%, p = 0.0390; 100 mg b.i.d. 6.5%, p = 0.0249) versus placebo (20.4%). The cumulative KM‐estimated event‐free rates up to Day 7 were numerically higher in the enpatoran treatment groups with estimated event‐free rates (95% CI) of 90.6% (78.8, 96.0) and 95.6% (83.5, 98.9) for the enpatoran 50 mg and 100 mg b.i.d. groups, respectively, compared with 81.6% (67.7, 90.0) for the placebo group.
The proportion of patients in each WHO ordinal scale category from Day 1 to Day 60 is shown in Figure 3. Fewer disease progression events were observed with enpatoran compared to placebo. The observed proportion of patients with a score of >4 numerically increased until Day 5 in the placebo group and increased slightly less in the enpatoran 50 mg b.i.d. group, before decreasing until the end of the study. In the enpatoran 100 mg group, the observed proportion remained low and no patient had a score of ≥5 after Day 3.
FIGURE 3.
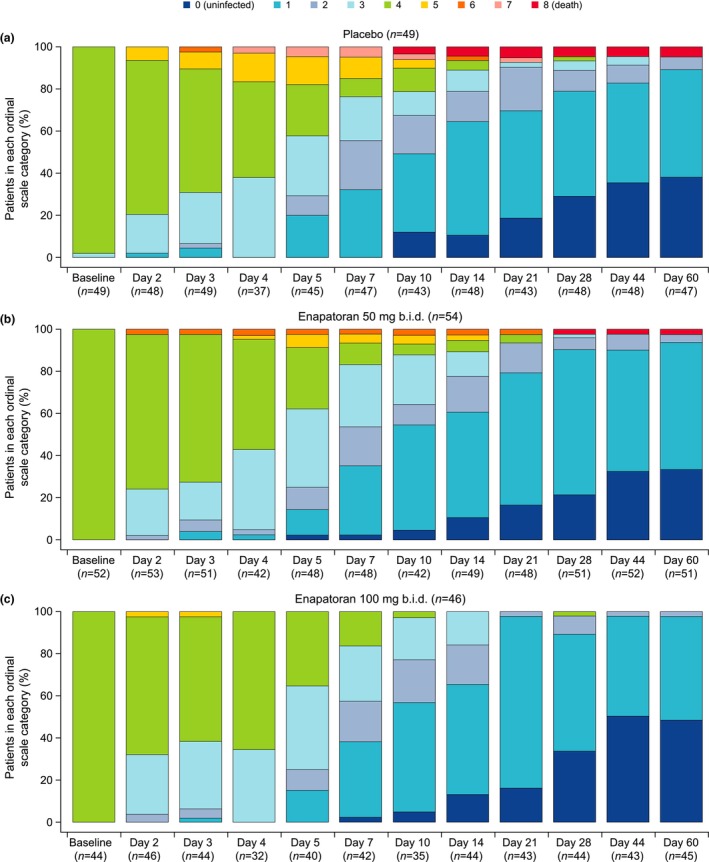
Proportion of patients in each ordinal scale category from Day 1 to Day 60 in the (a) placebo, (b) enpatoran 50 mg b.i.d., and (c) enpatoran 100 mg b.i.d. groups. Clinical score was recorded daily during hospitalization, at the time of hospital discharge, and on study visits following discharge up to Day 60, using the WHO 9‐point ordinal scale. Fewer disease progression events were observed with enpatoran compared to placebo. Safety analysis set, N = 149. Data presented as collected with no imputation of missing data. b.i.d., twice daily. Key: 0 = Uninfected; 1 = Ambulatory: no limitation of activities; 2 = Ambulatory: limitation of activities; 3 = Hospitalized, mild disease: no oxygen therapy; 4 = Hospitalized, mild disease: oxygen by mask or nasal prongs; 5 = Hospitalized, severe disease: noninvasive ventilation or high‐flow oxygen; 6 = Hospitalized, severe disease: intubation and mechanical ventilation; 7 = Hospitalized, severe disease: ventilation plus additional organ support; 8 = Death.
All‐cause mortality (95% CI) through Day 60 was 1.9% (0.3, 9.8), 0.0% (0.0, 7.7), and 4.1% (1.1, 13.7) for the enpatoran 50 mg b.i.d., enpatoran 100 mg b.i.d., and placebo groups, respectively.
Exploratory outcome
In the subgroup with high IFN‐GS scores at baseline, the KM‐estimated cumulative recovery rates up to Day 14 were numerically higher for patients who received enpatoran (50 mg b.i.d. n = 11, 78.6% [95% CI 55.2, 94.8]; 100 mg b.i.d. n = 15, 88.2% [95% CI 68.8, 98.0]) compared with those who received placebo (n = 8, 53.3% [95% CI 31.1, 78.8]; Figure 4). This was consistent to Day 28, when recovery rates were 73.3% (p = 0.031) and 88.2% (p = 0.031) with enpatoran 50 mg b.i.d. and enpatoran 100 mg b.i.d., respectively, and 53.3% with placebo.
FIGURE 4.
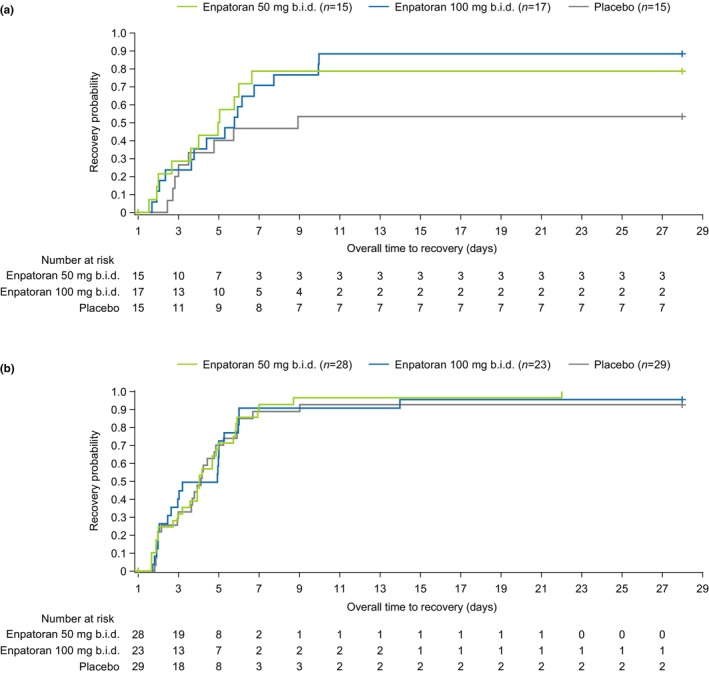
Cumulative distribution function of time to recovery for patients with (a) high and (b) low interferon‐gene signature (IFN‐GS) scores at baseline. IFN‐GS scores were evaluated at baseline using DxTerity's IFN‐I Test. The cut‐off for high and low IFN‐GS was predefined based on expression profiles in previous studies (data on file). Time to recovery was defined as the time from Day 1 to first occurrence of WHO 9‐point ordinal scale of ≤3. In the subgroup with high IFN‐GS, the cumulative recovery rates were higher for patients who received enpatoran compared to those who received placebo. From Day 1 through Day 28, recovery rates in the high IFN‐GS group were 73.3% (n = 11/15; p = 0.031) and 88.2% (n = 15/17; p = 0.031) with enpatoran 50 mg b.i.d. and enpatoran 100 mg b.i.d., respectively, and 53.3% (n = 8/15) with placebo. In the subgroup with low IFN‐GS, there was a lack of differentiation between placebo and enpatoran. From Day 1 through Day 28, recovery rates in the low IFN‐GS group were 100% (n = 28/28; p = 0.236) and 91.3% (n = 21/23; p = 0.458) with enpatoran 50 mg b.i.d. and enpatoran 100 mg b.i.d., respectively, and 86.2% (n = 25/29) with placebo. Kaplan–Meier curves are shown. High IFN‐GS score subgroup, N = 47; low IFN‐GS score subgroup, N = 80. b.i.d., twice daily.
Placebo‐treated patients in the subgroup with low IFN‐GS scores at baseline had higher recovery rates at Day 28 (n = 25, 86.2%) than those in the high IFN‐GS subgroup, and there was a lack of differentiation between placebo and enpatoran (50 mg b.i.d. n = 28, 100%, p = 0.236; 100 mg b.i.d. n = 21, 91.3%, p = 0.458).
Biomarkers
Serum concentrations of inflammatory biomarkers were assessed from baseline to Day 28 (Figure S2). The baseline concentrations of CRP (median [Q1–Q3]: 30.04 [11.40–98.02] mg/L), ferritin (993 [496–1636] μg/L) and D‐dimer (0.62 [0.39–1.01] mg/L) were above normal levels and were variable but similar across the treatment groups. Mean levels of CRP and ferritin decreased from baseline to Day 28 in all treatment groups, with no discernable differences across groups. The levels of D‐dimer were highly variable with no apparent meaningful change over time.
Pharmacokinetics
Mean plasma enpatoran concentration profiles, based on sparsely collected PK samples, increased with increasing dose (Figure S3). Mean enpatoran exposure (Cmax and Ctrough) appeared to be dose‐proportional. Comparable Cmax (42.1 and 44.0 ng/mL with 50 mg b.i.d.) and Ctrough (14.6 and 16.4 ng/mL) were observed on Days 3 and 7 after multiple b.i.d. enpatoran dosing (Table S3), indicating that enpatoran PK steady state was achieved as early as Day 3, which is consistent with the half‐life of 7–12 h demonstrated previously. 14
DISCUSSION
The primary goal of this randomized, exploratory trial was to investigate the safety profile of enpatoran in patients hospitalized with COVID‐19 pneumonia. In this population, enpatoran up to 100 mg b.i.d. for 14 days was well tolerated, while enpatoran exposure and PK parameters were generally consistent with those observed in healthy participants. 14 Trends in clinical improvement were also evaluated. Although the primary efficacy end point was not met as the time to recovery (classed as no longer requiring oxygen therapy) was not improved with enpatoran compared with placebo, trends towards improved outcomes in some secondary efficacy end points were observed, including decreased time to worsening of symptoms with enpatoran versus placebo.
The study was conducted during the early phase of the COVID‐19 pandemic, when the pre‐delta and delta variants were prevalent in participating countries and the treatment landscape was rapidly evolving. 23 The impact of enpatoran treatment on subsequent SARS‐CoV‐2 variants, which differ in the levels/severity of hyperinflammation and the predominant area of respiratory system infection, were not evaluated. Placebo‐treated patients recovered on average within 3.4 days, which was faster than originally anticipated in this study, and the 10 days observed in the remdesivir COVID‐19 trial, 21 supporting the decision to change the primary efficacy end point during the study. This may have been due to the enrollment of younger patients who had fewer comorbidities than in similar COVID‐19 trials, 21 , 24 , 25 as well as improvements in SoC including the high (89%) use of corticosteroids following the RECOVERY trial results, may have contributed to the faster than expected recovery rates in this study. 26 In addition, the patients presented late in their disease course (mean time since symptom onset was 10 days).
TLR7/8 are a key part of the first line of defense against viral infections, including the detection and clearance of SARS‐CoV‐2. TLR7 recognizes SARS‐CoV‐2 in the respiratory tract and initiates the early immune response (type I IFN) that could halt development of severe disease. 27 In addition, loss‐of‐function TLR7 mutations have been identified that are associated with severe disease in young, previously healthy men. 28 TLR7 and TLR8 have essential, non‐redundant roles in the immune response to ssRNA from viruses and their activation results in the production of proinflammatory cytokines, including IFN‐α, IL‐6, and TNF‐α. 7 , 13 , 29
Given the protective roles of TLR7/8 in the viral response phase of COVID‐19 infection, the timing of dosing with a TLR7/8 inhibitor should halt progression to the host inflammatory phase and severe immunopathology without compromising viral clearance. Therefore, patients with abnormal chest imaging who were expected to be in the pulmonary phase of COVID‐19 infection were selected. The patients had begun to progress to a hyperinflammatory state, as demonstrated by high CRP, D‐dimer, and ferritin levels, and TLR7/8 inhibition did not exacerbate COVID‐19 pneumonia, suggesting that enpatoran did not impact viral clearance, or increase the risk of development of other infections. Further, there was some evidence that TLR7/8 inhibition provided measurable treatment effects, particularly with the highest enpatoran dose tested. A numerical trend towards decreased likelihood of clinical deterioration was observed in the overall study population, and the time to recovery was numerically improved in patients with high IFN‐GS scores at baseline; a biomarker associated with certain autoimmune disorders including lupus. 30
This study has several limitations: (i) the number of patients was relatively small (particularly the IFN‐GS subgroups), although the study was powered to show an effect on the primary outcome; (ii) SoC differed across hospital sites but this was not found to impact the results; and (iii) anti‐SARS‐CoV‐2 antibody titers were not measured at study entry or prospectively, which may have provided greater insight into patients' immune responses and treatment outcomes. The patients recovered quickly on average, therefore, the results are not generalizable to all populations of hospitalized patients. All statistical analyses were considered exploratory and there was no adjustment for multiplicity.
CONCLUSIONS
ANEMONE was the first trial to evaluate the safety and efficacy of a TLR7/8 inhibitor in an infectious disease. Enpatoran treatment up to 100 mg b.i.d. for 14 days was well tolerated by hospitalized patients acutely ill with COVID‐19 pneumonia and although the primary efficacy end point was not met, potential beneficial effects on prevention of disease progression were observed, supporting further evaluation of enpatoran in patients with hyperinflammation due to infection or autoimmunity. The results do not raise any safety concerns that treatment with enpatoran might put patients at higher risk of adverse effects, including worsening of COVID‐19 pneumonia.
AUTHOR CONTRIBUTIONS
J.E.M., D.Y., F.M., L.K.‐S., J.Sh., and A.H.K. designed the research. J.Sa. and C.M.d.O. performed the research. M.K., F.M., L.K.‐S., J.Sh., S.R., and A.H.K. analyzed the data. J.E.M., J.Sa., C.M.d.O., D.Y., M.K., F.M., L.K.‐S., J.Sh., S.R., and A.H.K. wrote the manuscript.
FUNDING INFORMATION
This study was sponsored by the healthcare business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945), which funded medical writing support by Bioscript Group.
CONFLICT OF INTEREST STATEMENT
F.M., J.Sh., and A.H.K. are employees of EMD Serono, L.K.‐S. is an employee of the healthcare business of Merck KGaA, Darmstadt, Germany, and S.R. is an employee of Ares Trading SA, Eysins, Switzerland, an affiliate of Merck KGaA, Darmstadt, Germany. D.Y. and M.K. were employees of EMD Serono and Merck Serono Ltd., Feltham, UK, an affiliate of Merck KGaA, Darmstadt, Germany, respectively, at the time of the study. J.E.M. has served as a consultant for EMD Serono.
Supporting information
Data S1
ACKNOWLEDGMENTS
We would like to thank those who took part in the ANEMONE trial, including the patients, study investigators, and study team (See Appendix S1 in the Supporting Information for a list of principal investigators and study team members.) We thank Shinji Okitsu, Bharat Vaidyanathan, Julie DeMartino, and Elizabeth Adams (who were all employed by EMD Serono at the time of the study) for their contributions to the planning and execution of the study. Medical writing support was provided by Samantha Lommano, PhD, of Bioscript Group Ltd., Macclesfield, UK.
McKinnon JE, Santiaguel J, Murta de Oliveira C, et al. Enpatoran in COVID‐19 pneumonia: Safety and efficacy results from a phase II randomized trial. Clin Transl Sci. 2023;16:2640‐2653. doi: 10.1111/cts.13658
Contributor Information
John E. McKinnon, Email: mckinjoh@musc.edu.
the ANEMONE Study Team:
Richard Barbers, Roland El Ghazal, Srikanth Ramachandruni, Marcus Zervos, Benjamin De La Rosa, Marina Andrade Lima, Valeria Cristina Aguiar, Caroline Candida, Felipe Dal Pizzol, Antonio de Faria Freire, Ivan Silva Marinho, Victor Augusto Hamamoto Sato, Tamara Newman Lobato Souza, Arnold Germar, Lawrence O. Raymond, Joel M. Santiaguel, Pavankumar Bhagat, Zaida Rodriguez Docampo, Katrin Zaragoza Dörr, Janice Englund, Marco Antonio Ferraz Nogueira Filho, Marcela Garrot, Amy Kao, Jean‐Pierre Labaune, Melanie Knuth, Noelia Martinez‐Lopez, Thomas Mondrup, Zuzana Murgasova, Christopher Rojik, Joerg Ruoff, Simone Schicker, Beate Schneider‐Olt, Angela Thomas, Erik Thomas, Özkan Yalkinoglu, and Marie Yeager
REFERENCES
- 1. Jiang Y, Rubin L, Peng T, et al. Cytokine storm in COVID‐19: from viral infection to immune responses, diagnosis and therapy. Int J Biol Sci. 2022;18:459‐472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xia H, Cao Z, Xie X, et al. Evasion of type I interferon by SARS‐CoV‐2. Cell Rep. 2020;33:108234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lei X, Dong X, Ma R, et al. Activation and evasion of type I interferon responses by SARS‐CoV‐2. Nat Commun. 2020;11:3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Eijk LE, Binkhorst M, Bourgonje AR, et al. COVID‐19: immunopathology, pathophysiological mechanisms, and treatment options. J Pathol. 2021;254:307‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. de la Rica R, Borges M, Gonzalez‐Freire M. COVID‐19: in the eye of the cytokine storm. Front Immunol. 2020;11:558898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krieg AM, Vollmer J. Toll‐like receptors 7, 8, and 9: linking innate immunity to autoimmunity. Immunol Rev. 2007;220:251‐269. [DOI] [PubMed] [Google Scholar]
- 7. Bender AT, Tzvetkov E, Pereira A, et al. TLR7 and TLR8 differentially activate the IRF and NF‐kappaB pathways in specific cell types to promote inflammation. Immunohorizons. 2020;4:93‐107. [DOI] [PubMed] [Google Scholar]
- 8. Fillatreau S, Manfroi B, Dörner T. Toll‐like receptor signalling in B cells during systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17:98‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lood C, Arve S, Ledbetter J, Elkon KB. TLR7/8 activation in neutrophils impairs immune complex phagocytosis through shedding of FcgRIIA. J Exp Med. 2017;214:2103‐2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bagheri‐Hosseinabadi Z, Rezazadeh Zarandi E, Mirabzadeh M, Amiri A, Abbasifard M. mRNA expression of toll‐like receptors 3, 7, 8, and 9 in the nasopharyngeal epithelial cells of coronavirus disease 2019 patients. BMC Infect Dis. 2022;22:448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naqvi I, Giroux N, Olson L, et al. DAMPs/PAMPs induce monocytic TLR activation and tolerance in COVID‐19 patients; nucleic acid binding scavengers can counteract such TLR agonists. Biomaterials. 2022;283:121393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li Y, Chen M, Cao H, Zhu Y, Zheng J, Zhou H. Extraordinary GU‐rich single‐strand RNA identified from SARS coronavirus contributes an excessive innate immune response. Microbes Infect. 2013;15:88‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vlach J, Bender AT, Przetak M, et al. Discovery of M5049: a novel selective TLR7/8 inhibitor for treatment of autoimmunity. J Pharmacol Exp Ther. 2020;376:397‐409. [DOI] [PubMed] [Google Scholar]
- 14. Port A, Shaw JV, Klopp‐Schulze L, et al. Phase 1 study in healthy participants of the safety, pharmacokinetics and pharmacodynamics of enpatoran (M5049), a dual antagonist of toll‐like receptor 7 and 8. Pharmacol Res Perspect. 2021;9:e00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sherer B, Bender AT, Pereira A, et al. O37 M5049, a novel potent and selective inhibitor of toll‐like receptors 7 and 8 (TLR 7/8). Lupus Sci Med. 2020;7:A28. [Google Scholar]
- 16. Klopp‐Schulze L, Shaw JV, Dong JQ, Khandelwal A, Vazquez‐Mateo C, Goteti K. Applying modeling and simulations for rational dose selection of novel toll‐like receptor 7/8 inhibitor enpatoran for indications of high medical need. Clin Pharmacol Ther. 2022;112:297‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gopalakrishnan S, Krebs‐Brown A, Nogueira Filho M, et al. POS0755 Safety, tolerability, pharmacokinetics, and pharmacodynamics of a single orally administered dose of enpatoran in a phase I study of healthy Japanese and Caucasian participants. Ann Rheum Dis. 2022;81(Suppl. 1):663‐664. [Google Scholar]
- 18. The Belmont Report . Ethical principles and guidelines for the protection of human subjects of research. J Am Coll Dent. 2014;81:4‐13. [PubMed] [Google Scholar]
- 19. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20:e192‐e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — final report. N Engl J Med. 2020;383:1813‐1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Broman N, Rantasärkkä K, Feuth T, et al. IL‐6 and other biomarkers as predictors of severity in COVID‐19. Ann Med. 2021;53:410‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. World Health Organization . Tracking SARS‐CoV‐2 variants. 2022. Accessed November 3, 2022. http://who.int/activities/tracking‐SARS‐CoV‐2‐variants
- 24. Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID‐19 (COV‐BARRIER): a randomised, double‐blind, parallel‐group, placebo‐controlled phase 3 trial. Lancet Respir Med. 2021;9:1407‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid‐19. N Engl J Med. 2020;384:795‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The RECOVERY Collaborative Group . Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2020;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asano T, Boisson B, Onodi F, et al. X‐linked recessive TLR7 deficiency in ~1% of men under 60 years old with life‐threatening COVID‐19. Sci Immunol. 2021;6:eabl4348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xavier S, Vargas‐Parra G, van der Made CI, et al. Genetic screening for TLR7 variants in young and previously healthy men with severe COVID‐19. Front Immunol. 2021;12:719115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mohammad HA, Majidi J, Baradaran B, Yousefi M. Toll‐like receptors in the pathogenesis of autoimmune diseases. Adv Pharm Bull. 2015;5:605‐614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sinicato NA, Postal M, Appenzeller S, Niewold TB. Defining biological subsets in systemic lupus erythematosus: progress toward personalized therapy. Pharm Med. 2017;31:81‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


