Abstract
Lidocaine is classified as a class Ib anti‐arrhythmic that blocks voltage‐ and pH‐dependent sodium channels. It exhibits well investigated anti‐arrhythmic effects and has been the anti‐arrhythmic of choice for the treatment of ventricular arrhythmias for several decades. Lidocaine binds primarily to inactivated sodium channels, decreases the action potential duration, and increases the refractory period. It increases the ventricular fibrillatory threshold and can interrupt life‐threatening tachycardias caused by re‐entrant mechanisms, especially in ischemic tissue. Its use was pushed into the background in the era of amiodarone and modern electric device therapy. Recently, lidocaine has come back into focus for the treatment of acute sustained ventricular tachyarrhythmias. In this brief overview, we review the clinical pharmacology including possible side effects, the historical course, possible indications, and current Guideline recommendations for the use of lidocaine.
INTRODUCTION
Lidocaine was synthesized as a local anesthetic in 1943 1 by Loefgren and initially administered as an anti‐arrhythmic drug by Southworth et al. 2 Over the last 70 years, the indication has undergone many changes – once the drug of choice for ventricular arrhythmias (VAs), the indication has increasingly receded into the background. Recently, lidocaine has come back into focus for the treatment of acute sustained VA. 3 Are there arguments for a renaissance of the drug in the clinical setting of VA? In this brief overview, we review the clinical pharmacology including possible side effects, the historical course, possible indications, and actual recommendations for the use of lidocaine.
HISTORY OF THE USE OF LIDOCAINE AS AN ANTI‐ARRHYTHMIC DRUG
Lidocaine was first synthetized in 1943 4 as an anesthetic agent and first used in 1950 as an anti‐arrhythmic drug. 1 , 5 Up to the late 1980s, it has been the anti‐arrhythmic of choice for VA, mostly due to lack of alternatives. There was a theoretical and experimental basis for its routine use. In 1988, MacMahon et al. 6 published an overview of results from randomized, controlled trials investigating the effectiveness of lidocaine. In summary, lidocaine was associated with a reduction of ventricular fibrillation (VF) of about one third, but there was a lack of evidence for any beneficial effect on mortality. In addition, the analyzed trials had small numbers of reported events and relatively short follow‐up periods. 6 Reports on side effects, especially sinus arrest, also led to questioning the use of lidocaine. 7 The use of external defibrillators was not widespread up to the late 1970s. However, with improving defibrillation technology their use became more common. Due to an increase in lidocaine's affinity to sodium channel receptors in an acidotic environment, lidocaine may increase defibrillation thresholds. Therefore, lidocaine's effects on defibrillation were questioned because of a possible reduction of shock efficacy of ventricular tachyarrhythmias.
CLINICAL PHARMACOLOGY AND EXPERIMENTAL DATA
Lidocaine blocks voltage‐ and pH‐dependent sodium channels which results in a decrease in conduction velocity. 8 The electrophysiological effects have been studied extensively in experimental studies and are summarized as follows: lidocaine has got dose‐dependent effects on the automaticity of pacemaker cells as it decreases automaticity by slowing the rate of spontaneous phase 4 depolarization. 9 It prolongs the effective refractory period relatively compared to the action potential duration. Sodium channels have got three different states: a closed conformation (resting), an open conformation (activated), and a non‐conduction conformation (inactivated). The resting state occurs during phase 4 of the action potential. Membrane depolarization opens the activation gate and sodium ions move into the cell (phase 0), the inward sodium movement is terminated with maintained depolarization and closing of the inactivation gate. As the membrane potential repolarizes during phase III, the cell is refractory. At the end of phase III, the sodium channels begin to transition back into their resting state. 10 Lidocaine binds, not exclusively, but primarily to inactivated sodium channels and therefore effects the action potential duration. Because of this blockade, the recovery of the fast sodium channels is prolonged and by that the effective refractory period is increased. Of note, lidocaine exerts a negligible effect on voltage‐gated potassium channels. 11 In summary, lidocaine binds primarily to inactivated sodium channels, decreases action potential duration, and increases the refractory period (Figure 1). Decreasing the action potential duration and increasing the effective refractory period can interrupt tachycardias caused by re‐entrant mechanisms. 12 Besides, the ventricular fibrillatory threshold is increased at therapeutic perfusion concentrations, in acutely ischemic hearts at even lower doses. The therapeutic levels in humans range between 1.5 and 5 μg/mL. 9 According to experimental data, the most prominent effect is noted on Purkinje fibers, followed by ventricular cells. The effect on atrial tissue is rather low and seen only at higher levels of perfusion concentration. 13
FIGURE 1.
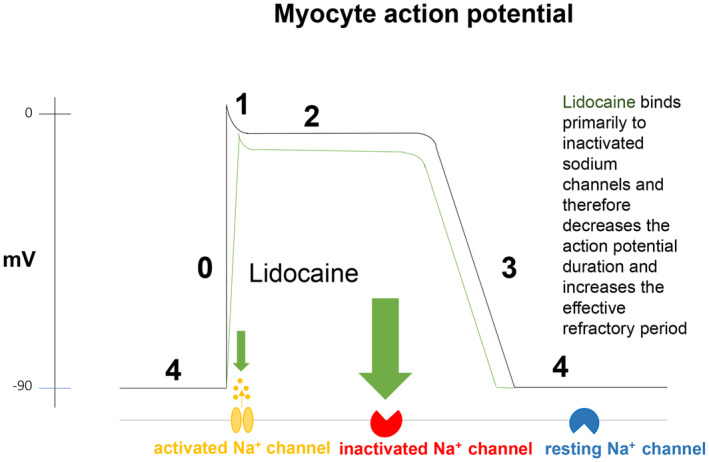
Cellular mechanisms of lidocaine.
Pharmacokinetics
The plasma concentration of lidocaine can be described in a biphasic curve model that can be fitted in two exponential components: early fall, followed by a later slower decrease in plasma concentration. The first rapid phase is due to changes in distribution of lidocaine within the two compartments. The first compartment is the central compartment, including the intravascular space, and the second compartment is the peripheral compartment. 14 Approximately 70% of lidocaine is extracted in the liver. The second slow phase is dependent on the slower net transfer of drug from the larger peripheral compartment to the smaller central compartment. VA have been observed to return within 15 to 20 min after a lidocaine injection and a constant infusion alone may not provide effective blood levels in life‐threatening VA. 15 Thus, the clinical approach is to give a bolus dose with constant infusion simultaneously. To maintain therapeutic blood levels, a bolus of 1–2 mg/kg is recommended, followed by a 55 μg/kg/min infusion. Because lidocaine is mainly eliminated via the liver and the hepatic blood flow is related to the cardiac output, the dosing should be adjusted in special populations. In acute myocardial infarction with moderately to severely reduced cardiac output, the initial injection should, for example, be 1.5 mg/kg, followed by a 30 μg/kg/min infusion. Patients with cardiogenic shock should be managed with an initial injection of 0.75 mg/kg, followed by 10–20 μg/kg/min. In this patient group, monitoring of lidocaine plasma levels is highly recommended. 16 The average half‐life of lidocaine is about 8 to 17 min for the early rapid fall and the half‐life for the slow decrease is about 90 to 110 min. After discontinuing an infusion at a steady‐state level, the dominant elimination half‐life is ~1.5 to 2 h. 17 This implies that reduction of possible toxic effects may take several hours. On the other hand, reaching higher plasma levels requires repetitive bolus injection, for example, in case of an incessant ventricular tachyarrhythmia. 9 , 14 , 18
Effects on atrial tissue
Several experimental data demonstrated that lidocaine is most effective on Purkinje fibers in the presence of ischemia. Kostis et al. 19 investigated the effects of lidocaine (applicated in a dosage of 2 and 3 mg/kg) on the threshold of atrial fibrillation in anesthetized dogs. Lidocaine confirmed effects on atrial tissue, but only on higher than usual doses of 3 mg/kg, which on the other hand were accompanied by a higher rate of side effects. Due to well‐established alternatives, lidocaine's use on atrial tachyarrhythmias is therefore not recommended 20 and it appears no longer in the European Society of Cardiology (ESC) and American Heart Association (AHA) guidelines for the treatment of atrial fibrillation. 21 , 22
Effects on ischemic tissue
Several studies demonstrated that the effect on the maximum rate of depolarization and membrane responsiveness is not only dose‐dependent but also depends of the extracellular potassium concentration. 13 , 23 , 24 At physiological potassium concentrations, the maximum rate of depolarization and membrane responsiveness is decreased in the presence of recommended therapeutic lidocaine concentrations. In hypokalemia, up to tenfold of the recommended lidocaine concentrations are needed due to hyperpolarization of the cell membrane. 23 , 25 During acute ischemia there is an anaerobic metabolism with intracellular and interstitial acidosis and potassium efflux leading to increase in extracellular potassium. These effects result in progressive loss of resting membrane potential, reduction of action potential upstroke velocity and refractoriness, and a transient increase followed by a decrease in excitability leading to heterogeneities in refractoriness. This may consequently enable re‐entrant mechanisms. In addition, Purkinje fibers surviving in an infarcted area may give rise to abnormal automaticity. The release of endogenous catecholamine may also trigger activity secondary to delayed afterdepolarizations. In this setting, lidocaine binds to sodium channels, inactivates them during a low membrane potential of the plateau phase, and dissociates from channel receptors after repolarization. The metabolic effects of ischemia with increased potassium levels lead to an increase of the affinity of lidocaine to sodium channels and the acidosis potentiates the action of lidocaine. 26
Effects on the atrioventricular node and intraventricular conduction time
In experimental animal studies, no significant changes on the conduction time were demonstrated with therapeutic doses between 1 and 2 mg/kg. In the presence of higher doses of 5–20 mg/kg, an increased atrioventricular node conduction time in dogs was reported. 27 , 28 Weaver et al. 29 observed a 25% occurrence rate of asystole with repeated lidocaine administration for VF after a first defibrillation shock. In a randomized trial, Haynes et al. 30 reported an increased incidence of asystole after the administration of lidocaine compared with bretylium, a potent potassium channel blocker. The respective drug was administered after the initial shock unless the post shock rhythm was asystole. Possible mechanisms of asystole after lidocaine administration include decreases in conduction and automaticity and the simultaneous use of other cardio depressant drugs. 31 Mechanisms other than direct electrophysiologic depression are also discussed: In anesthetized dogs, Aidonidis et al. 32 demonstrated that lidocaine induced asystole after defibrillation was associated with decrease in quantitative postganglionic cardiac sympathetic nerve activity. Previous studies have also shown that defibrillation in the absence of lidocaine was associated with enhanced activation of the sympathetic nervous system. 33
Hemodynamic effects
In animal experiments, lidocaine depresses ventricular contractility. Rapidly applied doses of 4 and 8 mg/kg produced dose‐dependent, significant transient depression of ventricular contractility, arterial pressure, heart rate, and cardiac output in anesthetized dogs with myocardial infarction. In contrast, given continuous infusion, the circulatory changes are described as minimal and bolus doses of lidocaine up to 2 mg/kg were shown to be safe with minimal changes in arterial pressure or heart rate. 34 , 35 , 36
Effects on defibrillation
Chow et al. 37 investigated the effect of lidocaine (2 mg/kg) and bretylium (5 mg/kg) on defibrillation thresholds in anesthetized dogs undergoing cardiopulmonary resuscitation (CPR). In these experiments, lidocaine acutely elevated the defibrillation threshold. Echt et al. 38 investigated the pH dependence of lidocaine on internal defibrillation energy requirements in dogs and demonstrated that the effects of increasing the energy required for termination of ventricular defibrillation were exacerbated in acidosis. 38 Therefore, the use of lidocaine in the presence of ischemia‐related acidosis may have unwanted effects on shock efficacy.
CLINICAL DATA
Regarding prophylactic use of lidocaine on VA, lidocaine suppressed ventricular ectopy in the setting of acute myocardial infarction in a historic meta‐analysis of clinical studies. 39 Of note, in contrast to, for example, flecainide it does not alter QRS duration in sinus rhythm. In a double‐blinded randomized study in 1974, it was shown that the effectiveness was related to the dosage used; the use of a high dosage of 3 mg/min preceded by an i.v. bolus of 100 mg was effective in preventing VF but was also associated with high rates of side effects in 15% in the patients receiving lidocaine in that dosage, more common in the older age group (>60 years). Of note, patients older than 69 years were excluded. 40 There is a lack of randomized studies investigating the effect of prophylactic use of lidocaine on mortality. In contrast, Cairns et al. 41 reported in the randomized, double‐blinded CAMIAT trial a decrease in mortality in patients with myocardial infarction with non‐sustained ventricular tachycardia (VT) or premature ventricular complexes with the use amiodarone. Yoshie et al. 42 investigated the role of lidocaine on refractory VA in a retrospective study. This study suggested that combining lidocaine and amiodarone might be effective in terminating refractory ventricular tachyarrhythmias in patients with reduced cardiac output. Forty‐four percent of the 42 studied patients were effectively treated with lidocaine, 28.6% of these were already treated with amiodarone at the start of the lidocaine therapy. With or without amiodarone, the left ventricular function was higher in the effective group. In conclusion, in patients with preserved cardiac output, the administration of lidocaine even without amiodarone was suggested. In a recent, randomized, double‐blinded trial amiodarone, lidocaine, and placebo along with standard care were investigated in adults with out‐of‐hospital cardiac arrest, shock‐refractory ventricular defibrillation or pulseless VT with regard to survival to hospital discharge, and favorable neurologic function at discharge. 43 Both anti‐arrhythmic drugs failed to increase long‐term survival or survival with favorable neurological outcome. However, administration of lidocaine (60–180 mg) compared to placebo showed a higher level of survival at hospital admission without an effect on hospital discharge. Amiodarone compared to lidocaine showed no difference in survival to hospital admission and discharge (Table 1).
TABLE 1.
Clinical studies of role of lidocaine in cardiac arrest.
| Design | End points | Results | |
|---|---|---|---|
| Wagner et al. 2022 60 |
Retrospective cohort study, 14,630 patients were enrolled. Patients receiving amiodarone or lidocaine for VF/VT in‐hospital cardiac arrest refractory to CPR were analyzed. |
Primary end point: ROSC, secondary outcomes: 24 h survival, survival to hospital discharge and favorable neurologic outcome | Compared with amiodarone, lidocaine was associated with significantly higher odds of ROSC (OR: 1.15), 24 h survival (OR: 1.16), survival to discharge (OR: 1.19) |
| Kudenchuk et al. 2016 43 |
Randomized, double‐blind trial, 3026 patients were enrolled. In patients with out‐of‐hospital cardiac arrest, shock‐refractory VF or pulseless VT after at least one shock, amiodarone, lidocaine, and placebo were compared. |
Primary end point: Survival to hospital discharge. Secondary end point: Favorable neurologic function | Amiodarone had a survival rate compared to placebo by 3.2 percentage points. For lidocaine versus placebo, the difference survival rate was 2.6 percentage points. For amiodarone versus lidocaine, the difference survival rate was 0.7 percentage points. None of the differences were statistically significant. Neurologic outcome at discharge was similar in the three groups. |
| Yoshie et al. 2014 42 |
Retrospective cohort study, 42 patients were enrolled. Clinical data of patients receiving lidocaine for the treatment of VF/VT were analyzed. |
Primary end point: Effectiveness in terminating refractory ventricular arrhythmias | LVEF was significantly higher in the effective group (51 ± 16% vs. 32 ± 9%). Regardless of the LVEF, combination of amiodarone and lidocaine was more effective than admission of lidocaine only. |
| Kudenchuck et al. 2013 61 | Retrospective cohort study, 1721 patients were enrolled. Patients with witnessed out‐of‐hospital‐cardiac‐arrest and VT/VF who did or did not receive prophylactic lidocaine at first ROSC were analyzed. | Primary end point: Frequency of re‐arrest from recurrent VF/VT after initial ROSC, admission alive to hospital, survival to hospital discharge | Prophylactic lidocaine was associated with reduced odds of re‐arrest from VF/VT (OR: 0.34) and from nonshockable arrhythmias (0.47), a higher hospital admission rate (1.88) and improved survival to discharge (1.49). |
| Shiga et al. 2010 62 |
Prospective, observational study, 55 patients were enrolled. Patients with in‐hospital VF or VT resistant to at least two shocks were analyzed after participating hospitals were pre‐registered either to nifekalant or lidocaine. |
Primary end point: Termination of VF or VT with/without additional shock. Secondary endpoints: ROSC, 1‐month survival, and survival to hospital discharge |
Patients with nifekalant therapy showed significantly higher termination rates of VF or VT as compared with patients treated with lidocaine (OR: 3.8). There was no difference in 1‐month survival between the two groups. There was a higher incidence of asystole with lidocaine (7 of 28 patients) than with nifekalant (0 of 27 patients). |
| Rea et al. 2006 63 | Multicenter retrospective study, 194 patients were enrolled. Hospitalized patients who received amiodarone, lidocaine, or a combination for pulseless VT/VF were analyzed. | Primary end point: Proportion of patients alive 24 h post‐cardiac arrest |
Among the lidocaine group, the amiodarone‐group, and the combination‐group, there were no differences in the proportion of patients alive 24 hs post‐cardiac arrest (p = 0.39). The likelihood of survival in patients who received amiodarone was decreased as compared with lidocaine. |
| Dorian et al. 2002 64 |
Randomized, double‐blinded study, 347 patients were enrolled. Patients with out‐of‐hospital VF resistant to three shocks, i.v. epinephrine, and a further shock, or recurrent VF after initially successful defibrillation, were assigned to receive amiodarone + lidocaine placebo or i.v. lidocaine + amiodarone placebo. |
Primary end point: Proportion of patients who survived to be admitted to the hospital | 22.8% of the patients treated with amiodarone survived to hospital admission as compared with 12% of the patients treated with lidocaine (p = 0.009) |
| Herlitz et al. 1997 65 |
Retrospective cohort study, 1212 patients were enrolled. Patients with out‐of‐hospital‐cardiac arrest found in VF, with and without lidocaine treatment, were analyzed. |
Primary end point: Survival to hospital discharge | In case of sustained VF, as well as after conversion to a pulse‐generating rhythm, patients treated with lidocaine had a higher rate of ROSC and were more likely to hospitalized alive (p < 0.01 for ROSC and being hospitalized alive). The proportion of patients being discharged did not significantly defer between the lidocaine and the no lidocaine groups. |
Abbreviations: CPR, cardiopulmonary resuscitation; LVEF, left ventricular ejection fraction; OR, odds ratio; ROSC, return of spontaneous circulation; VF, ventricular fibrillation; VT, ventricular tachycardia.
ROLE OF LIDOCAINE IN CURRENT INTERNATIONAL GUIDELINES
In current ESC, AHA/American College of Cardiology (ACC)/ Heart Rhythm Society (HRS), and Canadian Cardiovascular Society (CCS) guidelines for the management of patient with VA recommendations for the use of lidocaine differ 44 , 45 but similar to mexiletine 46 the role of lidocaine is very limited (Table 2): The recent ESC Guideline 47 considers lidocaine solely as second‐line therapy for the treatment of VA associated with an acute coronary syndrome (class IIb). In addition, the 2017 AHA/ACC/HRS 48 guideline gives a class IIa recommendation for the use of lidocaine in case of a witnessed cardiac arrest due to VF or polymorphic VT that is unresponsive to CPR, defibrillation, and vasopressor therapy. Similarly, the 2020 CCS/Canadian Heart Rhythm Society (CHRS) 49 position statement suggests a strong recommendation for the use of lidocaine in patients with shock refractory VT/VF or recurrent polymorphic VT/VF and additionally considers lidocaine as second‐line alternative to procainamide for the acute treatment of stable monomorphic VT in patients with structural heart disease. All guidelines agree on initial bolus followed by continuous maintenance infusion or repeat bolus in case of shock refractory arrhythmias, although recommended doses differ. 47
TABLE 2.
Indications for the use of lidocaine as an antiarrhythmic drug in the current international guidelines.
| 2022 ESC guideline | 2017 AHA/ACC/HRS guideline | 2020 CCS/CHRS position statement | |
|---|---|---|---|
| General indication | Polymorphic VT/VF associated with ACS | VT/VF | monomorphic VT, polymorphic VT/VF |
| Specific recommendations for the use of i.v. lidocaine (class of recommendation) | Recurrent PVT/VF not responding to beta‐blockers or amiodarone, or if amiodarone is contraindicated during the acute phase of ACS (II b) |
|
|
| Dose (i.v.) | 50–200 mg bolus, then 2–4 mg/min |
1 mg/kg bolus, 1–3 mg/min 1–1.5 mg/kg, repeat 0.5–0.75 mg/kg bolus every 5–10 min (max. cumulative dose 3 mg/kg) Maintenance infusion is (0.5)‐1–4 mg/min |
Shock refractory VT/VF: 100 mg bolus (50 mg if <45 kg) with a subsequent dose of 50 mg bolus in the event of failure of another shock Acute treatment of sustained monomorphic VT: 1 mg/kg bolus, followed by 1–2 mg/min infusion |
| Recommendations for pregnancy | Use only if potential benefit outweighs potential risks | – | – |
Abbreviations: ACC, American College of Cardiology; ACS, American Community Survey; AHA, American Heart Association; CCS, Canadian Cardiovascular Society; CHRS, Canadian Heart Rhythm Society; ESC, European Society of Cardiology; HRS, Heart Rhythm Society; PVT, portal vein thrombosis; SHD, structural heart disease; VF, ventricular fibrillation; VT, ventricular tachycardia.
SIDE EFFECTS OF LIDOCAINE
Side effects mostly occur when plasma concentrations rise to toxic levels. They mainly include effects on the central nervous system and on intraventricular conduction. Sinus bradycardia may be further slowed or induced, and sinus arrest related to lidocaine is discussed in several case reports. 6 , 50 , 51 , 52 Focal and grand mal seizures, psychosis, respiratory arrests, drowsiness, decreased hearing, paraesthesia, disorientation, muscle twitching, and agitation are additional side effects. 53 The metabolites of lidocaine (N‐dealkylation metabolites, monoethylglycerine‐xylidide, and glycine‐xylidide) may be responsible for central nervous system symptoms. 54 , 55 The therapy of these neurological side effects consists of lidocaine withdrawal and therapy with sedatives, for example, barbiturates and/or diazepam. An increased incidence of lidocaine toxicity, including central nervous system disturbances, is seen in patients with severe liver disease and congestive heart failure as the clearance of lidocaine is limited by hepatic blood flow, resulting in elevated blood concentrations. Thus, depressed cardiac output with the consequence of reduced hepatic blood flow in patients with acute myocardial infarction must also be considered while applying lidocaine. 15
CONCLUSION
Lidocaine has got well investigated anti‐arrhythmic effects, especially on ischemic tissue. It has been the anti‐arrhythmic of choice for the treatment of VA in acute myocardial infarction for several decades. According to the Vaughan Williams classification, it is classified as a class Ib anti‐arrhythmic drug 56 that blocks voltage‐ and pH‐dependent sodium channels, resulting in decreased conduction. The ventricular fibrillatory threshold is increased. Tachycardias caused by re‐entrant mechanisms can be interrupted by decreasing action potential duration and increasing the effective refractory period. This effect is most prominent in the presence of myocardial ischemia. Due to controversial experimental and clinical data, reporting both efficacy 39 , 57 , 58 and ineffectiveness 59 of lidocaine in preventing life‐threatening VA during acute myocardial infarction, the use of lidocaine has declined and the era of amiodarone has emerged at the same time. As pointed out by MacMahon et al., 6 in an overview of randomized, controlled trials of prophylactic lidocaine use in suspected myocardial infarction, “there was no evidence of any beneficial effect on early mortality.” Thus, a prophylactic use of lidocaine is no longer recommended. However, lidocaine has returned as an alternative to amiodarone or as second‐line therapy for refractory VT. But more trials are needed to investigate the role of lidocaine in the era of amiodarone and modern electric device therapy.
FUNDING INFORMATION
No funding was received for this work.
CONFLICT OF INTEREST STATEMENT
The authors declared no competing interests for this work.
ACKNOWLEDGMENTS
Open Access funding enabled and organized by Projekt DEAL.
Güler S, Könemann H, Wolfes J, et al. Lidocaine as an anti‐arrhythmic drug: Are there any indications left? Clin Transl Sci. 2023;16:2429‐2437. doi: 10.1111/cts.13650
REFERENCES
- 1. Lidocaine GT. The origin of a modern local anesthetic. 1949. Anesthesiology. 2010;113(6):1433‐1437. [DOI] [PubMed] [Google Scholar]
- 2. Southworth JL, McKusick VA, Pierce EC, Rawson FL. Ventricular fibrillation precipitated by cardiac catheterization; complete recovery of the patient after 45 minutes. JAMA. 1950;143(8):717‐720. [DOI] [PubMed] [Google Scholar]
- 3. Panchal AR, Berg KM, Kudenchuk PJ, et al. American Heart Association focused update on advanced cardiovascular life support use of antiarrhythmic drugs during and immediately after cardiac arrest: an update to the American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2018;138(23):e740‐e746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Löfgren N. Studies on Local Anesthetics: Xylocaine: A New Synthetic Drug. Hæggströms Boktr; 2012. [Google Scholar]
- 5. Southworth JL. Ventricular fibrillation precipitated by cardiac catheterization. JAMA. 1950;143(8):717‐720. [DOI] [PubMed] [Google Scholar]
- 6. MacMahon S, Collins R, Peto R, Koster RW, Yusuf S. Effects of prophylactic lidocaine in suspected acute myocardial infarction. An overview of results from the randomized, controlled trials. JAMA. 1988;260(13):1910‐1916. [PubMed] [Google Scholar]
- 7. Wesley RC, Resh W, Zimmerman D. Reconsiderations of the routine and preferential use of lidocaine in the emergent treatment of ventricular arrhythmias. Crit Care Med. 1991;19(11):1439‐1444. [DOI] [PubMed] [Google Scholar]
- 8. Bekheit S, Murtagh JG, Morton P, Fletcher E. Effect of lignocaine on conducting system of human heart. Heart. 1973;35(3):305‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Collinsworth KA, Kalman SM, Harrison DC. The clinical pharmacology of lidocaine as an antiarrhythymic drug. Circulation. 1974;50(6):1217‐1230. [DOI] [PubMed] [Google Scholar]
- 10. Grant AO, Starmer CF, Strauss HC. Antiarrhythmic drug action. Blockade of the inward sodium current. Circ Res. 1984;55(4):427‐439. [DOI] [PubMed] [Google Scholar]
- 11. Rolf S, Haverkamp W, Borggrefe M, et al. Effects of antiarrhythmic drugs on cloned cardiac voltage‐gated potassium channels expressed in Xenopus oocytes. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(1):22‐31. [DOI] [PubMed] [Google Scholar]
- 12. Kodama I, Toyama J, Takanaka C, Yamada K. Block of activated and inactivated sodium channels by class‐I antiarrhythmic drugs studied by using the maximum upstroke velocity (Vmax) of action potential in Guinea‐pig cardiac muscles. J Mol Cell Cardiol. 1987;19(4):367‐377. [DOI] [PubMed] [Google Scholar]
- 13. Bigger JT, Mandel WJ. Effect of lidocaine on the electrophysiological properties of ventricular muscle and Purkinje fibers. J Clin Investig. 1970;49(1):63‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rowland M, Thomson PD, Guichard A, Melmon KL. Disposition kinetics of Lidocaine in normal subjects. Ann NY Acad Sci. 1971;179:383‐398. [DOI] [PubMed] [Google Scholar]
- 15. Boyes RN, Scott DB, Jebson PJ, Godman MJ, Julian DG. Pharmacokinetics of lidocaine in man. Clin Pharmacol Ther. 1971;12(1):105‐116. [DOI] [PubMed] [Google Scholar]
- 16. Thomson PD, Rowland M, Melmon KL. The influence of heart failure, liver disease, and renal failure on the disposition of lidocaine in man. Am Heart J. 1971;82(3):417‐421. [DOI] [PubMed] [Google Scholar]
- 17. https://labeling.pfizer.com/ShowLabeling.aspx?id=4497; 2023. [cited 2023 Sep 7]. Available from: https://labeling.pfizer.com/ShowLabeling.aspx?id=4497.
- 18. Stenson RE, Constantino RT, Harrison DC. Interrelationships of hepatic blood flow, cardiac output, and blood levels of Lidocaine in man. Circulation. 1971;43(2):205‐211. [DOI] [PubMed] [Google Scholar]
- 19. Kostis JB, Goodking MJ, Gotzoyannis S, Gerber NH, Kuo PT. Effect of lidocaine on the atrial fibrillation threshold. Am Heart J. 1977;94(6):764‐768. [DOI] [PubMed] [Google Scholar]
- 20. Marrouche NF, Reddy RK, Wittkowsky AK, Bardy GH. High‐dose bolus lidocaine for chemical cardioversion of atrial fibrillation: a prospective, randomized, double‐blind crossover trial. Am Heart J. 2000;139(6):E8‐E11. [DOI] [PubMed] [Google Scholar]
- 21. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373‐498. [DOI] [PubMed] [Google Scholar]
- 22. January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the Management of Patients with Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(2):e125‐e151. [DOI] [PubMed] [Google Scholar]
- 23. Singh BN, Williams EM. Effect of altering potassium concentration on the action of lidocaine and diphenylhydantoin on rabbit atrial and ventricular muscle. Circ Res. 1971;29(3):286‐295. [DOI] [PubMed] [Google Scholar]
- 24. Wittig J, Harrison LA, Wallace AG. Electrophysiological effects of lidocaine on distal Purkinje fibers of canine heart. Am Heart J. 1973;86(1):69‐78. [PubMed] [Google Scholar]
- 25. Keenaghan JB, Boyes R. The tissue distribution, metabolism and excretion of lidocaine in rats, Guinea pigs, dogs and man. J Pharmacol Exp Ther. 1972;180:454‐463. [PubMed] [Google Scholar]
- 26. Wendt DJ, Starmer CF, Grant AO. pH dependence of kinetics and steady‐state block of cardiac sodium channels by lidocaine. Am J Physiol. 1993;264(5 Pt 2):H1588‐H1598. [DOI] [PubMed] [Google Scholar]
- 27. Rosen KM, Lau SH, Weiss MB, Damato AN. The effect of lidocaine on atrioventricular and intraventricular conduction in man. Am J Cardiol. 1970;25(1):1‐5. [DOI] [PubMed] [Google Scholar]
- 28. Lieberman NA, Harris RS, Katz RI, Lipschutz HM, Dolgin M, Fisher VJ. The effects of lidocaine on the electrical and mechanical activity of the heart. Am J Cardiol. 1968;22(3):375‐380. [DOI] [PubMed] [Google Scholar]
- 29. Weaver WD, Fahrenbruch CE, Johnson DD, Hallstrom AP, Cobb LA, Copass MK. Effect of epinephrine and lidocaine therapy on outcome after cardiac arrest due to ventricular fibrillation. Circulation. 1990;82(6):2027‐2034. [DOI] [PubMed] [Google Scholar]
- 30. Haynes RE, Chinn TL, Copass MK, Cobb LA. Comparison of bretylium tosylate and lidocaine in management of out of hospital ventricular fibrillation: a randomized clinical trial. Am J Cardiol. 1981;48(2):353‐356. [DOI] [PubMed] [Google Scholar]
- 31. Rosen MR, Hoffman BF. Mechanisms of action of antiarrhythmic drugs. Circ Res. 1973;32(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 32. Aidonidis I, Brachmann J, Seller H, Demowsky K, Czachurski J, Kübler W. Cardiac sympathetic nervous activity during myocardial ischemia, reperfusion and ventricular fibrillation in the dog—effects of intravenous lidocaine. Cardiology. 1992;80(3–4):196‐204. [DOI] [PubMed] [Google Scholar]
- 33. Pansegrau DG, Abboud FM. Hemodynamic effects of ventricular defibrillation. J Clin Invest. 1970;49(2):282‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Austen W, Moran JM. Cardiac and peripheral vascular effects of lidocaine and procainamide. Am J Cardiol. 1965;16(5):701‐707. [DOI] [PubMed] [Google Scholar]
- 35. Robison SL, Schroll M, Harrison DC. The circulatory response to Lidocaine in experimental myocardial infarction. Am J Med Sci. 1969;258(4):260‐269. [PubMed] [Google Scholar]
- 36. Grossman JI, Cooper JA, Frieden J. Cardiovascular effects of infusion of lidocaine on patients with heart disease. Am J Cardiol. 1969;24(2):191‐197. [DOI] [PubMed] [Google Scholar]
- 37. Chow MS, Ronfeld RA, Hamilton RA, Helmink R, Fieldman A. Effect of external cardiopulmonary resuscitation on lidocaine pharmacokinetics in dogs. J Pharmacol Exp Ther. 1983;224(3):531‐537. [PubMed] [Google Scholar]
- 38. Echt DS, Cato EL, Coxe DR. pH‐dependent effects of lidocaine on defibrillation energy requirements in dogs. Circulation. 1989;80(4):1003‐1009. [DOI] [PubMed] [Google Scholar]
- 39. Lown B. Lidocaine to prevent ventricular fibrillation: easy does it. N Engl J Med. 1985;313(18):1154‐1156. [DOI] [PubMed] [Google Scholar]
- 40. Lie KI, Wellens HJ, van Capelle FJ, Durrer D. Lidocaine in the prevention of primary ventricular fibrillation. A double‐blind, randomized study of 212 consecutive patients. N Engl J Med. 1974;291(25):1324‐1326. [DOI] [PubMed] [Google Scholar]
- 41. Cairns JA, Connolly SJ, Roberts R, Gent M. Randomised trial of outcome after myocardial infarction in patients with frequent or repetitive ventricular premature depolarisations: CAMIAT. Canadian amiodarone myocardial infarction arrhythmia trial investigators. Lancet. 1997;349(9053):675‐682. [DOI] [PubMed] [Google Scholar]
- 42. Yoshie K, Tomita T, Takeuchi T, et al. Renewed impact of lidocaine on refractory ventricular arrhythmias in the amiodarone era. Int J Cardiol. 2014;176(3):936‐940. [DOI] [PubMed] [Google Scholar]
- 43. Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, Lidocaine, or placebo in out‐of‐hospital cardiac arrest. N Eng J Med. 2016;374(18):1711‐1722. [DOI] [PubMed] [Google Scholar]
- 44. Könemann H, Ellermann C, Zeppenfeld K, Eckardt L. Management of Ventricular Arrhythmias Worldwide: comparison of the latest ESC, AHA/ACC/HRS, and CCS/CHRS guidelines. JACC Clin Electrophysiol. 2023;9(5):715‐728. [DOI] [PubMed] [Google Scholar]
- 45. Könemann H, Dagres N, Merino JL, et al. Spotlight on the 2022 ESC guideline management of ventricular arrhythmias and prevention of sudden cardiac death: 10 novel key aspects. Europace. 2023;25(5):7‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frommeyer G, Garthmann J, Ellermann C, et al. Broad antiarrhythmic effect of mexiletine in different arrhythmia models. Europace. 2018;20(8):1375‐1381. [DOI] [PubMed] [Google Scholar]
- 47. ESC Guidelines on Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death. 2022. [cited 2022 Dec 19]. Available from: https://www.escardio.org/Guidelines/Clinical‐Practice‐Guidelines/Ventricular‐Arrhythmias‐and‐the‐Prevention‐of‐Sudden‐Cardiac‐Death
- 48. Guidelines for cardiopulmonary resuscitation and emergency cardiac care. Emergency cardiac care committee and subcommittees, American Heart Association. Part III. Adult advanced cardiac life support. JAMA. 1992;268(16):2199‐2241. [PubMed] [Google Scholar]
- 49. Deyell MW, AbdelWahab A, Angaran P, et al. 2020 Canadian cardiovascular society/Canadian Heart Rhythm Society position statement on the Management of Ventricular Tachycardia and Fibrillation in patients with structural heart disease. Can J Cardiol. 2020;36(6):822‐836. [DOI] [PubMed] [Google Scholar]
- 50. Lichstein E, Chadda KD, Gupta PK. Atrioventricular block with lidocaine therapy. Am J Cardiol. 1973;31(2):277‐281. [DOI] [PubMed] [Google Scholar]
- 51. Jeresaty RM, Kahn AH, Landry AB. Sinoatrial arrest due to lidocaine in a patient receiving guinidine. Chest. 1972;61(7):683‐685. [DOI] [PubMed] [Google Scholar]
- 52. Cheng TO. Sinus standstill following intravenous Lidocaine administration. JAMA. 1973;223(7):790. [PubMed] [Google Scholar]
- 53. Daraz YM, Abdelghffar OH. Lidocaine infusion: an antiarrhythmic with neurologic toxicities. Cureus. 2022;14(3):e23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Smith E, Duce B. The acute antiarrhythmic and toxic effects in mice and dogs of 2‐ethylamino‐2′,6‐‘acetoxylidine (L‐86), a metabolite of lidocaine. J Pharmacol Exp Ther. 1971;179:580‐585. [PubMed] [Google Scholar]
- 55. Blumer J, Strong J, Atkinson AJ. The convulsant potency of lidocaine and its N‐dealkylated metabolites. J Pharmacol Exp Ther. 1973;186:31‐36. [PubMed] [Google Scholar]
- 56. Frumin H, Kerin NZ, Rubenfire M. Classification of antiarrhythmic drugs. J Clin Pharmacol. 1989;29(5):387‐394. [DOI] [PubMed] [Google Scholar]
- 57. King FG, Addetia AM, Peters SD, Peachey GO. Prophylactic lidocaine for postoperative coronary artery bypass patients, a double‐blind, randomized trial. Can J Anaesth. 1990;37(3):363‐368. [DOI] [PubMed] [Google Scholar]
- 58. Dunn HM, Kinney CD, Campbell NP, Shanks RG, Adgey AA. Prophylactic lidocaine in suspected acute myocardial infarction. Int J Cardiol. 1984;5(1):96‐98. [DOI] [PubMed] [Google Scholar]
- 59. Hine LK, Laird N, Hewitt P, Chalmers TC. Meta‐analytic evidence against prophylactic use of lidocaine in acute myocardial infarction. Arch Intern Med. 1989;149(12):2694‐2698. [PubMed] [Google Scholar]
- 60. Wagner D, Kronick SL, Nawer H, Cranford JA, Bradley SM, Neumar RW. Comparative effectiveness of amiodarone and Lidocaine for the treatment of in‐hospital cardiac arrest. Chest. 2022;163:1109‐1119. [DOI] [PubMed] [Google Scholar]
- 61. Kudenchuk PJ, Newell C, White L, Fahrenbruch C, Rea T, Eisenberg M. Prophylactic lidocaine for post resuscitation care of patients with out‐of‐hospital ventricular fibrillation cardiac arrest. Resuscitation. 2013;84(11):1512‐1518. [DOI] [PubMed] [Google Scholar]
- 62. Shiga T, Tanaka K, Kato R, et al. Nifekalant versus lidocaine for in‐hospital shock‐resistant ventricular fibrillation or tachycardia. Resuscitation. 2010;81(1):47‐52. [DOI] [PubMed] [Google Scholar]
- 63. Rea RS, Kane‐Gill SL, Rudis MI, et al. Comparing intravenous amiodarone or lidocaine, or both, outcomes for inpatients with pulseless ventricular arrhythmias*. Crit Care Med. 2006;34(6):1617‐1623. [DOI] [PubMed] [Google Scholar]
- 64. Dorian P, Cass D, Schwartz B, Cooper R, Gelaznikas R, Barr A. Amiodarone as compared with Lidocaine for shock‐resistant ventricular fibrillation. N Engl J Med. 2002;346(12):884‐890. [DOI] [PubMed] [Google Scholar]
- 65. Herlitz J, Ekström L, Wennerblom B, et al. Lidocaine in out‐of‐hospital ventricular fibrillation. Does it improve survival? Resuscitation. 1997;33(3):199‐205. [DOI] [PubMed] [Google Scholar]


