Abstract
Oral corticosteroid use is limited by side effects, some caused by off‐target actions on the mineralocorticoid receptor that disrupt electrolyte balance. AZD9567 is a selective, nonsteroidal glucocorticoid receptor modulator. The efficacy, safety, and tolerability of AZD9567 and prednisolone were assessed in a phase IIa study. Anti‐inflammatory mechanism of action was also evaluated in vitro in monocytes from healthy donors. In this randomized, double‐blind, parallel‐group, multicenter study, patients with active rheumatoid arthritis were randomized 1:1 to AZD9567 40 mg or prednisolone 20 mg once daily orally for 14 days. The primary end point was change from baseline in DAS28‐CRP at day 15. Secondary end points included components of DAS28‐CRP, American College of Rheumatology (ACR) response criteria (ACR20, ACR50, and ACR70), and safety end points, including serum electrolytes. Overall, 21 patients were randomized to AZD9567 (n = 11) or prednisolone (n = 10), and all completed the study. As anticipated, AZD9567 had a similar efficacy profile to prednisolone, with no clinically meaningful (i.e., >1.0) difference in change from baseline to day 15 in DAS28‐CRP between AZD9567 and prednisolone (least‐squares mean difference: 0.47, 95% confidence interval: −0.49 to 1.43). Similar results were observed for the secondary efficacy end points. In vitro transcriptomic analysis showed that anti‐inflammatory responses were similar for AZD9567, prednisolone, and dexamethasone. Unlike prednisolone, AZD9567 had no effect on the serum sodium:potassium ratio. The safety profile was not different from that of prednisolone. Larger studies of longer duration are required to determine whether AZD9567 40 mg may in the future be an alternative to prednisolone in patients with inflammatory disease.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
AZD9567, a first‐in‐class, selective, nonsteroidal glucocorticoid receptor modulator, has an improved anti‐inflammatory–dysglycemic side‐effect profile compared with prednisolone and shows greater selectivity for the glucocorticoid receptor over the mineralocorticoid receptor than prednisolone.
WHAT QUESTION DID THIS STUDY ADDRESS?
This study assessed the anti‐inflammatory efficacy and safety of AZD9567 versus prednisolone in patients with active rheumatoid arthritis (RA). Preclinical evaluation aimed to elucidate the underlying mechanisms driving the anti‐inflammatory effects for AZD9567 versus prednisolone.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
AZD9567 40 mg and prednisolone 20 mg had a similar efficacy profile in patients with active RA. In vitro transcriptomic analysis suggests that the anti‐inflammatory response of AZD9567 is consistent with that of prednisolone and dexamethasone. Unlike prednisolone, AZD9567 had no effect on the serum sodium: potassium ratio, consistent with its higher selectivity for the glucocorticoid receptor over the mineralocorticoid receptor.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
AZD9567 40 mg may be a mechanistically differentiated alternative to prednisolone in patients with inflammatory disease.
INTRODUCTION
Oral corticosteroids, such as prednisolone, are potent anti‐inflammatory drugs used widely to treat chronic inflammatory diseases, including rheumatoid arthritis (RA). 1 , 2 However, the duration and dose of oral corticosteroid therapy are limited by serious side effects from unwanted actions on the glucocorticoid receptor, such as hyperglycemia and reduced bone density, and off‐target actions on the mineralocorticoid receptor that disrupt electrolyte balance and increase water retention. 3 In over 60 years of corticosteroid use, the uncoupling of their therapeutic anti‐inflammatory effects from their side effects, by identifying novel selective ligands of the glucocorticoid receptor, has not been successfully demonstrated in the clinic. 4 An anti‐inflammatory medication with similar efficacy to prednisolone but with a reduced side effect risk would therefore be greatly beneficial to patients requiring long‐term oral corticosteroid treatment.
AZD9567 is a first‐in‐class, oral, selective, nonsteroidal glucocorticoid receptor modulator being developed as an alternative to oral corticosteroids for inflammatory disease. In vitro, AZD9567 has higher affinity for the glucocorticoid receptor and 104‐fold lower affinity for the mineralocorticoid receptor than prednisolone, 5 suggesting that it could be less disruptive to electrolyte balance. AZD9567 binds the glucocorticoid receptor differently from steroids. 5 In preclinical experiments, AZD9567 had similar anti‐inflammatory effects to prednisolone, both in vivo in a rat model of joint inflammation and ex vivo by inhibition of lipopolysaccharide‐stimulated tumor necrosis factor α (TNFα) release in human whole blood. 5 However, AZD9567 has been shown to have a less deleterious effect than prednisolone on glucose homeostasis in vitro: it does not upregulate transcription of gluconeogenic enzymes in human hepatocytes, unlike prednisolone, 5 , 6 and inhibition of glucose‐stimulated insulin secretion in human pancreatic islets is twofold lower with AZD9567. 6 Phase I study data in healthy volunteers support preclinical findings: ex vivo inhibition of lipopolysaccharide‐stimulated TNFα release in whole blood and results of oral glucose tolerance tests indicate that AZD9567 has an improved anti‐inflammatory–dysglycemic side‐effect profile versus prednisolone. 6 Furthermore, AZD9567 had no clinically meaningful effects on serum electrolytes in healthy volunteers. 6
The aim of this proof‐of‐principle phase IIa study was to assess the efficacy and safety of AZD9567 versus prednisolone in patients with active RA, using a clinical disease activity score to evaluate efficacy. Preclinical evaluation aimed to elucidate underlying mechanisms that drive anti‐inflammatory effects for AZD9567, prednisolone, and dexamethasone.
METHODS
Phase IIa clinical study
Study design, participants, and procedures
This was a phase IIa randomized double‐blind parallel‐group multicenter study in patients aged 18–80 years with active RA, defined as a disease activity score in 28 joints with serum C‐reactive protein (DAS28‐CRP) of greater than or equal to 3.2 despite stable treatment with conventional disease‐modifying antirheumatic drugs. The study was conducted across five sites: University Medical Center Utrecht, Maastricht University Medical Center, and Medisch Spectrum Twente, The Netherlands, and Sahlgrenska University Hospital and Skåne University Hospital Lund in Sweden. The study was conducted according to the principles of the Declaration of Helsinki and the Good Clinical Practice Guideline of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Independent ethics committees at University Medical Center Utrecht (for sites in The Netherlands) and Regionala etikprövningsnämnden i Göteborg (for sites in Sweden) prospectively approved the study protocol before it was reviewed at participating sites. All participants provided written informed consent before inclusion. This trial is registered with ClinicalTrials.gov, number NCT03368235. The study protocol is available online via https://clinicaltrials.gov/ct2/show/NCT03368235.
AZD9567 and prednisolone were administered at doses predicted to be equipotent, based on a dose–response analysis of ex vivo inhibition of TNFα release in whole blood from phase I studies in healthy volunteers. 7 Within 7 days post‐screening, eligible participants were randomized 1:1 to either AZD9567 40 mg or prednisolone 20 mg once daily orally for 14 days. On day 5, study staff checked participants' well‐being via telephone. Participants attended clinic visits on days 8 and 15 and a follow‐up visit ~14 days after the last dose.
Full details of study inclusion criteria, assessments, and analyses are in the Data S1.
Randomization and masking
Randomization was performed using a computer‐generated randomization code supplied by the sponsor. Randomization was done via a centralized interactive voice/web response system. Patients were block randomized 1:1 to either AZD9567 or prednisolone. The study was conducted in a double‐blind manner, with patients, study site personnel, and sponsor personnel blinded to treatment assignment.
AZD9567 was provided as a suspension with a matching placebo. Prednisolone was provided as a capsule with a matching placebo. Because AZD9567 and prednisolone were not similar in appearance, a double dummy method was used: at each dosing occasion, participants receiving AZD9567 were also given a placebo for prednisolone, and participants receiving prednisolone were also given a placebo for AZD9567.
End points
The primary end point was change from baseline to day 15 in DAS28‐CRP. Secondary end points included: proportions of patients achieving American College of Rheumatology (ACR) 20, 50, and 70 response criteria at day 15; change from baseline in 68 tender joint count (TJC68) and 66 swollen joint count (SJC66); change from baseline in scores of the individual components of DAS28‐CRP and ACR response; safety and tolerability, including adverse events, clinical chemistry (including, but not limited to, fasting plasma glucose and serum electrolytes), and vital signs; and AZD9567 pharmacokinetics. Exploratory end points included: prednisolone pharmacokinetics; anti‐inflammatory effects on lipopolysaccharide‐stimulated cytokine release in whole blood ex vivo; and hypothalamic–pituitary–adrenal axis activity using serum cortisol levels. Bone formation/resorption balance was evaluated using the following serum biomarkers: procollagen‐1N‐terminal peptide (P1NP) and osteocalcin (bone formation), C‐terminal telopeptide of type 1 collagen (CTX‐1; bone resorption), and metabolites of collagens type 1, 3, and 4 (C1M, C3M, and C4M; soft tissue turnover).
DAS28‐CRP was evaluated using the formula from Wells et al. 8 at screening, baseline, on days 8 and 15, and at follow‐up. A clinically meaningful change in DAS28‐CRP was defined as a reduction of greater than or equal to one point. 9 ACR response criteria, including TJC68 and SJC66, were evaluated according to Felson et al. 10 at the same timepoints. Adverse events were monitored throughout the study. Full details of the sampling schedule are in Data S1 and Table S1.
Statistical analyses
A sample size of 36 participants (18 per arm) was originally planned, based on a Lalonde‐type go/no‐go decision framework 11 using a reliability threshold for the DAS28 index of 0.6 12 and an assumed standard deviation of 2.3 for change from baseline in DAS28‐CRP. However, blinded data review and monitoring by the study sponsor revealed that data variability was lower than expected, indicating that a smaller sample size of greater than or equal to 10 participants per arm would be sufficient to address the study's primary objective. Recruitment was therefore stopped when each treatment group reached greater than or equal to 10 participants.
The primary end point, difference in DAS28‐CRP units (i.e., difference between the mean change from baseline with AZD9567 40 mg and prednisolone 20 mg), was used to estimate average difference in DAS28‐CRP between the two treatment groups. This difference was calculated using a mixed model with baseline DAS28‐CRP as a covariate and categorical fixed effects of treatment, visit, treatment‐by‐visit interaction, and country. Changes from baseline in secondary efficacy variables were also analyzed using mixed models. Each model included the baseline value for the variable of interest as a covariate, with categorical fixed effects of treatment, visit, treatment‐by‐visit interaction, and country. The study was not powered for inferential hypothesis testing.
Calculated pharmacokinetic parameters for AZD9567 and prednisolone were area under the concentration–time curve from time 0 to 6 h postdose (AUC(0–6 h)), time to maximum concentration (T max), maximum concentration (C max), and the last plasma concentration measured before the next dose. Inhibition of ex vivo cytokine release by AZD9567 and prednisolone was assessed separately for each cytokine using a sigmoid maximum effect model from which half‐maximal inhibitory concentration values were estimated. AUC(0–6 h) was calculated for serum osteocalcin. Bone balance, a measure incorporating bone resorption and formation, 13 was calculated as the ratio of serum concentrations of CTX‐1 to P1NP or osteocalcin.
The efficacy analysis population was the intention‐to‐treat population, including all randomized patients who received greater than or equal to one dose of study treatment. All participants who received greater than or equal to one dose of study drug were included in the safety analyses. The pharmacokinetic analysis set included all participants with greater than or equal to one quantifiable plasma AZD9567 concentration.
Statistical analyses were performed using SAS version 9.4 or higher (SAS Institute). Pharmacokinetic parameters were derived using noncompartmental methods with Phoenix WinNonlin version 8.0 (Certara).
Preclinical methods
To compare molecular mechanisms, monocytes isolated from the blood of six healthy donors were split and treated in vitro with four different single doses of AZD9567 (9–949 nM), prednisolone (32–3162 nM), or dexamethasone (3–316 nM), and incubated for 4 h with and without TNFα stimulation. The comparable doses used were based on half‐maximal effective concentration values from a dose‐setting experiment that assessed transcriptional effects on a set of glucocorticoid receptor‐regulated genes. Isolated RNA was transcriptionally characterized by RNA sequencing, using a paired‐end sequencing approach on an Illumina NovaSeq 6000 platform (Illumina).
Full details on preclinical methods are provided in Data S1 and Tables S2 and S3.
RESULTS
The phase IIa study took place from January 18, 2018 to November 12, 2019. Of 27 screened patients, 21 were randomized (AZD9567, n = 11; prednisolone, n = 10). All 21 participants completed the study and were included in the efficacy, pharmacokinetic, and safety analyses (Figure S1). There were slight imbalances between the AZD9567 and prednisolone groups at baseline (Table 1), with higher mean age, more women, and slightly greater disease severity (indicated by higher mean DAS28‐CRP, a higher proportion of patients with radiographic erosions, slightly higher functional class, and a higher number of patients treated with anti‐TNFα therapies) in the AZD9567 group.
TABLE 1.
Participant baseline demographics and disease characteristics.
| AZD9567 (n = 11) | Prednisolone (n = 10) | Overall (N = 21) | |
|---|---|---|---|
| Age, years | 64.5 (8.4) | 55.5 (13.6) | 60.2 (11.8) |
| Age group, years | |||
| 18–40 (%) | 0 | 2 (20.0) | 2 (9.5) |
| 41–65 (%) | 7 (63.6) | 6 (60.0) | 13 (61.9) |
| >65 (%) | 4 (36.4) | 2 (20.0) | 6 (28.6) |
| Female (%) | 8 (72.7) | 5 (50.0) | 13 (61.9) |
| White (%) | 11 (100) | 10 (100) | 21 (100) |
| Height, cm | 169.3 (9.4) | 171.0 (10.9) | 170.1 (9.9) |
| Weight, kg | 78.43 (13.28) | 80.67 (23.34) | 79.50 (18.29) |
| Years since onset of RA symptoms | 14.73 (14.59) | 13.35 (10.91) | 14.07 (12.67) |
| Years since RA diagnosis | 13.20 (15.24) | 12.79 (11.21) | 13.01 (13.14) |
| Presence of radiographic erosions (%) | 7 (63.6) | 5 (50.0) | 12 (57.1) |
| Rheumatoid factor positive (%) | 9 (81.8) | 9 (90.0) | 18 (85.7) |
| Functional capacity class (%) | |||
| Class I | 0 | 2 (22.2) | 2 (10.5) |
| Class II | 7 (70.0) | 5 (55.6) | 12 (63.2) |
| Class III | 3 (30.0) | 1 (11.1) | 4 (21.1) |
| Class IV | 0 | 1 (11.1) | 1 (5.3) |
| Previously treated with TNFα antagonist | 6 (54.5) a | 3 (30.0) | 9 (42.9) |
| Reason for TNFα antagonist discontinuation (%) | |||
| No response | 1 (20.0) | 1 (33.3) | 2 (25.0) |
| Subsequent loss of response | 2 (40.0) | 1 (33.3) | 3 (37.5) |
| Adverse effect/intolerance | 1 (20.0) | 1 (33.3) | 2 (25.0) |
| Other | 1 (20.0) | 0 | 1 (12.5) |
| DAS28‐CRP | 5.26 (0.98) | 4.90 (0.74) | 5.09 (0.87) |
| Comorbidities b (%) | |||
| Hypertension | 3 (27.3) | 4 (40.0) | 7 (33.3) |
| Hypothyroidism | 3 (27.3) | 1 (10.0) | 4 (19.0) |
| Hematuria | 0 | 3 (30.0) | 3 (14.3) |
| Concomitant medications (%) | |||
| Folic acid and derivatives | 8 (72.7) | 7 (70.0) | 15 (71.4) |
| Immunosuppressants, including methotrexate | 8 (72.7) | 7 (70.0) | 15 (71.4) |
| Anilides, including paracetamol/acetaminophen | 4 (36.4) | 3 (30.0) | 7 (33.3) |
| Nonsteroidal anti‐inflammatory and anti‐rheumatic agents, including hydroxychloroquine | 5 (45.5) | 2 (20.0) | 7 (33.3) |
Note: Data are n (%) or mean (SD).
Abbreviations: DAS28‐CRP, disease activity score in 28 joints with C‐reactive protein; RA, rheumatoid arthritis; SD, standard deviation; TNFα, tumor necrosis factor α.
One patient who was previously treated with TNFα antagonist continued treatment during the study.
Reported by at least three patients overall.
Efficacy results
In the primary efficacy analysis, the least‐squares mean difference in improvement from baseline to day 15 in DAS28‐CRP between the AZD9567 and prednisolone groups was 0.47 (95% confidence interval [CI]: −0.49 to 1.43), with the numerical difference between the groups being clinically non‐meaningful (i.e., <1.0; Figure 1a, Table S4). At all timepoints, least‐squares mean DAS28‐CRP overlapped with the 95% CI for the comparator group (Figure 1b, Table S4). Similar results were observed for the change from baseline in the four individual components of DAS28‐CRP: 28 tender joint count [TJC28], 28 swollen joint count [SJC28], global health, and CRP levels (Figure 1c–f, Table S4).
FIGURE 1.
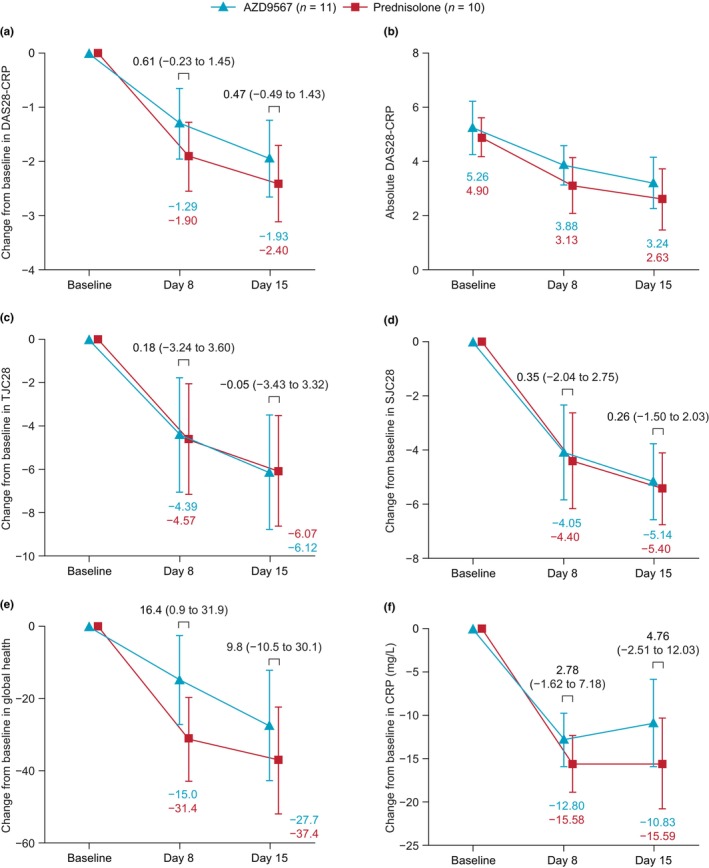
DAS28‐CRP and components. Change from baseline in: (a) DAS28‐CRP (primary endpoint); (b) absolute DAS28‐CRP; and individual components of DAS28‐CRP: (c) TJC28; (d) SJC28; (e) global health; and (f) CRP. Data are LS means with 95% CIs. Comparisons are LS mean differences for AZD9567 − prednisolone, with 95% CIs. Supporting data are shown in Table S4. CI, confidence interval; CRP, C‐reactive protein; DAS28‐CRP, disease activity score in 28 joints with C‐reactive protein; LS, least‐squares; SJC28, 28 swollen joint count; TJC28, 28 tender joint count.
Similar proportions of patients in both treatment groups achieved the ACR20, ACR50, and ACR70 response criteria, although proportions were numerically lower with AZD9567 (Figure S2a). Improvements in TJC68 and SJC66 from baseline to day 15 were similar in each group (Figure S2b,c,; Table S4); however, the reduction was numerically greater with AZD9567 for TJC68 and with prednisolone for SJC66. Similar results were observed for change from baseline in the three other individual components of the ACR response: pain score, disease activity, and physical function (Figure S2d–f, Table S4).
Serum sodium:potassium ratio
Nuclear hormone receptor binding profiles of AZD9567, prednisolone, and dexamethasone in the preclinical radioligand binding study showed that AZD9567 was more selective for the glucocorticoid receptor than for the mineralocorticoid receptor (Table S5). Significantly greater selectivity for the glucocorticoid receptor was observed with AZD9567 than for prednisolone (p = 0.0001) and dexamethasone (p = 0.0003).
Consistent with this, the serum sodium:potassium ratio was unchanged from baseline to day 15 in the AZD9567 group in the phase IIa study, but increased in the prednisolone group (Figures 2 and S3) because decreased potassium in the prednisolone group was not reported in the AZD9567 group. No changes in sodium levels were reported in either group (Figures 2 and S3). Both the serum potassium level and sodium:potassium ratio returned to baseline values at follow‐up in the prednisolone group.
FIGURE 2.
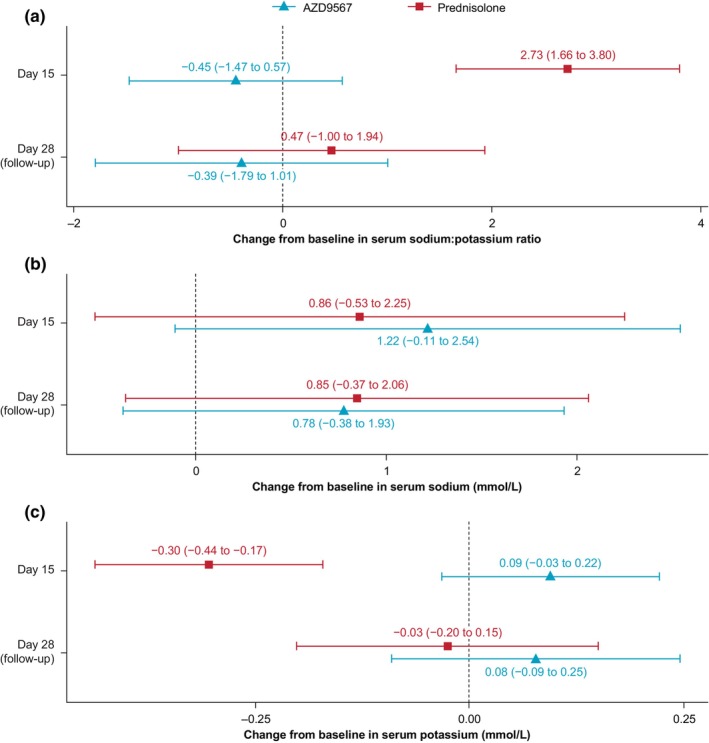
Morning serum sodium and potassium levels. Change from baseline in: (a) sodium:potassium ratio; (b) serum sodium; and (c) serum potassium. Data are least‐squares means with 95% confidence intervals.
Clinical chemistry
Fasting plasma glucose levels were similar between the AZD9567 and prednisolone groups throughout the study, with a mean decrease from baseline of ~0.5 mmol/L at day 8 and day 15 in both groups. There were no other clinically relevant findings in clinical chemistry, hematology, or urinalysis. There were also no clinically relevant findings in electrocardiographic or physical assessments, including body weight, or in vital signs, including systolic and diastolic blood pressure (although there was a slight increase from baseline in mean systolic blood pressure in the AZD9567 group at day 15; Figure S4).
AZD9567 and prednisolone pharmacokinetics
Pharmacokinetic analyses showed that AZD9567 and prednisolone were rapidly absorbed, with a median T max of 0.7 h and 1.5 h, respectively (Table 2). Following C max, elimination of both compounds appeared monophasic and plasma concentrations remained quantifiable until the last sampling time at 6 h postdose.
TABLE 2.
Pharmacokinetic parameters of AZD9567.
| Parameter | Summary statistic | AZD9567 (n = 11) | Prednisolone (n = 10) |
|---|---|---|---|
| AUC(0–6 h) (h·nmol/L) | Geometric mean (CV%) | 17,740 (35) | 3591 (22) |
| T max (h) | Median (min, max) | 0.7 (0.3, 1.0) | 1.5 (1.0, 1.5) |
| C max (nmol/L) | Geometric mean (CV%) | 4468 (27) | 980 (26) |
| C trough (nmol/L) | Geometric mean (CV%) | 382 (96) | 8 (102) |
Abbreviations: AUC(0–6 h), area under the concentration–time curve from time zero to 6 h after dose; C max, maximum observed concentration; C trough, observed trough plasma concentration; CV, coefficient of variation; T max, time to maximum observed concentration.
Lipopolysaccharide‐stimulated cytokine release
After lipopolysaccharide stimulation of whole blood ex vivo, both AZD9567 and prednisolone inhibited the release of all cytokines assessed (TNFα, interferon‐γ, interleukins 6 and 8, and macrophage inflammatory protein [MIP]‐1α and ‐1β); the relative inhibitory potency of AZD9567 versus prednisolone was similar for each cytokine (albeit with wide CIs; Figure S5).
Serum cortisol levels
Morning serum cortisol levels were reduced at day 15 versus baseline in both treatment groups, and the reduction was more pronounced with AZD9567 (Figure S6). Cortisol levels returned to near baseline values at follow‐up in both groups, without intervention.
Bone and soft tissue turnover biomarkers
No differences in individual bone and soft tissue biomarker levels in serum were observed between treatment groups other than for P1NP, which was decreased from baseline at day 15 with AZD9567 versus prednisolone, and C1M, which was decreased from baseline at day 15 with prednisolone versus AZD9567 (Figure S7). Bone balance, assessed as change from baseline to day 15 in CTX‐1:P1NP and CTX‐1:osteocalcin ratios, was similar with AZD9567 and prednisolone.
Safety
Similar numbers of participants in each group reported treatment‐emergent adverse events (AZD9567, n = 10 and prednisolone, n = 9; Table 3). Most adverse events were mild in severity. Six patients in the AZD9567 group and three patients in the prednisolone group reported adverse events assessed by the investigator as related to study treatment. The most common adverse events were cough (AZD9567, 2 patients; and prednisolone, 1 patient), fatigue (AZD9567, 3 patients), headache (AZD9567, 2 patients; and prednisolone, 1 patient), and hot flash (AZD9567, 3 patients). After completion of treatment, one serious adverse event of severe suicidal depression was reported by the patient's physician as related to AZD9567. The event resolved after ~1 month, and the patient was not hospitalized nor given any medical intervention.
TABLE 3.
Summary of participants with AEs.
| AZD9567 (n = 11), % | Prednisolone (n = 10), % | |
|---|---|---|
| Any AE | 10 (90.9) | 9 (90.0) |
| Mild | 6 (54.5) | 8 (80.0) |
| Moderate | 3 (27.3) | 1 (10.0) |
| Severe | 1 (9.1) | 0 |
| Any serious AE | 1 (9.1) a | 0 |
| Any treatment‐related AE | 6 (54.5) | 3 (30.0) |
| Any AE leading to discontinuation | 0 | 0 |
| AE by preferred term b | ||
| Abdominal pain (upper) | 2 (18.2) | 0 |
| Cough | 2 (18.2) | 1 (10.0) |
| Dry mouth | 2 (18.2) | 0 |
| Eye pain | 2 (18.2) | 0 |
| Fatigue | 3 (27.3) | 0 |
| Headache | 2 (18.2) | 1 (10.0) |
| Hot flash | 3 (27.3) | 0 |
| Increased appetite | 1 (9.1) | 1 (10.0) |
| Insomnia | 2 (18.2) | 0 |
| Nasopharyngitis | 1 (9.1) | 1 (10.0) |
| Treatment‐related AE by preferred term b | ||
| Abdominal pain (upper) | 2 (18.2) | 0 |
| Dry mouth | 2 (18.2) | 0 |
| Hot flash | 2 (18.2) | 0 |
| Increased appetite | 1 (9.1) | 1 (10.0) |
Note: Data are n (%). Includes AEs that started on or after the date of the first dose, up to and including 14 days after the date of last dose of study treatment (i.e., the follow‐up period).
Abbreviation: AE, adverse event.
One event of severe suicidal depression was reported in the AZD9567 group, reported by the patient's physician as related to study treatment.
AEs reported by at least two patients overall; patients with multiple events of the same preferred term are counted only once in that preferred term; preferred terms were coded by the Medical Dictionary for Regulatory Activities (MedDRA) version 22.1.
Human monocytes
Transcriptional profiling demonstrated clear dose–response data for AZD9567, prednisolone, and dexamethasone (Figure S8) and revealed that AZD9567 gene regulation exhibited a comprehensive overlap with the two corticosteroids (Figure 3a, Table S6). Predicted upstream regulator analysis and pathway analyses of differentially expressed genes confirmed that AZD9567, prednisolone, and dexamethasone exhibited similar pharmacological profiles (glucocorticoid receptor activation) typical of steroids, and predicted a similar anti‐inflammatory response in terms of leukocyte activation (Figure 3b).
FIGURE 3.
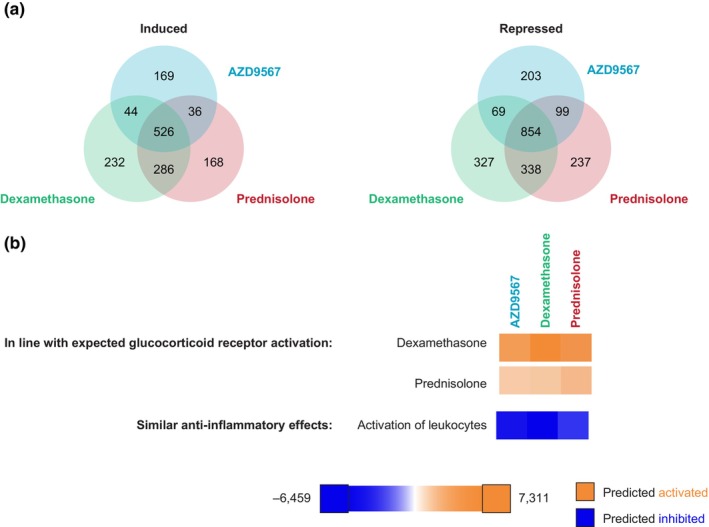
Overall AZD9567 treatment effects on gene transcription in primary monocytes stimulated with tumor necrosis factor α at 4 h (preclinical study). (a) Venn diagrams of protein‐coding genes induced or repressed by AZD9567, prednisolone, and dexamethasone at their highest concentrations (949, 3162, and 316 nM, respectively; false discovery rate < 0.05); (b) predicted upstream regulator analysis and pathway analyses of differentially expressed genes. A full list of protein‐coding genes induced or repressed by AZD9567, prednisolone, and dexamethasone at their highest concentrations is included in Table S6. Color by z‐score: blue for predicted inhibition (negative z‐score) and orange for predicted activation (positive z‐score).
A global view of all differentially expressed genes induced by both dexamethasone and prednisolone treatment showed that the AZD9567 and prednisolone responses correlated strongly with the dexamethasone response, with R 2 values of 0.968 for prednisolone log2 fold‐change versus dexamethasone log2 fold‐change, and 0.841 for AZD9567 log2 fold‐change versus dexamethasone log2 fold‐change (Figure 4). For the downregulated genes, R 2 values were 0.901 and the slope (b) was 0.87 for prednisolone log2 fold‐change versus dexamethasone log2 fold‐change, and R 2 values were 0.717 and the slope (b) was 0.77 for AZD9567 log2 fold‐change versus dexamethasone log2 fold‐change. For the upregulated genes, R 2 values were 0.931 and the slope (b) was 0.93 for prednisolone log2 fold‐change versus dexamethasone log2 fold‐change, and R 2 values were 0.658 and the slope (b) was 0.54 for AZD9567 log2 fold‐change versus dexamethasone log2 fold‐change. The data suggest that AZD9567 acts as a partial agonist in gene activation, inducing lower fold‐change in those genes while acting as a full agonist for gene repression, indicated by overlapping fold‐changes.
FIGURE 4.
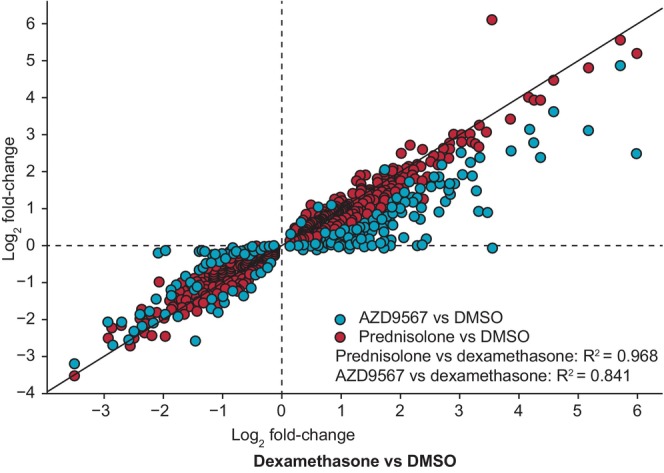
Log2 fold‐change of AZD9567 or prednisolone versus dexamethasone for differentially expressed genes common to prednisolone and dexamethasone (preclinical study). AZD9567 (blue) or prednisolone (red) effects compared with dexamethasone (highest concentration, respectively) for all differentially expressed genes (false discovery rate < 0.05) common to prednisolone and dexamethasone treatments in primary human monocytes stimulated with TNFα, at 4 h. DMSO, dimethyl sulfoxide; TNFα, tumor necrosis factor α.
Transcript analysis revealed that, although primary monocytes exhibited significant expression of glucocorticoid receptor, the other analyzed steroid receptor family members, including mineralocorticoid receptor, were not expressed (Figure S9).
DISCUSSION
In this phase IIa study in patients with active RA, the selective glucocorticoid receptor modulator AZD9567 40 mg had a similar efficacy profile to prednisolone 20 mg based on clinical disease activity measures. AZD9567 40 mg had previously been predicted to be equipotent to prednisolone 20 mg based on pharmacokinetic/pharmacodynamic modeling of anti‐inflammatory biomarkers in phase I studies. 7
Consistent with these data, AZD9567 and prednisolone performed similarly on multiple measures of anti‐inflammatory efficacy in patients with active RA, including reductions in the number of tender joints and swollen joints, reductions in serum CRP levels, and improvements in global health and treatment response (ACR20, ACR50, and ACR70). Moreover, AZD9567 and prednisolone inhibited the release of TNFα and other pro‐inflammatory cytokines assessed after lipopolysaccharide stimulation of whole blood ex vivo, with a similar relative potency against each cytokine, demonstrating that AZD9567 40 mg has a similar broad anti‐inflammatory profile to prednisolone 20 mg and further confirming previous biomarker findings in healthy volunteers. 6 , 7 Although many of the improvements in efficacy measures were numerically smaller in the AZD9567 group than in the prednisolone group, differences were not clinically meaningful, suggesting a similar efficacy profile. Numerical differences may have resulted from the imbalance between the groups in age, sex, and disease severity at baseline, as well as from the small sample size. The differences may also have resulted from the choice of AZD9567 dose. This was based on an estimate of ex vivo equipotency of AZD9567 40 mg with prednisolone 20 mg in healthy volunteers, 7 which was accompanied by a 95% CI from 29 to 54 mg.
An in vitro transcriptomic analysis (preclinical data) suggests an anti‐inflammatory response of AZD9567 consistent with those of prednisolone and dexamethasone. A radioligand binding study using overexpressed receptors determined that AZD9567 has a higher selectivity for the glucocorticoid receptor over the mineralocorticoid receptor. Consistent with this, and unlike prednisolone, AZD9567 had no effect on the serum sodium: potassium ratio. However, it should be noted that, in general, very few mineralocorticoid receptor transcriptomic published data exist, 14 and many genes are regulated by both glucocorticoid and mineralocorticoid receptors. 15 , 16 Indazole ethers are great tools to explore glucocorticoid receptor‐exclusive profiles, 17 but there are currently no equivalent tools for mineralocorticoid receptor‐exclusive profiles, and even aldosterone binds to the glucocorticoid receptor, albeit at a lower affinity than to the mineralocorticoid receptor. 18
In the clinical study, serum cortisol levels were reversibly reduced with both AZD9567 and prednisolone (more so in the AZD9567 group). Both AZD9567 and prednisolone were well tolerated, and there were no new safety findings of concern. Together with the reduced dysglycemia observed with AZD9567 40 mg compared with prednisolone 20 mg in previous phase I studies, 6 these findings suggest that AZD9567 is mechanistically differentiated from prednisolone. Further studies are warranted to support these preliminary data.
Transcriptomic and in vitro analysis in monocytes (preclinical data) revealed an anti‐inflammatory response to AZD9567 similar to that of prednisolone and dexamethasone. AZD9567 gene regulation exhibited a comprehensive overlap with the two corticosteroids, and AZD9567, prednisolone and dexamethasone exhibited similar pharmacological profiles (glucocorticoid receptor activation), typical of steroids, and predicted a similar anti‐inflammatory response with regard to leukocyte activation. This is in stark contrast to the functional profiling in primary hepatocytes, in which AZD9567 showed a clearly differentiated profile, suggestive of a reduced risk for hyperglycemia as measured by mRNA levels of tyrosine aminotransferase (a key enzyme in gluconeogenesis). 5
Some of the side effects of corticosteroids, such as edema, result from off‐target actions on the mineralocorticoid receptor that disrupt electrolyte balance and increase water retention. 3 , 19 The absence of AZD9567 effects on serum potassium levels in the present study and in previous phase I studies 6 is consistent with its demonstrated higher affinity for the glucocorticoid receptor and lower affinity for the mineralocorticoid receptor than prednisolone. 5 Together, these data support a mechanistic differentiation between AZD9567 and prednisolone.
Corticosteroid treatment also disrupts the regulation of endogenous cortisol concentrations via constant activation of the glucocorticoid receptor, suppressing hypothalamic–pituitary–adrenal axis activity and thus reducing levels of cortisol. 19 , 20 Here, both AZD9567 and prednisolone reduced morning cortisol levels, demonstrating activation of the glucocorticoid receptor. Cortisol suppression appeared more pronounced in the AZD9567 group than in the prednisolone group. These changes were reversible, and levels had spontaneously returned to near baseline values at follow‐up, 2 weeks after discontinuation of treatment.
Similar effects on fasting plasma glucose were reported with AZD9567 and prednisolone, although observable differences in this parameter between the drugs were not expected based on previous findings. 6 , 21 Reduced disruption of glycemic control with AZD9567 versus prednisolone was evident in a healthy volunteer study: with AZD9567 doses up to 80 mg, plasma glucose after an oral glucose tolerance test was similar to that observed with prednisolone 5 mg. 6 The effects of the study drugs on glycemic control were not assessed in the present phase IIa study; however, a phase IIa study in adults with type 2 diabetes has been conducted to evaluate these effects (NCT04556760).
Similar changes from baseline in serum CTX‐1:P1NP and CTX‐1:osteocalcin ratios were observed with AZD9567 and prednisolone. This may indicate that these drugs have similar effects on bone balance, a measure of overall bone metabolism that assesses the equilibrium between bone formation and resorption using ratios of biomarker levels (such as osteocalcin or P1NP for formation and CTX‐1 for resorption). 13 Safety and exploratory end point findings for AZD9567 in this phase IIa study should be interpreted with caution, owing to the small sample size and the imbalance in demographics and disease severity between treatment groups; however, findings are consistent with previous studies reporting the effects of cortisol on bone biomarkers in healthy volunteers. 3
Systemic exposure to AZD9567 and prednisolone was 60% and 30% higher, respectively, in patients with RA in the present study versus healthy volunteers from a previous study. 6 Differences in body composition, organ capacity, 22 and presence of an inflammatory condition 23 among the participants of the two studies are factors potentially affecting metabolism‐dependent drug elimination, and there were also differences in participant body weight and age. Interpretation of this finding is limited by the short pharmacokinetic profile of AZD9567 generated in this study due to participants' limited time in the clinic. Thus, the elimination half‐life of AZD9567 could not be evaluated.
The main limitation of the phase IIa study is the small sample size, such that it was not powered to evaluate non‐inferiority of AZD9567; therefore, larger studies are needed to assess this. Additionally, the small sample size may have contributed to an imbalance in baseline population and disease characteristics between the randomized groups, although the analyses were performed using models adjusted for baseline values. Nevertheless, the participants' demographics were sufficiently representative of the intended study population to confirm that AZD9567 has similar anti‐inflammatory effects to prednisolone in a population with active RA. Although the study design was sufficient to observe an anti‐inflammatory effect, another limitation of this study was the short duration of treatment, which did not permit the assessment of adverse effects that have a low incidence or may take longer to manifest, such as bone remodeling. Despite these limitations, the findings were consistent with a similar efficacy profile and a potentially improved safety profile of AZD9567 versus prednisolone in terms of mineralocorticoid receptor‐mediated effects on serum potassium. In addition, findings from the preclinical study support the findings of this phase IIa study.
In conclusion, these results demonstrate that AZD9567 is mechanistically differentiated from prednisolone, showing consistent anti‐inflammatory response without any impact on electrolyte balance. AZD9567 40 mg had a similar efficacy profile to prednisolone 20 mg in patients with active RA in this phase IIa study. In vitro transcriptomic analysis suggests that the anti‐inflammatory response of AZD9567 is consistent with that of the steroid comparators, prednisolone and dexamethasone. Additionally, AZD9567 showed broad overlap with prednisolone and dexamethasone gene regulation and similar glucocorticoid receptor activation, predicting a comparable anti‐inflammatory (leukocyte activation) response. Unlike prednisolone, AZD9567 had no effect on the morning serum sodium:potassium ratio, which is consistent with the higher selectivity of AZD9567 for the glucocorticoid receptor over the mineralocorticoid receptor. Both drugs were well tolerated, with no new safety findings of concern. These results support further clinical trials of AZD9567 in patients with chronic inflammatory disease.
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript. J.M.vL., A.L., M.S.‐K., S.N., S.P., K.E., L.Ö., and G.B. designed the research. J.M.vL., M.S.‐K., K.E., L.Ö., G.B., I.Di., B.R.A., and P.B. performed the research. J.M.vL., A.L., M.S‐K., J.A., G.B., K.E., L.O., B.A., I.D., P.B., D.E., I.D., C.A., M.B., S.N., A.P., S.P., S.S., P.S., and C.K. analyzed the data.
FUNDING INFORMATION
This study is supported by AstraZeneca, Gothenburg, Sweden.
CONFLICT OF INTEREST STATEMENT
J.M.vL. has received grants from AstraZeneca, MSD, Roche, and Thermo Fisher, and honoraria from AbbVie, Arxx Therapeutics, Boehringer Ingelheim, Galapagos, Gesynta, Leadiant, Magenta, and Sanofi Genzyme. M.S.‐K. received a student grant from AstraZeneca. A.L., J.A., G.B., K.E., L.Ö., B.R.A., I.Di., P.B., D.E., I.Da., C.A., M.G.B., S.N., A.P., S.P., S.S., P.S., and C.K. are employees of, and may own shares in, AstraZeneca.
Supporting information
Data S1
ACKNOWLEDGMENTS
The authors thank the clinical study volunteers and their families, as well as the investigators and site staff. We also thank Joanne Betts, Evelina Björnsson, Graham Clarke, David R. Close, Cyrus Ghobadi, Gudmundur Johannsson, Marjolein De Hair, Mahdi Hashemi, Kicki Johansson, Jacob Leander, Morten A Karsdal, Katarina Korsback, Maarten Kraan, Katerina Pardali, Naimish Patel, Anna Rudin, and Per‐Erik Strömstedt for their contributions to this work. In addition, we thank Richard Claes, PhD, of PharmaGenesis London, London, UK, for providing medical writing support, funded by AstraZeneca, Gothenburg, Sweden, in accordance with Good Publication Practice 3 (GPP3) guidelines (http://www.ismpp.org/gpp3). AZD9567 is an investigational medical product with no approved indication. AstraZeneca develops and markets treatments for inflammatory diseases.
Appendix A. A. APPENDIX: OTHER MEMBERS OF THE SEMRA STUDY GROUP
J. W. G. Jacobs and Ellen Kaan (University Medical Center Utrecht, Utrecht, The Netherlands); Astrid van Tubergen and Kasper Hermans (Maastricht University Medical Center, Maastricht, The Netherlands); Harald Vonkeman and Marjan Ghiti Moghadam (Medisch Spectrum Twente, Enschede, The Netherlands); Inger Gjertsson and Anna‐Karin Ekwall (Sahlgrenska University Hospital, Gothenburg, Sweden); Meliha Kapetanovic and Jon Thorkell Einarsson (Skåne University Hospital Lund, Lund, Sweden); and Lena T. Axelsson, Richard Olsson, Tove Hegelund Myrbäck, Ziad Taib, and Kerstin Vikman (AstraZeneca, Gothenburg, Sweden).
van Laar JM, Lei A, Safy‐Khan M, et al. AZD9567 versus prednisolone in patients with active rheumatoid arthritis: A phase IIa, randomized, double‐blind, efficacy, and safety study. Clin Transl Sci. 2023;16:2494‐2506. doi: 10.1111/cts.13624
Alejhandra Lei and Mary Safy‐Khan contributed equally to this work.
The names of the people in the SEMRA study group are detailed in Appendix A.
Contributor Information
Jacob M. van Laar, Email: j.m.vanlaar@umcutrecht.nl.
the SEMRA study group:
J. W. G. Jacobs, Ellen Kaan, Astrid van Tubergen, Kasper Hermans, Harald Vonkeman, Marjan Ghiti Moghadam, Inger Gjertsson, Anna‐Karin Ekwall, Meliha Kapetanovic, Jon Thorkell Einarsson, Lena T. Axelsson, Richard Olsson, Tove Hegelund Myrbäck, Ziad Taib, and Kerstin Vikman
DATA AVAILABILITY STATEMENT
The data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
REFERENCES
- 1. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease‐modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79:685‐699. [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL Jr, et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016;68:1‐26. [DOI] [PubMed] [Google Scholar]
- 3. McKay LI, Cidlowski JA. Physiologic and pharmacologic effects of corticosteroids. In: Kufe DW, Pollock RE, Weichselbaum RR, eds. Holland‐Frei Cancer Medicine. 6th ed. BC Decker; 2003. [Google Scholar]
- 4. Clark AR, Belvisi MG. Maps and legends: the quest for dissociated ligands of the glucocorticoid receptor. Pharmacol Ther. 2012;134:54‐67. [DOI] [PubMed] [Google Scholar]
- 5. Ripa L, Edman K, Dearman M, et al. Discovery of a novel oral glucocorticoid receptor modulator (AZD9567) with improved side effect profile. J Med Chem. 2018;61:1785‐1799. [DOI] [PubMed] [Google Scholar]
- 6. Myrbäck T, Prothon S, Edman K, et al. Effects of a selective glucocorticoid receptor modulator (AZD9567) versus prednisolone in healthy volunteers: two phase 1, single‐blind, randomised controlled trials. Lancet Rheumatol. 2020;2:e31‐e41. [DOI] [PubMed] [Google Scholar]
- 7. Almquist J, Sadiq MW, Eriksson UG, Hegelund Myrbäck T, Prothon S, Leander J. Estimation of equipotent doses for anti‐inflammatory effects of prednisolone and AZD9567, an oral selective nonsteroidal glucocorticoid receptor modulator. CPT Pharmacometrics Syst Pharmacol. 2020;9:444‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wells G, Becker JC, Teng J, et al. Validation of the 28‐joint disease activity score (DAS28) and European league against rheumatism response criteria based on C‐reactive protein against disease progression in patients with rheumatoid arthritis, and comparison with the DAS28 based on erythrocyte sedimentation rate. Ann Rheum Dis. 2009;68:954‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ward MM, Guthrie LC, Alba MI. Clinically important changes in individual and composite measures of rheumatoid arthritis activity: thresholds applicable in clinical trials. Ann Rheum Dis. 2015;74:1691‐1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felson DT, Smolen JS, Wells G, et al. American College of Rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70:404‐413. [DOI] [PubMed] [Google Scholar]
- 11. Frewer P, Mitchell P, Watkins C, Matcham J. Decision‐making in early clinical drug development. Pharm Stat. 2016;15:255‐263. [DOI] [PubMed] [Google Scholar]
- 12. van Gestel AM, Prevoo ML, van't Hof MA, van Rijswijk MH, van de Putte LB, van Riel PL. Development and validation of the European league against rheumatism response criteria for rheumatoid arthritis. Comparison with the preliminary American College of Rheumatology and the World Health Organization/international league against rheumatism criteria. Arthritis Rheum. 1996;39:34‐40. [DOI] [PubMed] [Google Scholar]
- 13. Karsdal MA, Schett G, Emery P, et al. IL‐6 receptor inhibition positively modulates bone balance in rheumatoid arthritis patients with an inadequate response to anti‐tumor necrosis factor therapy: biochemical marker analysis of bone metabolism in the tocilizumab RADIATE study (NCT00106522). Semin Arthritis Rheum. 2012;42:131‐139. [DOI] [PubMed] [Google Scholar]
- 14. Konu O, Targen S. Investigating the role of mineralocorticoid receptor signaling in cancer biology in the genomic era. In: Harvey B, Jaisser F, eds. Aldosterone‐Mineralocorticoid Receptor—Cell Biology to Translational Medicine. IntechOpen; 2019. [Google Scholar]
- 15. Sharma P, Banerjee R, Narayan KP. Mineralocorticoid receptor mediated liposomal delivery system for targeted induction of apoptosis in cancer cells. Biochim Biophys Acta. 2016;1858:415‐421. [DOI] [PubMed] [Google Scholar]
- 16. Chadwick JA, Hauck JS, Gomez‐Sanchez CE, Gomez‐Sanchez EP, Rafael‐Fortney JA. Gene expression effects of glucocorticoid and mineralocorticoid receptor agonists and antagonists on normal human skeletal muscle. Physiol Genomics. 2017;49:277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hemmerling M, Edman K, Lepistö M, et al. Discovery of indazole ethers as novel, potent, non‐steroidal glucocorticoid receptor modulators. Bioorg Med Chem Lett. 2016;26:5741‐5748. [DOI] [PubMed] [Google Scholar]
- 18. Gaeggeler HP, Gonzalez‐Rodriguez E, Jaeger NF, et al. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone‐stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005;16:878‐891. [DOI] [PubMed] [Google Scholar]
- 19. Yasir M, Goyal A, Sonthalia S. Corticosteroid adverse effects. StatPearls [Internet]. StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 20. Younes AK, Younes NK. Recovery of steroid induced adrenal insufficiency. Transl Pediatr. 2017;6:269‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burt MG, Roberts GW, Aguilar‐Loza NR, Frith P, Stranks SN. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab. 2011;96:1789‐1796. [DOI] [PubMed] [Google Scholar]
- 22. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41:67‐76. [DOI] [PubMed] [Google Scholar]
- 23. Schmitt C, Kuhn B, Zhang X, Kivitz AJ, Grange S. Disease‐drug‐drug interaction involving tocilizumab and simvastatin in patients with rheumatoid arthritis. Clin Pharmacol Ther. 2011;89:735‐740. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Data Availability Statement
The data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.


