Abstract
Vericiguat, a novel soluble guanylate cyclase (sGC) stimulator, is approved for the treatment of heart failure (HF) with reduced ejection fraction (HFrEF). Decreased nitric oxide (NO) availability, sGC desensitization to NO, sGC deficiency, and reduced cyclic guanosine monophosphate (cGMP) signaling are potential contributing factors for HF disease progression. Vericiguat works via stimulation of sGC in the critical NO‐sGC‐cGMP pathway. Vericiguat is primarily metabolized by glucuronidation via uridine diphosphate‐glucuronosyltransferase (UGT) isoforms UGT1A1 and UGT1A9. Urinary excretion and renal clearance of vericiguat are low. No intrinsic factor had a clinically relevant effect on vericiguat exposure. Vericiguat has low drug–drug interaction potential with no clinically relevant pharmacokinetic or pharmacodynamic interactions observed with warfarin, digoxin, aspirin, or sacubitril/valsartan. The global phase III study VICTORIA included patients with HFrEF who had a recent HF hospitalization or intravenous diuretic treatment for HF. Treatment with vericiguat on top of standard of care resulted in a 10% relative reduction in the primary composite outcome of death from cardiovascular causes or first hospitalization for HF. Vericiguat was well‐tolerated with low incidence of symptomatic hypotension and syncope compared to placebo. Given its positive benefit–risk profile, vericiguat is an important option for high‐risk patients with HFrEF who are already on guideline‐directed medical therapy and had recent worsening of HF. Future efforts to develop additional effective therapies are needed to further reduce morbidity and mortality in patients with HF.
Short abstract
Clinical and Translational Card for Vericiguat.
Mechanism of action: sGC stimulator.
Indication(s): In the US, vericiguat is indicated for the treatment of adults with symptomatic chronic HF and EF less than 45% following a hospitalization for HF or need for outpatient IV diuretics.
Dosage and administration: A starting dose of 2.5 mg is administered orally once daily with food. The dose is then doubled approximately every 2 weeks to reach the target maintenance dose of 10 mg once daily with food, as tolerated by the patient.
Major metabolic pathway: Vericiguat primarily undergoes glucuronidation by UGT1A9 and to a lesser extent by UGT1A1. CYP‐mediated metabolism is a minor clearance pathway (<5%).
Key PK characteristics: The time to maximum concentration of vericiguat is approximately 4 hours when taken with food; the half‐life is 30 hours in patients with HFrEF. The peak serum concentrations (Cmax) and % coefficient of variation (CV) for vericiguat 2.5 mg, 5 mg, and 10 mg are 120 μg/L (29%), 201 μg/L (29%), and 350 μg/L (29%), respectively. The areas under the curve (AUC) and % CV for vericiguat 2.5 mg, 5 mg, and 10 mg are 2300 μg×h/L (33.9%), 3850 μg×h/L (33.9%), and 6680 μg×h/L (33.9%), respectively.
INTRODUCTION
Vericiguat, a novel soluble guanylate cyclase (sGC) stimulator, is approved for the treatment of heart failure (HF) with reduced ejection fraction (HFrEF). 1 HF is a disease that affects ~64 million people worldwide, resulting in HF hospitalizations totaling over 35 million between 2010 and 2017 in the United States alone. 2 , 3 HF is most prevalent in those over 65 years of age, and, as the population ages, its prevalence is expected to increase by 46% from 2012 to 2030. 4 Approximately 25% of patients with HF die within 1 year of admission to the hospital and the majority die within the following 5 years. 5 Furthermore, the total direct and indirect medical cost of patients with HF was estimated to be $30.7 billion in 2012 in the United States and, with increased prevalence, this is projected to increase to $69.8 billion by 2030. 6 Therefore, there is a significant unmet need to develop new medications to treat HF. At least 50% of all patients with HF have HFrEF and hence vericiguat, currently approved for treatment of patients with HFrEF, provides an important new tool to alleviate the burden of HF. 7 In this review, we describe the regulatory journey, mechanism of action, and clinical data of vericiguat including efficacy, safety, pharmacokinetic (PK), and pharmacodynamic (PD) data.
Regulatory Approval
For nearly a decade, Merck (Rahway, NJ) and Bayer AG (Wuppertal, Germany) have collaborated to co‐develop vericiguat. Following the completion of the phase III study, Vericiguat Global Study in Patients With Heart Failure With Reduced Ejection Fraction (VICTORIA), a New Drug Application was submitted to the US Food and Drug Administration (FDA) in May 2020. After a comprehensive priority review of its benefit–risk profile, the FDA approved vericiguat in January 2021 for once‐daily oral administration to reduce the risk of cardiovascular death and HF hospitalization following either hospitalization for HF, or the need for outpatient intravenous (i.v.) diuretics in adults with symptomatic HF and ejection fraction (EF) less than 45%. 1 Marketing authorization applications were submitted to the European Medicines Agency (EMA) and the Pharmaceuticals and Medical Devices Agency (PMDA) in May 2020 and June 2020, respectively, and these were followed by approvals in July 2021 and June 2021, respectively. As of June 5, 2023, vericiguat has been approved in ~75 countries/regions around the world, including the United States, Europe, Japan, Australia, Switzerland, India, China, Tanzania, Saudi Arabia, and Canada. Across the globe, the prescribing information for vericiguat is generally similar, with some variation in terms of the indication, dosage and administration, contra‐indications, and warnings and precautions (Table 1). A case in point is the selection of the marketed dosing regimen for vericiguat. All regulatory agencies were aligned with the overall dose selection for vericiguat which consists of a starting dose of 2.5 mg followed by dose doublings approximately every 2 weeks as tolerated to achieve the targeted therapeutic dose of 10 mg. Although the overall dosing regimen (starting dose, titration, and target dose) is the same across the globe, there is a slight difference in dosage and administration instructions in terms of when to initiate vericiguat treatment and in the implementation of down‐titration or treatment interruption, as evident in the FDA, the EMA, and the PMDA labels (Table 1).
TABLE 1.
Key prescribing information for vericiguat and differences amongst global health authorities (the FDA, the EMA, and the PMDA).
| Topic | FDA Label | EMA Label | PMDA Label | Comment |
|---|---|---|---|---|
| Indication | To reduce the risk of cardiovascular death and HF hospitalization, following a hospitalization for HF or need for outpatient i.v. diuretics, in adults with symptomatic chronic HF and ejection fraction <45% | Treatment of symptomatic chronic HF in adult patients with reduced ejection fraction who are stabilized after a recent decompensation event requiring i.v. | Chronic HF (only in patients who are receiving standard treatment for chronic HF) |
|
| Contraindications |
|
Any medicine that contains another sGC stimulator | During treatment with any sGC stimulator | All three labels contraindicate concomitant use of other sGC stimulators. FDA label also specifies contraindication in pregnancy. |
| Dosage and administration | The recommended starting dose is 2.5 mg orally once daily with food. Double the dose every 2 weeks to reach the maintenance dose of 10 mg once daily, as tolerated by the patient. |
|
|
EMA and PMDA labels mention down‐titration or dose interruption in case of hypotension; EMA label is very specific about not initiating therapy in patients with low SBP. |
| Warning and precautions | Embryo‐fetal toxicity |
Healthcare provider should be consulted before taking vericiguat if patients have:
|
Hypotension is an important identified risk | FDA label focuses on potential reproductive harm, while PMDA and EMA labels focus on blood pressure‐related issues |
| Other | Not recommended for co‐administration with PDE5 inhibitors. | Not recommended for co‐administration with PDE5 inhibitors; not recommended in severe renal‐ or hepatic‐impaired patients | Administration of vericiguat should be judged carefully for severe hepatic‐ or renal‐impaired patients | FDA and PMDA labels do not explicitly discourage use in severe hepatic‐ or renal‐impaired patients |
Abbreviations: EMA, European Medicines Agency; FDA, U.S. Food and Drug Administration; HF, heart failure; i.v., intravenous; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PDE5, phosphodiesterase type 5; PMDA, Pharmaceuticals and Medical Devices Agency; SBP, systolic blood pressure; sGC, soluble guanylate cyclase.
Mechanism of Action
In HF there is an inability of the heart muscle to pump enough blood and oxygen to meet the body's metabolic demands. One approach to categorizing patients with HF is according to their EF, which is a measurement of the percentage of the left ventricle's volume that is pumped out with each contraction. Patients with HF and EF less than or equal to 40%, 41%–49%, or greater than or equal to 50% are designated as reduced EF (HFrEF), mildly reduced (referred to as mid‐range), or preserved EF (HFpEF), respectively. 8
Vericiguat is approved for the treatment of patients with HFrEF and works via stimulation of sGC in the nitric oxide (NO)‐sGC‐cyclic guanosine monophosphate (cGMP) pathway. 1 , 9 In this pathway, NO binds to and stimulates the heme‐containing moiety of the sGC enzyme and catalyzes the conversion of guanosine triphosphate to generate cGMP. 10 The cGMP acts a second messenger whose signaling is essential to maintaining normal vascular tone and cardiac contractility. 10 Decreased NO availability, sGC desensitization to NO, sGC deficiency, and reduced cGMP signaling are potential contributing factors for HF disease progression. 11 Vericiguat directly stimulates sGC independent of NO involvement (Figure 1). 11 It also sensitizes sGC to NO by stabilizing the nitrosyl‐heme complex. 10 Vericiguat restores the signaling of the diminished NO‐sGC‐cGMP pathway, even under conditions of low NO availability and oxidative stress, leading to improvements in cardiovascular functions. 10 Targeting NO‐sGC‐cGMP signaling with organic nitrates (i.e., NO donors), which directly stimulate sGC, has been used to treat HF for many years. Unfortunately, nitrate use is associated with side effects including headache, dizziness, and hypotension. More importantly, development of tolerance to nitrates is common, limiting the beneficial effects of this therapy with long‐term use. 10 Other drugs targeting this pathway include phosphodiesterase type 5 (PDE5) inhibitors, which limit the degradation of cGMP but require the availability of NO, which is decreased in patients with HF. 10 PDE5 inhibitors are not recommended for the treatment of HF. 10
FIGURE 1.
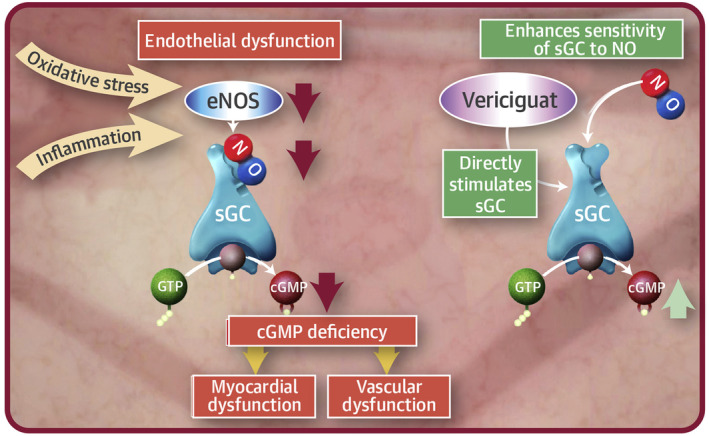
Mechanism of action of vericiguat. In heart failure (orange), oxidative stress and inflammation contribute to endothelial dysfunction in part through decreases in eNOS resulting in decreased NO availability. This decrease in NO leads to decreases in sGC activity and cGMP production. cGMP deficiency leads to myocardial and vascular dysfunction. Treatment with vericiguat (green) both increases the sensitivity of sGC to activation by NO and directly stimulates sGC activity, ameliorating the deficiency in cGMP, thereby improving cardiovascular functions. From Armstrong PW et al. 11 Copyright © 2018, with permission from Elsevier. cGMP, cyclic guanylate monophosphate; eNOS, endothelial nitric oxide synthase; GTP, guanosine‐5′‐triphosphate; NO, nitric oxide; sGC, soluble guanylate cyclase.
Other HF pharmacotherapies work on molecular pathways that are distinct from vericiguat. These therapies include drugs that target the renin‐angiotensin‐aldosterone system, including angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers, angiotensin receptor‐neprilysin inhibitors (ARNIs; such as sacubitril/valsartan), and mineralocorticoid receptor antagonists (MRAs), as well as sodium‐glucose cotransporter type 2 (SGLT‐2) inhibitors, beta‐blockers, and diuretics. Several of these therapies were developed to counteract adaptive neurohumoral pathways that are dysregulated in response to HF. In contrast, vericiguat restores a deficient pathway that is required to maintain normal cardiovascular functioning. 10
In practice, ACEIs, ARNIs, beta‐blockers, MRAs, and the SGLT‐2 inhibitors dapagliflozin and empagliflozin are considered first‐line treatment options for HFrEF. 8 Vericiguat may be added to treat those who continue to worsen while on the standard of care. 8 Unlike some drugs currently considered as standard of care, vericiguat can be administered once daily, does not require laboratory testing for dose adjustment, and does not increase the risk of electrolyte imbalance or worsen renal function in the setting of hypovolemia. Furthermore, vericiguat effectively engages the NO‐sGC pathway with minimal impact to blood pressure and without the development of tolerance seen with nitrates. 12
Pharmacokinetic/Pharmacodynamic characteristics
PK and PD properties of vericiguat have been characterized in 29 phase I, three phase II, and one phase III study with additional studies ongoing (Figure 2).
FIGURE 2.
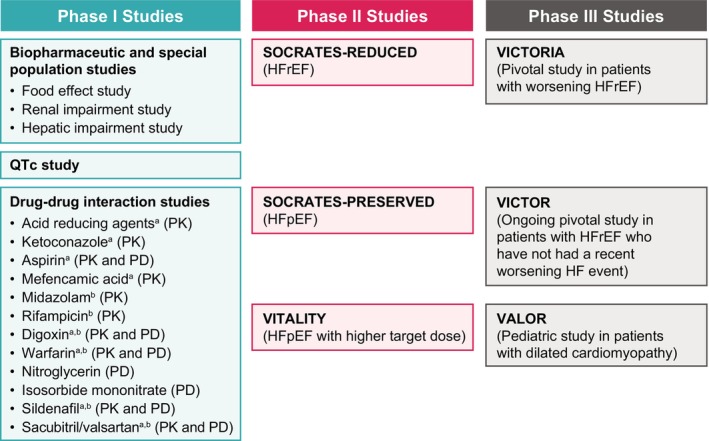
Key clinical trials of vericiguat throughout phases of development. HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PD, pharmacodynamics; PK, pharmacokinetics. aA study to assess vericiguat as victim of PK drug–drug interactions. bA study to assess vericiguat as perpetrator of PK drug–drug interactions.
Pharmacokinetics
Following oral administration of tablets, vericiguat is rapidly absorbed, with a time to reach maximum plasma concentration that ranges from about 1 h (fasted condition) to about 4 h (fed condition), and demonstrates slightly less than linear PK, with an approximate 30‐h half‐life. 1 Administration of vericiguat with food reduces PK variability and increases exposure; patients are therefore instructed to take vericiguat with food. 1 When taken with food, the absolute bioavailability of vericiguat is 93%. 1 Vericiguat primarily undergoes glucuronidation by UGT1A9 and, to a lesser extent, by UGT1A1 to form an inactive N‐glucuronide metabolite. Cytochrome P‐mediated metabolism is a minor clearance pathway (<5%) of vericiguat. 1
In addition to intensive PK data in clinical pharmacology studies, sparse PK data were collected in the phase II trial SOCRATES‐REDUCED (Soluble Guanylate Cyclase Stimulator in Heart Failure Study) and the phase III trial VICTORIA. 13 , 14 PK data from these two studies were used to develop a population PK model to characterize vericiguat PK in patients with HFrEF. 14 Results from phase I studies along with this integrated phase II/III population PK model were considered in assessing the impact of intrinsic factors on vericiguat PK in patients with HFrEF. The magnitudes of effects of intrinsic factors, such as age, body weight, or race/ethnicity, on vericiguat exposure are not clinically relevant. The impacts of mild‐to‐moderate renal or hepatic impairment on vericiguat exposure are also not clinically meaningful and do not necessitate dose adjustment. 1 Vericiguat has not been studied in patients with severe renal or hepatic impairment.
Pharmacodynamics
During the clinical development of vericiguat, N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) was used as a biomarker of efficacy since its level has been shown to be predictive of clinical outcome in HF. 15 Dose‐ and exposure‐dependent decreases in NT‐proBNP were observed in the SOCRATES‐REDUCED trial. 13 , 16
Consistent with its mechanism of action as an sGC stimulator, vericiguat exhibits PD effects in the vasculature leading to small hemodynamic changes. The mean reduction in systolic blood pressure was ~1 to 2 mmHg greater in patients who received vericiguat compared with placebo. 1 Correlation between vericiguat plasma concentrations and mild hemodynamic effects on blood pressure in patients with HFrEF was evident after the first dose of vericiguat, a relationship that was no longer present upon repeated dosing. Therefore, a titration regimen (starting dose of 2.5 mg and target dose of 10 mg) was implemented in VICTORIA. 11 Vericiguat showed no proarrhythmic potential in a dedicated phase Ib QTc study in patients with chronic coronary syndrome. 9
Drug–drug interactions
Vericiguat has low potential for drug–drug interaction mediated by cytochrome enzymes or transporters. Concomitant use of acid‐reducing agents also does not meaningfully impact vericiguat PK. Vericiguat does not interact with warfarin, digoxin, aspirin, or sacubitril/valsartan PK and PD. 1 In addition, concomitant administration of vericiguat with nitroglycerin (a short‐acting nitrate) is well‐tolerated in patients with HF. 17 Although no PK interaction is observed between vericiguat and sildenafil or isosorbide mononitrate, there is limited experience with their concomitant use in patients with HF. 9 The current FDA label for vericiguat does not exclude concomitant use of nitrates, but recommends against concurrent use of a PDE5 inhibitor. 1
Key clinical trials
After robust evaluation in phase I studies, vericiguat was studied in patients with HF in two phase II studies: SOCRATES‐REDUCED and SCORATES‐PRESERVED. In the dose‐ranging phase II trial SOCRATES‐PRESERVED, vericiguat did not improve NT‐proBNP nor left atrial volume at 12 weeks compared with placebo, but there was an association with improvements in quality of life for patients with HFpEF as measured by changes from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score. 18 However, the phase IIb VITALITY trial failed to confirm this improvement in KCCQ score; based on the results of this trial, vericiguat has not been developed further for the treatment of patients with HFpEF. 19
SOCRATES‐REDUCED was a phase II dose‐ranging study conducted in patients with HFrEF with worsening HF. 16 Although vericiguat did not demonstrate a statistically significant reduction in NT‐proBNP levels in the pooled dose arms compared to placebo, an exploratory secondary analysis did show that vericiguat decreased NT‐proBNP levels in a dose‐dependent manner, with higher doses leading to greater reductions. Based on these data, vericiguat was subsequently evaluated in the phase III VICTORIA trial in patients with HFrEF with worsening HF. 11 Dose titration was used, with patients starting at 2.5 mg daily, doubling their dose to 5 mg daily as tolerated (based on symptoms and blood pressure measurements), and doubling again after an additional 2 weeks to the target dose of 10 mg daily.
The VICTORIA trial enrolled patients who had either been hospitalized for HF in the prior 6 months or required treatment with i.v. diuretics during the prior 3 months. The rationale for this approach was a desire to study patients with worsening HF who have a very high risk of a recurrent HF event and therefore present the greatest unmet need. Additional criteria for enrollment included an EF of less than 45%; a New York Heart Association (NYHA) functional class II‐IV; brain natriuretic peptide (BNP) greater than or equal to 300 pg/mL or NT‐proBNP levels greater than or equal to 1000 pg/mL in patients without established atrial fibrillation or; BNP greater than or equal to 500 pg/mL or NT‐proBNP levels greater than or equal to 1600 pg/mL in patients with established atrial fibrillation; and an estimated glomerular filtration rate greater than or equal to 15 mL/min/1.73 m2. 20 As a result, the 5050 patients enrolled in the VICTORIA trial generally had a higher level of acuity compared to patients enrolled in other recently conducted pivotal trials in HF including PARADIGM‐HF (sacubitril/valsartan), DAPA‐HF, and EMPEROR‐Reduced (SGLT2 inhibitor therapies). 12 Importantly, a high proportion of VICTORIA participants received optimal standard of care treatment with so‐called triple therapy (ACEI or ARNI, beta‐blocker, and MRA inhibitor).
In this high‐risk population, treatment with vericiguat on top of standard of care resulted in a 10% relative reduction (Figure 3) compared to placebo in the risk of the primary outcome of a composite of death from cardiovascular causes or first hospitalization for HF after a median treatment period of 10.8 months. 20 This 10% relative reduction corresponded to an absolute risk reduction of 4.2%, which translates to an annualized number needed to treat of 24 patients to prevent one primary end point event. Significant reductions in total hospitalizations due to HF and the risk of the composite of hospitalization due to HF or all‐cause death were also observed. A 7% relative reduction in the risk of cardiovascular death was seen but this result was not statistically significant. The lower risk reduction for cardiovascular death compared to that seen for the primary end point may have reflected the relatively short treatment period, which was a consequence of the higher‐than‐expected primary event rate in this event‐driven trial. There was general consistency in the primary end point results across subgroups, including sex, race, geographic region, index event, use of sacubitril‐valsartan, NYHA class, and EF. Heterogeneity was seen in the prespecified subgroup of baseline NT‐proBNP by quartile; those in the highest quartile (>5314 pg/mL) did not appear to benefit from treatment with vericiguat. A subsequent post hoc analysis of the impact of baseline NT‐proBNP level analyzed along the continuum of NT‐proBNP level revealed a treatment benefit for the primary composite end point and cardiovascular death up to a level of 8000 pg/mL. 21
FIGURE 3.
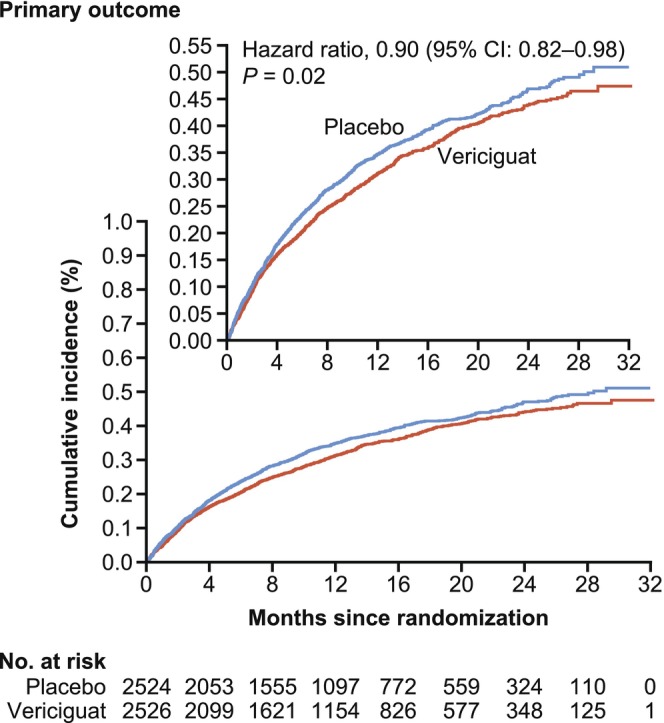
Estimates of the cumulative incidence of the primary outcome of the VICTORIA study. The primary outcome in VICTORIA was a composite of death from cardiovascular causes or first hospitalization for heart failure. The inset is the same data plotted on an enlarged y‐axis. From Armstrong PW et al. 20 Copyright © 2020 Massachusetts Medical Society, with permission from Massachusetts Medical Society. CI, confidence interval.
In VICTORIA, the prespecified adverse events of clinical interest were symptomatic hypotension and syncope. The incidences of these events were similarly low in the vericiguat and the placebo arms, with symptomatic hypotension occurring in 9.1% of the participants treated with vericiguat versus 7.9% of the participants treated with placebo (p = 0.12); syncope occurred in 4.0% of the participants on vericiguat versus 3.5% of the participants on placebo (p = 0.30). Treatment with vericiguat was well‐tolerated with 90.3% of patients reaching the 10 mg target dose after ~12 months (89.2% in the vericiguat group and 91.4% in the placebo group). Additional post hoc analysis of VICTORIA examined hypotension in vulnerable subpopulations, such as older patients (>75 years of age), those with lower baseline systolic blood pressure (100–110 mmHg), or those concurrently taking other vasoactive medications. These analyses showed no significant differences between vericiguat and placebo within these subgroups. 22 Taken together, these results suggest vericiguat is safe and well‐tolerated in a broad population of patients with HFrEF.
The phase III study VICTOR (VerICiguaT in adults with chrOnic heart failure and Reduced EF, NCT05093933), a pivotal trial enrolling patients with chronic HF and an EF less than or equal to 40% without recent hospitalization or in need of outpatient i.v. diuretic treatment, is ongoing. 23 The goal of this study is to evaluate the benefit of vericiguat in patients with HFrEF who have not had a recent worsening HF event and whose disease is therefore more stable than that of patients enrolled in the VICTORIA study. 23
In addition, the phase II/III VALOR study has been initiated in pediatric participants with dilated cardiomyopathy (NCT05714085). 24 This study will evaluate the appropriate dose in children as well as the ability of vericiguat to reduce levels of NT‐proBNP after 16 weeks of treatment in the pediatric population. Initial doses for this study were selected to achieve plasma levels of vericiguat exposure consistent with those seen in adult patients with HF. 24
Drugs under development for the HFrEF indication
Over the last 20 years, there have been many additions to therapies available for patients with HF. These advances have culminated in a guideline‐directed medical therapy (GDMT) that includes four different classes of medications that are taken for the purpose of decreasing mortality and improving the quality of life. 8 In addition to these GDMT therapies, vericiguat is now an option for high‐risk patients with HFrEF and recent worsening of HF. 8 If results from VICTOR and VALOR are favorable, use of vericiguat may be extended to more stable patients as well as to children. In HF, the identification of novel mechanisms with a favorable risk–benefit profile has been challenging. 25 However, persistent efforts to identify inroads for therapy continue as there is much room to improve the quality and duration of life for patients with HF.
Some of the therapies currently under investigation include those that modulate pathways known to affect HF prognosis, such as IONIS‐AGT‐LRx, which utilizes antisense technology for targeted inhibition of angiotensinogen activity (NCT04836182); ADP418, a selective β3‐adrenergic receptor inhibitor being evaluated in a phase II study (NCT05139615); or finerenone, a nonsteroidal MRA, currently in phase III (EudraCT Number: 2020‐000306‐29). In addition, novel cell‐based therapies providing stem cells to aid in the repair and restoration of cardiac tissue are being explored. 26 Thus, efforts to develop novel and more effective therapies continue despite the challenges.
CONCLUSION
Vericiguat, a novel oral sGC simulator, is an option for high‐risk patients with HFrEF already on GDMT with recent worsening of HF. Despite available therapies, there remains a significant unmet need. Future efforts to develop additional effective therapies targeting novel mechanisms are expected to result in improved patient care and reduced morbidity/mortality in patients with HF.
AUTHOR CONTRIBUTIONS
M.E.T., F.G., R.O.B., and S.A. wrote the manuscript.
FUNDING INFORMATION
This work was supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
CONFLICT OF INTEREST STATEMENT
M.E.T., S.A., R.O.B., and F.G. are employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA.
ACKNOWLEDGMENTS
Editorial support, including fact checking, referencing, figure preparation, formatting, and proofreading was provided by Fiona Van, PhD, Eva Giannakouri, PhD, and Melissa Ward, BSc, all of Scion, London, UK, supported by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA according to Good Publication Practice guidelines (Link).
Trujillo ME, Ayalasomayajula S, Blaustein RO, Gheyas F. Vericiguat, a novel sGC stimulator: Mechanism of action, clinical, and translational science. Clin Transl Sci. 2023;16:2458‐2466. doi: 10.1111/cts.13677
REFERENCES
- 1. Food and Drug Administration . Verquvo™ (vericiguat) tablets. Highlights of prescribing information. Accessed May 11, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214377s000lbl.pdf
- 2. GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789‐1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal MA, Fonarow GC, Ziaeian B. National trends in heart failure hospitalizations and readmissions from 2010 to 2017. JAMA Cardiol. 2021;6:952‐956. doi: 10.1001/jamacardio.2020.7472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson SL, Tong X, King RJ, et al. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11:e004873. doi: 10.1161/CIRCHEARTFAILURE.117.004873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor CJ, Ordonez‐Mena JM, Roalfe AK, et al. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000‐2017: population based cohort study. BMJ. 2019;364:l223. doi: 10.1136/bmj.l223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heidenreich PA, Albert NM, Allen LA, et al. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606‐619. doi: 10.1161/HHF.0b013e318291329a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murphy SP, Ibrahim NE, Januzzi JL Jr. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324:488‐504. doi: 10.1001/jama.2020.10262 [DOI] [PubMed] [Google Scholar]
- 8. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895‐e1032. doi: 10.1161/cir.0000000000001063 [DOI] [PubMed] [Google Scholar]
- 9. European Medicines Agency . Verquvo summary of product characteristics. Accessed July 31, 2023. https://www.ema.europa.eu/en/documents/product‐information/verquvo‐epar‐product‐information_en.pdf
- 10. Breitenstein S, Roessig L, Sandner P, Lewis KS. Novel sGC stimulators and sGC activators for the treatment of heart failure. Handb Exp Pharmacol. 2017;243:225‐247. doi: 10.1007/164_2016_100 [DOI] [PubMed] [Google Scholar]
- 11. Armstrong PW, Roessig L, Patel MJ, et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial. JACC Heart Fail. 2018;6:96‐104. doi: 10.1016/j.jchf.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 12. Rao VN, Diez J, Gustafsson F, et al. Practical patient care considerations with use of vericiguat after worsening heart failure events. J Card Fail. 2023;29:389‐402. doi: 10.1016/j.cardfail.2022.10.431 [DOI] [PubMed] [Google Scholar]
- 13. Ruehs H, Klein D, Frei M, et al. Population pharmacokinetics and pharmacodynamics of vericiguat in patients with heart failure and reduced ejection fraction. Clin Pharmacokinet. 2021;60:1407‐1421. doi: 10.1007/s40262-021-01024-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Trujillo ME, Arrington L, Patel Y, et al. Population pharmacokinetics of vericiguat in patients with heart failure with reduced ejection fraction: an integrated analysis. Clin Pharmacol Ther. 2022;112:1061‐1069. doi: 10.1002/cpt.2712 [DOI] [PubMed] [Google Scholar]
- 15. Schmitt W, Rühs H, Burghaus R, et al. NT‐proBNP qualifies as a surrogate for clinical endpoints in heart failure. Clin Pharmacol Ther. 2021;110:498‐507. doi: 10.1002/cpt.2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gheorghiade M, Greene SJ, Butler J, et al. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES‐REDUCED randomized trial. JAMA. 2015;314:2251‐2262. doi: 10.1001/jama.2015.15734 [DOI] [PubMed] [Google Scholar]
- 17. Boettcher M, Düngen HD, Donath F, et al. Vericiguat in combination with short‐acting nitroglycerin in patients with chronic coronary syndromes: the randomized, phase Ib, VENICE study. Clin Pharmacol Ther. 2022;111:1239‐1247. doi: 10.1002/cpt.2574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pieske B, Maggioni AP, Lam CSP, et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: results of the SOluble guanylate cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES‐PRESERVED) study. Eur Heart J. 2017;38:1119‐1127. doi: 10.1093/eurheartj/ehw593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong PW, Lam C, Anstrom K, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY‐HFpEF randomized clinical trial. JAMA. 2020;324:1512‐1521. doi: 10.1001/jama.2020.15922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armstrong PW, Pieske B, Anstrom KJ, et al. Vericiguat in patients with heart failure and reduced ejection fraction. N Engl J Med. 2020;382:1883‐1893. doi: 10.1056/NEJMoa1915928 [DOI] [PubMed] [Google Scholar]
- 21. Ezekowitz JA, O'Connor CM, Troughton RW, et al. N‐terminal pro‐B‐type natriuretic peptide and clinical outcomes: vericiguat heart failure with reduced ejection fraction study. JACC Heart Fail. 2020;8:931‐939. doi: 10.1016/j.jchf.2020.08.008931-939 [DOI] [PubMed] [Google Scholar]
- 22. Lam CSP, Mulder H, Lopatin Y, et al. Blood pressure and safety events with vericiguat in the VICTORIA trial. J Am Heart Assoc. 2021;10:e021094. doi: 10.1161/jaha.121.021094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. ClinicalTrials.gov . A study of vericiguat (MK‐1242) in participants with chronic heart failure with reduced ejection fraction (HFrEF) (MK‐1242‐035) (VICTOR). Accessed April 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05093933
- 24. ClinicalTrials.gov . Efficacy, safety, and pharmacokinetics of vericiguat in pediatric participants with heart failure due to left ventricular systolic dysfunction (MK‐1242‐036). Accessed April 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05714085
- 25. Teerlink JR, Diaz R, Felker GM, et al. Effect of ejection fraction on clinical outcomes in patients treated with omecamtiv mecarbil in GALACTIC‐HF. J Am Coll Cardiol. 2021;78:97‐108. doi: 10.1016/j.jacc.2021.04.065 [DOI] [PubMed] [Google Scholar]
- 26. Biocardia . BioCardia announces US patent on bone marrow derived neurokinin‐1 receptor positive (NK1R+) mesenchymal stem cells for therapeutic applications. Accessed June 22, 2023. https://www.globenewswire.com/news‐release/2023/05/09/2664254/0/en/BioCardia‐Announces‐US‐Patent‐on‐Bone‐Marrow‐Derived‐Neurokinin‐1‐Receptor‐Positive‐NK1R‐Mesenchymal‐Stem‐Cells‐for‐Therapeutic‐Applications.html


