Abstract
This ethnic sensitivity analysis used data from the phase III POLARIX study (NCT03274492) to assess polatuzumab vedotin pharmacokinetics (PKs) in Asian versus non‐Asian patients with previously untreated diffuse large B‐cell lymphoma and examined the appropriateness of extrapolating global study findings to Asian patients. PK and population PK (PopPK) analyses assessed polatuzumab vedotin analyte exposures by ethnicity (Asian [n = 84] vs. non‐Asian [n = 345] patients) and region (patients enrolled from Asia [n = 80] vs. outside Asia [n = 349]). In patients from Asia versus outside Asia, observed mean antibody‐conjugated monomethyl auristatin E (acMMAE) concentrations were comparable (1.2% lower at cycle [C]1 postdose, 4.4% higher at C4 predose; and 6.8% lower at C4 postdose in patients from Asia). Observed mean unconjugated MMAE was lower in patients from Asia by 6.5% (C1 postdose), 20.0% (C4 predose), and 15.3% (C4 postdose). In the PopPK analysis, C6 area under the curve and peak plasma concentrations were also comparable for acMMAE (6.3% and 3.0% lower in Asian vs. non‐Asian patients, respectively) and lower for unconjugated MMAE by 19.1% and 16.7%, respectively. By region, C6 mean acMMAE concentrations were similar, and C6 mean unconjugated MMAE concentrations were lower, in patients enrolled from Asia versus outside Asia, by 3.9%–7.0% and 17.3%–19.7%, respectively. In conclusion, polatuzumab vedotin PKs were similar between Asian and non‐Asian patients by ethnicity and region, suggesting PKs are not sensitive to Asian ethnicity and dose adjustments are not required in Asian patients to maintain efficacy and safety.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Clinically meaningful differences in PKs/pharmacodynamics between patients of different ethnicities can necessitate dose adjustments in patient subpopulations, to maintain the intended drug efficacy and safety.
WHAT QUESTION DID THIS STUDY ADDRESS?
An ethnic sensitivity assessment was conducted to explore any differences in polatuzumab vedotin PK between Asian and non‐Asian patients from the PK‐evaluable patient population in POLARIX treated with polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola‐R‐CHP), in order to determine whether findings from the POLARIX global study population could be extrapolated to the Asian patient subpopulation of the study.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
Overall, no clinically meaningful differences in polatuzumab vedotin PK were found in patients of Asian versus non‐Asian ethnicity and in those enrolled in Asian versus non‐Asian countries.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These results suggest that no dose adjustments are required when giving polatuzumab vedotin to Asian patients.
INTRODUCTION
Diffuse large B‐cell lymphoma (DLBCL) is the most common type of B‐cell non‐Hodgkin's lymphoma (NHL) and has typically been treated with a combination of chemotherapy with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R‐CHOP). 1 Polatuzumab vedotin is an antibody–drug conjugate comprising a humanized IgG1 antibody against CD79b, a signaling component of the B‐cell receptor, conjugated to a potent cytotoxin, monomethyl auristatin E (MMAE). After binding CD79b, polatuzumab vedotin delivers MMAE to B cells, resulting in anticancer activity against B‐cell malignancies. 2 , 3 MMAE is then partly metabolized by cytochrome P450 3A4 (CYP3A4) and is otherwise eliminated unchanged. 1 In the phase III POLARIX study (NCT03274492), the novel combination of polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone (Pola‐R‐CHP) prolonged progression‐free survival (PFS) compared with R‐CHOP therapy in patients with previously untreated intermediate‐ or high‐risk DLBCL. 4 Pola‐R‐CHP has since received approval as treatment for newly diagnosed DLBCL in more than 50 countries, 5 including in the European Union, 6 China, 7 and Japan. 8 The same dosing regimen is recommended for use globally irrespective of region or race. As part of the Pola‐R‐CHP combination, polatuzumab vedotin is administered at 1.8 mg/kg via intravenous (i.v.) infusion once every 21 days (q3w) for six cycles. 4
Drug efficacy and safety can potentially vary between patients of different ethnicities due to factors including bodyweight, genetic polymorphisms in key metabolic enzymes, and regional standard‐of‐care practices. 1 , 9 For example, between Asian and non‐Asian patients, such differences can necessitate dose adjustments across patient subpopulations. 1 It is therefore important for clinical trial designs to consider ethnic diversity by ensuring that patients are recruited from different ethnicities and regions globally, to identify any significant covariates in drug exposure that may impact the dosing regimen. A previous ethnic sensitivity analysis using a population pharmacokinetic (PopPK) modeling approach provided initial clinical evidence supporting similar polatuzumab vedotin pharmacokinetics (PKs) for Asian versus non‐Asian patients with B‐cell NHL. 1 This previous analysis included PK data from patients with relapsed/refractory (R/R) and newly diagnosed B‐cell NHL from five phase Ib/II studies assessing polatuzumab vedotin as monotherapy or in combination with other treatments. However, only one of the five studies used PK data from patients with newly diagnosed B‐cell NHL receiving Pola‐R‐CHP, and data from this patient subpopulation were therefore limited (n = 82 for non‐Asian patients; no Asian patients by ethnicity or region were included). Therefore, whereas the previous evaluation demonstrated similar PKs for polatuzumab vedotin as monotherapy or as part of multiple combination regimens, particularly in the R/R setting, further evaluation of Asian ethnic sensitivity for PKs in patients receiving Pola‐R‐CHP in the first‐line setting was needed. We aimed to explore whether there were significant differences between the PK profile of polatuzumab vedotin in Asian and non‐Asian patients with previously untreated DLBCL receiving Pola‐R‐CHP in POLARIX, and determine the appropriateness of extrapolating the global study data to Asian patients.
METHODS
POLARIX study design
The methods used in the POLARIX study have been published previously. 4 Briefly, patients with newly diagnosed DLBCL were randomized 1:1 to receive polatuzumab vedotin at 1.8 mg/kg by i.v. infusion q3w in combination with R‐CHP, for six cycles, or the standard R‐CHOP regimen for six cycles. During cycles 7 and 8, patients received rituximab monotherapy. The current Asian subpopulation ethnic sensitivity assessment explores analyses conducted using data from the PK population in the Pola‐R‐CHP arm.
PK data were stratified based on ethnicity and the country of enrollment. In POLARIX, 440 patients were assigned to receive Pola‐R‐CHP. Patient demographics and clinical characteristics of the study population have been reported previously. 4 The PK analysis population included 84 patients of Asian ethnicity (10 [12%] of Chinese descent, 48 [57%] of Japanese descent, 17 [20%] of South Korean descent, and 9 [11%] of Taiwanese descent) and 345 patients of non‐Asian ethnicity, which included patients whose ethnicity was unknown. By region of enrollment, 80 Asian patients were enrolled from countries in Asia (9 [11%] from China, 45 [56%] from Japan, 17 [21%] from South Korea, and 9 [11%] from Taiwan), and 349 patients (including 4 Asian patients) were enrolled from non‐Asian countries. All patients in the PK population enrolled from Asia were of Asian ethnicity.
Per the recommended approach for bioanalysis of antibody–drug conjugates, 10 , 11 polatuzumab vedotin PK is typically profiled in clinical studies by examining three analytes: total antibody (including conjugated and unconjugated antibody), antibody‐conjugated MMAE (acMMAE), and unconjugated MMAE. 12 During the POLARIX study, acMMAE and unconjugated MMAE were measured in human plasma using liquid chromatography with tandem mass spectrometry with and without immunoaffinity capture, respectively. The total antibody concentrations in human serum were measured using an enzyme‐linked immunosorbent assay. Serum samples to examine total antibody concentration only were taken on day 1 of cycle 1 before polatuzumab vedotin administration and at a 3‐month post‐treatment follow‐up visit. Additional PK samples for all three analytes (serum samples for total antibody concentration and plasma samples for acMMAE and unconjugated MMAE) were taken at cycle 1 day 1 (30 min postdose), at cycle 4 day 1 (predose and 30 min postdose), and at treatment completion (or treatment end if treatment was terminated early).
PK analyses
Direct data observations alongside a PopPK approach were used to assess the impact of ethnicity (Asian compared with non‐Asian) on the PK profile of polatuzumab vedotin with POLARIX PK data. Previously, a two‐analyte (acMMAE‐MMAE) integrated PopPK analysis model, using PK data from 460 patients with NHL from four studies, was established to characterize the PK properties of acMMAE and unconjugated MMAE. 12 The legacy PopPK model structure used PopPK analyses to characterize polatuzumab vedotin PK data from POLARIX. 13 Post hoc exposure, assessed by cycle 6 area under the curve (AUC) and peak plasma concentration (C max), was stratified based on ethnicity and region. Both the cycle 6 acMMAE and unconjugated MMAE exposures were projected based on the integrated PopPK model after the six cycles of polatuzumab vedotin (1.8 mg/kg q3w) were completed.
RESULTS
Patient demographics and baseline characteristics were generally similar for Asian and non‐Asian patients receiving Pola‐R‐CHP, including age, sex, Ann Arbor Stage, International Prognostic Index score, bone marrow involvement at diagnosis, and some baseline disease biomarkers (double expressor lymphoma and double‐ or triple‐hit lymphoma; Table 1). However, Asian patients had lower average bodyweight, and fewer had an Eastern Cooperative Oncology Group Performance Status of 1 or 2, or bulky disease, compared with non‐Asian patients. Cell of origin as a baseline disease biomarker showed more Asian patients had the activated B‐cell (ABC) subtype versus germinal center B‐cell (GCB), and more non‐Asian patients had the GCB subtype versus ABC (Table 1).
TABLE 1.
Key demographic and baseline clinical characteristics in Asian and non‐Asian patients in the Pola‐R‐CHP treatment arm in POLARIX (intent‐to‐treat population).
| Asian | Non‐Asian | |
|---|---|---|
| Pola‐R‐CHP (n = 81) | Pola‐R‐CHP (n = 359) | |
| Median age, years | 63.0 | 66.0 |
| Male, n (%) | 42 (51.9) | 197 (54.9) |
| Mean weight at baseline, kg (SD) | 60.4 (13.6) | 79.5 (19.6) |
| ECOG PS at baseline, n (%) | ||
| 0 | 42 (51.9) | 133 (37.0) |
| 1 | 29 (35.8) | 170 (47.4) |
| 2 | 10 (12.3) | 56 (15.6) |
| Ann Arbor stage, n (%) | ||
| I | 1 (1.2) | 1 (0.3) |
| II | 12 (14.8) | 33 (9.2) |
| III | 17 (21.0) | 107 (29.8) |
| IV | 51 (63.0) | 218 (60.7) |
| IPI score, n (%) | ||
| 2 | 31 (38.3) | 136 (37.9) |
| 3–5 | 50 (61.7) | 223 (62.1) |
| Bulky disease status, n (%) | ||
| Absent | 55 (67.9) | 192 (53.5) |
| Present | 26 (32.1) | 167 (46.5) |
| Bone marrow involvement at diagnosis, n (%) | n = 80 | n = 349 |
| Indeterminate | 0 | 11 (3.1) |
| Negative | 64 (79.0) | 278 (77.4) |
| Positive | 16 (19.8) | 60 (16.7) |
| DEL by IHC, n (%) | n = 69 | n = 293 |
| DEL | 24 (34.8) | 115 (39.2) |
| Non‐DEL | 45 (65.2) | 178 (60.8) |
| Double/triple‐hit lymphoma, n (%) | n = 67 | n = 264 |
| DH/TH+ | 3 (4.5) | 23 (8.7) |
| DH/TH− | 64 (95.5) | 241 (91.3) |
| COO, n (%) | n = 53 | n = 277 |
| ABC | 26 (49.1) | 76 (27.4) |
| GCB | 21 (39.6) | 163 (58.8) |
| Unclassified | 6 (11.3) | 38 (13.7) |
Abbreviations: ABC, activated B‐cell; COO, cell of origin; DEL, double‐expressor lymphoma; DH, double‐hit; GCB, germinal center B‐cell; ECOG PS, Eastern Cooperative Oncology Group Performance Status; IHC, immunohistochemistry; IPI, International Prognostic Index; Pola‐R‐CHP, polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, prednisone; SD, standard deviation; TH, triple‐hit.
PK comparisons based on observed data
Polatuzumab vedotin PK, covariate effects, and interindividual variability are well‐characterized in patients with DLBCL. 12 Overall, the observed data showed that mean acMMAE concentrations in patients enrolled from Asia were similar to those in patients enrolled from outside of Asia (Table 2). The concentrations did not notably differ across cycles; at day 1 of cycle 1, patients enrolled from Asia had an ~1.2% lower postdose mean acMMAE C max than patients enrolled from outside of Asia. At day 1 of cycle 4, the predose mean acMMAE concentration (C trough) was around 4.4% higher and the 30‐min postdose mean C max was around 6.8% lower in patients enrolled from Asia than in those enrolled outside of Asia. Similarly, mean total antibody concentration was generally comparable across cycles. At day 1 of cycle 1 30 min postdose, patients enrolled from Asia had an ~1.1% lower mean total antibody C max than patients enrolled from outside of Asia. At day 1 of cycle 4, the predose mean total antibody concentration (C trough) was around 7.8% lower and the 30‐min postdose mean C max was around 6.2% lower in patients enrolled from Asia than in those enrolled outside of Asia. In contrast, the mean unconjugated MMAE concentrations were lower in patients enrolled from Asia than in those enrolled outside of Asia (Table 2). At day 1 of cycle 1, postdose mean unconjugated MMAE C max was ~6.5% lower in patients enrolled from Asia than in those enrolled outside of Asia. Similarly, day 1 of cycle 4 unconjugated MMAE was around 20% lower predose and 15.3% lower postdose in patients enrolled from Asia than in those enrolled outside of Asia. Boxplots confirmed similar trends for the cycle 1 and cycle 4 pre‐ and postdose median and range values for all three analytes, in patients enrolled from Asia and outside of Asia (Figures 1, 2, 3).
TABLE 2.
Mean (standard deviation) concentrations of acMMAE, total antibody and unconjugated MMAE by region measured in patients from the POLARIX PK‐evaluable population following polatuzumab vedotin at 1.8 mg/kg as part of the Pola‐R‐CHP regimen.
| Region of enrollment | |||
|---|---|---|---|
| Asia | Non‐Asia | All | |
| Mean acMMAE concentration, ng/mL | |||
| Cycle 1 day 1 | |||
| C max 30 min postdose (SD) | 597 (104) | 604 (162) | 603 (153) |
| n | 68 | 294 | 362 |
| Cycle 4 day 1 | |||
| C trough predose (SD) | 18.8 (23.7) | 18.0 (6.6) | 18.2 (12.0) |
| n | 78 | 324 | 402 |
| C max 30 min postdose (SD) | 621 (110) | 666 (186) | 657 (175) |
| n | 68 | 292 | 360 |
| Mean total antibody concentration, μg/mL | |||
| Cycle 1 day 1 | |||
| C trough predose (SD) | NR (NR) | NR (NR) | NR (NR) |
| n | 78 | 340 | 418 |
| C max 30 min postdose (SD) | 35.8 (7.7) | 36.2 (11.7) | 36.1 (11.0) |
| n | 76 | 319 | 395 |
| Cycle 4 day 1 | |||
| C trough predose (SD) | 5.1 (2.4) | 5.5 (3.4) | 5.4 (3.2) |
| n | 77 | 330 | 407 |
| C max 30 min postdose (SD) | 42.1 (10.2) | 44.9 (15.3) | 44.4 (14.5) |
| n | 77 | 321 | 398 |
| Mean unconjugated MMAE concentration, ng/mL | |||
| Cycle 1 day 1 | |||
| C max30 min postdose (SD) | 0.401 (0.2) | 0.429 (0.4) | 0.424 (0.3) |
| n | 80 | 323 | 403 |
| Cycle 4 day 1 | |||
| C trough predose (SD) | 0.120 (0.1) | 0.150 (0.1) | 0.144 (0.1) |
| n | 79 | 327 | 406 |
| C max 30 min postdose (SD) | 0.194 (0.1) | 0.229 (0.1) | 0.222 (0.1) |
| n | 79 | 319 | 398 |
Note: Values are rounded to 1 decimal point. C trough values were measured in samples taken predose and C max values were measured in samples taken 30 min postdose. The number of evaluable patients for each PK variable ranged between 68 and 80 for patients from Asia, 292–340 for patients from outside of Asia, and 360–418 for patients from all regions.
Abbreviations: acMMAE, antibody‐conjugated monomethyl auristatin E; C max, maximum concentration; C trough, last concentration prior to dosing; MMAE, monomethyl auristatin E; NR, not reached; PK, pharmacokinetic; Pola‐R‐CHP, polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin, and prednisone; SD, standard deviation.
FIGURE 1.
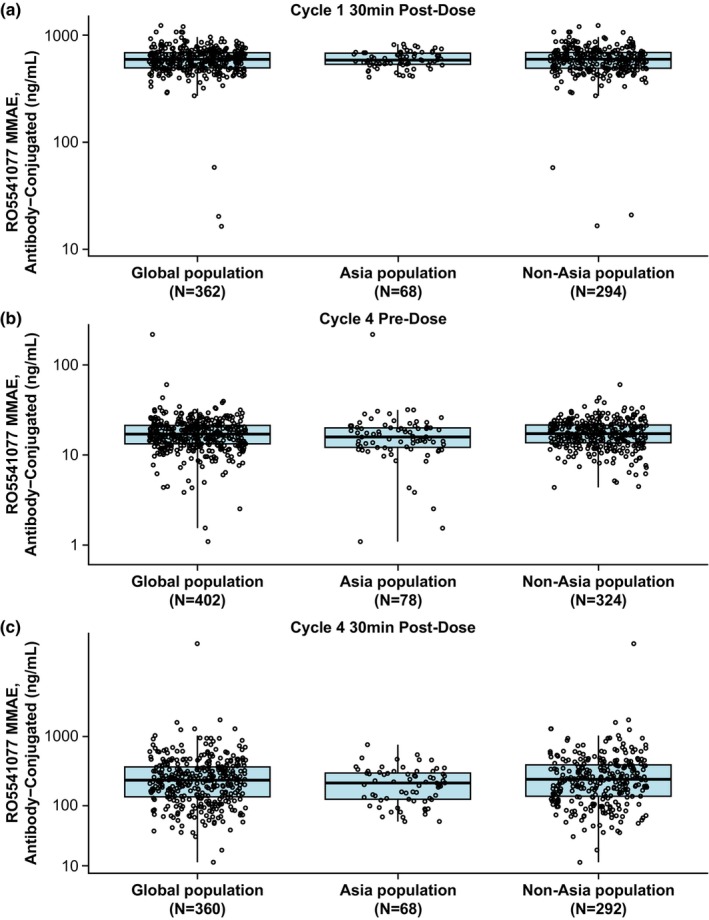
Boxplots of acMMAE concentrations by region in patients from the POLARIX PK‐evaluable population receiving polatuzumab vedotin at 1.8 mg/kg as part of the Pola‐R‐CHP regimen, measured at (a) cycle 1 30 min postdose (C max), (b) cycle 4 predose (C trough), and (c) cycle 4 30 min postdose (C max). Box, first and third quartiles; middle bar, median; black circle, observed data points; whiskers, largest observed value within the upper fence (third quartile plus 1.5 × IQR); smallest observed value within the lowest fence (first quartile plus 1.5 × IQR). acMMAE, antibody‐conjugated monomethyl auristatin E; C max, maximum concentration; C trough, last concentration prior to dosing; IQR, interquartile range; min, minutes; MMAE, monomethyl auristatin E; PK, pharmacokinetics; Pola‐R‐CHP, polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin and prednisone.
FIGURE 2.
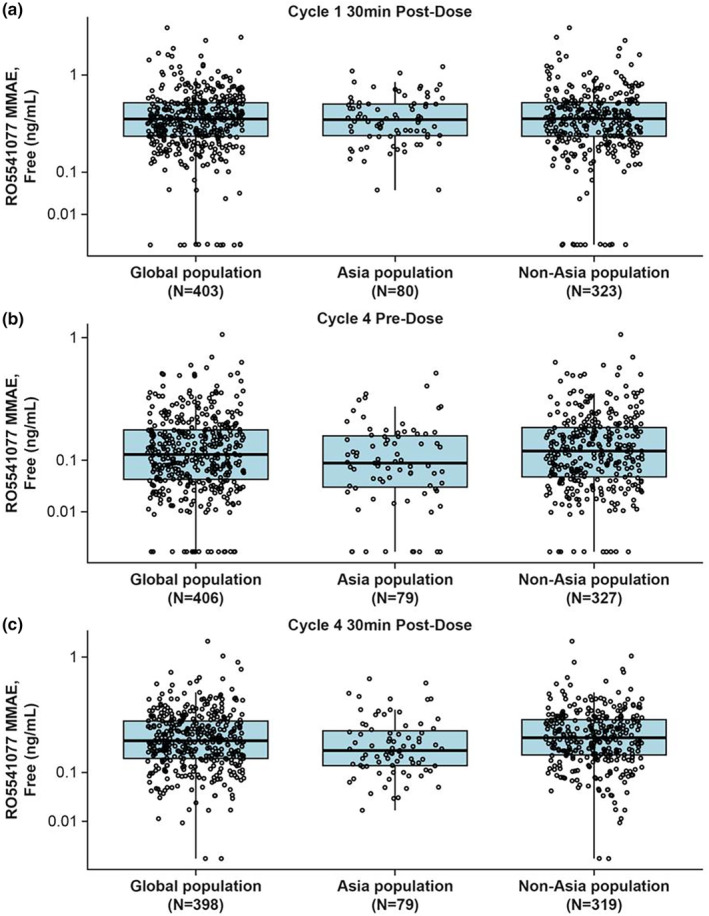
Boxplots of unconjugated MMAE concentrations by region in patients from the POLARIX PK‐evaluable population receiving polatuzumab vedotin at 1.8 mg/kg as part of the Pola‐R‐CHP regimen, measured at (a) cycle 1 30 min postdose (C max), (b) cycle 4 predose (C trough), and (c) cycle 4 30 min postdose (C max). Box, first and third quartiles; middle bar, median; black circle, observed data points; whiskers, largest observed value within the upper fence (third quartile plus 1.5 × IQR); smallest observed value within the lowest fence (first quartile plus 1.5 × IQR). C max, maximum concentration; Ctrough, last concentration prior to dosing; IQR, interquartile range; min, minutes; MMAE, monomethyl auristatin E; PK, pharmacokinetic; Pola‐R‐CHP, polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin and prednisone.
FIGURE 3.
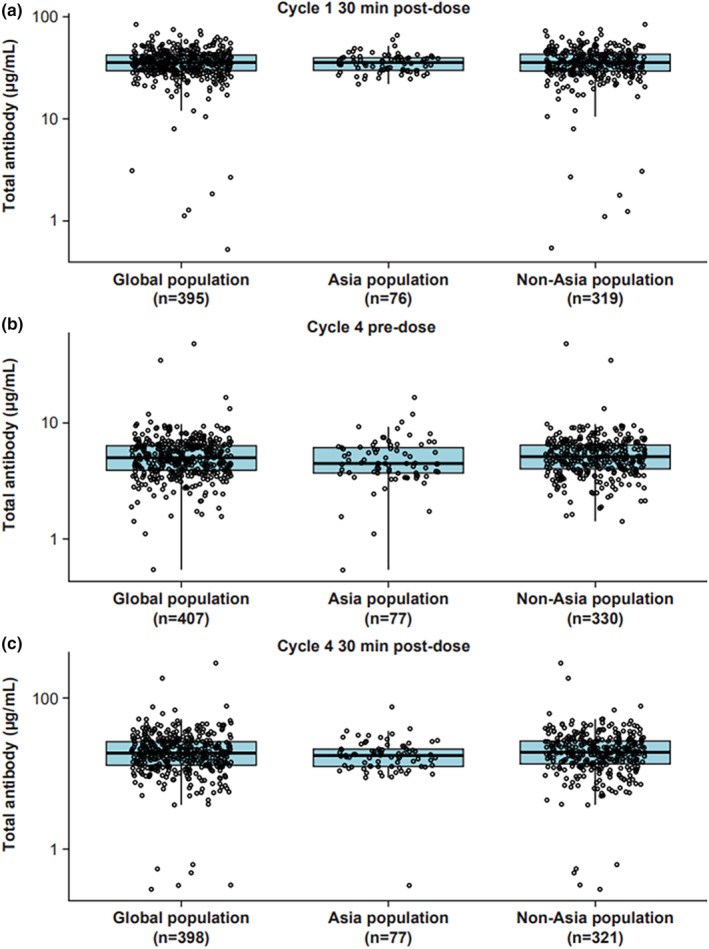
Boxplots of total antibody concentrations by region in patients from the POLARIX PK‐evaluable population receiving polatuzumab vedotin at 1.8 mg/kg as part of the Pola‐R‐CHP regimen, measured at (a) cycle 1 30 min postdose (C max), (b) cycle 4 predose (C trough), and (c) cycle 4 30 min postdose (C max). Box, first and third quartiles; middle bar, median; black circle, observed data points; whiskers, largest observed value within the upper fence (third quartile plus 1.5 × IQR); smallest observed value within the lowest fence (first quartile plus 1.5 × IQR). C max, maximum concentration; C trough, last concentration prior to dosing; IQR, interquartile range; min, minutes; PK, pharmacokinetic; Pola‐R‐CHP, polatuzumab vedotin with rituximab, cyclophosphamide, doxorubicin and prednisone.
PK comparisons based on PopPK simulations
Asian compared with non‐Asian ethnicity
The legacy PopPK analyses showed that bodyweight accounted for 36.1% and 43.1% of the interindividual variability in clearance and central volume, respectively. Bodyweight‐based dosing is expected to reduce PK exposure variability compared with a hypothetical flat‐dose approach. 14 The PopPK analyses also showed that Asian patients had a 7.1% (95% confidence interval: 0.5%, 14.8%) lower volume of distribution than non‐Asian patients. Similarly, the isolated effect of ethnicity on the AUC and C max for both acMMAE and unconjugated MMAE was minimal (~8%) based on ethnic sensitivity analyses to assess the cycle 6 exposures for a typical patient. In the POLARIX PopPK analyses, patients were of Asian ethnicity (n = 84 [19.6%]), non‐Asian/White (n = 228 [53.1%]), or unknown/other (n = 117 [27.3%]). The analyses compared cycle 6 exposures for Asian and non‐Asian patients, finding that the exposures in both groups were similar; acMMAE AUC was only 6.3% lower, and acMMAE C max was only 3.0% lower in Asian patients than non‐Asian patients (Table 3). Unconjugated MMAE exposures in cycle 6 were lower in Asian patients than in non‐Asian patients, by 19.1% for AUC and 16.7% for C max (Table 3).
TABLE 3.
Summary of acMMAE and unconjugated MMAE exposures at cycle 6 in the POLARIX PopPK analysis by ethnicity and region of enrollment.
| Exposure a | Statistics | Ethnicity | Region of enrollment | ||
|---|---|---|---|---|---|
| Asian (n = 84) | Non‐Asian (n = 345) | Within Asia d (n = 80) | Outside of Asia e (n = 349) | ||
| acMMAE AUC, ng/day/mL | Geometric mean (CV%) b | 2330 (12) | 2490 (14) | 2310 (12) | 2490 (14) |
| GMR c (90% CI) | 0.937 (0.913, 0.961) | 0.930 (0.906, 0.954) | |||
| acMMAE C max, ng/mL | Geometric mean (CV%) b | 596 (12) | 615 (15) | 592 (12) | 616 (15) |
| GMR c (90% CI) | 0.970 (0.945, 0.995) | 0.961 (0.937, 0.986) | |||
| Unconjugated MMAE AUC, ng/day/mL | Geometric mean (CV%) b | 11.2 (44) | 13.8 (49) | 11.1 (45) | 13.8 (49) |
| GMR c (90% CI) | 0.809 (0.739, 0.886) | 0.803 (0.731, 0.881) | |||
| Unconjugated MMAE C max, ng/mL | Geometric mean (CV%) b | 1.11 (38) | 1.34 (42) | 1.10 (38) | 1.33 (42) |
| GMR c (90% CI) | 0.833 (0.771, 0.900) | 0.827 (0.764, 0.896) | |||
Abbreviations: acMMAE, antibody‐conjugated monomethyl auristatin E; AUC, area under the concentration–time curve; C max, maximum concentration; CI, confidence interval; CV, coefficient of variation; GMR, geometric mean ratio; MMAE, monomethyl auristatin E; PopPK, population pharmacokinetics.
Individual exposures were computed for all patients following polatuzumab vedotin 1.8 mg/kg every 3 weeks for six cycles.
CV was computed as standard deviation of a log‐transformed variable.
GMR is defined as the mean ratio between Asian and non‐Asian patients, and between patients enrolled from Asia and those from outside of Asia.
Asian regions included China, Japan, (n = 54) South Korea (n = 17) and Taiwan (n = 9).
Non‐Asian regions included Western Europe (n = 159), North America (n = 117), Eastern Europe (n = 46), Pacific region (n = 21), and South/Central America (n = 6).
Patients enrolled in Asia compared with outside of Asia
Region was not included as a model covariate in the legacy PopPK model because of the strong correlation between region and ethnicity. In the POLARIX PopPK analysis, there were 429 patients, of whom 159 (37.1%) were from Western Europe, 117 (27.3%) were from North America, 80 (18.6%) were from Asia, 46 (10.7%) were from Eastern Europe, 21 (4.9%) were from Pacific regions, and six (1.4%) were from Central and South America. Cycle 6 exposures in patients enrolled in Asia compared with those in patients enrolled from outside of Asia are presented in Table 3; acMMAE exposures were similar between the two groups (3.9%–7.0% lower in patients enrolled from Asia) and unconjugated MMAE exposures were lower in patients enrolled from Asia than in those enrolled from outside of Asia (17.3%–19.7% lower). Additional comparisons of cycle 6 exposures showed similar results for patients enrolled from Asia and patients enrolled from Western countries (Western Europe, North America, and Australia) or the rest of the world (all countries except Asian and Western countries; data not shown). Further detailed analyses comparing patients in POLARIX enrolled from Taiwan and all other countries, and patients enrolled from South Korea and all other countries, are presented in the Appendix S1.
DISCUSSION
This ethnic sensitivity assessment based on the POLARIX study found minimal differences between the PKs of polatuzumab vedotin in previously untreated patients with DLBCL enrolled from Asia compared with those enrolled from outside of Asia (3.9%–7.0% lower acMMAE and 17.3%–19.7% lower unconjugated MMAE). Minimal differences were also observed for patients of Asian ethnicity compared with those of non‐Asian ethnicity (3–6.3% lower acMMAE and 16.7%–19.1% lower unconjugated MMAE). These differences would not be expected to affect clinical outcomes. The results add to the existing evidence that polatuzumab vedotin dose adjustment is not warranted in Asian patients with DLBCL. For example, in a previous ethnic sensitivity analysis of PK data from five phase Ib/II studies of patients with R/R or previously untreated DLBCL receiving polatuzumab vedotin as monotherapy or in combination with other treatments, the mean analyte exposures (acMMAE, MMAE, and total antibody) were generally lower in Asian patients by ~9.9%–17.5%; however, this was not considered clinically meaningful. 1 Similarly, a small study (JO29138) comparing seven Japanese patients with 95 non‐Japanese patients from the DCS4968G study with R/R B‐cell NHL found that there were no clinically significant differences in polatuzumab vedotin exposure, treatment outcome, and safety between the two patient groups. 15 , 16 Together, these data suggest that the slight differences in PK exposure based on ethnicity and region observed in the present study are not clinically relevant and are not expected to adversely affect the safety of Asian patients or patients enrolled from Asia.
Ethnic sensitivity analyses for other antibody–drug conjugates had similar findings. Assessments by Li et al. (2016), including PopPK analyses, for trastuzumab emtansine, an antibody–drug conjugate indicated for previously treated human epidermal growth factor receptor 2‐positive (HER2‐positive) breast cancer, demonstrated generally similar efficacy and safety in Asian patients compared with the global population. The study concluded that the approved dosing regimen was appropriate across ethnicities. 17 Similarly, a PopPK study for the antibody–drug conjugate brentuximab vedotin, indicated for cutaneous T‐cell lymphomas, found race (defined as “White/Black/Asian/other” ethnicities) was not a significant covariate for exposure–response or clearance. 18
The current study contributes additional information to the ethnic sensitivity landscape in a number of ways. It includes data from a broader Asian patient population than in previous studies; Shi et al. 1 used data from Japanese and South Korean patients, whereas here, data from Chinese, Japanese, South Korean, and Taiwanese patients were used. This is further supported by the POLAROSE study (NCT04236141), which was dedicated to analyzing data from Chinese patients with R/R DLBCL receiving polatuzumab vedotin. 19 The present analysis also included a greater number of Asian patients (Shi et al. 1 : n = 18 Asian patients; present analysis: n = 84 Asian patients), offering greater representation of the safety and efficacy of polatuzumab vedotin in the Asian patient population. Furthermore, whereas Shi et al. (2020) evaluated patient data from multiple studies using a range of doses and dosing schedules, the POLARIX study included patients treated with the same regimen/protocol at the recommended dose. This approach led to fewer variables that could impact treatment outcome, and isolated the impact of Asian ethnicity on PKs.
Similar to the findings previously reported by Shi et al., 1 ethnicity‐related polymorphisms in CYP3A4 were not considered to meaningfully impact the metabolism of MMAE in Asian patients. In the POLARIX study, postdose unconjugated MMAE levels in Asian patients were similar to, or only marginally lower than, those of patients enrolled from outside of Asia. The observation may be confounded by large interindividual variabilities in unconjugated MMAE and a relatively small sample size. Nonetheless, variation in CYP3A4 expression is considered largely non‐genetic and is unlikely to affect MMAE metabolism in Asian patients. MMAE is also only partially metabolized; therefore, the impact of CYP3A4 variation on metabolism and clearance is expected to be low for polatuzumab vedotin compared with drugs exclusively metabolized by CYP3A4. 1
Deng et al. (2022) previously described analyses of the exposure–safety and exposure–efficacy relationships for acMMAE, and exposure–safety relationship for unconjugated MMAE after polatuzumab vedotin 1.8 mg/kg q3w in the POLARIX global population. 13 Briefly, the exposure–response results and the favorable benefit:risk profile supported polatuzumab vedotin dosing at 1.8 mg/kg q3w for six cycles in combination with R‐CHP (Pola‐R‐CHP) in patients with previously untreated DLBCL. 13 The previous analyses also found that a decrease in polatuzumab vedotin exposure, represented by AUC and C max for acMMAE, may be associated with poorer efficacy as indicated by shorter PFS and event‐free survival. 13 Although the present study showed that AUC and C max for acMMAE were numerically lower in Asian patients than in non‐Asian patients, a clinically meaningful impact on efficacy would not be expected. Similarly, the reduced unconjugated MMAE AUC and C max in Asian patients compared with non‐Asian patients are not expected to meaningfully impact efficacy due to the low circulating levels of acMMAE and unconjugated MMAE (<3% of acMMAE levels), as well as the lack of tumor targeting once MMAE is deconjugated from the CD79b‐directed antibody. 20 In support of these results, efficacy in Asian patients in the POLARIX study was similar to that in the global study population. 19
In examining covariates within the exposure–safety analyses, Asian ethnicity was identified as a significant factor correlated with an increased probability of grade greater than or equal to 3 neutropenia. However, this did not lead to clinical consequences, such as dose reductions, modifications, or discontinuations, and, overall, the safety profile was generally comparable between global and Asian subgroups, with some numerical differences that were not deemed significant. 19 A possible explanation is that the higher incidence of physician‐reported grade greater than or equal to 3 neutropenia (excluding febrile neutropenia, infections, thrombocytopenia, or dose modifications/dose reductions) reported in both the R‐CHOP and Pola‐R‐CHP arms in the Asia subgroup 4 could be related to other components of the R‐CHP regimen, polatuzumab vedotin itself, or regional differences in event‐reporting practices.
Variations in drug efficacy and safety can occur between patients of different ethnicities, which can lead to dose adjustments being required across patient subpopulations. 1 Although these analyses showed no meaningful differences in exposure between Asian and non‐Asian patients receiving polatuzumab vedotin, patient recruitment diversity for clinical studies remains crucial to ensuring clinical understanding of new therapies is representative of real‐world patient populations. Adequate representation ensures the valid translation of clinical trial findings across patient ethnicities. Widespread adoption of this approach across clinical studies could help physicians alleviate treatment inequalities across patient subpopulations to improve patient outcomes globally. 21
CONCLUSIONS
In conclusion, this analysis of previously untreated patients with DLBCL from the POLARIX study showed polatuzumab vedotin PKs in Asian patients were similar to those for non‐Asian patients, and the PKs for patients enrolled from Asia were similar to those for patients enrolled from outside of Asia. This indicates dose adjustment for polatuzumab vedotin is not required to maintain efficacy and safety for Asian patients.
AUTHOR CONTRIBUTIONS
C.Le, M.Z.L., R.Den, L.G., T.L., P.A., R.Der, J.H., C.H., G.S., D.M., and C.Li wrote the manuscript. C.Le, J.H., C.H., and G.S. designed the study. C.Le, M.Z.L., R.Den, R.Der, J.H., C.H., and G.S. performed the research. C.Le, M.Z.L., R.Den, L.G., T.L., P.A., R.Der, J.H., D.M., and C.Li analyzed the data.
FUNDING INFORMATION
This study was sponsored by Genentech, Inc. and F. Hoffmann‐La Roche Ltd.
CONFLICT OF INTEREST STATEMENT
M.Z.L., T.L., P.A., R.Der, C.Le, J.H., C.Li, and D.M. are employees of Genentech, Inc. and receive F. Hoffmann‐La Roche Ltd. stocks/stock options. R.Den and L.G. are paid consultants for Genentech, Inc. C.H. has received research funding from Takeda, honoraria from F. Hoffmann‐La Roche Ltd., Janssen‐Cilag, AbbVie, and Gilead Sciences, and has received travel, accommodation, and expenses from F. Hoffman‐La Roche Ltd., Janssen‐Cilag, and AbbVie. G.S. has had a consulting or advisory role with F. Hoffmann‐La Roche Ltd., Genentech, Inc. Janssen, Novartis, Morphosys, Epizyme, Genmab, Debiopharm Group, Velosbio, Bristol‐Myers Squibb, BeiGene, Incyte, Miltenyi Biotec, Ipsen, AbbVie, Kite/Gilead Sciences, Loxo/Lilly, Molecular Partners, Nordic Nanovector, RAPT Therapeutics, Takeda, and Incyte, has received honoraria from AbbVie, Bayer, Regeneron, and Incyte, and receives Owkin stocks/stock options.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
The authors thank the patients who participated in POLARIX, their families, and the research nurses, study coordinators, and operations staff. POLARIX, and the current analysis, were sponsored by Genentech, Inc. and F. Hoffmann‐La Roche Ltd. Third‐party medical writing assistance, under the direction of the authors, was provided by Leen Al‐Mohammad, BSc, of Ashfield MedComms, an Inizio company, and was funded by F. Hoffmann‐La Roche Ltd.
Liao MZ, Deng R, Gibiansky L, et al. Ethnic sensitivity assessment: Polatuzumab vedotin pharmacokinetics in Asian and non‐Asian patients with previously untreated diffuse large B‐cell lymphoma in POLARIX. Clin Transl Sci. 2023;16:2744‐2755. doi: 10.1111/cts.13669
DATA AVAILABILITY STATEMENT
Qualified researchers may request access to individual patient level clinical data through a data request platform. At the time of writing, this request platform is Vivli https://vivli.org/ourmember/roche/. For up‐to‐date details on Roche's Global Policy on the Sharing of clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re‐identification.
REFERENCES
- 1. Shi R, Lu T, Ku G, et al. Asian race and origin have no clinically meaningful effects on polatuzumab vedotin pharmacokinetics in patients with relapsed/refractory B‐cell non‐Hodgkin lymphoma. Cancer Chemother Pharmacol. 2020;86:347‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dornan D, Bennett F, Chen Y, et al. Therapeutic potential of an anti‐CD79b antibody‐drug conjugate, anti‐CD79b‐vc‐MMAE, for the treatment of non‐Hodgkin lymphoma. Blood. 2009;114:2721‐2729. [DOI] [PubMed] [Google Scholar]
- 3. Sehn LH, Herrera AF, Flowers CR, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B‐cell lymphoma. J Clin Oncol. 2020;38:155‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tilly H, Morschhauser F, Sehn LH, et al. Polatuzumab Vedotin in previously untreated diffuse large B‐cell lymphoma. N Engl J Med. 2022;386:351‐363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Genentech Inc . Genentech presents new and updated data for polivy in previously untreated diffuse large B‐cell lymphoma at ASH 2022 . 2022. Accessed August 2023. https://www.gene.com/media/press‐releases/14976/2022‐12‐11/genentech‐presents‐new‐and‐updated‐data
- 6. European Medicines Agency . POLIVY® summary of product characteristics . 2022. Accessed August 2023. https://www.ema.europa.eu/en/documents/product‐information/polivy‐epar‐product‐information_en.pdf
- 7. Genentech, Inc . FDA advisory committee votes in favor of the clinical benefit of genentech's polivy combination for people with previously untreated diffuse large B‐cell lymphoma . 2023; Accessed August 2023 https://www.gene.com/media/press‐releases/14984/2023‐03‐09/fda‐advisory‐committee‐votes‐in‐favor‐of
- 8. Chugai . Chugai obtains regulatory approval for POLIVY for additional indication of previously untreated diffuse large B‐cell lymphoma . 2022. Accessed August 2023. https://www.chugai‐pharm.co.jp/english/news/detail/20220824150000_944.html
- 9. Tyson RJ, Park CC, Powell JR, et al. Precision dosing priority criteria: drug, disease, and patient population variables. Front Pharmacol. 2020;11:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorovits B, Alley SC, Bilic S, et al. Bioanalysis of antibody‐drug conjugates: American Association of Pharmaceutical Scientists antibody‐drug conjugate working group position paper. Bioanalysis. 2013;5:997‐1006. [DOI] [PubMed] [Google Scholar]
- 11. Kaur S, Xu K, Saad OM, Dere RC, Carrasco‐Triguero M. Bioanalytical assay strategies for the development of antibody‐drug conjugate biotherapeutics. Bioanalysis. 2013;5:201‐226. [DOI] [PubMed] [Google Scholar]
- 12. Lu D, Lu T, Shi R, et al. Application of a two‐analyte integrated population pharmacokinetic model to evaluate the impact of intrinsic and extrinsic factors on the pharmacokinetics of polatuzumab vedotin in patients with non‐Hodgkin lymphoma. Pharm Res. 2020;37:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng R, Gibiansky L, Lu T, et al. Polatuzumab vedotin population pharmacokinetics (popPK) and exposure‐response (ER) analyses from the POLARIX study in previously untreated diffuse large B‐cell lymphoma (DLBCL). American Conference on Pharmacometrics. 2022.
- 14. Lu D, Lu T, Gibiansky L, et al. Integrated two‐analyte population pharmacokinetic model of polatuzumab vedotin in patients with non‐Hodgkin lymphoma. CPT Pharmacometrics Syst Pharmacol. 2020;9:48‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palanca‐Wessels MC, Czuczman M, Salles G, et al. Safety and activity of the anti‐CD79B antibody‐drug conjugate polatuzumab vedotin in relapsed or refractory B‐cell non‐Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16:704‐715. [DOI] [PubMed] [Google Scholar]
- 16. Hatake K, Kinoshita T, Terui Y, et al. A phase I pharmacokinetic and safety study of polatuzumab vedotin in Japanese patients with relapsed/refractory B‐cell non‐Hodgkin lymphoma: a comparison with non‐Japanese DCS4968g study. Jpn J Clin Oncol. 2016;34:e19070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li C, Wang B, Lu D, et al. Ethnic sensitivity assessment of the antibody‐drug conjugate trastuzumab emtansine (T‐DM1) in patients with HER2‐positive locally advanced or metastatic breast cancer. Cancer Chemother Pharmacol. 2016;78:547‐558. [DOI] [PubMed] [Google Scholar]
- 18. Suri A, Mould DR, Liu Y, Jang G, Venkatakrishnan K. Population PK and exposure–response relationships for the antibody–drug conjugate brentuximab Vedotin in CTCL patients in the phase III ALCANZA study. Clin Pharmacol Therapeut. 2018;104:989‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Song Y, Tilly H, Rai S, et al. Polatuzumab vedotin in previously untreated DLBCL: an Asia subpopulation analysis from the phase 3 POLARIX trial. Blood. 2023;141:1971‐1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li D, Lee D, Dere RC, et al. Evaluation and use of an anti‐cynomolgus monkey CD79b surrogate antibody–drug conjugate to enable clinical development of polatuzumab vedotin. Br J Pharmacol. 2019;176:3805‐3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kadam RA, Borde SU, Madas SA, Salvi SS, Limaye SS. Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res. 2016;7:137‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Data Availability Statement
Qualified researchers may request access to individual patient level clinical data through a data request platform. At the time of writing, this request platform is Vivli https://vivli.org/ourmember/roche/. For up‐to‐date details on Roche's Global Policy on the Sharing of clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re‐identification.


